Abstract
Objective
To investigate the association between pre-treatment thrombocytosis and prognosis in patients with ovarian cancer (OC).
Methods
PubMed, EMBASE, and the Cochrane Library were searched for articles regarding the prognosis of OC patients with pre-treatment thrombocytosis by the end of March 2018. Pooled estimates for overall survival (OS) and progression-free survival (PFS) events were calculated as hazard ratios (HRs) either on a fixed or random effect model by Stata 13.0 software. Funnel plot and Egger's test were applied to evaluate publication bias and sensitivity analyses were undertaken to estimate the strength of outcomes.
Results
Eleven studies that met the inclusion criteria were enrolled, including a total of 4,953 patients. Pooled results showed that pre-treatment thrombocytosis was significantly associated with OS (HR=1.722; 95% confidence interval [CI]=1.437–2.064) and PFS (HR=1.452; 95% CI=1.323–1.593) in the cohort. Significant correlation was found in OS and PFS between pre-treatment thrombocytosis and both epithelial OC (all stages and differentiation degrees of OC) and advanced epithelial OC (III or IV) by subgroup analyses, which were performed according to publication year, country, case numbers, OC category, International Federation of Gynecology and Obstetrics stage, and cut-off value. However, subgroup analyses indicated no significant correlation between pre-treatment thrombocytosis and OS for patients with high-grade serous (poorly differentiated or undifferentiated) OC (HR=1.220; 95% CI=0.946–1.573; p=0.125). Egger's test demonstrated no obvious publication bias in the articles enrolled in this study (OS: p=0.226; PFS: p=0.071).
Conclusion
Pre-treatment thrombocytosis might be taken as an independent prognostic indicator for patients with OC.
Keywords: Thrombocytosis, Prognosis, Ovarian Cancer, Meta-analysis
INTRODUCTION
Ovarian cancer (OC) is one of the most common female malignancies. In 2016, OC has become the fifth leading cause of female cancer deaths in the United States [1]. Considering that early stage OC patients generally show no visible symptoms and there is no available early screening method for this population, 60% of the patients are identified in advanced stage [1,2,3]. As a result, the five-year survival rate for OC is relatively low. CA125/HE4 can be used as indicators for diagnosis, therapeutic effect monitor, and follow-up of OC patients [4], however, the pathogenesis and cure rate of OC still need to be studied. If there was a marker that could be used as a useful prognostic indicator for OC, or for subgroup classification in the treatment, the therapeutic effect might be improved by the drugs selected according to the markers enrolled.
Pretreatment thrombocytosis is an unfavorable prognostic factor for many solid tumors including colorectal cancer, breast cancer, pancreatic cancer, and lung cancer [5,6,7,8,9]. Platelet could promote metastasis by improving disseminated tumor cell survival in circulation system and extravasation and angiogenesis in target sites [10]. Additionally, platelets promote the survival of cancer cells by preventing immune surveillance, stimulating angiogenesis, and arresting cancer cell cycles [11]. Tumor-derived interleukin-6 could increase hepatic thrombopoietin and stimulate the production of transforming growth factor (TGF)-β1, hence activating TGF-β1/smad proliferation pathway in tumor cells [12]. A recent systematic review and meta-analysis confirmed that pretreatment thrombocytosis is an independent adverse prognostic factor for cervical cancer [13]. However, it is still controversial whether pretreatment thrombocytosis is an independent adverse prognostic factor for OC [14,15,16,17,18]. To this end, meta-analysis was performed by systematically searching for relevant literatures.
MATERIALS AND METHODS
1. Search strategy
A literature search was conducted using PubMed, EMBASE, and Cochrane Library databases from their inception to March 2018 to identify relevant studies. In addition, references of the included studies were also reviewed to include as many studies as possible. The search terms used were: 1) “thrombocytosis,” “thrombocythemia,” or “platelet count”; and 2) “ovarian cancer,” “ovarian neoplasm,” “ovarian carcinoma.” In this study, language for the searching and inclusion studies were not limited. Detailed information of the inclusion and exclusion criteria were described as follows.
2. Selection criteria
Researches that satisfied the following conditions were included in this meta-analysis: 1) Subjects that were about OC patients, with the prognosis reported; 2) Comparing the survival of OC patients between pretreatment thrombocytosis and without pretreatment thrombocytosis; 3) Clearly described survival outcomes such as overall survival (OS) and progression-free survival (PFS); 4) The relevant hazard ratios (HRs) and their 95% confidence intervals (CIs) were available or available after conversion.
3. Exclusion criteria
Exclusion criteria are as follows: 1) studies that did not involve prognosis of OC; 2) unanalyzed platelet count or thrombocytosis; 3) case report, review, meta-analysis, comments, guideline, vitro, or animal trials; and 4) no data available or still unavailable after conversion. Two researchers simultaneously searched the studies and extracted data. When there were inconsistencies in literatures or data, they discussed and reached an agreement.
4. Data extraction and quality assessment
Basic information of the studies included in this meta-analysis was extracted, including: author, publication year, country, sample size, clinical stage, age, cut-off, and follow-up time. For subgroup analysis, OC are OC of all stages and different degrees of differentiation, epithelial OC are epithelial OC with all stages and different degrees of differentiation, advanced epithelial stage OC is epithelial OC of stage III or IV, and high grade serous OC are poorly differentiated or undifferentiated OC. The primary objective of this study was OS; and PFS was a secondary outcome. As for HR extraction, the HR calculated by multivariable analyses was preferentially extracted, followed by univariate analyses, and Kaplan-Meier survival curve conversion which was performed by Engauge Digitizer 4.1. The quality of the included studies was evaluated using a non-randomized studies (NRS) rating scale [19].
5. Statistical analysis
All statistical analyses were carried out by Stata statistical software, version 13.0 (STATA Corporation, College Station, TX, USA) and p<0.05 was considered statistically significant. Cochran's Q test and I2 statistic were used to quantify heterogeneity of HR. When heterogeneity was more than 50%, we chose a random-effects model to pool analysis; otherwise, a fixed effects model was chosen. In addition, a sub-analysis of OS based on different cut-off values, publication year, and pathological types was also performed. The publication bias was assessed using Egger's test and funnel plot. A sensitivity analysis was undertaken to examine the effect of a single study on the results.
RESULTS
1. Literature search, screening and quality assessment
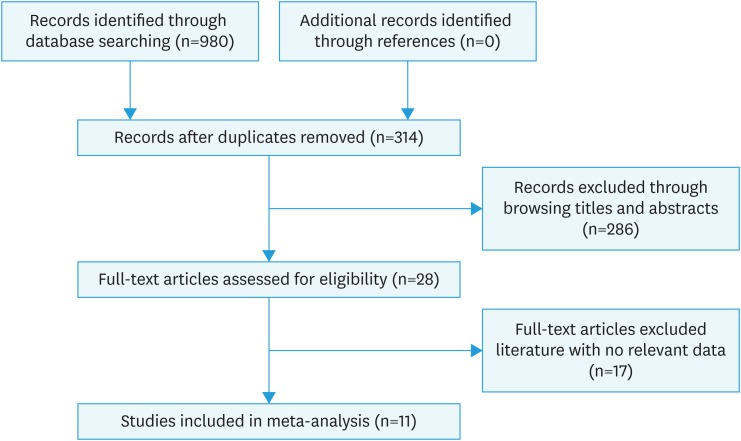
The entire searching and screening process of the literature is shown in Fig. 1. A total of 924 related articles were obtained through searching PubMed, EMBASE, and Cochrane Library. Duplicate articles and studies such as case report, review, guideline, and animal trials that do not meet the inclusion criteria were excluded by browsing abstract. Finally, 11 articles met the inclusion criteria by carefully reading the full text were enrolled in this study. All the 11 studies included in the analysis were nonrandomized and retrospectively designed [15,20,21,22,23,24,25,26,27,28,29]. These studies were published between 2004 and 2016, mainly in China and the United States, involving a total of 4,953 participants. Cozzi et al. [20] used three different cut-off values (>350, >400, >450) for the same study subjects for survival analysis, similarly, for this study we conducted pooled analysis according to three independent studies. Of the 11 studies, 10 literatures reported HR which was adjusted according to other relevant variables. The detailed general information of the other is listed in Table 1. To identify the quality of these studies, a quality assessment on NRS rating scale was conducted. The specific score of each study quality evaluation is shown in Table 2. Only one study was with quality assessment of 6 points, and the rest were higher than 6 points, indicating that the quality of the included literatures was high.
Fig. 1. Flowchart for literature selection process.
Table 1. The basic characteristics of the 11 studies included.
| Study | Year | Country | Case number | FIGO stage | Age | Cut-off | Follow-up time | Category | Adjusted† | Survival analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Cozzi et al. [20] | 2016 | USA | 304 | I–IV | 59.9±14.8 | >350 | Unclear | Ovarian cancer | Yes | OS |
| 59.1±14.8 | >400 | |||||||||
| 59.8±14.8 | >450 | |||||||||
| Feng et al. [21] | 2016 | China | 875 | I–IV | 56 (30–90)* | >450 | 29 (1–115) | High-grade serous ovarian cancer | No | OS, PFS |
| Matsuo et al. [22] | 2015 | USA | 1,308 | I–IV | Unclear | >400 | 31.3 | Epithelial ovarian cancer | Yes | OS, PFS |
| Chen et al. [23] | 2015 | China | 816 | I–IV | 53 (21–79)* | >400 | 65 (0–140) | Epithelial ovarian cancer | Yes | OS, PFS |
| Man et al. [24] | 2015 | China | 190 | I–IV | 66 (27–90)* | >300 | 48 (2–150) | Epithelial ovarian cancer | Yes | OS, PFS |
| Digklia and Voutsadakis [25] | 2014 | Canada | 91 | III–IV | Unclear | >350 | 32.5 (1–140) | Serous ovarian cancer | Yes | OS, PFS |
| Allensworth et al. [26] | 2013 | USA | 578 | I–IV | Unclear | >450 | 35 (9–59) | Epithelial ovarian cancer | Yes | OS, PFS |
| Qiu et al. [27] | 2012 | China | 136 | I–IV | Unclear | >400 | Unclear | Epithelial ovarian cancer | Yes | OS, PFS |
| Lee et al. [28] | 2011 | Korea | 179 | III–IV | 54.3±11.1, 55.3±11.2 | >400 | Unclear | Advanced epithelial ovarian cancer | Yes | OS |
| Gungor et al. [29] | 2017 | Turkey | 293 | I–IV | 57.4±3.8, 58.2±2.9 | >400 | Unclear | Epithelial ovarian cancer | Yes | OS |
| Li et al. [15] | 2004 | USA | 183 | III–IV | 59.8 | >400 | Unclear | Advanced epithelial ovarian cancer | Yes | OS, PFS |
| 59.0 |
FIGO, International Federation of Gynecology and Obstetrics; OS, overall survival; PFS, progression-free survival.
*Median (range); †Adjusted: data from multivariate analysis with Cox proportional hazards regression model.
Table 2. Quality assessment for the including study.
| No. | Study | Year | Grouping method | Blinding | ITT | Baseline data | Diagnostic criteria | Confrontation factor control | Quality level |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cozzi et al. [20] | 2016 | 2 | 0 | 1 | 1 | 2 | 0 | 6 |
| 2 | Feng et al. [21] | 2016 | 2 | 0 | 2 | 0 | 2 | 2 | 8 |
| 3 | Matsuo et al. [22] | 2015 | 2 | 0 | 2 | 1 | 2 | 2 | 9 |
| 4 | Chen et al. [23] | 2015 | 2 | 0 | 2 | 0 | 2 | 2 | 8 |
| 5 | Man et al. [24] | 2015 | 2 | 0 | 2 | 2 | 2 | 2 | 10 |
| 6 | Digklia and Voutsadakis [25] | 2014 | 2 | 0 | 2 | 1 | 2 | 2 | 9 |
| 7 | Allensworth et al. [26] | 2013 | 2 | 0 | 2 | 0 | 2 | 2 | 8 |
| 8 | Qiu et al. [27] | 2012 | 2 | 0 | 1 | 1 | 2 | 2 | 9 |
| 9 | Lee et al. [28] | 2011 | 2 | 0 | 1 | 1 | 2 | 2 | 8 |
| 10 | Gungor et al. [29] | 2017 | 2 | 0 | 1 | 1 | 2 | 2 | 8 |
| 11 | Li et al. [15] | 2004 | 2 | 0 | 1 | 0 | 2 | 2 | 7 |
ITT, intention to treat.
2. OS pooled analysis
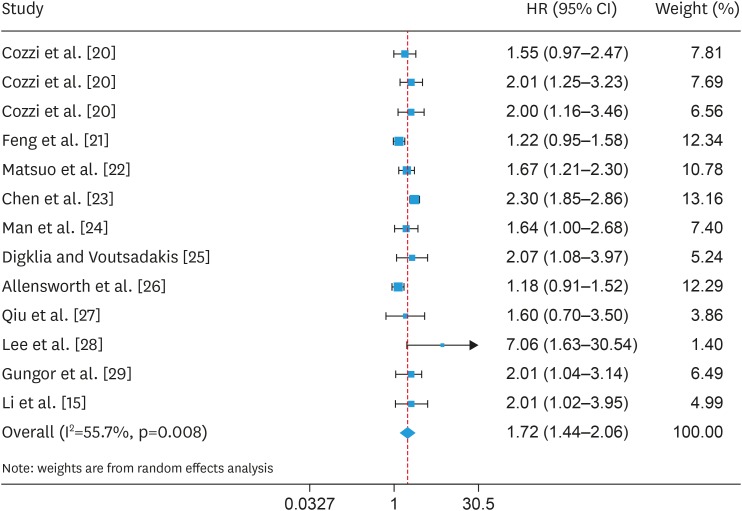
All the 11 articles included in this pooled analysis reported the relationship between OS and thrombocytosis for OC patients. Among these studies, Cozzi et al. [20] reported 3 HRs based on three different platelet count cut-off values (Table 1). We herein performed a meta-analysis on 13 HRs that reflecting associations between OS and pretreatment thrombocytosis. Taking into account the relatively large heterogeneity, we chose a random effect model for aggregation analysis (I2=55.7 percent, p=0.008). The results showed that pre-treatment thrombocytosis had a poor prognostic factor for OS in OC patients (HR=1.722; 95% CI=1.437–2.064; p<0.001; Fig. 2).
Fig. 2. Forest plot of studies evaluating the association between pretreatment thrombocytosis and overall survival.
CI, confidence interval; HR, hazard ratio.
3. PFS pooled analysis
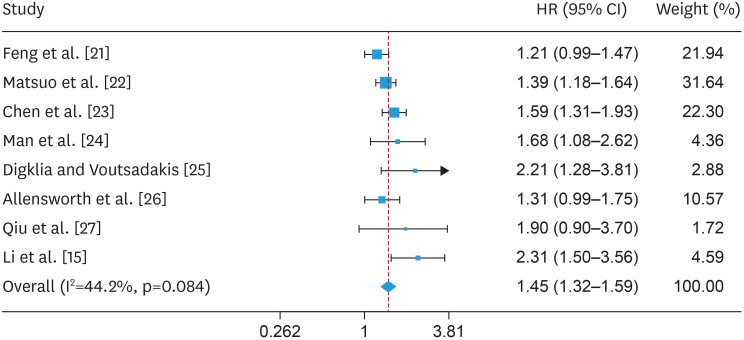
Eight studies enrolled in the current analysis reported the relationship between pre-treatment thrombocytosis and PFS, including a total of 4,177 patients with OC (Table 1) [15,21,22,23,24,25,26,27]. The heterogeneity for PFS was relatively low (I2=44.2 percent, p=0.084), therefore, a fixed effect model was applied to pool analysis. The meta-analysis demonstrated that the associations between pre-treatment thrombocytosis and PFS were statistical significant (HR=1.452; 95% CI=1.323–1.593; p<0.001; Fig. 3). This result further illustrated that pretreatment thrombocytosis was associated with poor PFS in OC.
Fig. 3. Forest plot of studies evaluating the association between pretreatment thrombocytosis and progression-free survival.
CI, confidence interval; HR, hazard ratio.
4. Subgroup analysis
As the studies enrolled in the present study applied different categories, namely OC, epithelial OC, advanced epithelial OC, and high-grade serous OC. In order to investigate whether the classification could influence the conclusion of the relationship between pretreatment thrombocytosis and OS, subgroup analyses were performed according to publication year, country, case numbers, OC category, International Federation of Gynecology and Obstetrics (FIGO) stage, and cut-off value. The results showed that for patients with high-grade serous OC (OS: HR=1.220; 95% CI=0.946–1.573; p=0.125; PFS: HR=1.210; 95% CI=0.993–1.474; p=0.059), there was no significant correlation between pretreatment thrombocytosis and OS or PFS. However, preoperatively thrombocytosis was significantly associated with OS and PFS in patients with OC for different years of onset, country, number of studies, clinical stage, and cut-off. In addition, we performed a different cut-off subgroup analysis for only I–IV patients (Tables 3 and 4). The results showed that when thrombocytosis was defined as >350 and >400, it was significantly associated with OS in patients with OC, while it was highly linked to PFS in patients when the cut-off was set as >300, >400, and >450 (Tables 3 and 4).
Table 3. Subgroup analyses of pooled HRs for overall survival.
| Study | No. | HR (95% CI) | Z | p-value | I-squared (%) | p-value | |
|---|---|---|---|---|---|---|---|
| Publication year | |||||||
| 2004–2014 | 6 | 1.782 (1.243–2.064) | 3.15 | 0.002 | 52.0 | 0.064 | |
| 2015–2016 | 7 | 1.727 (1.394–2.141) | 4.99 | 0.000 | 59.1 | 0.023 | |
| Country | |||||||
| Asia | 5 | 1.815 (1.211–2.720) | 2.89 | 0.004 | 76.9 | 0.000 | |
| Europe | 1 | 2.012 (1.159–3.492) | 2.48 | 0.013 | - | - | |
| America | 7 | 1.605 (1.331–1.937) | 4.94 | 0.000 | 23.7 | 0.248 | |
| Case number | |||||||
| <500 | 9 | 1.822 (1.549–2.286) | 6.36 | 0.000 | 0.0 | 0.805 | |
| >500 | 4 | 1.537 (1.097–2.154) | 2.50 | 0.013 | 85.2 | 0.000 | |
| Category | |||||||
| Ovarian cancer | 3 | 1.823 (1.372–2.423) | 4.14 | 0.000 | 0.0 | 0.692 | |
| Epithelial ovarian cancer | 6 | 1.697 (1.298–2.217) | 3.87 | 0.000 | 67.6 | 0.009 | |
| Advanced epithelial ovarian cancer | 3 | 2.359 (1.403–3.968) | 3.24 | 0.001 | 20.1 | 0.286 | |
| High-grade serous ovarian cancer | 1 | 1.220 (0.946–1.573) | 1.53 | 0.125 | - | - | |
| FIGO stage | |||||||
| I–IV | 9 | 1.733 (1.430–2.100) | 5.61 | 0.000 | 50.9 | 0.038 | |
| III–IV | 4 | 1.886 (1.117–3.186) | 2.37 | 0.018 | 63.8 | 0.040 | |
| Cut-off | |||||||
| >300 | 1 | 1.671 (1.314–2.127) | 1.97 | 0.049 | - | - | |
| >350 | 2 | 1.710 (1.170–2.500) | 2.77 | 0.006 | 0.0 | 0.479 | |
| >400 | 7 | 2.070 (1.775–2.413) | 9.29 | 0.000 | 0.0 | 0.455 | |
| >450 | 3 | 1.292 (1.031–1.618) | 2.23 | 0.026 | 34.6 | 0.217 | |
| Cut-off* | |||||||
| >300 | 1 | 1.640 (1.003–2.682) | 1.97 | 0.049 | - | - | |
| >350 | 1 | 1.550 (0.971–2.473) | 1.84 | 0.066 | - | - | |
| >400 | 5 | 2.043 (1.744–2.395) | 8.83 | 0.000 | 0.0 | 0.558 | |
| >450 | 2 | 1.450 (0.876–2.403) | 1.44 | 0.149 | 65.9 | 0.087 | |
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio.
*Only for I–IV patients.
Table 4. Subgroup analyses of pooled HRs for progression-free survival.
| Study | No. | HR (95% CI) | Z | p-value | I-squared (%) | p-value | |
|---|---|---|---|---|---|---|---|
| Publication year | |||||||
| 2004–2014 | 4 | 1.804 (1.299–2.506) | 3.52 | 0.000 | 50.8 | 0.107 | |
| 2015–2016 | 4 | 1.409 (1.237–1.606) | 5.16 | 0.000 | 31.1 | 0.226 | |
| Country | |||||||
| Asia | 4 | 1.457 (1.202–1.767) | 3.83 | 0.000 | 39.8 | 0.173 | |
| America | 4 | 1.613 (1.253–2.075) | 3.71 | 0.000 | 59.5 | 0.060 | |
| Case number | |||||||
| <500 | 4 | 2.015 (1.567–2.595) | 5.46 | 0.000 | 0.0 | 0.762 | |
| >500 | 4 | 1.377 (1.229–1.544) | 5.50 | 0.000 | 21.5 | 0.281 | |
| Category | |||||||
| Ovarian cancer | 3 | 1.625 (1.057–2.496) | 2.22 | 0.027 | 60.7 | 0.111 | |
| Epithelial ovarian cancer | 4 | 1.529 (1.319–1.774) | 5.61 | 0.000 | 0.0 | 0.611 | |
| Advanced epithelial ovarian cancer | 1 | 2.310 (1.499–3.559) | 3.80 | 0.000 | - | - | |
| High-grade serous ovarian cancer | 1 | 1.210 (0.993–1.474) | 1.89 | 0.059 | - | - | |
| Clinical stage | |||||||
| I–IV | 6 | 1.401 (1.267–1.550) | 6.58 | 0.000 | 5.3 | 0.383 | |
| III–IV | 2 | 2.271 (1.618–3.186) | 4.75 | 0.000 | 0.0 | 0.901 | |
| Cut-off | |||||||
| >300 | 1 | 1.681 (1.079–2.619) | 2.29 | 0.049 | - | - | |
| >350 | 1 | 2.210 (1.281–3.813) | 2.85 | 0.004 | - | - | |
| >400 | 4 | 1.603 (1.329–1.934) | 4.94 | 0.000 | 43.4 | 0.151 | |
| >450 | 2 | 1.242 (1.056–1.461) | 2.61 | 0.009 | 0.0 | 0.653 | |
| Cut-off* | |||||||
| >300 | 1 | 1.681 (1.079–2.619) | 2.29 | 0.022 | - | - | |
| >400 | 4 | 1.481 (1.308–1.676) | 6.20 | 0.000 | 0.0 | 0.462 | |
| >450 | 2 | 1.242 (1.056–1.461) | 2.61 | 0.009 | 0.0 | 0.653 | |
CI, confidence interval; HR, hazard ratio.
*Only for I–IV patients.
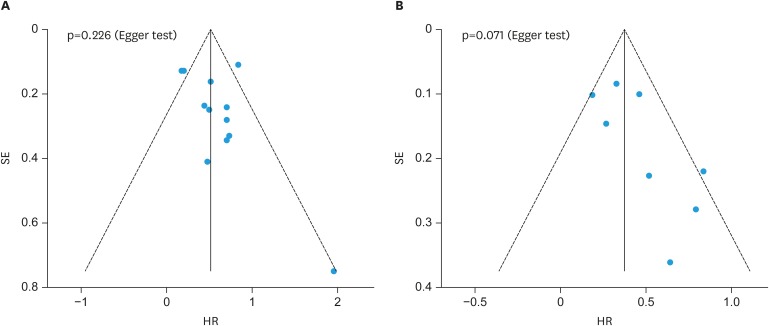
5. Publication bias and sensitivity analysis
Funnel and Egger's test was applied to evaluate the potential publication bias which might affect the accuracy of the results. No obvious evidence of asymmetry was revealed in the funnel plots. Furthermore, Egger's test confirmed the result as that of funnel plots (OS: p=0.226; PFS: p=0.071, Fig. 4). Sensitivity analysis found no significant effect on the results in a single study, suggesting that the results were robust (Supplementary Fig. 1).
Fig. 4. Funnel plots and Egger's test of studies evaluating the potential publication bias. (A) overall survival, (B) progression-free survival.
HR, hazard ratio; SE, standard error.
DISCUSSION
A certain correlation between thrombocytosis and the pathology of cancers is revealed [5,6,7,8,9]. In this study, we used 11 retrospective studies including a total of 4,953 OC patients to assess the association of pretreatment thrombocytosis with OS and PFS. As far as we know, this research is the first systematic review and meta-analysis of this topic. Overall pooled results showed that pre-treatment thrombocytosis is significantly associated with poor OS and PFS in patients with OC. Of the 11 literatures included in this study, six were about epithelial OC and three were regarding advanced OCs which referring to stage III–IV patientes [15,22,23,24,25,26,27,28,29]. Epithelial OC often means high mortality, as the patients are mostly found in late stage. Because of this, looking for an independent predictor of survival outcomes for the patients is particularly important. The results of this subgroup analysis confirmed that pretreatment thrombocytosis was an independent risk factor for prognosis of patients with epithelial OC or advanced OC. It is possible to assume that thrombocytosis could be used as the basis for subgroup classification in the treatment. The therapeutic effect might be improved by corresponding therapy, for example, it is demonstrated that heparin could improve the prognosis of patients [30]. In addition, pretreatment thrombocytosis does not appear to be related to prognosis for high-grade serous OC which is defined by World Health Organization diagnostic criteria. However, it should be pointed out that only one high-grade serous OC was included in the subgroup analysis. And thereby, more studies are needed to verify this relationship between pretreatment thrombocytosis and the prognosis for high-grade serous OC
According to the range of the threshold of thrombocytosis, 20%–50% of patients with OC have thrombocytosis [20]. Platelet plays crucial roles for coagulation system. Generally, thrombocytosis is defined as having a platelet count greater than 300×109/L [15,20,21,22,23,24,25,26,27,28,29]. However, the threshold for the diagnosis of thrombocytosis varies in the range of 300–450, usually is defined as above 400 [31]. In this study, we performed subgroup analysis based on different diagnostic thresholds and the results showed that pretreatment thrombocytosis was associated with poor OS and PFS under different thresholds. In order to eliminate the effect of different stages, we analyzed the studies of stage I–IV patients. It is noteworthy that only one study set the threshold to be greater than 300 and 350 (24, HR=1.671; 95% CI=1.314–2.127; p=0.049; 20, HR=1.550; 95% CI=0.971–2.473; p=0.066). Therefore, whether pretreatment thrombocytosis is related to the prognosis of OC patients with the threshold above 300 and above 350 remains to be further verified.
Several studies have shown that thrombocytosis is associated with advanced FIGO stage and worse prognosis in patients with OC [12,32,33]. These results were further confirmed in this study. In our study, pretreatment thrombocytosis had a worse prognosis in patients with stage III–IV compared with stage I–IV for OC (HR for OS, 2.359 vs. 1.651). Cozzi et al. [20] have shown that as the threshold for the diagnosis of thrombocytosis increases, the proportion of advanced OC increases accordingly. This means that as the threshold increases, HR for OS should also increase. However, it is interesting to note that for I–IV patients with OC, there is no difference in HR at a cut-off value of >450, which requires further verification.
Our study has the following advantages. First, we strictly followed the guidelines of PRISMA for literature search, screening, data extraction, and analysis [34]. Second, publication bias was evaluated with funnel plots and Egger's test. Additionally, sensitivity analysis was used to estimate the effect of single research on our results, which ensured the reliability of this study. Third, subgroup analysis was performed to investigate more detailed results. For example, pretreatment thrombocytosis might not be associated with OS in high-grade serous OC or advanced epithelial OC. However, our research also has some disadvantages. For example, only one study was about high-grade serous OC in the subgroup analysis, indicating that the pooled results still need to be further verified.
In conclusion, this systematic review and meta-analysis showed that pretreatment thrombocytosis is significantly associated with poor OS and PFS in OC. We consider that pretreatment thrombocytosis might serve as a ready-made biomarker to provide routine clinicopathological variables to improve the clinical prognosis of OC patients.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.D.
- Data curation: Y.M., C.J., L.D.
- Formal analysis: C.J., Y.M.
- Methodology: C.J., L.D.
- Writing - original draft: Y.Q.
- Writing - review & editing: C.J.
SUPPLEMENTARY MATERIAL
Graphs showing the sensitivity of a single study to the results. (A) overall survival, (B) progression-free survival.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 4.Goff BA, Agnew K, Neradilek MB, Gray HJ, Liao JB, Urban RR. Combining a symptom index, CA125 and HE4 (triple screen) to detect ovarian cancer in women with a pelvic mass. Gynecol Oncol. 2017;147:291–295. doi: 10.1016/j.ygyno.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106:887–891. doi: 10.1002/jso.23163. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36:192–200. doi: 10.1007/s00268-011-1329-7. [DOI] [PubMed] [Google Scholar]
- 7.Stravodimou A, Voutsadakis IA. Pretreatment thrombocytosis as a prognostic factor in metastatic breast cancer. Int J Breast Cancer. 2013;2013:289563. doi: 10.1155/2013/289563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang SG, Kim KM, Cheong JH, Kim HI, An JY, Hyung WJ, et al. Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol. 2012;38:562–567. doi: 10.1016/j.ejso.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, Hideghéty K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33:1725–1729. [PubMed] [Google Scholar]
- 10.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AN, Afshar-Kharghan V, Sood AK. Platelet effects on ovarian cancer. Semin Oncol. 2014;41:378–384. doi: 10.1053/j.seminoncol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J, Zeng Z, Ye Q, Zhang Y, Yan R, Liang C, et al. The association of pretreatment thrombocytosis with prognosis and clinicopathological significance in cervical cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:24327–24336. doi: 10.18632/oncotarget.15358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friebe Z, Watrowski R, Bembnista M, Rokowska A, Włosiińska J, Dembińska M. Correlation between platelet count and CA-125 in ovarian cancer. Ginekol Pol. 2005;76:187–194. [PubMed] [Google Scholar]
- 15.Li AJ, Madden AC, Cass I, Leuchter RS, Lagasse LD, Karlan BY. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol Oncol. 2004;92:211–214. doi: 10.1016/j.ygyno.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Menczer J, Schejter E, Geva D, Ginath S, Zakut H. Ovarian carcinoma associated thrombocytosis. Correlation with prognostic factors and with survival. Eur J Gynaecol Oncol. 1998;19:82–84. [PubMed] [Google Scholar]
- 17.Soonthornthum T, Suraseraneewong V, Kengsakol K, Wijaithum K, Kasemsan P, Prommatt S. Thrombocytosis in advanced epithelial ovarian cancer. J Med Assoc Thai. 2007;90:1495–1500. [PubMed] [Google Scholar]
- 18.Zeimet AG, Marth C, Müller-Holzner E, Daxenbichler G, Dapunt O. Significance of thrombocytosis in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1994;170:549–554. doi: 10.1016/s0002-9378(94)70225-x. [DOI] [PubMed] [Google Scholar]
- 19.Ts Z. Applied methodology for evidence-based medicine. Changsha Hunan: Central South University; 2014. [Google Scholar]
- 20.Cozzi GD, Samuel JM, Fromal JT, Keene S, Crispens MA, Khabele D, et al. Thresholds and timing of pre-operative thrombocytosis and ovarian cancer survival: analysis of laboratory measures from electronic medical records. BMC Cancer. 2016;16:612. doi: 10.1186/s12885-016-2660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Z, Wen H, Bi R, Duan Y, Yang W, Wu X. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer. 2016;16:43. doi: 10.1186/s12885-016-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, Stone RL, et al. Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma. Eur J Cancer. 2015;51:1978–1988. doi: 10.1016/j.ejca.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Zhang L, Liu WX, Liu XY. Prognostic significance of preoperative anemia, leukocytosis and thrombocytosis in Chinese women with epithelial ovarian cancer. Asian Pac J Cancer Prev. 2015;16:933–939. doi: 10.7314/apjcp.2015.16.3.933. [DOI] [PubMed] [Google Scholar]
- 24.Man YN, Wang YN, Hao J, Liu X, Liu C, Zhu C, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer. 2015;25:24–32. doi: 10.1097/IGC.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 25.Digklia A, Voutsadakis IA. Thrombocytosis as a prognostic marker in stage III and IV serous ovarian cancer. Obstet Gynecol Sci. 2014;57:457–463. doi: 10.5468/ogs.2014.57.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allensworth SK, Langstraat CL, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2013;130:499–504. doi: 10.1016/j.ygyno.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38:651–657. doi: 10.1111/j.1447-0756.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee M, Kim SW, Nam EJ, Yim GW, Kim S, Kim YT. The impact of pretreatment thrombocytosis and persistent thrombocytosis after adjuvant chemotherapy in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2011;122:238–241. doi: 10.1016/j.ygyno.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Gungor T, Kanat-Pektas M, Sucak A, Mollamahmutoglu L. Erratum to: The role of thrombocytosis in prognostic evaluation of epithelial ovarian tumors. Arch Gynecol Obstet. 2017;296:847. doi: 10.1007/s00404-017-4483-9. [DOI] [PubMed] [Google Scholar]
- 30.Borsig L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb Res. 2010;125(Suppl 2):S66–S71. doi: 10.1016/S0049-3848(10)70017-7. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen LM, Milman N. Diagnostic significance of platelet count and other blood analyses in patients with lung cancer. Oncol Rep. 2003;10:213–216. [PubMed] [Google Scholar]
- 32.Allensworth S, Dowdy S, Martin J, Lemens M, Mcgree M, Weaver A, et al. The prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2012;127:17–18. doi: 10.1016/j.ygyno.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Wang Y, Sheng H, Tian W, Qi Z, Teng F, et al. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obstet Gynaecol Res. 2014;40:178–183. doi: 10.1111/jog.12151. [DOI] [PubMed] [Google Scholar]
- 34.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphs showing the sensitivity of a single study to the results. (A) overall survival, (B) progression-free survival.