Abstract
As medicine advances, cancer is still among one of the major health problems, posing significant threats to human health. New anticancer agents features with novel scaffolds and/or unique mechanisms of action are highly desirable for the treatment of cancers, especially those highly aggressive and drug-resistant ones. Nitrogen mustard has been widely used as an anticancer drug since the discovery of its antitumor effect in the 1942. However, the lack of selectivity to cancer cells restricts the wide usage of a mass of nitrogen mustard agents to achieve further clinical significance. Discovery of antitumor hybrids using nitrogen mustards as key functional groups has exhibited enormous potential in the drug development. Introduction of nitrogen mustards resulted in improvement in the activity, selectivity, targetability, safety, pharmacokinetics and pharmacodynamics properties of corresponding lead compounds or agents. Herein, the recently developed nitrogen mustard based hybrids have been introduced in the cancer therapy.
Keywords: antitumor, nitrogen mustard, hybrids, side effects, drug discovery
Introduction
In recent years, malignant tumors have become a serious threat to human health due to their worldwide rising incidence and mortality. Second to cardiovascular diseases, cancer contributed the second most mortalities among all diseases (Torre et al., 2015; Ryerson et al., 2016; Lallukka et al., 2017). In recent decades, development of antitumor drugs has achieved significant progress in the treatment of cancer. Since nitrogen mustard, known as an alkylating agent, was proven effective in the treatment of malignant lymphoma in the 1940s, the usage of nitrogen mustard drugs in cancer chemotherapy has a history of over 70 years. At present, nitrogen mustard agents are still used clinically, and targeted modification of nitrogen mustards is an important strategy for the discovery of anticancer drugs. The development of nitrogen mustard derivatives originated from bis(2-chloroethyl) sulfide, which was used as a poison gas during World War II (Gilman, 1963; DeVita and Chu, 2008). After a terrible accident, it was found that bis(2-chloroethyl) sulfide exhibited therapeutic potential on leukemia. Because of its severe toxicity, bis(2-chloroethyl) sulfide was not applied as a antitumor drug for clinical use. However, nitrogen mustard antitumor drugs were developed based on the leukocyte killing effect of bis(2-chloroethyl) sulfide (Figure 1).
FIGURE 1.

Origin of nitrogen mustards.
Nitrogen mustard is a kind of bio-alkylating agent, which can form active electron-deficient intermediates or other compounds with active electrophilic groups in vivo. The active intermediates can react electrophilically with some electron-rich groups in bio-macromolecules by forming covalent bonds, and results in activity inhibition of corresponding bio-macromolecules. The mechanism of nitrogen mustards includes DNA binding and cross-linking, thus preventing DNA replication and cell proliferation. Since its binding to the N7 nitrogen-atoms on DNA guanines with poor selectivity, nitrogen mustard agents are revealed to be toxic to normal cells (Kohn et al., 1987; Bank et al., 1989; Povirk and Shuker, 1994; Di Antonio et al., 2014).
Clinical application of nitrogen mustard compounds has a long history, but the present and future application of nitrogen mustards is limited by disadvantages including poor selectivity and severe adverse reactions (Frei et al., 1988; Sanderson and Shield, 1996; Schobert et al., 2009; Chen et al., 2014). Therefore, enormous effort has been made in the development of nitrogen mustard derivatives, aiming to obtain antitumor nitrogen mustard drugs with high activity and low toxicity (Zarytova et al., 1990). In recent years, the discovery of nitrogen mustard drugs and derivatives has become attractive field in the anticancer therapy. Development of nitrogen mustard based hybrid molecules by introducing druggable fragment, has been considered to be effective strategy in the antitumor drug discovery. Herein, recently development of nitrogen mustard based hybrids was reviewed and provided suggestions for the future study of bifunctional and multitargeted antitumor drugs.
Nitrogen Mustard Drugs
According to different carriers, nitrogen mustard drugs can be classified into several classes, including fatty nitrogen mustard, aromatic nitrogen mustard, amide nitrogen mustard, amino acid and polypeptide nitrogen mustard, and heterocyclic nitrogen mustard.
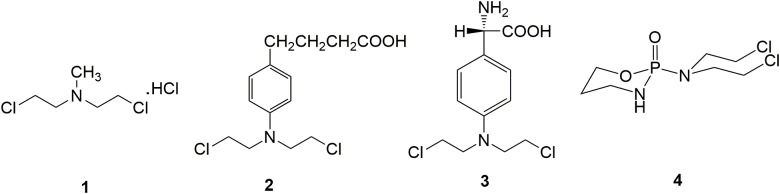
Chlormethine 1 (Figure 2), a fatty nitrogen mustard, is now rarely used for clinic due to its poor selectivity and severe toxicity. The introduction of aromatic rings into nitrogen mustard causes the decrease of electrophilicity of the nitrogen atom. Consequently, aromatic nitrogen mustards are characterized with reduced reactivity and toxicity compared with fatty nitrogen mustard (Goodman and Wintrobe, 1946). Chlorambucil 2 (Figure 2) is used clinically for the treatment of ovarian cancer, Hodgkin’s disease, chronic lymphocytic leukemia and lymphosarcoma (Galton et al., 1961). Clinical application of chlorambucil is also limited by adverse effects including nausea, vomiting, anemia, bone marrow suppression and neurotoxicity (Springer et al., 1990; Nicolle et al., 2004). Melphalan 3 (Figure 2), which takes phenylalanine as the carrier, has exhibited clinical effects on ovarian cancer, breast cancer, lymphoid sarcoma and multiple myeloma (Sarosy et al., 1988). Cyclophosphamide 4 (Figure 2), a heterocyclic amide nitrogen mustard, features a board spectrum of anti-malignancy activity, and is commonly utilized in the management of malignant lymphoma, acute lymphoblastic leukemia, multiple myeloma, lung cancer, neuroblastoma, breast cancer, ovarian cancer and nasopharyngeal cancer (Hughes et al., 2018). Moreover, cyclophosphamide has been discovered to be less toxicity than other types of nitrogen mustard drugs, due to the specific metabolic pathway.
FIGURE 2.
Representative nitrogen mustard agents.
Nitrogen Mustard Based Hybrids
In recent years, it has been revealed that the conjugation of targeted antitumor drugs or natural molecules with nitrogen mustard drugs provides novel strategies for the discovery of antitumor molecules with improved antitumor effect, selectivity, and reduced toxicity.
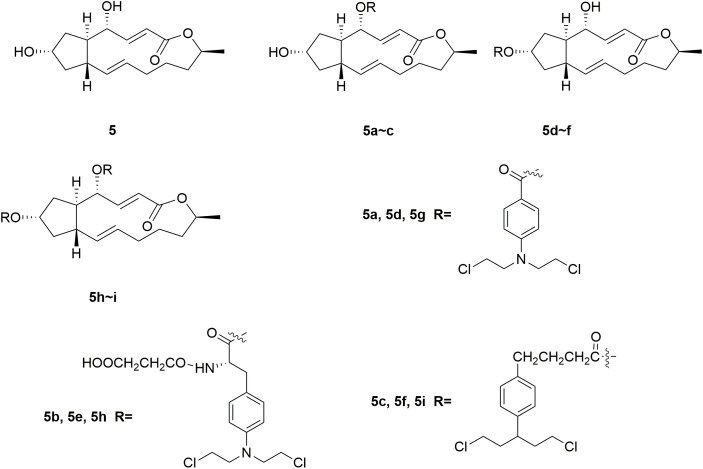
Brefeldin A (BFA) 5 (Figure 3) is a 16-member macrolide antibiotic with a broad range of pharmacological activities, including antitumor, antiviral and antifungal effects (Rajamahanty et al., 2010; Moon et al., 2012; South et al., 2013; Toda et al., 2015; Grose and Klionsky, 2016; Huang et al., 2017). In the antiproliferative activity assay, BFA exhibited GI50 (half maximal growth inhibitory concentration) value of 40 nM against the national cancer institute NCI-60 cancer cell line (Anadu et al., 2006). Although BFA has great potentials to serve as a cancer chemotherapeutic drug, its development is still restricted by major limitations including severe undesirable effects and relatively low selectivity on tumor cells over normal ones (Kikuchi et al., 2003; Seehafer et al., 2013). Several novel BFA-nitrogen mustard conjugates were derived by introducing nitrogen mustards at 4-OH and/or 7-OH of BFA (Han et al., 2018). All the synthesized BFA-nitrogen mustard compounds 5a-i (Figure 3) were assessed for their effectiveness against different tumor cell lines. Several hybrid molecules exhibited potent cytostatic activities and improved selectivity on malignant cells over normal ones. It is revealed that almost all the new BFA-nitrogen mustards showed stronger cytotoxic activities against one or more cell lines than nitrogen mustards and even 5-FU. Among all the tested compounds, molecule 5a exhibited the most potent antiproliferative effects against various tumor cell lines (with IC50 (half maximal inhibitory concentration) values of 4.48, 9.37, 0.2, and 0.84 μM against human leukemia HL-60, human prostate PC-3, human hepatocellular carcinoma Bel-7402 and drug-resistant Bel-7402/5-FU cell lines), respectively. Molecule 5a also displayed much lower cytotoxicity (IC50 < 0.001 μM) than BFA (IC50= 9.74 μM) against normal human hepatic L-O2 cells. Therefore, introduction of nitrogen mustard to toxic natural products could be significant in the improvement the potency and safety of lead compounds.
FIGURE 3.
Structures of Brefeldin A-nitrogen mustard hybrids.
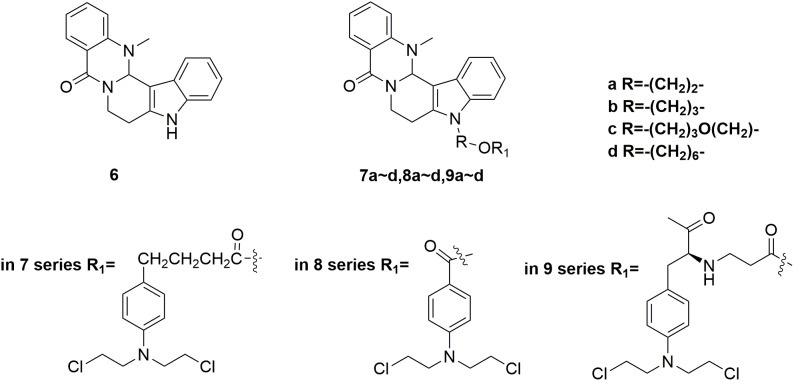
Evodiamine 6 (Figure 4) is a natural quinolone alkaloid widely studied for the treatment of diverse human disorders including Alzheimer’s disease, inflammation and especially cancer (Ogasawara et al., 2002; Yu et al., 2013; Lv et al., 2015; Shi L. et al., 2016; Wang et al., 2016; Wu et al., 2016; Fan et al., 2017; Shi et al., 2017). By targeting topoisomerase I and II, evodiamine has induced apoptosis and cell cycle arrest of a broad spectrum of tumor cell lines (Shyu et al., 2006). However, it is revealed that evodiamine is cytotoxic to human normal cells, such as peripheral blood mononuclear cells (PBMC). In discovery of antitumor agent with improved potency and reduced adverse side effects, conjunct of evodiamine to nitrogen mustards was carried out by Li and coworkers (Hu et al., 2017). The synthesized nitrogen mustard-evodiamine hybrids were evaluated in the antitumor activity assay. Compared with evodiamine (IC50 values of 22.87 μM against PBMC cells), all the tested mustard-evodiamine hybrids 7a-d, 8a-d, 9a-d (Figure 4) showed improved safety properties with IC50 values of more than 200 μM in inhibition the proliferation of PBMC cells. Remarkably, molecule 9c revealed potent antiproliferative effects against human liver cancer HepG2, human leukemic THP-1 and HL-60 cell lines with IC50 value of 17.04 μM, 4.05 μM and 0.50 μM, respectively. The involved investigations indicated that further drug discovery based on 9c is promising in the treatment of tumor, such as leukemia. Collectively, introduction of nitrogen mustard moiety has shown significance in the improvement of potency and safety, and the nitrogen mustard hybridation strategy could be productive for the optimization of lead compounds.
FIGURE 4.
Structures of evodiamine-nitrogen mustard hybrids.
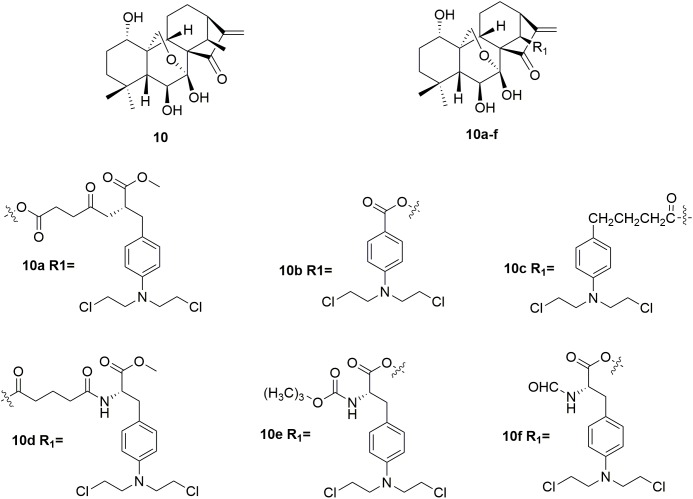
Oridonin 10 (Figure 5) is a kind of natural diterpenoids, which has a unique, safe, broad antitumor activity (Sun et al., 2006; Cui et al., 2007; Zhou et al., 2007; Bao et al., 2014; Li Y. et al., 2015; Ding et al., 2016; Dong et al., 2016; Shi M. et al., 2016; Liu et al., 2017). However, the utilization of oridonin in cancer chemotherapy was limited by its relatively low potency (Wang et al., 2012; Xu S.T. et al., 2014). Development of oridonin-nitrogen mustard conjugates used for antitumor application has been demonstrated to be promising in the drug discovery (Ding et al., 2013a,b). Several synthetic oridonin-nitrogen mustard conjugates 10a∼f (Figure 5), and their anticancer activities evaluated in four human malignant cell lines (human leukemia K562 cells, human breast cancer MCF-7 cells, human hepatocellular carcinoma Bel-7402 cells, and human gastric cancer MCG-803 cells) were reported by Xu and coworkers (Xu S. et al., 2014). All the tested compounds exhibited better antiproliferative effects comparing to the positive control drugs, melphalan, chlorambucil and benzoic acid mustard. Among the synthetic oridonin mustards, compound 10b was the most potent hybrid against MCF-7 and Bel-7402 cells with IC50 values of 0.68 μM and 0.50 μM, respectively. It is also revealed that 10b and 10c could inhibit the growth of drug-resistant cancer cells. Notably, molecule 10b exhibits approximately eight-fold higher selectivity for cancer cells over normal cells, which is significantly higher than its parent oridonin compound and clinically available nitrogen mustard drugs. Collectively, the derived oridonin-nitrogen mustard conjugates exhibited improved activity and safety than the parent fragments, and introduction of nitrogen mustard make contributions to the potency and selectivity of oridonin based hybrids.
FIGURE 5.
Structures of oridonin-nitrogen mustard hybrids.
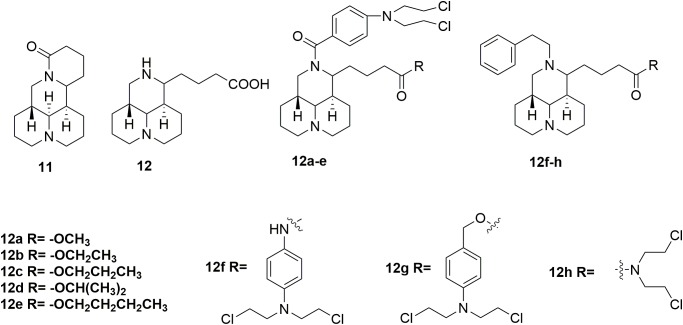
In addition to evodiamine, another alkaloid, sophoridine 11 (Figure 6) evaluated in detailed for its antitumor potency, was approved by the CFDA in 2005 for treatment several types of cancer, including liver, gastric and lung cancer (Sun et al., 2012; Liu and Liu, 2013; Wang et al., 2014). The sophoridine which could cause apoptotic cell death by inhibiting DNA topoisomerase I activity and initiate cell cycle arrest at the G0/G1 phase, has high solubility and good safety profiles (Quo et al., 2013). However, the moderate anticancer activity of sophoridine limit its clinical application. Therefore, development of sophoridine derivatives was performed in discovery of more effective drug candidates. The D-ring of sophridine has been opened to generate sophoridinic acid 12 (Figure 6) for further structural modification. A series of sophoridinic acid-nitrogen mustard deivatives 12a-h (Figure 6) were derived by modifying 12 nitrogen atom and carboxyl groups of 12 (Li D.D. et al., 2015). Compared with sophoridine (IC50 > 80 μM against human liver cancer HepG2 cells), several new synthesized hybrids showed improved antitumor activity. Especially compound 12f showed IC50 value of 0.47 μM compared with melphalan (IC50 value of 0.41 μM) in the inhibition of HepG2 cells. SAR analysis indicated two promising substituents on the 12-nitrogen atom and carboxyl region, which were helpful for maintaining potent antitumor activity. Moreover, various decorating various substituents may be introduced to these two moieties, regulating the pharmacological effects of the compounds. Introduction of the cyclophosphamide metabolite (phosphamide mustard A) analogs also resulted in hybrids with significantly improved activities compared with sophoridine (Li D. et al., 2018). It is demonstrated that the introduction of nitrogen mustard on sophoridine could significantly improve interactions between sophoridine and DNA-Topo I, and subsequently increase the antitumor activity. Therefore, the study of nitrogen mustard as the parent drug is of great significance in the design and synthesis of antitumor drugs.
FIGURE 6.
Structures of sophoridine-nitrogen mustard hybrids.
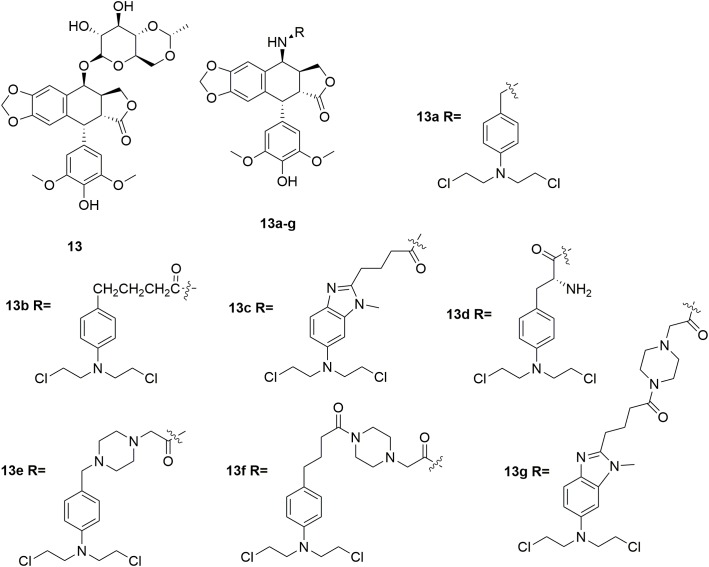
Etoposide 13 (Figure 7) is a topoisomerase II inhibitor effective in the treatments of various types of cancer including testicular cancer, lung cancer, lymphoma, leukemia, neuroblastoma, and ovarian cancer (Nitiss, 2009; Pommier and Marchand, 2011; Pommier, 2013). In discovery of etoposide analogs, glycoside moiety of etoposide was replaced by nitrogen mustard moiety designed to alkylate either protein residues on topoisomerase II, or the DNA bases on the DNA-topoisomeraseII complex (Deweese and Osheroff, 2009; Pommier et al., 2010; Wu et al., 2011). Seven N-mustard–epipodophyllotoxin hybrid compounds 13a-g (Figure 7) were synthesized, and demonstrated to target topoisomerase II by kDNA decatenation assay, DNA cleavage assay, cellular ICE assay and the cell cycle analyses (Yadav et al., 2014). The derived molecules also exhibited nitrogen mustard-alike activity as it crosslinked DNA. In the in vitro antiproliferative assay, molecule 13e exhibited the best antiproliferative activity with IC50 values of 0.27 μM and 0.85 μM against human leukemia K562 cells and etoposide-resistant K/VP.5 cells, and GI50 of 0.71 μM against NCI-60 cells in contrast to the control melphalan (IC50 values of 12 μM and 5.3 μM against K562 cells and K/VP.5 cells, and GI50 of 29 μM against NCI-60 cells) and etoposide (IC50 values of 0.29 μM and 4.9 μM against K562 cells and K/VP.5 cells, and GI50 of 12 μM against NCI-60 cells). The results suggested that hybridization of etoposide and nitrogen mustards is promising in the development of highly potent antitumor molecules both by topoisomerase II inhibition as well as DNA alkylation.
FIGURE 7.
Structures of etoposide-nitrogen mustard hybrids.
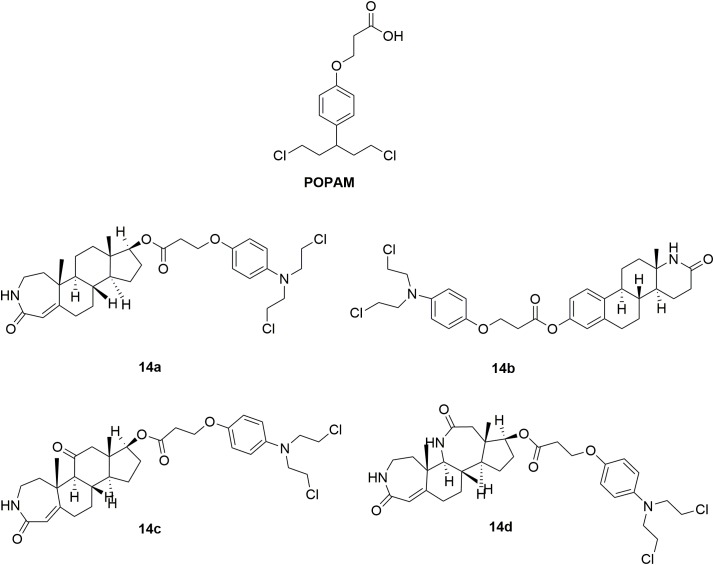
In order to decrease toxicity of nitrogen mustards, steroids have been tested as a vehicle to deliver the mustard drugs to a specific target tissue via interaction with steroid receptors (Wall et al., 1969; Catane, 1978). Such conjugates improved the lipophilicity and solubility of the resulting drugs. Development of steroidal alkylating agents has been reviewed by Bérubé and coworkers (Trafalis et al., 2016). Herein, the recently derived novel steroidal lactam derivatives and 3-(4-(bis(2-chloroethyl)amino)phenoxy)propanoic acid (POPAM) (Figure 8) conjunctions were described (Trafalis et al., 2016). Four new ester conjugates 14a-d (Figure 8) of steroidal lactams with POPAM were synthesized and tested against human leukemia cell lines in vitro. Molecule 14c was discovered to be the most potent hybrid with IC50 values of 90 μM, 65 μM, 80 μM, and 85 μM against human leukemia MOLT-4, CCRF-CEM, JURKAT and SUP-B15 cells compared with melphalan (IC50 > 100 μM against all the test cell lines) and POPAM (IC50 > 100 μM against all the test cell lines), respectively. In the in vivo antiproliferative assay, 14c also exhibited improved antileukemic activity compared with their alkylating component alone (POPAM). Moreover, in the in vivo acute toxicity test, all the derived hybrids had significantly lower acute toxicity (LD10 (10% lethal dose) > 80 mg/kg), in contrast to the non-steroidal alkylators POPAM (LD10 = 14 mg/kg) and melphalan (LD10 = 15 mg/kg). Further investigation revealed that the chemical linkage between the nitrogen mustard and the lactam-steroids seems to both decreased the toxicities of the nitrogen mustards and improved the bioactivity and antitumor effects.
FIGURE 8.
Structures of steroid-nitrogen mustard hybrids.
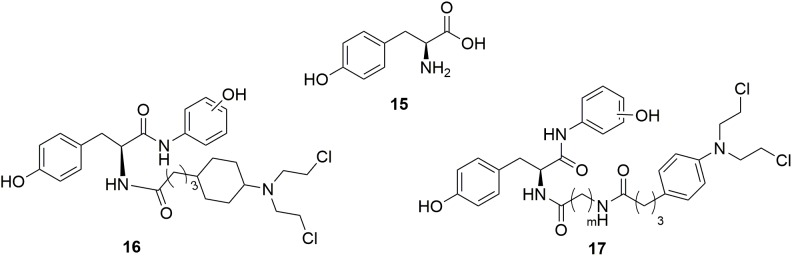
Tyrosine 15 (Figure 9), a natural amino acid, has been reported to share some structural similarities to that of the phenol group of estradiol (Anstead et al., 1997). Molecular modeling study indicated that the phenol group of tyrosine also interact with the estrogen receptor binding site in the same manner as the A-ring phenol of estradiol. Therefore, the tyrosine was modified to mimic the structure of estradiol (Muthyala et al., 2003; Descoteaux et al., 2012b). Tyrosinamide, combined tyrosine with hydroxyaniline, was proved to be structurally similar to estradiol. A series of tyrosinamide-nitrogen mustard derivatives were synthesized and tested by Bérubé and coworkers (Descoteaux et al., 2012a). It is revealed that all new compounds showed potent antitumor activities. Among the derived tyrosinamide-chlorambucil hybrids, compound m-16 (Figure 9), showed IC50 values of 48.61 and 31.25 μM against human breast cancer MDA-MB-231 cells and MCF-7 cells compared with the parent compounds chloramucil (IC50 values of 136.85 and 130.36 μM against MDA-MB-231 cell and MCF-7 cells), respectively. Compared with chloramucil (IC50 values of 63.17, 66.11, 100.48 and 131.83 μM against human ovarian carcinoma A2780 cells, OVCAR-3 cells, human breast cancer ZR-75-1 cells and MDA-MB-468 cells, respectively), the m-17 (Figure 9) showed potent antitumor activity with IC50 values of 31.79, 35.42, and 52.10 μM against OVCAR-3 cells, MDA-MB-468 cells and ZR-75-1 cells, respectively. It is found that all the synthesized tyrosinamide-chlrambucil molecules exhibited improved inhibitory activity in the inhibition of breast, ovarian and uterine cancer cells than the parental chlorambucil. Introduction of tyrosine entity to nitrogen mustards was considered to make contributes to the increased antitumor activity of the derived hybrid molecules.
FIGURE 9.
Structures of Tyrosine-nitrogen mustard hybrids.
Platinum-based antineoplastic drugs are usually considered as another class of alkylating agents with high antitumor potency (Jamieson and Lippard, 1999; Wang and Lippard, 2005). Notably, cisplatin is one of the most potent platinum(II) complexes used in cancer chemotherapy by binding to DNA and subsequently interfere with replication and transcription, eventually leading to cellular apoptosis (Abu-Surrah and Kettunen, 2006; Wheate et al., 2010). However, clinical application of cisplatin is limited by its severe adverse effects, including nephrotoxicity, hepatotoxicity, ototoxicity and neurotoxicity, etc. Acquired resistance is also a concern (Brabec et al., 2017). In discovery of more potent and safe antitumor compounds, conjunction of two different types of DNA-damaging drugs by combining chlorambucil with platinum(IV) complexes was performed by Gou and coworkers (Qin et al., 2017). In the in vitro activity assay, molecule 18 (Figure 10), a hybrid of cisplatin and chlorambucil, displayed potent antiproliferative activities with IC50 values of 3.99 μM, 4.37 μM, 4.97 μM, 2.97 μM, and 4.23 μM against human breast cancer MCF-7, human colon cancer HCT-116, human liver cancer HepG-2, human gastric cancer SGC7901, and cisplatin-resistant SGC7901/CDDP cells, respectively. Compared with chlorambucil, cisplatin, and oxaliplatin, molecule 18 exhibited improved activity in the inhibition of cisplatin resistant SGC7901/CDDP cells. Further studies revealed that molecule 18 induced cell cycle arrest at S/G2 phases (distinct from those of cisplatin and chlorambucil), and revealed ability of overcome drug resistance. Collectively, hybridization of nitrogen mustards and platinum(IV) complexes resulted in conjunctions with improved antitumor potency, and with advantage of overcoming drug resistance of tumor cells.
FIGURE 10.
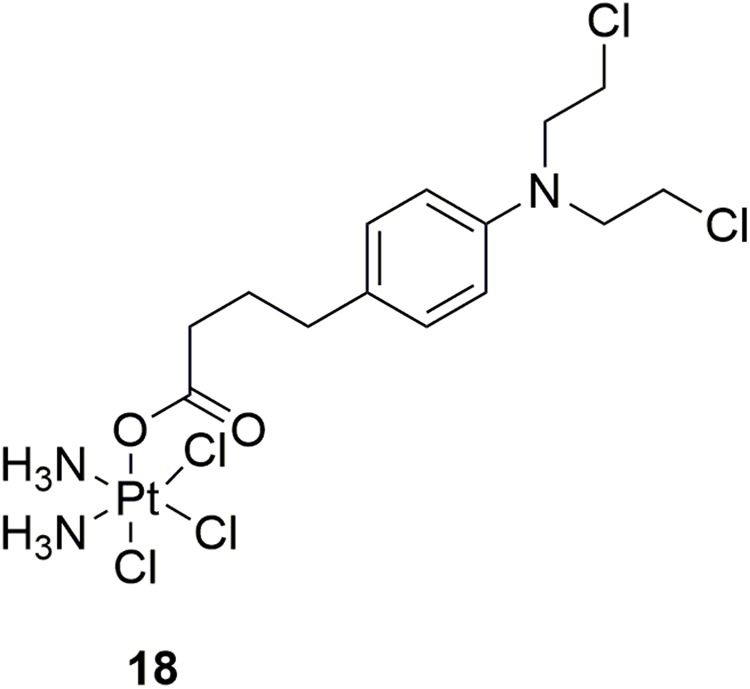
Structure of Platinum-nitrogen mustard hybrid.
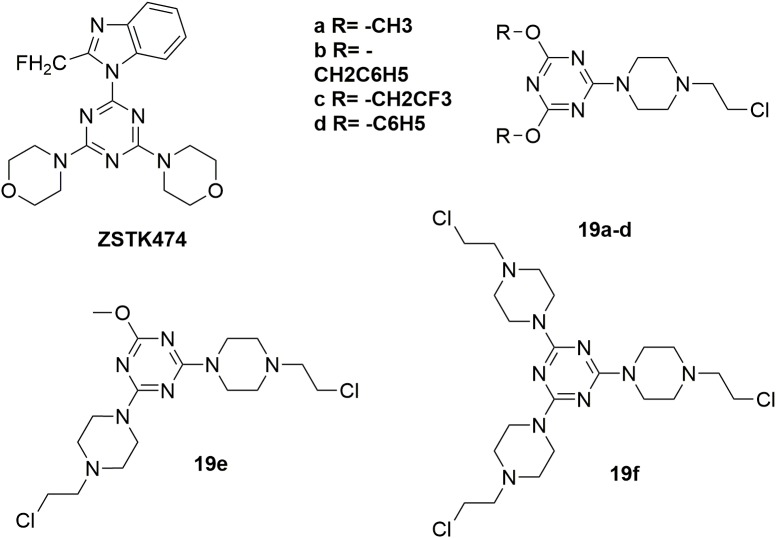
Several highly potent 1,3,5-triazine scaffold-carrying cytostatic agents have been previously reported as inhibitors of cell proliferation-involved enzymes (Maeda et al., 2000; Riou et al., 2002; Gomez et al., 2003; Kaminski et al., 2004; Mandal et al., 2007; Paquin et al., 2008). Among them, ZSTK474 (Figure 11) was discovered to inhibit the growth of tumor cells in human cancer xenografts without toxic effects on critical organs by targeting PI3K (Di Francesco et al., 2000; Vedejs et al., 2003). In the structural modification of current melamine derivatives, a series of melamine-nitrogen mustard derivatives 19a-f (Figure 11) were synthesized by introducing one or more 2-chloroethylamine groups (Kolesinska et al., 2012). It is revealed that all synthesized molecules showed potent antitumor activities. Compared with the positive control chlorambucil (IC50 value of 29.14 μM against human breast cancer MCF-7 cells), the obtained molecule 19f showed potent antitumor activity with IC50 value of 18.70 μM against MCF-7 cells. Compound 19a also exhibited potent antitumor activities with IC50 value of 0.62 μM, 0.99 μM, 1.40 μM, 2.06 μM and 3.45 μM against human leukemia Jurkat, human prostate adenocarcinoma LNCaP, human breast adenocarcinoma T47D, human lung adenocarcinoma A549 and human colorectal carcinoma SW707 cells, respectively. Further biological studies suggested that introducing nitrogen mustard into triazine is promising in the increase of antitumor activity by promoting alkylation. Accordingly, introduction of nitrogen mustard is regarded to make contribution to improve the selectivity, activity and lipophilicity of current drug-like cytotoxic derivatives.
FIGURE 11.
Structures of melamine-nitrogen mustard hybrids.
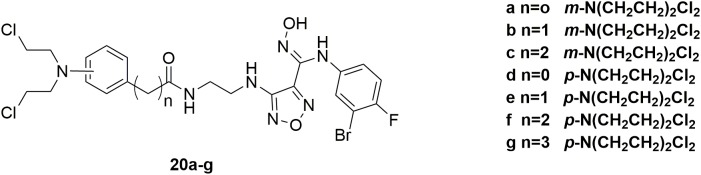
IDO1, a heme-containing enzyme, plays an important role in carcinogenesis and its progression by converting Trp to Kyn (Jiang et al., 2015). Both Trp degradation and Kyn accumulation are associated with immune tolerance by affecting T cell activity and altering the tumor microenvironment (Moon et al., 2015). A number of studies showed that the combination of IDO1 inhibitors along with cytotoxic chemotherapeutic agents is an effective strategy in cancer treatment (Muller et al., 2005). However, such a simple combination will inevitably be limited by the severe adverse effects induced by the highly toxic cytotoxic agents and possible drug-drug interactions. Therefore, in discovery of potent antitumor molecules with reduced toxicity, a series of hybrid molecules 20a-g (Figure 12) were synthesized by including the pharmacophores of both IDO1 inhibitors and nitrogen mustards (Fang et al., 2018). All the compounds showed potent antitumor activities compared with the positive drug chlorambucil in the inhibition of murine colorectal carcinoma CT-26, human lung adenocarcinoma A549, human colon cancer HCT116 and human colorectal adenocarcinoma HT-29 cells. Obviously, compound 20a significantly inhibited IDO1 activity in tumor tissues and reduced Kyn level in plasma with IDO1 inhibitory IC50 value of 0.13 μM and antiproliferative EC50 (half maximal effect concentration) value of 0.27 μM aganist HeLa cells. Moreover, molecule 20a exhibited high potent in vivo antitumor efficacy (tumor growth inhibition (TGI) = 58.2%) compared with clinical candidate IDO1 inhibitor epacadostat (TGI = 47.5%) in the allograft animal model with CT-26 without remarkable body weight loss or adverse effects. In conclusion, it is revealed that introduction of nitrogen mustard into pharmacophores of IDO1 inhibitors could significantly improve the antitumor activity and reduce toxicity of parent compound in the antitumor evaluation.
FIGURE 12.
Structures of IDO1-nitrogen mustard hybrids.
Conclusion and Perspective
Nitrogen mustards represent the earliest studied DNA cross-linking agents, and application of DNA alkylating agents has been widely utilized for the treatment of cancer for more than 70 years. In spite of their long history, several nitrogen mustard drugs, including cyclophosphamide, chlorambucil, and melphalan, still remained as first line antitumor agents in the management of various types of tumors. However, the clinical application of nitrogen mustards was restricted by their undesired adverse effects, relatively low efficacy compared with targeted antitumor drugs, and drug resistance caused by enhanced drug inactivation, decreased cellular uptake, enhanced DNA repair and/or DNA damage tolerance. It is generally accepted that cancer has its pathological root in genetical mutations, affecting cell replication. Thus, targeting different proliferative mechanisms by the construction of hybrid anticancer drugs seem to be a promising strategy. Development of nitrogen mustard based hybrids has been revealed to be effective strategy in discovery of antitumor drugs with increased activity, reduced toxicity, and improved physicochemical properties such as the lipophilicity and the solubility.
With N,N-bis(2-chloroethyl)amine as functional group, nitrogen mustards has been hybridized with various drug-like fragments (Figure 13). Novel hybrids have been derived with improved potency, selectivity, safety, pharmacokinetics, pharmacodynamics properties and/or broader range of therapeutic activities. Current achievements make development of nitrogen mustard based conjunctions to be attractive area in the cancer treatment. Despite the reported advantages, unexpected side effects caused by introduction of nitrogen mustards also require careful attention in the drug design and biological evaluation. As questioned on the efficacy of twin drugs and prodrugs, it is also necessary to demonstrate advantages of the conjugates linked by ester bond or more stable bonds in comparison with the combined therapy with parental drugs.
FIGURE 13.
Summary of the nitrogen mustard based hybridization strategy involved in the current article.
Author Contributions
YC and YJ participated in most of the literature retrieval and article writing. LZ and WS guided the review and revised the writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Some of the materials in this work were supported by National Natural Science Foundation of China (Youth Found, Grant No. 81803343).
References
- Abu-Surrah A. S., Kettunen M. (2006). Platinum group antitumor chemistry: design and development of new anticancer drugs complementary to cisplatin. Curr. Med. Chem. 13 1337–1357. 10.2174/092986706776872970 [DOI] [PubMed] [Google Scholar]
- Anadu N. O., Davisson V. J., Cushman M. (2006). Synthesis and anticancer activity of brefeldin A ester derivatives. J. Med. Chem. 49 3897–3905. 10.1021/jm0602817 [DOI] [PubMed] [Google Scholar]
- Anstead G. M., Carlson K. E., Katzenellenbogen J. A. (1997). The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 62 268–303. 10.1016/S0039-128X(96)00242-5 [DOI] [PubMed] [Google Scholar]
- Bank B. B., Kanganis D., Liebes L. F., Silber R. (1989). Chlorambucil pharmacokinetics and DNA binding in chronic lymphocytic leukemia lymphocytes. Cancer Res. 49 554–559. [PubMed] [Google Scholar]
- Bao R. F., Shu Y. J., Wu X. S., Weng H., Ding Q., Cao Y., et al. (2014). Oridonin induces apoptosis and cell cycle arrest of gallbladder cancer cells via the mitochondrial pathway. BMC Cancer 14:217. 10.1186/1471-2407-14-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec V., Hrabina O., Kasparkova J. (2017). Cytotoxic platinum coordination compounds. DNA binding agents. Coordin. Chem. Rev. 351 2–31. 10.1016/j.ccr.2017.04.013 [DOI] [Google Scholar]
- Catane R. (1978). Clinical experience with estramustine phosphate and predinimustine, two steroidal alkylating agents compounds [proceedings]. Cancer Treat. Rep. 62 1264–1265. [PubMed] [Google Scholar]
- Chen W. B., Balakrishnan K., Kuang Y. Y., Han Y. Y., Fu M., Gandhi V., et al. (2014). Reactive Oxygen Species (ROS) inducible DNA cross-linking agents and their effect on cancer cells and normal lymphocytes. J. Med. Chem. 57 4498–4510. 10.1021/jm401349g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q., Tashiro S., Onodera S., Minami M., Ikejima T. (2007). Oridonin induced autophagy in human cervical carcinoma HeLa cells through Ras. JNK, and P38 regulation. J. Pharmacol. Sci. 105 317–325. 10.1254/jphs.FP0070336 [DOI] [PubMed] [Google Scholar]
- Descoteaux C., Brasseur K., Leblanc V., Parent S., Asselin E., Berube G. (2012a). Design of novel tyrosine-nitrogen mustard hybrid molecules active against uterine, ovarian and breast cancer cell lines. Steroids 77 403–412. 10.1016/j.steroids.2011.12.021 [DOI] [PubMed] [Google Scholar]
- Descoteaux C., Brasseur K., Leblanc V., Parent S., Asselin E., Berube G. (2012b). SAR study of tyrosine-chlorambucil hybrid regioisomers; synthesis and biological evaluation against breast cancer cell lines. Amino Acids 43 923–935. 10.1007/s00726-011-1152-3 [DOI] [PubMed] [Google Scholar]
- DeVita V. T., Jr., Chu E. (2008). A history of cancer chemotherapy. Cancer Res. 68 8643–8653. 10.1158/0008-5472.CAN-07-6611 [DOI] [PubMed] [Google Scholar]
- Deweese J. E., Osheroff N. (2009). The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 37 738–748. 10.1093/nar/gkn937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Antonio M., McLuckie K. I. E., Balasubramanian S. (2014). Reprogramming the mechanism of action of chlorambucil by coupling to a G-Quadruplex ligand. J. Am. Chem. Soc. 136 5860–5863. 10.1021/ja5014344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco A. M., Hargreaves R. H., Wallace T. W., Mayalarp S. P., Hazrati A., Hartley J. A., et al. (2000). The abnormal cytotoxicities of 2,5-diaziridinyl-1,4-benzoquinone-3-phenyl esters. Anti Cancer Drug Des. 15 347–359. [PubMed] [Google Scholar]
- Ding C. Y., Zhang Y. S., Chen H. J., Yang Z. D., Wild C., Chu L. L., et al. (2013a). Novel nitrogen-enriched oridonin analogues with thiazole-fused a-ring: protecting group-free synthesis, enhanced anticancer profile, and improved aqueous solubility. J. Med. Chem. 56 5048–5058. 10.1021/jm400367n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C. Y., Zhang Y. S., Chen H. J., Yang Z. D., Wild C., Ye N., et al. (2013b). Oridonin ring a-based diverse constructions of enone functionality: identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J. Med. Chem. 56 8814–8825. 10.1021/jm401248x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Ding C. Y., Ye N., Liu Z. Q., Wold E. A., Chen H. Y., et al. (2016). Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 122 102–117. 10.1016/j.ejmech.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. J., Liu F. Y., Li M. L. (2016). Inhibition of nuclear factor kappa B transcription activity drives a synergistic effect of cisplatin and oridonin on HepG2 human hepatocellular carcinoma cells. Anticancer Drugs 27 286–299. 10.1097/CAD.0000000000000329 [DOI] [PubMed] [Google Scholar]
- Fan X., Zhu J. Y., Sun Y., Luo L., Yan J., Yang X., et al. (2017). Evodiamine inhibits Zymosan-induced inflammation in vitro and in vivo: inactivation of NF-kappa B by inhibiting I kappa B alpha phosphorylation. Inflammation 40 1012–1027. 10.1007/s10753-017-0546-0 [DOI] [PubMed] [Google Scholar]
- Fang K., Dong G. Q., Wang H. Y., He S. P., Wu S. C., Wang W., et al. (2018). Improving the potency of cancer immunotherapy by dual targeting of IDO1 and DNA. ChemMedChem 13 30–36. 10.1002/cmdc.201700666 [DOI] [PubMed] [Google Scholar]
- Frei E., III, Teicher B. A., Holden S. A., Cathcart K. N., Wang Y. Y. (1988). Preclinical studies and clinical correlation of the effect of alkylating dose. Cancer Res. 48 6417–6423. [PubMed] [Google Scholar]
- Galton D. A., Wiltshaw E., Szur L., Dacie J. V. (1961). The use of chlorambucil and steroids in the treatment of chronic lymphocytic leukaemia. Br. J. Haematol. 7 73–98. 10.1111/j.1365-2141.1961.tb00321.x [DOI] [PubMed] [Google Scholar]
- Gilman A. (1963). The initial clinical trial of nitrogen mustard. Am. J. Surg. 105 574–578. 10.1016/0002-9610(63)90232-0 [DOI] [PubMed] [Google Scholar]
- Gomez D., Aouali N., Londono-Vallejo A., Lacroix L., Megnin-Chanet F., Lemarteleur T., et al. (2003). Resistance to the short term antiproliferative activity of the G-quadruplex ligand 12459 is associated with telomerase overexpression and telomere capping alteration. J. Biol. Chem. 278 50554–50562. 10.1074/jbc.M308440200 [DOI] [PubMed] [Google Scholar]
- Goodman L. S., Wintrobe M. M. (1946). Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 132 126–132. 10.1001/jama.1946.02870380008004 [DOI] [PubMed] [Google Scholar]
- Grose C., Klionsky D. J. (2016). Alternative autophagy, brefeldin A and viral trafficking pathways. Autophagy 12 1429–1430. 10.1080/15548627.2016.1203489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T., Tian K. T., Pan H. Q., Liu Y. X., Xu F. X., Li Z. L., et al. (2018). Novel hybrids of brefeldin A and nitrogen mustards with improved antiproliferative selectivity: design, synthesis and antitumor biological evaluation. Eur. J. Med. Chem. 150 53–63. 10.1016/j.ejmech.2018.02.088 [DOI] [PubMed] [Google Scholar]
- Hu X., Wang Y., Xue J. J., Han T., Jiao R. W., Li Z. L., et al. (2017). Design and synthesis of novel nitrogen mustard-evodiamine hybrids with selective antiproliferative activity. Bioorg. Med. Chem. Lett. 27 4989–4993. 10.1016/j.bmcl.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Huang H. R., Liu T., Guo J. X., Yu L., Wu X. F., He Y., et al. (2017). Brefeldin A enhances docetaxel-induced growth inhibition and apoptosis in prostate cancer cells in monolayer and 3D cultures. Bioorg. Med. Chem. Lett. 27 2286–2291. 10.1016/j.bmcl.2017.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E., Scurr M., Campbell E., Jones E., Godkin A., Gallimore A. (2018). T-cell modulation by cyclophosphamide for tumour therapy. Immunology 154 62–68. 10.1111/imm.12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson E. R., Lippard S. J. (1999). Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 99 2467–2498. 10.1021/cr980421n [DOI] [PubMed] [Google Scholar]
- Jiang T. Z., Sun Y. Y., Yin Z. C., Feng S., Sun L. P., Li Z. Y. (2015). Research progress of indoleamine 2,3-dioxygenase inhibitors. Future Med. Chem. 7 185–201. 10.4155/fmc.14.151 [DOI] [PubMed] [Google Scholar]
- Kaminski Z. J., Kolesinska B., Markowicz S. W. (2004). Synthesis and cytostatic properties of monoterpene derivatives of cyanuric and isocyanuric acids. Acta. Pol. Pharm. 61(Suppl.), 29–32. [PubMed] [Google Scholar]
- Kikuchi S., Shinpo K., Tsuji S., Yabe I., Niino M., Tashiro K. (2003). Brefeldin A-induced neurotoxicity in cultured spinal cord neurons. J. Neurosci. Res. 71 591–599. 10.1002/jnr.10479 [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Hartley J. A., Mattes W. B. (1987). Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 15 10531–10549. 10.1093/nar/15.24.10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesinska B., Barszcz K., Kaminski Z. J., Drozdowska D., Wietrzyk J., Switalska M. (2012). Synthesis and cytotoxicity studies of bifunctional hybrids of nitrogen mustards with potential enzymes inhibitors based on melamine framework. J. Enzyme Inhib. Med. Chem. 27 619–627. 10.3109/14756366.2011.604482 [DOI] [PubMed] [Google Scholar]
- Lallukka T., Millear A., Pain A., Cortinovis M., Giussani G. (2017). GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specifi c mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015 (vol 388, pg 1459, 2016). Lancet 389:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Dai L., Zhao X., Zhi S., Shen H., Yang Z. (2018). Novel sophoridine derivatives bearing phosphoramide mustard moiety exhibit potent antitumor activities in vitro and in vivo. Molecules 23:E1960. 10.3390/molecules23081960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. D., Dai L. L., Zhang N., Tao Z. W. (2015). Synthesis, structure-activity relationship and biological evaluation of novel nitrogen mustard sophoridinic acid derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 25 4092–4096. 10.1016/j.bmcl.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang Y., Wang S. H., Gao Y. J., Zhang X. F., Lu C. H. (2015). Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med. Oncol. 32:365. 10.1007/s12032-014-0365-1 [DOI] [PubMed] [Google Scholar]
- Liu J. B., Liu Y. Q. (2013). Influence of Erbanxiao solution on inhibiting angiogenesis in stasis toxin stagnation of non-small cell lung cancer. J. Tradit. Chin. Med. 33 303–306. 10.1016/S0254-6272(13)60169-7 [DOI] [PubMed] [Google Scholar]
- Liu M., Wang W. G., Sun H. D., Pu J. X. (2017). Diterpenoids from Isodon species: an update. Nat. Prod. Rep. 34 1090–1140. 10.1039/c7np00027h [DOI] [PubMed] [Google Scholar]
- Lv Q. L., Xue Y., Li G. D., Zou L. F., Zhang X., Ying M. F., et al. (2015). Beneficial effects of evodiamine on P2X(4)-mediated inflammatory injury of human umbilical vein endothelial cells due to high glucose. Int. Immunopharmacol. 28 1044–1049. 10.1016/j.intimp.2015.08.020 [DOI] [PubMed] [Google Scholar]
- Maeda M., Ligo M., Tsuda H., Fujita H., Yonemura Y., Nakagawa K., et al. (2000). Antimetastatic and antitumor effects of 2,4-diamino-6-(pyridine-4-yl)-1,3,5-triazine (4PyDAT) on the high lung metastatic colon 26 tumor in mice. Anti Cancer Drug Des. 15 217–223. [PubMed] [Google Scholar]
- Mandal S., Berube G., Asselin E., Mohammad I., Richardson V. J., Gupta A., et al. (2007). A novel series of potent cytotoxic agents targeting G2/M phase of the cell cycle and demonstrating cell killing by apoptosis in human breast cancer cells. Bioorg. Med. Chem. Lett. 17 4955–4960. 10.1016/j.bmcl.2007.06.033 [DOI] [PubMed] [Google Scholar]
- Moon J. L., Kim S. Y., Shin S. W., Park J. W. (2012). Regulation of brefeldin A-induced ER stress and apoptosis by mitochondrial NADP(+)-dependent isocitrate dehydrogenase. Biochem. Bioph. Res. Commun. 417 760–764. 10.1016/j.bbrc.2011.12.030 [DOI] [PubMed] [Google Scholar]
- Moon Y. W., Hajjar J., Hwu P., Naing A. (2015). Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer 3:51. 10.1186/s40425-015-0094-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. J., DuHadaway J. B., Donover P. S., Sutanto-Ward E., Prendergast G. C. (2005). Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11 312–319. 10.1038/nm1196 [DOI] [PubMed] [Google Scholar]
- Muthyala R. S., Carlson K. E., Katzenellenbogen J. A. (2003). Exploration of the bicyclo[3.3.1]nonane system as a template for the development of new ligands for the estrogen receptor. Bioorg. Med. Chem. Lett. 13 4485–4488. 10.1016/j.bmcl.2003.08.061 [DOI] [PubMed] [Google Scholar]
- Nicolle A., Proctor S. J., Summerfield G. P. (2004). High dose chlorambucil in the treatment of lymphoid malignancies. Leuk. Lymphoma 45 271–275. 10.1080/10428190310001595704 [DOI] [PubMed] [Google Scholar]
- Nitiss J. L. (2009). Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9 338–350. 10.1038/nrc2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara M., Matsunaga T., Takahashi S., Saiki I., Suzuki H. (2002). Anti-invasive and metastatic activities of evodiamine. Biol. Pharm. Bull. 25 1491–1493. 10.1248/bpb.25.1491 [DOI] [PubMed] [Google Scholar]
- Paquin I., Raeppel S., Leit S., Gaudette F., Zhou N., Moradei O., et al. (2008). Design and synthesis of 4-[(s-triazin-2-ylamino)methyl]-N-(2-aminophenyl)-benzamides and their analogues as a novel class of histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 18 1067–1071. 10.1016/j.bmcl.2007.12.009 [DOI] [PubMed] [Google Scholar]
- Pommier Y. (2013). Drugging topoisomerases: lessons and challenges. ACS Chem. Biol. 8 82–95. 10.1021/cb300648v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Leo E., Zhang H., Marchand C. (2010). DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17 421–433. 10.1016/j.chembiol.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Marchand C. (2011). Interfacial inhibitors: targeting macromolecular complexes. Nat. Rev. Drug Discov. 11 25–36. 10.1038/nrd3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Shuker D. E. (1994). DNA damage and mutagenesis induced by nitrogen mustards. Mutat. Res. 318 205–226. 10.1016/0165-1110(94)90015-9 [DOI] [PubMed] [Google Scholar]
- Qin X. D., Fang L., Chen F. H., Gou S. H. (2017). Conjugation of platinum(IV) complexes with chlorambucil to overcome cisplatin resistance via a “joint action” mode toward DNA. Eur. J. Med. Chem. 137 167–175. 10.1016/j.ejmech.2017.05.056 [DOI] [PubMed] [Google Scholar]
- Quo L., Xue T. Y., Xu W., Gao J. Z. (2013). Matrine promotes G(0)/G(1) arrest and down-regulates cyclin D1 expression in human rhabdomyosarcoma cells. Panminerva Med. 55 291–296. [PubMed] [Google Scholar]
- Rajamahanty S., Alonzo C., Aynehchi S., Choudhury M., Konno S. (2010). Growth inhibition of androgen-responsive prostate cancer cells with brefeldin A targeting cell cycle and androgen receptor. J. Biomed. Sci. 17:5. 10.1186/1423-0127-17-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J. F., Guittat L., Mailliet P., Laoui A., Renou E., Petitgenet O., et al. (2002). Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc. Natl. Acad. Sci. U.S.A. 99 2672–2677. 10.1073/pnas.052698099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson A. B., Eheman C. R., Altekruse S. F., Ward J. W., Jemal A., Sherman R. L., et al. (2016). Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 122 1312–1337. 10.1002/cncr.29936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson B. J., Shield A. J. (1996). Mutagenic damage to mammalian cells by therapeutic alkylating agents. Mutat. Res. 355 41–57. 10.1016/0027-5107(96)00021-8 [DOI] [PubMed] [Google Scholar]
- Sarosy G., Leyland-Jones B., Soochan P., Cheson B. D. (1988). The systemic administration of intravenous melphalan. J. Clin. Oncol. 6 1768–1782. 10.1200/JCO.1988.6.11.1768 [DOI] [PubMed] [Google Scholar]
- Schobert R., Biersack B., Dietrich A., Knauer S., Zoldakova M., Fruehauf A., et al. (2009). Pt(II) complexes of a combretastatin A-4 analogous chalcone: effects of conjugation on cytotoxicity, tumor specificity, and long-term tumor growth suppression. J. Med. Chem. 52 241–246. 10.1021/jm801001d [DOI] [PubMed] [Google Scholar]
- Seehafer K., Rominger F., Helmchen G., Langhans M., Robinson D. G., Ozata B., et al. (2013). Synthesis and biological properties of novel brefeldin a analogues. J. Med. Chem. 56 5872–5884. 10.1021/jm400615g [DOI] [PubMed] [Google Scholar]
- Shi C. S., Li J. M., Chin C. C., Kuo Y. H., Lee Y. R., Huang Y. C. (2017). Evodiamine induces cell growth arrest, apoptosis and suppresses tumorigenesis in human urothelial cell carcinoma cells. Anticancer. Res. 37 1149–1159. 10.21873/anticanres.11428 [DOI] [PubMed] [Google Scholar]
- Shi L., Yang F., Luo F., Liu Y., Zhang F., Zou M. J., et al. (2016). Evodiamine exerts anti-tumor effects against hepatocellular carcinoma through inhibiting beta-catenin-mediated angiogenesis. Tumor Biol. 37 12791–12803. 10.1007/s13277-016-5251-3 [DOI] [PubMed] [Google Scholar]
- Shi M., Lu X. J., Zhang J., Diao H., Li G. M., Xu L., et al. (2016). Oridonin, a novel lysine acetyltransferases inhibitor, inhibits proliferation and induces apoptosis in gastric cancer cells through p53-and caspase-3-mediated mechanisms. Oncotarget 7 22623–22631. 10.18632/oncotarget.8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu K. G., Lin S., Lee C. C., Chen E., Lin L. C., Wang B. W., et al. (2006). Evodiamine inhibits in vitro angiogenesis: implication for antitumorgenicity. Life Sci. 78 2234–2243. 10.1016/j.lfs.2005.09.027 [DOI] [PubMed] [Google Scholar]
- South P. F., Harmeyer K. M., Serratore N. D., Briggs S. D. (2013). H3K4 methyltransferase Set1 is involved in maintenance of ergosterol homeostasis and resistance to Brefeldin A. Proc. Natl. Acad. Sci. U.S.A. 110 E1016–E1025. 10.1073/pnas.1215768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer C. J., Antoniw P., Bagshawe K. D., Searle F., Bisset G. M., Jarman M. (1990). Novel prodrugs which are activated to cytotoxic alkylating agents by carboxypeptidase G2. J. Med. Chem. 33 677–681. 10.1021/jm00164a034 [DOI] [PubMed] [Google Scholar]
- Sun H. D., Huang S. X., Han Q. B. (2006). Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 23 673–698. 10.1039/b604174d [DOI] [PubMed] [Google Scholar]
- Sun Q., Sattayakhom A., Backs J., Stremmel W., Chamulitrat W. (2012). Role of myocyte enhancing factor 2B in epithelial myofibroblast transition of human gingival keratinocytes. Exp. Biol. Med. 237 178–185. 10.1258/ebm.2011.011261 [DOI] [PubMed] [Google Scholar]
- Toda T., Watanabe M., Kawato J., Kadin M. E., Higashihara M., Kunisada T., et al. (2015). Brefeldin a exerts differential effects on anaplastic lymphoma kinase positive anaplastic large cell lymphoma and classical Hodgkin lymphoma cell lines. Br. J. Haematol. 170 837–846. 10.1111/bjh.13508 [DOI] [PubMed] [Google Scholar]
- Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. (2015). Global cancer statistics, 2012. Cancer J. Clin. 65 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- Trafalis D., Geromichalou E., Dalezis P., Nikoleousakos N., Sarli V. (2016). Synthesis and evaluation of new steroidal lactam conjugates with aniline mustards as potential antileukemic therapeutics. Steroids 115 1–8. 10.1016/j.steroids.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Vedejs E., Naidu B. N., Klapars A., Warner D. L., Li V. S., Na Y., et al. (2003). Synthetic enantiopure aziridinomitosenes: preparation, reactivity, and DNA alkylation studies. J. Am. Chem. Soc. 125 15796–15806. 10.1021/ja030452m [DOI] [PubMed] [Google Scholar]
- Wall M. E., Abernethy G. S., Jr., Carroll F. I., Taylor D. J. (1969). The effects of some steroidal alkylating agents on experimental animal mammary tumor and leukemia systems. J. Med. Chem. 12 810–818. 10.1021/jm00305a021 [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Bai X. Y., Wang C. H. (2014). Traditional chinese medicine: a treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 42 543–559. 10.1142/S0192415X14500359 [DOI] [PubMed] [Google Scholar]
- Wang D., Lippard S. J. (2005). Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 4 307–320. 10.1038/nrd1691 [DOI] [PubMed] [Google Scholar]
- Wang L., Li D. H., Xu S. T., Cai H., Yao H. Q., Zhang Y. H., et al. (2012). The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 52 242–250. 10.1016/j.ejmech.2012.03.024 [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Liu J. G., Li H., Yang H. M. (2016). Pharmacological effects of active components of chinese herbal medicine in the treatment of Alzheimer’s disease: a review. Am. J. Chin. Med. 44 1525–1541. 10.1142/S0192415X16500853 [DOI] [PubMed] [Google Scholar]
- Wheate N. J., Walker S., Craig G. E., Oun R. (2010). The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 39 8113–8127. 10.1039/c0dt00292e [DOI] [PubMed] [Google Scholar]
- Wu C. C., Li T. K., Farh L., Lin L. Y., Lin T. S., Yu Y. J., et al. (2011). Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science 333 459–462. 10.1126/science.1204117 [DOI] [PubMed] [Google Scholar]
- Wu W. S., Chien C. C., Chen Y. C., Chiu W. T. (2016). Protein Kinase RNA-like endoplasmic reticulum kinase-mediated Bcl-2 protein phosphorylation contributes to evodiamine-induced apoptosis of human renal cell carcinoma cells. PLoS One 11:e0160484. 10.1371/journal.pone.0160484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Pei L. L., Wang C. Q., Zhang Y. K., Li D. H., Yao H. Q., et al. (2014). Novel hybrids of natural oridonin-bearing nitrogen mustards as potential anticancer drug candidates. ACS Med. Chem. Lett. 5 797–808. 10.1021/ml500141f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. T., Pei L. L., Li D. H., Yao H., Cai H., Yao H. Q., et al. (2014). Synthesis and antimycobacterial evaluation of natural oridonin and its enmein-type derivatives. Fitoterapia 99 300–306. 10.1016/j.fitote.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Yadav A. A., Wu X., Patel D., Yalowich J. C., Hasinoff B. B. (2014). Structure-based design, synthesis and biological testing of etoposide analog epipodophyllotoxin-N-mustard hybrid compounds designed to covalently bind to topoisomerase II and DNA. Bioorg. Med. Chem. 22 5935–5949. 10.1016/j.bmc.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Jin H., Gong W., Wang Z., Liang H. (2013). Pharmacological actions of multi-target-directed evodiamine. Molecules 18 1826–1843. 10.3390/molecules18021826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarytova V. F., Ivanova E. M., Chasovskikh M. N. (1990). [Synthesis of steroid-containing oligonucleotides and their alkylating derivatives]. Bioorg. Khim. 16 610–616. [PubMed] [Google Scholar]
- Zhou G. B., Kang H., Wang L., Gao L., Liu P., Xie J., et al. (2007). Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 109 3441–3450. 10.1182/blood-2006-06-032250 [DOI] [PMC free article] [PubMed] [Google Scholar]