Abstract
Since 2013 the efficacy of new live Salmonella Enteritidis (SE) vaccines for chickens needs to be demonstrated according to European Pharmacopoeia Monograph 04/2013:2520 to receive approval in the EU. The purpose of this study was to determine whether a vaccine licensed since 1999 could also fulfil the required tests of the current guideline. For this, Salmonella-free chickens (n = 50) were vaccinated on their 2nd, 46th and 84th day of life with the live attenuated S. Enteritidis strain IDT No. 441/014. Non-vaccinated control animals (n = 50) were kept accordingly. To demonstrate the duration of immunity 20 animals of each group were challenge infected 65 weeks after the last vaccination with a virulent SE (PT 4) strain. According to the monograph, cloacal swabs were taken 3, 5, 7, 10 and 14 days post challenge (dpc). Tissue samples of liver, spleen, caeca, ovaries and oviduct were collected during necropsy of 10 animals per group on 7 and 14 dpc, respectively. All samples were analysed bacteriologically regarding the presence of the challenge strain. The number of challenge strain positive tissue samples and cloacal swabs was significantly reduced in vaccinated animals (p < 0.05). Therefore, the vaccine strain complied with the EP guideline. This study is the first that demonstrates the efficacy of this vaccine according to the current regulations. However, efficacy could also be shown during the development of the vaccine but by use of another animal model that comprised fewer animals per group. The use of this model is no longer accepted by EU regulatory authorities. The results need discussion in context with the 3R principle.
Keywords: Microbiology, Immunology, Veterinary medicine, Vaccines
1. Introduction
Human Salmonellosis is mainly caused by contaminated food derived from animals of carrier state. Despite a tendency of dropping incidence in recent years still a total of 94,530 human Salmonellosis cases were confirmed in the EU in 2016 [1]. Herein S. Enteritidis was the most dominant serovar with more than half (59.0 %) of the reported cases caused by this serovar. Furthermore, the number of S. Enteritidis associated cases continued to increase in 2016 compared with 2014 and 2015 [1].
Since poultry products have been identified as one major source of Salmonella Enteritidis (SE) and Salmonella Typhimurium (STM) in human food the EU has implemented the obligation of vaccination of chicken flocks as one important part of the program for control of Salmonella in poultry [2]. Several live attenuated or killed vaccines are currently licenced for this purpose in the EU.
The efficacy of SE or STM live vaccines has to be demonstrated as per two specific guidelines of the European Pharmacopoeia (EP): 04/2013:2520 and 04/2013:2521. Both describe the animal trials including the challenge model to be applied. This challenge model is based on a qualitative bacteriological examination of cloacal swabs and internal organs (liver, spleen, caeca, ovary and oviduct) and requires a minimum of 20 animals per group. In contrast, a different challenge model based on quantitative bacteriological examinations of the target organs by tenfold serial dilution of the samples (Koch's spread plate method) has been widely published by numerous authors in the last 3 decades [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. By determining the bacterial burden per gram tissue, this model allows a demonstration of statistically relevant differences between vaccinated and non-vaccinated animals using much lower numbers of animals per group (n = 4–10) [3–13]. It is therefore more in line with the ‘3R’ principle in animal experiments.
The aim of this study was to examine whether an already licensed live SE vaccine, which was developed by means of a quantitative challenge model [3] during the late 90ies, could also comply with the current guideline released in 2013.
2. Methods
2.1. Study design, animals and vaccination
Dekalb white chickens (n = 100) from a Salmonella-free herd were included. During the study the animals were housed in biosecurity level 1 rooms according to the German law of animal welfare of laboratory animals and received commercial feed according to their age. Water was provided ad libitum.
Chickens (n = 50, group 1) were individually vaccinated orally by use of a buttoned cannula on their 2nd, 46th and 84th day of life with the live attenuated S. Enteritidis strain IDT No. 441/014 (batch #1490315) (registered under the trade names Salmovac 440®/Salmovac SE®/Zoosal 440®, IDT Biologika GmbH, Germany). The vaccine was reconstituted in sterile water and adjusted at the minimum effective dose (1 × 108 cfu/bird). Non-vaccinated controls (group 2, n = 50) were housed separately to avoid transmission of the vaccine strain onto the controls. Cloacal swabs were taken from 10 animals per group each month and analysed regarding the presence of SE/STM according to DIN ISO 6579-1:2017.
At the hatchery the chickens were vaccinated with live vaccines against Infectious Bronchitis (Nobilis® IB Primo QX, MSD), Infectious Bursitis and Marek's Disease (Vaxxitek® HVT+IBD, Merial).
All animals received further vaccinations during the trial according to the SPC of the vaccines: Infectious Bronchitis (Poulvac® IB QX, Zoetis; Avipro IB H52, Elanco), Newcastle Disease (Avinew®, Merial; Avipro® ND LaSota, Elanco), Gumboro Disease (Hipragumboro®, Hipra), Chicken Anaemia Virus (Thymovac®, Elanco), Avian Pneumovirus (Nemovac®, Merial), Infectious Laryngotracheitis (Nobilis ILT, MSD), Reovirus-Infection (Nobilis® Reo, MSD; Nobilis® Reo inac, MSD), Avian Rhinotracheitis, Infectious Bronchitis, Infectious Bursitis, Newcastle Disease (Nobilis® RT+IBmulti+G+ND, MSD), Avian Encephalomyelitis and Fowlpox (Nobilis® AE+POX, MSD).
A notification of the study was approved by the local animal welfare authority (Landesverwaltungsamt Sachsen-Anhalt, reference no. 42502-3-759 IDT).
2.2. Challenge trial
The challenge trial was performed as a blinded study. Directly prior to the challenge 20 chickens per group were transferred to a biosecurity level 2 facility. They were challenged in their 77th week of life (455 days post 3rd vaccination) by intraingluvial administration of 5 × 108 cfu of a virulent wild-type S. Enteritidis 147Nalr (PT 4) strain in 1.0 ml physiological saline solution via buttoned cannula. Cloacal swabs (CS) were sampled from each animal prior to the infection as well as 3, 5, 7, 10 and 14 days post challenge (dpc). Necropsies were performed at 7 and 14 dpc on n = 10 randomly selected animals per group (sampling of liver, spleen, caeca, oviduct, ovary). The animals were euthanized by exsanguination after mechanical head stunning.
Blood samples were taken from each animal at 4 days prior to infection and during necropsy. They were analysed for Salmonella-specific antibodies with a commercial ELISA kit (‘Salmonella Group B and D Antibody test kit’, BioChek Ltd., UK) at Anicon GmbH (Hoeltinghausen, Germany) according to the manufacturer's instructions. Samples with a titre < 1:654 were assigned as negative.
2.3. Bacteriological evaluation
CS and tissue samples (TS) were qualitatively examined for the presence of Salmonella spp. according to DIN ISO 6579-1:2017. In brief, CS were pre-enriched in buffered peptone water (16–20 h at 37 ± 1 °C) followed by cultivation on modified semi-solid rappaport vassiliadis agar (24 ± 3h at 41.5 ± 1 °C) and subsequent plating on XLD- and Rambach agar (24 ± 3 h at 37 ± 1 °C).
TS received pre-enrichment in buffered peptone water (16–20 h at 37 ± 1 °C). After this, 100 μl of this culture were inoculated in RVS-Bouillon and 1 ml MKTTn-Bouillon (24 ± 3 h at 37 ± 1 °C). Samples from both Bouillons were plated XLD- and Rambach-agar (24 ± 3 h at 37 ± 1 °C). Salmonella spp. positive samples were further cultivated on Desoxycholate-Citrate-Agar (LEIFSON) supplemented with 50 μg/ml Nalidixic acid to confirm the presence of the challenge strain.
2.4. Determination of efficacy and statistical analysis
The treatment groups were compared by the number of cloacal swabs and tissue samples containing challenge organisms. The statistical analysis was done with the Wilcoxon Mann-Whitney test at a level of two-sided significance ≤ 0.05 (SAS, North Carolina, USA).
3. Results
3.1. Serology
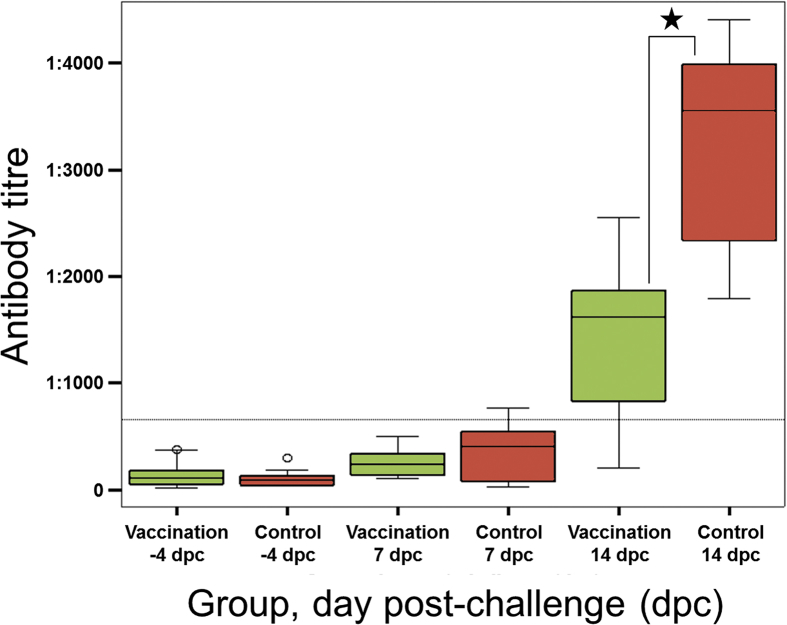
Prior to the challenge all chickens were serologically negative. In response to challenge a seroconversion was detected (7 days post vaccination [dpc]: 2/20 chickens positive, 14 dpc: 19/20 chickens positive). The controls showed higher mean antibody values than the vaccinates post challenge, but significant differences were only observed at 14 dpc (7 dpc: group 1 = 264.0 ± 123.8, group 2 = 528.4 ± 760.4 [p = 0.645], 14 dpc: group 1 = 1478.9 ± 741.7, group 2 = 3237.2 ± 926.7 [p = 0.001]) (Fig. 1).
Fig. 1.
Kinetics of the antibody response to challenge infection according to the treatment group and day of sampling. Prior to the infection all animals were serologically negative (threshold < 1:654, dashed line). Seven days after infection a mild increase in the antibody titres was detected with nearly equivalent values in both groups. The amount of anti-Salmonella antibodies further increased during the infection period and showed a further gain at 14 dpc with significant higher values in the controls (p = 0.001, asterisk).
3.2. Bacteriological examination of faecal swabs and tissue samples
All animals were bacteriologically negative for SE/STM during the vaccination period and until challenge. Furthermore, no Salmonella spp. were found prior to the infection. Post-infection, in both the CS and the TS the mean number of challenge strain positive samples was found to be lower in vaccinates (Table 1). These differences were statistically significant (CS p < 0.0001, TS p = 0.0240). When comparing the mean rate of challenge strain positive TS on both necropsies it was obvious that at 7 dpc (group 1 = 3.2, group 2 = 4.1) more organs per animal were positive than at 14 dpc (group 1 = 2.2, group 2 = 3.1).
Table 1.
Results of bacteriological examination of tissue and cloacal swab samples (frequency of challenge strain 'positive' samples per animal and challenge strain ‘positive’ cloacal swabs per day post-challenge).
| Group | N | Min. | Max. | Median | 95 % CI* | Mean | SD | P** | |
|---|---|---|---|---|---|---|---|---|---|
| Tissue samples | |||||||||
| 1 (Vaccination) | 20 | 0 | 5 | 3 | 2.0 | 3.0 | 2.7 | 1.34 | 0.0240 |
| 2 (Control) | 20 | 1 | 5 | 4 | 3.0 | 4.0 | 3.6 | 1.23 | |
| Cloacal swab samples | |||||||||
| 1 (Vaccination) | 20 | 0 | 4 | 1 | 1.0 | 2.0 | 1.6 | 1.10 | <0.0001 |
| 2 (Control) | 20 | 1 | 5 | 3 | 3.0 | 4.0 | 3.2 | 1.20 | |
| Positive cloacal swab samples per group and day post-challenge | ||||||
|---|---|---|---|---|---|---|
| Group | 0 dpc | 3 dpc | 5 dpc | 7 dpc | 10 dpc | 14 dpc |
| 1 (Vaccination) | 0 | 16 | 8 | 4 | 2 | 1 |
| 2 (Control) | 0 | 18 | 18 | 16 | 7 | 4 |
*CI: confidence interval of the median.
**P: p-value, Wilcoxon Mann-Whitney test (level of significance p ≤ 0.05).
The frequency of SE positive internal organs depended on the type of organ: Caeca > Liver > Spleen > Ovaries > Oviduct (Table 2). A protective effect of the vaccine was present in all organs.
Table 2.
Results of bacteriological examination of tissue samples (frequency table of the number of 'positive' samples per organ and the number of animals by the number of 'positive' samples).
| Organ | Group | Npositive | %positive | 95 % CI* | Total | |
|---|---|---|---|---|---|---|
| Caeca | 1 (Vaccination) | 18 | 90 | 68.3 | 98.8 | 20 |
| 2 (Control) | 19 | 95 | 75.1 | 99.9 | 20 | |
| Liver | 1 (Vaccination) | 16 | 80 | 56.3 | 94.3 | 20 |
| 2 (Control) | 18 | 90 | 68.3 | 98.8 | 20 | |
| Ovaries | 1 (Vaccination) | 7 | 35 | 15.4 | 59.2 | 20 |
| 2 (Control) | 13 | 65 | 40.8 | 84.6 | 20 | |
| Oviduct | 1 (Vaccination) | 3 | 15 | 3.2 | 37.9 | 20 |
| 2 (Control) | 7 | 35 | 15.4 | 59.2 | 20 | |
| Spleen | 1 (Vaccination) | 10 | 50 | 27.2 | 72.8 | 20 |
| 2 (Control) | 15 | 75 | 50.9 | 91.3 | 20 | |
| Number (%) of animals with k 'positive' samples (k = 0–5) | |||||||
|---|---|---|---|---|---|---|---|
| Group | 0 | 1 | 2 | 3 | 4 | 5 | Total |
| 1 (Vaccination) | 1 (5) | 3 (15) | 4 (20) | 7 (35) | 3 (15) | 2 (10) | 20 |
| 2 (Control) | 0 (0) | 2 (10) | 2 (10) | 2 (10) | 10 (50) | 4 (20) | 20 |
*CI: confidence interval.
Furthermore, the number of animals with multiple positive tissue samples was reduced by vaccination (Table 2).
4. Conclusion
The present study is the first that demonstrates the efficacy of the vaccine strain IDT 441/014 against SE according to the current requirements specified in EP monograph 04/2013:2520. It could be demonstrated that this vaccine leads to a significant reduction of excretion and a reduced invasion of the internal organs by pathogenic SE. In particular, invasion of the ovaries and oviduct as well as the colonization of the intestine, which are regarded as reservoirs for Salmonella contaminated egg contents and egg shells [14], were considerably reduced in vaccinated animals. Overall, it has to be noted that the vaccine still complies with the current regulations, although it has been developed 20 years ago.
Despite this, a duration of immunity of 65 weeks after the last vaccination and the safety of the vaccine within short-term application (1 day) of other live vaccines could be demonstrated in this laboratory study for the first time.
The animal model used in this study is mandatory since the monograph has come into force in 2013. It is based on a qualitative bacteriological examination of several organs and cloacal swabs and is very different from the one used in the developmental phase of the vaccine in the late 1990's [3]. The latter model is based on a quantitative determination of the challenge strain content per gram of tissue [3, 15]. Due to this approach, a higher statistical power is achieved and fewer animals can be used in the experiments, without limiting the significance of the model [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. This should be considered with regard to European Directive 2010/63/EU which describes the protection of animals used for scientific purposes. Through this directive, the principle of the ‘3R’ was legally recognized in the EU in 2010 and subsequently transposed into national law in the member states. Why the later released EP guideline 04/2013:2520 is contrary to this and excludes animal models that are widely used in scientific work and are more in line with the ‘3R’ principle, remains unclear.
Declarations
Author contribution statement
Tobias Theuß, Sven Springer: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gerhard Woitow, Michael Bulang: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by IDT Biologika GmbH.
Competing interests statement
The authors declare the following conflict of interests: Tobias Theuß and Sven Springer are employed by the manufacturer of the vaccine. The other authors declare that they have no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to Dr. Ulrich Methner (Friedrich Loeffler Institut, Federal Research Institute for Animal Health, Institute of Bacterial Infections, Naumburger Straße 96a, 07743 Jena, Germany) for kindly providing the challenge strain. We gratefully acknowledge the laboratory staff of IDT Biologika GmbH for excellent technical assistance.
All authors attest they meet the ICMJE criteria for authorship.
References
- 1.The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15(12):148. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Commission of the European Parliament . 02.08.2006. COMMISSION REGULATION (EC) No 1177/2006 of 1 August 2006 Implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as Regards Requirements for the Use of Specific Control Methods in the Framework of the National Programmes for the Control of salmonella in Poultry.http://data.europa.eu/eli/reg/2006/1177/oj [cited 2018 Mar 16]. Available from: [Google Scholar]
- 3.Springer S., Lehmann J., Lindner T., Thielebein J., Alber G., Selbitz H.J. A new live Salmonella enteritidis vaccine for chickens–experimental evidence of its safety and efficacy [Ein neuer Salmonella Enteritidis-Lebendimpfstoff Fur Huhner–experimenteller Nachweis der Sicherheit und Wirksamkeit] Berl Munch Tierarztl Wochenschr. 2000;113(6):246–252. [PubMed] [Google Scholar]
- 4.Hassan J.O., Curtiss R.3. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect. Immun. 1994;62(12):5519–5527. doi: 10.1128/iai.62.12.5519-5527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin G., Methner U., Steinbach G., Meyer H. Immunization with potential Salmonella enteritidis mutants-- 2. Investigations on the attenuation and immunogenicity for mice and young hens [Untersuchungen zur Immunisierung mit geeigneten Salmonella Enteritidis-Mutanten--2. Untersuchung der Attenuierung und Immunogenitat Fur Mause und Huhnerkuken] Berl Munch Tierarztl Wochenschr. 1996;109(10):369–374. [PubMed] [Google Scholar]
- 6.Methner U., Barrow P.A., Martin G., Meyer H. Comparative study of the protective effect against Salmonella colonisation in newly hatched SPF chickens using live, attenuated Salmonella vaccine strains, wild-type Salmonella strains or a competitive exclusion product. Int. J. Food Microbiol. 1997;35(3):223–230. doi: 10.1016/s0168-1605(96)01236-6. [DOI] [PubMed] [Google Scholar]
- 7.Methner U. Univ.; Leipzig: 1992. Erarbeitung eines Modells zur experimentellen oralen Infektion des Geflügels mit epidemiologisch bedeutsamen Salmonella-Serovaren [Diss.] p. 88.http://d-nb.info/921042361 Available from: [Google Scholar]
- 8.Atterbury R.J., Allen V.M., Carrique-Mas J.J., Davies R.H. Salmonella colonisation of laying hens following vaccination with killed and live attenuated commercial Salmonella vaccines. Vet. Rec. 2009;165(17):493–496. doi: 10.1136/vr.165.17.493. [DOI] [PubMed] [Google Scholar]
- 9.Varmuzova K., Faldynova M., Elsheimer-Matulova M., Sebkova A., Polansky O., Havlickova H., Sisak F., Rychlik I. Immune protection of chickens conferred by a vaccine consisting of attenuated strains of Salmonella Enteritidis, Typhimurium and Infantis. Vet Res. 2016;47(1):887. doi: 10.1186/s13567-016-0371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braukmann M., Barrow P., Berndt A., Methner U. Combination of competitive exclusion and immunisation with a live Salmonella vaccine in newly hatched chickens: immunological and microbiological effects. Res. Vet. Sci. 2016;107:34–41. doi: 10.1016/j.rvsc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Cooper G.L., Venables L.M., Woodward M.J., Hormaeche C.E. Vaccination of chickens with strain CVL30, a genetically defined Salmonella enteritidis aroA live oral vaccine candidate. Infect. Immun. 1994;62(11):4747–4754. doi: 10.1128/iai.62.11.4747-4754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrow P.A., Lovell M.A., Berchieri A. The use of two live attenuated vaccines to immunize egg-laying hens against Salmonella enteritidis phage type 4. Avian Pathol. 1991;20(4):681–692. doi: 10.1080/03079459108418807. [DOI] [PubMed] [Google Scholar]
- 13.Methner U. Immunisation of chickens with live Salmonella vaccines – role of booster vaccination. Vaccine. 2018;36(21):2973–2977. doi: 10.1016/j.vaccine.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey T.J. Contamination of egg shell and contents with Salmonella enteritidis: a review. Int. J. Food Microbiol. 1994;21(1-2):31–40. doi: 10.1016/0168-1605(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 15.Springer S., Lindner T., Ahrens M., Woitow G., Prandini F., Selbitz H. Duration of immunity induced in chickens by an attenuated live Salmonella enteritidis vaccine and an inactivated Salmonella enteritidis/typhimurium vaccine. Berl Munch Tierarztl Wochenschr. 2011;124(3-4):89–93. [PubMed] [Google Scholar]