Abstract
Raw or minimally processed vegetables are popular for health reasons and for their unique textural and flavor attributes. While many aroma volatiles are produced in situ when plant tissues are mechanically disrupted, enzymes expressed in bacteria in oral microbiota such as cysteine-β-lyase (EC 4.4.1.13) may also contribute to aroma formation in-mouth during consumption. Interactions between raw cabbage and fresh human saliva (n = 21) were measured ex vivo by real-time monitoring of sulfur volatile production by proton transfer reaction mass spectrometry (PTR-MS). Inter-individual differences in the concentration of sulfur volatiles from the breakdown of S-methyl-L-cysteine sulfoxide (SMCSO) in fresh cabbage by saliva were characterized and a 10-fold difference in the extent of sulfur volatile production was measured across individuals. The overall intensity and garlic odor of raw cabbage was positively correlated with the concentration of sulfur volatiles after incubation with fresh human saliva. A buildup of SMSCO-derived sulfur volatiles in vivo, over twenty repeated mouthfuls was demonstrated, indicating that these reactions can affect sensory perception within the timescale of eating. These findings show the perceived odor experienced when eating cabbage differs, thus resulting in a unique flavor experience between individuals.
Keyword: Food science
1. Introduction
Many consumers seek out the unique texture and flavor of raw or minimally processed plant foods [1]. Raw or minimally processed salads or slaws are popular due to greater retention of some vitamins and nutrients compared to thermally processed equivalents and for their characteristic “fresh” flavor profile [2]. Complex enzyme-induced reactions rapidly generate odor volatiles when raw plant tissues are mechanically broken down during mastication. For example, lipoxygenases present in plant tissue produce a range of C-6 alcohols and aldehydes, associated with “green” flavors [3, 4]. Some volatiles may be present in the form of non-volatile glycosides, requiring glucosidase enzyme activity from α-amylase present in saliva, for release and perception [5]. Sulfur containing glucosinolates are well-characterized in brassica vegetables, constituting only a minor component (0.1–0.6% dry weight) [6]. Myrosinase (thioglucoside) enzyme activity present in plant tissue is essential to convert glucosinolates into their bioactive and volatile isothiocyanate form [6]. While glucosinolates are well-known in brassicas, the presence of S-alkyl-L-cysteine conjugates is less familiar, although the latter constitute up to 1–2% of dry weight [7]. S-methyl-L-cysteine sulfoxide (SMCSO, PubChem CID: 182092), is a non-volatile amino acid abundant in many brassica vegetables [7, 8]. The breakdown of SMCSO requires the activity of cysteine-S-conjugate-beta-lyase (CBL) enzyme (EC 4.4.1.13), naturally present in plant tissues [9, 10]. Various CBL-subtypes have also been characterized in human tissue extracts and play an important role in liver detoxification pathways [11, 12]. Considerable CBL activity is present within anaerobic bacteria naturally present in the oral cavity and saliva, such as Fusobacterium nucleatum, which contributes directly to the breakdown of L-cysteine-S-conjugates [8]. Cystathionine β-lyase enzyme (EC 4.4.1.8), present in Veillonela spp. bacteria in saliva also has CBL activity [13].
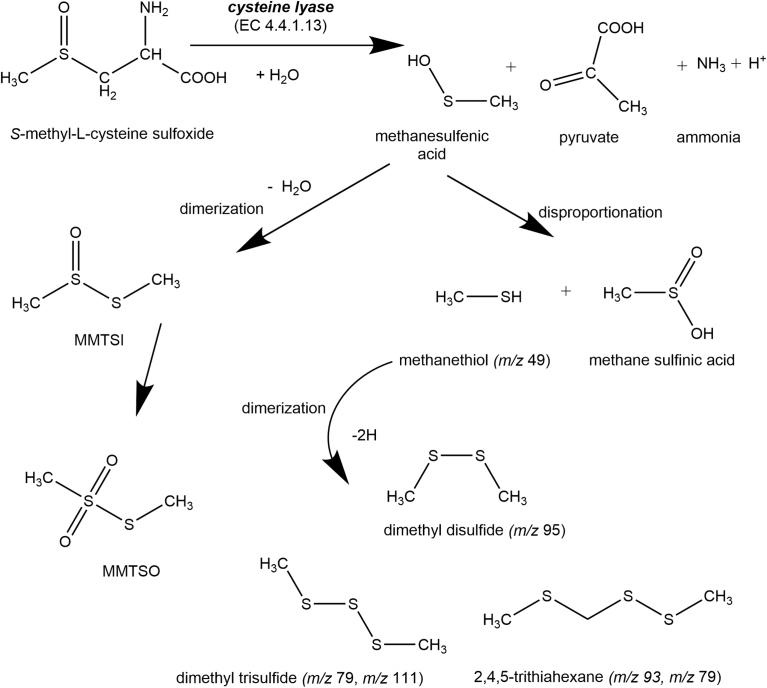
CBL catalyzes the cleavage of C–S bonds of L-cysteine-S-conjugates in the presence of pyridoxal-5-phosphate co-factor (P5P), to liberate methanesulfenic acid, ammonia and pyruvate (Fig. 1) [7]. Methanesulfenic acid is unstable and spontaneously undergoes disproportionation to generate the volatile compound methanethiol (MT, PubChem CID: 140171) which then dimerizes to form the odor active volatiles dimethyl disulfide (DMDS, PubChem CID: 12232) and dimethyl trisulfide (DMTS, PubChem CID: 19310) [14, 15]. SMSCO and its non-volatile decomposition products (S-methyl methanethiosulfinate (PubChem CID: 95200), S-methyl methanethiosulfonate (PubChem CID: 18064)) exhibit anti-microbial, anti-carcinogenic and other physiological effects [7, 12, 16, 17].
Fig. 1.
Diagram showing breakdown products of S-methyl-L-cysteine sulphoxide through the actions of cysteine lyase enzyme. Modified and adapted from (Edmands, Gooderham, Holmes, & Mitchell, 2013). MMTSO = S-methyl methanethiosulfonate, MMTSI = S-methyl methanethiosulfinate. Ion fragments (m/z) corresponding to volatiles measured by proton transfer reaction mass spectrometry denoted.
Differences in the composition of the human oral microbiome have been characterized, with most sites in the oral cavity having up to 20 to 30 different predominant species and the number of predominant species ranging from 34 to 72 between individuals. Species from genera Gemella, Granulicatella, Streptococcus, and Veillonella are common in the human oral microbiome [18]. We hypothesized that differences in the composition of individual oral microbiota would lead to individual differences in CBL activity, and hence, the degree of breakdown of SMSCO and the amount of sulfur volatile production in the mouth. This study characterized inter-individual differences in the extent of in-mouth sulfur volatile generation from plant material (raw cabbage) and subsequent aroma development using an ex vivo saliva monitoring technique and sensory evaluation. Build-up of sulfur volatiles in the mouth over repeated mouthfuls was also demonstrated in an in vivo experiment.
2. Materials and methods
2.1. Chemicals and reagents
Volatile reference standards were purchased from Sigma-Aldrich (Castle Hill, Australia); dimethyl disulfide, dimethyl trisulfide, hexanal, (E)-2-hexenal, 1-hexanol, allyl isothiocyanate (2-propenyl isothiocyanate) and 4-methyl-1-pentanol. 2,3,5-trithiahexane (PubChem CID: 93236) was supplied by Penta Manufacturing Corporation (Livingston, NJ, USA) and S-methyl-L-cysteine-sulfoxide was purchased from Cayman Chemicals (Sapphire Bioscience, Beaconsfield, NSW, Australia).
2.2. Ethics and saliva collection
Approval to collect and use human saliva in the ex vivo PTR-MS experiments was obtained from CSIRO low- risk ethics committee (LR-02-2016-F). Twenty one healthy subjects, 13 female (45 ± 12 years) and 8 male (42 ± 12 years) participated in the study and experiments were conducted one subject at a time over two separate two week periods. Saliva was collected between 9:00 and 11:00 hr. Subjects were instructed to have their usual breakfast and to brush their teeth using their normal dental care product and regime. Subjects were asked to refrain from using mouthwash and to stop eating and drinking (with the exception of water) one hour before collection. All subjects provided written informed consent before participation. Subjects were instructed to rinse their mouth twice with room temperature water (Pureau®, Noble Beverages, St Marys, Australia). After 5 minutes, subjects were asked to chew on a piece of 4 × 4 cm2 wax Parafilm® (Bemis, Oshkosh, WI, USA). Stimulated saliva (∼30 mL) was collected into 50 mL centrifuge tubes. During collection and handling (∼5–10 min), saliva was kept on ice. Half of the fresh saliva (∼15 mL) was deactivated by microwaving the loosely closed plastic tube in a beaker of water using defrost mode (Sharp R-230F, 800 W), until the beaker water was visibly boiling (∼10 s). The sample was removed and cooled and the microwaving process was repeated twice. Deactivated saliva was cooled to room temperature before using. Small loses in volume were corrected by addition of protein free artificial saliva buffer [19]. Using the ex vivo volatile method described below (Section 2.6), we demonstrated that the microwave conditions were sufficient to completely inhibit production of sulfur volatiles associated with CBL activity (data not shown). Deactivated saliva samples were required as controls for each subject, because mucin and amylase protein content varies considerably between individuals and both are known to affect volatile release [20, 21]. Prior to performing experiments, saliva samples were incubated for (15 min) to reach a temperature of 37 °C in a temperature controlled incubator (Sanyo, Japan).
2.3. Preparation of cabbage for experiments
The amount and distribution of gluscosinolates and SMSCO in brassica vegetables varies widely according cultivar and growing conditions [6]. To obtain consistent material for use across experiments, a homogeneous batch of cabbage powder was prepared. Fresh whole red cabbages (Brassica oleracea var. capitata f. rubra, L.) ∼2 kg, were purchased from a local supermarket. After washing and rinsing with Milli-Q water, the outer leaves were removed and discarded. The cabbages were cut into quarters and processed a quarter at a time. For the ex vivo assay, roughly chopped cabbage pieces (∼1 cm2) were transferred into liquid nitrogen (Linde Australia) and blended in a stainless steel vessel into a fine powder until the whole cabbage was processed. The cabbage powder was pooled, mixed and distributed into 20 separate plastic storage tubes (50 mL) sealed and stored frozen at −80 °C until later use. For the in vivo study, roughly fresh cut fresh cabbage pieces (2 cm2) were weighed into plastic cups (4 g). Cooked cabbage was prepared by steaming for 5 minutes, cooling and cutting into ∼2 cm2 pieces.
2.4. Quantitative measurement of SMSCO in cabbage
SMCSO was dissolved in acidified 70% methanol solution (formic acid 0.1 %) and a series of concentrations were used to construct an external calibration curve between 0.1 and 2 mg/mL. Raw and cooked cabbage was macerated in 70% acidified methanol (70%) using an Ultra-Turrax (T 25) followed by centrifugation. Samples were analyzed using a Dionex Ultimate-3000 liquid chromatograph coupled with triple quadrupole mass spectrometer (TSQ-Quantiva, Thermo Scientific, USA). The chromatographic separation was performed on an Intrada amino acid column (Imtakt Corporation, Japan) (3 mm × 150 mm) and the column oven was kept at 35 °C. Calibration standard solutions and extracts were injected by autosampler (2 μL injection volume). The mobile phases were 100 mM ammonium nitrate (A) and 0.1% formic acid in acetonitrile (B). The flow rate was 600 μL/min and the gradient program began at 14% B (3 min), then ramped to 100% B at 10 min and held for 1.5 min and then ramped to 14% B at 12.5 min and held for 2.5 min. The water content of raw cabbage was taken as 92% to calculate the SMSCO content on a dry weight basis [22]. The mass spectrometer was operated in negative electrospray ionization mode at a spray voltage of −2500 V and capillary temperature of 420 °C. The SMCSO precursor ion (m/z 150) and the following product ions (m/z 48, 63 & 86) with the corresponding collision energies (34.93, 10.25 & 10.25 V) were used for identification and quantification.
2.5. Solid phase microextraction (SPME) and gas chromatography-mass spectrometry
Frozen cabbage powder (1 g) was transferred quantitatively into headspace vials (20 mL) and 20 μL of 4-methylpentanol internal standard (40 μg/mL) and 1 mL of Milli-Q water (37 °C) were added. Immediately after collection, either fresh or deactivated saliva (2 mL) was immediately added and vials were sealed with a gas-tight Teflon® seal. Samples were incubated at 37 °C for 30 min. After incubation saturated calcium chloride solution (1 mL) was injected into the vials through the septum using a stainless steel cannula (24-gauge). To evaluate the effect of saliva on volatile development, replicate samples of cabbage incubated with fresh (n = 2) and deactivated (n = 2) saliva samples across the subjects (n = 10, 40 samples total) were measured. Headspace vials were placed into the auto sampler for the GC-MS analysis (AOC-5000 Plus, Shimadzu, Kyoto, Japan). The headspace was extracted using solid phase microextraction (SPME) (Carboxen/divinylbenzene/polydimethylsiloxane, StableflexTM (Supelco, USA), 50/30 μm, 23 gauge) fibers at 37 °C (30 min) with sample agitation and analyzed by gas chromatography-mass spectrometry (Shimadzu 2010 GC-MS). The SPME fiber was desorbed at 240 °C (splitless) for 5 min. Separation was achieved using a Zebron wax capillary column (0.25 mm × 30 m × 0.50 μm film, Phenomenex, Lane Cove, Australia). The GC oven was programmed from 100 °C (held 1 min) to 250 °C at 10 °C/min (held 3 min.). The MS was set to electron ionization (EI) mode to scan between 45-250 mass/charge ratio (m/z). Volatiles were initially identified through electron impact mass spectral matches in the National Institute of Standards and Technology (NIST) database (Version 2.0, United States of America, 2002) and in most cases identification was confirmed using reference standards and matching retention times. SPME volatile integrated area data were calculated using the LabSolutions® software (Shimadzu).
2.6. Ex vivo saliva PTR-MS protocol
Volatiles were measured using high-sensitivity quadrupole mode PTR-MS (Ionicon Analytic GmbH, Innsbruck, Austria). The inlet flow rate was set to 100 mL/m. The temperatures of inlet tube and reaction chamber were 70 °C and 80 °C, respectively. The drift tube voltage was 600 V and the pressure was 2.19 mbar. Frozen cabbage powder (4 g) was weighed into a sealed Schott bottle with a Teflon stir bar and thawed to room temperature (60 minutes). Immediately prior to experiments, 10 mL of protein-free artificial saliva buffer (37 °C) and was added to the vessel and connected to the PTR-MS via a Luer lock connection as described previously [23]. Fresh and deactivated saliva were kept on ice until placing in an incubator (37 °C) for 15 m prior to the experiment. The time between saliva collection and PTR-MS experiments was no more than 15 min. During piloting experiments, the headspace volatiles were measured in scan mode from m/z 40–150. A number of major ions increased after either macerating fresh cabbage samples (without addition of saliva) or after addition of fresh saliva to cabbage. Pure reference volatile standards (∼5 μg/L) in water were used to determine the PTR-MS fragmentation patterns for key volatile compounds identified by GC-MS (Table 1). Most of the volatiles had common ions. For example, the main fragment for DMDS was the M+H+ ion, m/z 95 (100%), however it also produced a significant amount of m/z 79 ((CH3)S2+, 49%), which was the most abundant ion for DMTS. Although no reference standard was available for methanethiol (MT), the fragment at m/z 49 (100%) was assigned to this molecule consistent with previous reports [24]. It should be noted that other non-characterized volatile species present in individual saliva samples may have also contributed to some of the PTR-MS ions monitored. A selected ion monitoring method was programmed such that a full acquisition cycle of ions of interest (Table 1) was completed every 4 s; m/z 49, m/z 51, m/z 57, m/z 59 (acetone), m/z 61, m/z 63, m/z 65, m/z 79, m/z 81, m/z 83, m/z 85, m/z 93, m/z 95, m/z 97, m/z 99, m/z 100 and m/z 127. For the ex vivo saliva measurements, cabbage powder solutions were scanned for 50 cycles (200 s) to reach a steady state baseline, before introduction of either fresh (8 mL) or deactivated (8 mL) saliva through a syringe via a cannula into the Schott bottle vessel [23]. Samples were then scanned for a further 100 cycles (400 s). The area under the curve (AUC) for the first 10 cycles (40 s), the first 20 cycles (80 s) and the full 100 cycles (400 s)) was calculated using Excel® (Microsoft). The AUC values for deactivated saliva samples were also measured. Two replicates for fresh and deactivated saliva were measured for 10 subjects. Further fresh saliva ex vivo samples for an additional 11 subjects were measured in duplicate, so that data for a total of 21 subjects were available to understand potential relationships between the degree of individual volatile production after incubation with fresh saliva and sensory quality.
Table 1.
Details of the main volatiles present in cabbage headspace, associated odor character, quantitative ion (m/z) monitored by gas chromatography- mass spectrometry (GC-MS) and main ions (m/z, %) measured for reference compounds by proton transfer reaction mass spectrometry (PTR-MS), odor thresholds in water and mean concentrations (n = 10 subjects) in cabbage powder incubated with either deactivated (Deact) or fresh saliva measured by GC-MS. P value for comparison of deactivated and fresh saliva.
| Volatile | Odor character | GC/MS m/z | Main PTR-MS ions m/z (%) | Odor threshold μg/L | Deact μg/kg | Fresh μg/kg | P value |
|---|---|---|---|---|---|---|---|
| Methanethiol (MT) | Sulfurous, putrid | 47 | 49 (100%) | 0.02a | 0.47 | 0.48 | ns |
| dimethyl sulfide (DMS) | Asparagus, cooked | 62 | 63 (100%), 65 (5%) | 1.0a | 3.0 | 2.5 | ns |
| dimethyl disulfide (DMDS) | Cabbage-like | 94 | 95 (100%), 79 (49%), 97 (12%) | 7.6a | 320 | 2160 | <0.001 |
| dimethyl trisulfide (DMTS) | Cabbage-like | 126 | 79 (100%), 81 (36%), 93 (32%), 61 (14%), 127 (12%) | 0.01a | 3410 | 4680 | 0.004 |
| (E)-2-hexenal | Marzipan, green | 69 | 57 (100%), 99 (24%), 81 (22%) | 316a | 340 | 370 | ns |
| hexanal | crushed leaves | 56 | 55 (100%), 83 (61%), 101 (3.2%) | 4.5a | 800 | 540 | ns |
| 1-hexanol | Fatty, green | 56 | 41 (100%), 43 (94%), 57 (36%), 85 (33%) | 2500a | 30 | 120 | <0.001 |
| allyl-isothiocyanate | Mustard, pungent | 99 | 41 (100%), 100 (55%) | 375a | 1220 | 1060 | ns |
| 2,3,5-trithiahexane (TTH) | Onion, penetrating | 61 | 93 (100%), 61 (40%) | 0.8b | 60 | 460 | <0.001 |
a (Belitz, Grosch, & Schieberle, 2009), b (Spadone, Matthey-Doret, & Blank, 2006).
2.7. Consecutive mouthful in vivo volatile release experiment
The purpose of the in vivo experiment was to test whether significant buildup of SMSCO-derived sulfur volatiles occurred within the timescale of a typical eating event, e.g. over 20 consecutive mouthfuls (24 s each) over a total consumption period of 480 s (8 min) period. Cooked cabbage (5 min steaming) was used as a control sample to confirm that there was no significant generation of sulfur volatiles from thermally processed material. Room temperature roughly chopped raw or cooked cabbage samples (∼4 g) were weighed into individual plastic cups (n = 20). An animated visual guide was programmed (Adobe Flash®) to coordinate the breath cycles and intake of twenty consecutive mouthfuls of either fresh or cooked cabbage [25]. A plastic disposable cannula was firmly placed in the subject's nostrils by tightening the plastic tubes at the back of the subject's head. The inlet of the PTR-MS was connected via peek tubing to the cannula and volatiles were measured in multiple ion monitoring mode as described in the previous section. The subject was asked to follow the animation on the computer screen for the consumption protocol. Initially they breathed for 5 cycles and then placed the cabbage sample in their mouth and chewed for 2–3 times prior to swallowing (24 s period). After another 5 breathe cycles another 19 consecutive cabbage samples were consumed according to the strict protocol. For the in vivo measurements a flow rate of 400 mL/min was used. To increase sensitivity, only m/z 49, m/z 79, m/z 95, m/z 111 and m/z 127 were measured (1 scan/s). Replicate fresh (n = 6) and cooked (n = 6) samples were consumed by one trained assessor according to the strict eating and breathing paradigm to ensure good temporal alignment of data. The area under the concentration curve (AUC) from the first to tenth mouthful (AUC-1-10) and from the eleventh to twentieth mouthful (AUC-11-20) were calculated and used in statistical comparisons.
2.8. Triangle testing and sensory evaluation
Within 30 min of completion of the ex vivo PTR-MS measurements, a volume (2 mL) of remaining samples from the deactivated and fresh saliva treatments were transferred into individual plastic cups and closed with a lid. Duplicate series of either; two deactivated saliva and one fresh saliva (BBA), or two fresh saliva and one deactivated saliva (AAB) samples were labelled with a random 3-digit code and presented to individual subjects (n = 21) as an alternative forced choice test (3-AFC) where subjects were required to choose the sample that differed from the other two presented in randomized order (a total of 4 assessments). Subjects were blindfolded in comfortable seated position. A technician removed the sample lids one at a time and held each of the three samples below the subject's nose and asked the subject to guess which sample was different. The total number of correct guesses for the four separate tests was calculated (0–4). Subjects were then asked to rate whether the following attributes were stronger or weaker in the fresh saliva sample compared to the deactivated saliva sample on a 100-mm line scale; green-odor, garlic-odor and overall odor intensity. The midpoint on the scale represented the same intensity or no difference. The left hand anchor was labeled weaker and the right hand anchor labelled stronger. The average intensity of each attribute was calculated for the fresh saliva samples and used to compare to volatile profiles.
2.9. Data processing and analysis
Volatile concentration (μg/L) was calculated using the PTR-MS software. PTR-MS data files were imported into Excel® (Microsoft Corporation). The area under the concentration curve (AUC) was calculated for the first 10 cycles (AUC-10, 40 s) and the first 20 cycles (AUC-20, 80 s) and for 200 complete cycles (AUC-200, 800 s). Replicate volatile data from GC-MS and PTR-MS experiments (n = 10 subjects) were subjected to Multivariate Analysis of Variance (MANOVA) using saliva (fresh, deactivated) and subject as fixed main factors. To understand relationships between various parameters, Pearson's correlation coefficients and associated two-sided p-values and correlation plots were generated using the standard procedures available in GenStat® (16th Edition, VSN International, Hemel Hempstead, UK). The one sample binomial test (Genstat) was used to analyze the data from the triangle tests. Principal components analysis was performed using the standard procedure in Genstat after normalizing data (1/standard deviation).
3. Results and discussion
3.1. Solid phase microextraction measurement of cabbage headspace volatiles
The SPME headspace profiles of fresh macerated cabbage (without addition of either fresh or deactivated saliva) was dominated by dimethyl trisulfide (DMTS) and dimethyl disulfide (DMDS) consistent with previous studies (Table 1) [26, 27]. Only trace amounts of methanethiol (MT) were measured by the SPME method (Table 1). 2,3,5-trithiahexane (TTH, or methyl methylthiomethyl disulfide) was also identified as a major volatile component in the raw cabbage headspace, previously reported only in cooked brassica vegetables [27, 28]. The low olfactory threshold concentrations and the measured concentration of MT, DMTS and TTH indicated that these sulfur volatiles had high odor impact relative to the other volatiles present in cabbage [28, 29]. In the presence of deactivated saliva, a background level of SMSCO-derived sulfur volatiles was measured, indicating a contribution of endogenous plant CBL enzyme activity. Allyl-isothiocyanate (2-propenyl isothiocyanate) was the major glucosinolate breakdown product present in the cabbage headspace, as expected [6, 26]. Typical C-6 volatiles generated from lipoxygenase pathways were also major components; hexanal, 1-hexanol and (E)-2-hexenal [3]. After incubation with fresh saliva, the concentration of DMDS, DMTS were significantly higher (p < 0.001), indicating that CBL activity also was present in human saliva (Table 1). No differences were measured for (E)-2-hexenal or hexanal. A significantly higher amount of 1-hexanol was measured after treatment with fresh saliva. The significantly higher concentration of 1-hexanol in fresh saliva may have indicated the presence of hexyl β-D-glucoside (not measured) in cabbage and release of 1-hexanol due to the activity of salivary α-amylase [5, 30, 31, 32]. Despite the higher amount of 1-hexanol in the fresh saliva, the concentration was still below the olfactory threshold and was considered unlikely to affect the sensory properties. The reason for the lower concentration of hexanal in the fresh saliva may have been due to a change in the confirmation of the denatured saliva proteins, leading to greater binding. The heat denatured saliva proteins in the deactivated saliva may have interacted with this volatile differently [5, 31]. In summary, the SPME GC-MS data demonstrated significant increases in key odor-active sulfur volatiles, typically generated from the breakdown of SMSCO through CBL enzyme activity [7, 8]. Few differences were measured for volatiles produced through lipoxygenase pathways, for example (E)-2-hexenal and hexanal.
3.2. Ex-vivo and saliva measurement using PTR-MS
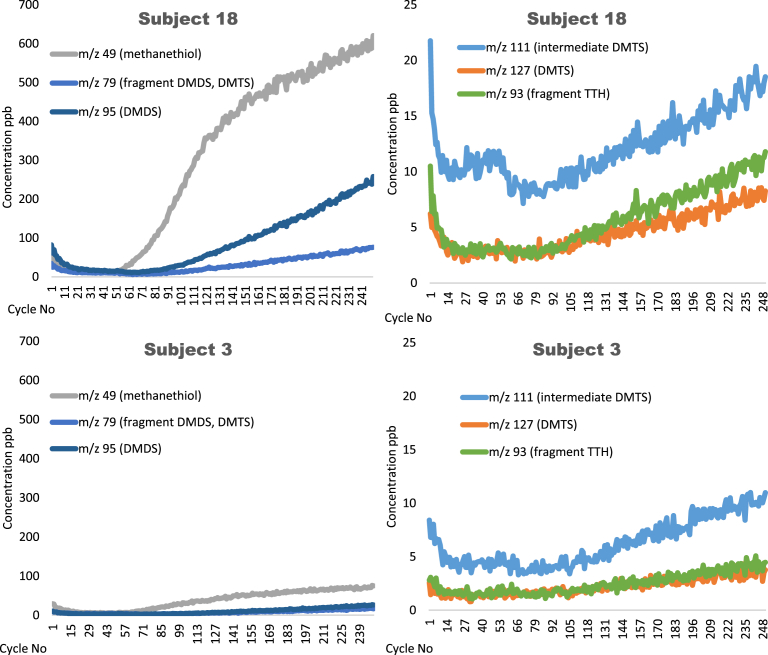
Typical PTR-MS volatile profiles for ex vivo fresh saliva experiments are shown for the most concentrated volatile ions for two individuals; a relative high (Subject 18) and low (Subject 3) producer of sulfur volatiles (Fig. 2). After the addition of saliva at (cycle 50) there was an almost immediate increase in the amount of MT (m/z 49), which was by far the most abundant sulfur volatile measured. After a short lag period a clear increase in m/z 95 (DMDS) and then m/z 79 (DMDS and DMTS) were measured. Significant increases in m/z 111, m/z 93 and m/z 127 were measured after a longer induction period. In previous studies, there has been speculation that DMDS and DMTS may form spontaneously when MT comes in contact with heated or metallic surfaces such as the injector inlet of a gas chromatograph and hence may be heat induced artifacts [33]. The PTR-MS data did not support this, confirming recent findings from other groups [24].
Fig. 2.
Representative real time ex vivo saliva PTR-MS profiles from two human subjects, showing increases in main volatile ions after addition of fresh saliva at 50 cycles. Methanethiol (m/z 49), fragment from dimethyl trisulfide and dimethyl disulfide (m/z 79), dimethyl disulfide (m/z 95), 2,3,5-trithiahexane and dimethyl trisulfide (m/z 93), unidentified ion (m/z 111) and dimethyl trisulfide (m/z 127).
The ion m/z 93 was the main PTR-MS fragment from TTH and also a major ion from DMTS. The m/z 127 ion corresponded to the M+H+ ion for DMTS. The ion m/z 111 increased in all samples after the addition of fresh saliva, although m/z 111 ion was not present in the reference PTR-MS spectra for either DMTS or TTH. In contrast, the electron impact mass spectrum of DMTS has a prominent ion at m/z 111 (16.2%) (National Center for Biotechnology Information. PubChem Compound Database; CID = 19310, https://pubchem.ncbi.nlm.nih.gov/compound/19310 (accessed Dec 11, 2018)) likely corresponding to the positive ion fragment (CH3)S3+. The presence of ion m/z 111 in ex vivo and in vivo PTR-MS data indicate that this may be an unstable chemical intermediate in the formation of DMTS from MT and DMDS. Addition of deactivated saliva did not result in the increase over time of any of the ions associated with the SMSCO-derived volatiles. The concentration of ions corresponding to other cabbage volatiles, for example (E)-2-hexenal (m/z 99), and 1-hexanol (m/z 57) decreased at similar rates over time after addition of both fresh and deactivated saliva ex vivo, indicating that these volatiles were not significantly increased by salivary enzymes. This was in contrast to the GC-MS result for 1-hexanol, in which a higher amount was measured in fresh saliva.
The AUC after 10 (40 s), 20 (80 s) and 200 (800 s) cycles for selected monitored ions for fresh and deactivated saliva for ten subjects were measured (Table 2). Significant differences (p < 0.05) were measured for most ions between fresh and deactivated saliva and also between individuals at each time point (Table 2). MT (m/z 49) was significantly higher in all samples with fresh saliva (p < 0.001). There were also clear differences in m/z 49 between individuals at each time point. For example, there was an almost 10-fold difference between the concentration of MT between subject 1 and 6 for AUC-200. Large differences in the concentration of m/z 51 in fresh saliva between individuals was also measured. The fragment m/z 51 was consistent with the 34S- isotope of methanethiol which has a natural abundance of around 4% [34]. Increases in the concentration of m/z 79, m/z 93, m/z 95 and m/z 111 in fresh saliva were measured after a period of time. Significantly higher m/z 127 was only measured after 200 cycles (800 s). The rapid initial formation of m/z 49 and m/z 95, indicated that the formation of MT and DMDS was under enzymatic control (CBL), whereas the formation of m/z 79 (DMDS, DMTS), m/z 93 (DMTS, MMTMDS), m/z 111 and m/z 127 (DMTS) was slower and appeared to be determined by chemical addition reactions. No consistent or large increases between fresh and deactivated saliva for m/z 57 (1-hexanol and other volatiles) or m/z 99 ((E)-2-hexenal) were measured (in contrast to m/z 49 and m/z 95) indicating that salivary enzymes did not significantly affect their generation. Enzymes (lipoxygenases) present mainly in plant tissues (not saliva) were expected to be responsible for the generation of these volatiles, hence these findings were not surprising.
Table 2.
Mean (n = 2) area under the concentration curve (AUC) data for volatile ions (m/z) after 10 cycles (AUC-10), 20 cycles (AUC-20) and 200 cycles (AUC-200) for 10 subjects for deactivated (deact) and fresh saliva measured by proton transfer reaction mass spectrometry. P values and standard errors of determination (SED) given for the effects of saliva (deact, fresh) and subject. m/z 49 (methanethiol); m/z 51 (34S-methanethiol), m/z 57 (1-hexanol), m/z 79 (dimethyl dilsufide, dimethyl trisulfide), m/z 93 (2,3,5-trithiahexane, dimethyl trisulfide), m/z 95 (dimethyl disulfide), m/z 99 (E)-2-hexenal, m/z 111 (CH3S3+) and m/z 127 (dimethyl trisulfide).
| AUC-10 |
||||||||||||||
| Ion |
Treatment |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Saliva |
Subject |
|
| m/z 49 | Deact | 96 | 89 | 141 | 72 | 122 | 66 | 84 | 76 | 261 | 112 | <0.001 | <0.001 | P value |
| Fresh | 895 | 397 | 214 | 89 | 239 | 86 | 247 | 144 | 476 | 169 | 113 | 26 | SED | |
| m/z 51 | Deact | 51 | 58 | 40 | 36 | 47 | 43 | 38 | 45 | 58 | 53 | <0.001 | <0.001 | P value |
| Fresh | 82 | 71 | 49 | 35 | 59 | 46 | 51 | 50 | 72 | 51 | 2.1 | 4.4 | SED | |
| m/z 57 | Deact | 555 | 784 | 537 | 636 | 725 | 477 | 498 | 639 | 483 | 810 | 0.534 | <0.001 | P value |
| Fresh | 601 | 732 | 519 | 508 | 757 | 479 | 539 | 571 | 561 | 695 | 29 | 61 | SED | |
| m/z 79 | Deact | 134 | 257 | 106 | 108 | 177 | 107 | 154 | 127 | 142 | 271 | 0.007 | <0.001 | P value |
| Fresh | 199 | 327 | 146 | 132 | 208 | 148 | 210 | 164 | 192 | 266 | 14 | 30 | SED | |
| m/z 93 | Deact | 33 | 57 | 24 | 28 | 35 | 21 | 34 | 25 | 29 | 56 | 0.017 | <0.001 | P value |
| Fresh | 43 | 71 | 31 | 30 | 42 | 29 | 46 | 32 | 40 | 54 | 3 | 6 | SED | |
| m/z 95 | Deact | 53 | 96 | 49 | 53 | 77 | 52 | 59 | 53 | 71 | 88 | 0.029 | 0.001 | P value |
| Fresh | 102 | 121 | 58 | 59 | 83 | 62 | 83 | 58 | 82 | 88 | 6.4 | 13.4 | SED | |
| m/z 99 | Deact | 159 | 193 | 159 | 218 | 192 | 132 | 129 | 201 | 117 | 194 | 0.028 | 0.008 | P value |
| Fresh | 152 | 165 | 135 | 155 | 187 | 122 | 125 | 156 | 139 | 155 | 9 | 19 | SED | |
| m/z 111 | Deact | 122 | 213 | 78 | 108 | 142 | 98 | 108 | 112 | 83 | 197 | 0.006 | <0.001 | P value |
| Fresh | 157 | 245 | 110 | 126 | 154 | 125 | 145 | 145 | 130 | 181 | 8.9 | 18.8 | SED | |
| m/z 127 |
Deact | 19 | 44 | 15 | 15 | 26 | 15 | 21 | 20 | 19 | 37 | 0.1 | <0.001 | P value |
| Fresh |
23 |
46 |
18 |
15 |
29 |
20 |
28 |
23 |
28 |
33 |
1.9 |
4 |
SED |
|
| AUC 20 |
||||||||||||||
| Ion |
Treatment |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Saliva |
Subject |
|
| m/z 49 | Deact | 203 | 178 | 290 | 127 | 247 | 120 | 154 | 143 | 514 | 221 | <0.001 | <0.001 | P value |
| Fresh | 4280 | 2349 | 878 | 344 | 1135 | 266 | 1217 | 692 | 2012 | 758 | 67 | 141 | SED | |
| m/z 51 | Deact | 142 | 152 | 86 | 74 | 96 | 83 | 93 | 93 | 114 | 113 | <0.001 | <0.001 | P value |
| Fresh | 300 | 255 | 125 | 87 | 169 | 97 | 135 | 122 | 197 | 131 | 6.5 | 14 | SED | |
| m/z 57 | Deact | 1002 | 1486 | 967 | 1128 | 1324 | 854 | 917 | 1152 | 854 | 1492 | 0.535 | <0.001 | P value |
| Fresh | 1105 | 1341 | 935 | 941 | 1392 | 846 | 977 | 1032 | 1006 | 1278 | 51 | 109 | SED | |
| m/z 79 | Deact | 267 | 493 | 201 | 190 | 331 | 191 | 279 | 231 | 261 | 509 | <0.001 | <0.001 | P value |
| Fresh | 539 | 726 | 283 | 248 | 415 | 258 | 406 | 306 | 394 | 503 | 26 | 56 | SED | |
| m/z 93 | Deact | 65 | 111 | 48 | 51 | 67 | 38 | 63 | 46 | 55 | 106 | 0.016 | <0.001 | P value |
| Fresh | 97 | 140 | 58 | 57 | 79 | 50 | 84 | 58 | 74 | 100 | 6 | 12 | SED | |
| m/z 95 | Deact | 103 | 183 | 88 | 89 | 138 | 87 | 107 | 92 | 132 | 160 | <0.001 | <0.001 | P value |
| Fresh | 422 | 441 | 140 | 117 | 213 | 109 | 199 | 124 | 236 | 196 | 12 | 25 | SED | |
| m/z 99 | Deact | 289 | 354 | 287 | 387 | 352 | 240 | 238 | 364 | 208 | 357 | 0.034 | 0.003 | P value |
| Fresh | 280 | 305 | 242 | 288 | 344 | 218 | 228 | 283 | 250 | 288 | 16 | 33 | SED | |
| m/z 111 | Deact | 232 | 406 | 144 | 195 | 266 | 180 | 197 | 206 | 153 | 368 | 0.006 | <0.001 | P value |
| Fresh | 314 | 467 | 200 | 237 | 288 | 221 | 265 | 263 | 238 | 335 | 17 | 35 | SED | |
| m/z 127 |
Deact | 38 | 89 | 28 | 29 | 50 | 28 | 37 | 36 | 35 | 69 | 0.087 | <0.001 | P value |
| Fresh |
51 |
90 |
34 |
29 |
54 |
35 |
51 |
42 |
52 |
61 |
3.4 |
7.2 |
SED |
|
| AUC-200 |
||||||||||||||
| Ion |
Treatment |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Saliva |
Subject |
|
| m/z 49 | Deact | 845 | 810 | 1421 | 646 | 1137 | 533 | 826 | 523 | 2543 | 968 | <0.001 | <0.001 | P value |
| Fresh | 51635 | 34423 | 15491 | 13769 | 17212 | 5164 | 17212 | 8606 | 34423 | 15491 | 1165 | 2457 | SED | |
| m/z 51 | Deact | 2264 | 2230 | 970 | 856 | 744 | 631 | 1349 | 699 | 878 | 1171 | <0.001 | <0.001 | P value |
| Fresh | 4282 | 4142 | 1886 | 1375 | 2348 | 957 | 1610 | 1387 | 2512 | 1702 | 96 | 202 | SED | |
| m/z 57 | Deact | 4197 | 6496 | 4312 | 5116 | 5789 | 3540 | 4016 | 4687 | 3616 | 6376 | 0.601 | <0.001 | P value |
| Fresh | 4849 | 5744 | 4117 | 4501 | 6091 | 3535 | 4215 | 4164 | 4194 | 5557 | 223 | 471 | SED | |
| m/z 79 | Deact | 1675 | 2812 | 1216 | 1269 | 1775 | 1017 | 1550 | 1027 | 1433 | 2651 | <0.001 | <0.001 | P value |
| Fresh | 9538 | 8614 | 2018 | 3518 | 1020 | 3555 | 1575 | 4761 | 2418 | 8614 | 345 | 727 | SED | |
| m/z 93 | Deact | 411 | 659 | 294 | 270 | 373 | 211 | 364 | 280 | 317 | 571 | <0.001 | <0.001 | P value |
| Fresh | 723 | 566 | 163 | 271 | 89 | 241 | 114 | 302 | 73 | 566 | 51 | 107 | SED | |
| m/z 95 | Deact | 578 | 911 | 460 | 425 | 630 | 369 | 512 | 392 | 708 | 727 | <0.001 | <0.001 | P value |
| Fresh | 11728 | 11481 | 3333 | 5556 | 864 | 5679 | 2099 | 6667 | 4198 | 11481 | 431 | 908 | SED | |
| m/z 99 | Deact | 1208 | 1499 | 1254 | 1614 | 1552 | 1017 | 1043 | 1577 | 893 | 1525 | 0.066 | <0.001 | P value |
| Fresh | 1244 | 1351 | 1064 | 1244 | 1523 | 927 | 998 | 1270 | 1056 | 1269 | 65 | 138 | SED | |
| m/z 111 | Deact | 1329 | 2216 | 792 | 1083 | 1396 | 932 | 1059 | 960 | 830 | 1847 | <0.001 | <0.001 | P value |
| Fresh | 2333 | 2717 | 1172 | 1405 | 1671 | 1088 | 1450 | 1277 | 1346 | 1764 | 95 | 200 | SED | |
| m/z 127 | Deact | 224 | 505 | 163 | 191 | 264 | 151 | 208 | 153 | 190 | 353 | <0.001 | <0.001 | P value |
| Fresh | 589 | 700 | 272 | 282 | 414 | 203 | 365 | 195 | 395 | 394 | 24 | 51 | SED |
3.3. Relationship between ex vivo saliva data and odor attributes
Significant differences (p < 0.001) were measured between individuals at AUC-200 (800 s) for m/z 49, m/z 51, m/z 79, m/z 93, m/z 95, m/z 111 and m/z 127 (Table 2). The data also clearly showed differences between individuals in the rate of production of the measured sulfur volatiles at different times during the oral processing of cabbage (10, 20 and 200 cycles). The varying concentration of sulfur volatiles in ex vivo saliva were expected to result in differences in sensory perception.
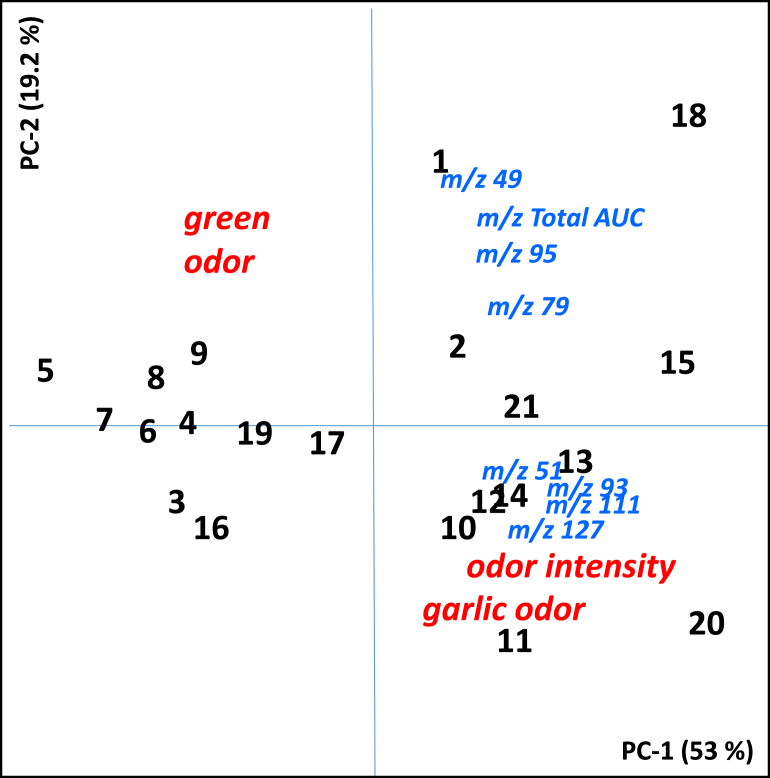
To determine whether differences in sensory perception, could be perceived triangle tests (3-AFC) were completed on cabbage incubated with fresh and deactivated saliva. Overall, there were 84 separate triangle tests performed (21 subjects, 4 triangle tests). A total of 61 tests were correct, significantly higher than chance (p < 0.001), indicating that sensory differences between deactivated and fresh saliva samples were able to be perceived by most assessors (Table 3). Individual AUC-200 sulfur volatiles (m/z 49, m/z 79, m/z 93, m/z 95, m/z 111 and m/z 127) across individuals (n = 21) were significantly (p < 0.05) positively correlated with each other (Table 4). MT (m/z 49) and DMDS (m/z 95) were strongly correlated (r = 0.76, p < 0.001). The odor intensity and garlic odor character were significantly correlated to each other (r = 0.89, p < 0.001). Although the intensity and garlic attributes were positively correlated with most volatiles, the strongest relationships were with higher mass sulfur compounds; e.g.; m/z 79 (DMTS), m/z 93 (DMTS, TTH) and m/z 95 (DMDS). Green character decreased significantly as the intensity and garlic odor increased (r = −0.70, p < 0.001). As no clear differences between deactivated and fresh saliva in background green volatile ions (e.g.; m/z 57, m/z 99) were measured by PTR-MS, masking of the green odor by the sulfur volatiles was indicated. Differences between the sulfur volatile composition and sensory attributes of ex vivo saliva samples for all subjects (n = 21) are summarized in a principal component bi-plot (Fig. 3). Low producers of sulfur volatiles (left hand side) generally reported greater green character than the garlic or odor intensity in ex vivo saliva samples. High sulfur volatile producers (right hand side) reported higher odor intensity and garlic odor character, particularly associated with m/z 93 and m/z 111, which are key ions from DMTS and TTH. Although based on only a small number of subjects (n = 21), these data suggest increased SMCSO derived sulfur volatile production was positively related to the perceived intensity and garlic odor and negatively associated with green odor character in ex vivo extracts.
Table 3.
Ex vivo mean (n = 2) fresh saliva data for 21 subjects. Evaluation of odor attributes (garlic, green, intensity) on a 100 mm line scale, number of correct guesses 3-alternative forced choice (3-AFC, triangle test, n = 4), mean area under concentration curve (AUC) data after 200 cycles for ions (m/z) corresponding to sulfur volatiles measured by proton transfer reaction mass spectrometry and total sum of volatiles (AUC total). m/z 49 (methanethiol); m/z 51 (34S-methanethiol), m/z 79 (CH3S2+, dimethyl disulfide, dimethyl trisulfide), m/z 93 (2,3,5-trithiahexane, dimethyl trisulfide), m/z 95 (dimethyl disulfide), m/z 111 (CH3S3+) and m/z 127 (dimethyl trisulfide).
| Subject No | Garlic odor | Green odor | Odor intensity | Correct (3-AFC) | m/z 49 | m/z 51 | m/z 79 | m/z 93 | m/z 95 | m/z 111 | m/z 127 | Total AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | 50 | 54 | 3 | 51635 | 4282 | 9538 | 723 | 11728 | 2333 | 365 | 80604 |
| 2 | 81 | 41 | 74 | 4 | 34423 | 4142 | 8614 | 566 | 11481 | 2717 | 194 | 62138 |
| 3 | 67 | 34 | 66 | 4 | 15491 | 1886 | 2018 | 163 | 3333 | 1172 | 91 | 24154 |
| 4 | 50 | 33 | 50 | 3 | 13769 | 1375 | 3518 | 271 | 5556 | 1405 | 149 | 26043 |
| 5 | 43 | 51 | 28 | 0 | 17212 | 2348 | 1020 | 89 | 864 | 1671 | 52 | 23256 |
| 6 | 61 | 51 | 62 | 3 | 5164 | 957 | 3555 | 241 | 5679 | 1088 | 157 | 16840 |
| 7 | 58 | 50 | 56 | 3 | 17212 | 1610 | 1575 | 114 | 2099 | 1450 | 42 | 24102 |
| 8 | 51 | 47 | 48 | 3 | 8606 | 1387 | 4761 | 302 | 6667 | 1277 | 205 | 23205 |
| 9 | 55 | 38 | 49 | 3 | 34423 | 2512 | 2418 | 73 | 4198 | 1346 | 41 | 45011 |
| 10 | 97 | 20 | 95 | 4 | 15491 | 1702 | 8614 | 566 | 11481 | 1764 | 194 | 39811 |
| 11 | 96 | 20 | 95 | 4 | 20929 | 9884 | 3138 | 942 | 5468 | 2133 | 709 | 43204 |
| 12 | 69 | 26 | 65 | 3 | 22114 | 6225 | 4893 | 1305 | 8671 | 3337 | 1077 | 47621 |
| 13 | 64 | 34 | 65 | 4 | 29494 | 6239 | 5082 | 1410 | 6719 | 4983 | 1186 | 55112 |
| 14 | 65 | 28 | 76 | 3 | 21354 | 7173 | 4446 | 1080 | 9337 | 3899 | 1291 | 48580 |
| 15 | 68 | 22 | 68 | 4 | 47100 | 9557 | 6842 | 1328 | 15126 | 3743 | 983 | 84679 |
| 16 | 68 | 41 | 51 | 3 | 9056 | 5419 | 1665 | 530 | 2253 | 1317 | 480 | 20722 |
| 17 | 58 | 38 | 42 | 2 | 17657 | 7774 | 3227 | 951 | 5460 | 2089 | 727 | 37885 |
| 18 | 57 | 43 | 69 | 2 | 74994 | 11410 | 6755 | 1244 | 20514 | 2480 | 984 | 118380 |
| 19 | 51 | 37 | 36 | 2 | 17258 | 6771 | 2306 | 671 | 4201 | 1982 | 561 | 33749 |
| 20 | 74 | 20 | 72 | 2 | 11964 | 15006 | 6027 | 1641 | 7477 | 5969 | 1372 | 49457 |
| 21 | 77 | 24 | 76 | 2 | 52431 | 6603 | 4306 | 1026 | 8088 | 2252 | 784 | 75490 |
Table 4.
Correlation plot (n = 21) showing correlation coefficient and associated p values (* = p < 0.05; **p < 0.01, *** = p < 0.001) for m/z 49 (methanethiol); m/z 51 (34S-methanethiol), m/z 79 (dimethyl dilsufide, dimethyl trisulfide), m/z 93 (2,3,5-trithiahexane, dimethyl trisulfide), m/z 95 (dimethyl disulfide), m/z 111 (CH3S3+), m/z 127 (dimethyl trisulfide), sum of total volatiles (Total AUC) and odor attributes (garlic, green, intensity) measured on a 100 mm line scale.
| methanethiol (m/z 49) | - | ||||||||||
| 34S-methanethiol (m/z 51) | 0.37 | - | |||||||||
| fragment DMDS, DMTS (m/z 79) | 0.49 * | 0.23 | - | ||||||||
| fragment TTH, DMTS (m/z 93) | 0.38 | 0.87 *** | 0.45 * | - | |||||||
| dimethyl disulfide (m/z 95) | 0.75 *** | 0.43 * | 0.81 *** | 0.55 ** | - | ||||||
| fragment DMTS(m/z 111) | 0.19 | 0.73 *** | 0.4 | 0.86 *** | 0.35 | - | |||||
| dimethyl trisulfide (m/z 127) | 0.28 | 0.85 *** | 0.26 | 0.96 *** | 0.42 | 0.85 *** | - | ||||
| Total AUC | 0.95 *** | 0.57 ** | 0.65 ** | 0.62 ** | 0.87 *** | 0.42 | 0.50 *** | - | |||
| Garlic odor | 0.005 | 0.28 | 0.38 | 0.34 | 0.24 | 0.22 | 0.23 | 0.15 | - | ||
| Green odor | -0.041 | -0.52 ** | -0.22 | -0.60 ** | -0.24 | -0.51 * | -0.57 ** | -0.22 | -0.70 *** | - | |
| Odor intensity | 0.19 | 0.3 | 0.49 * | 0.42 | 0.46 * | 0.32 | 0.34 | 0.34 | 0.90 *** | -0.67 *** | - |
| m/z 49 | m/z 51 | m/z 79 | m/z 93 | m/z 95 | m/z 111 | m/z 127 | Total AUC | garlic | green | intensity |
Fig. 3.
PCA plot for PT-RMS volatile production from cabbage incubated with fresh saliva and perceived sensory measures (green, garlic and intensity) for the 21 study participants (1–21).
3.4. In vivo consecutive mouthful experiment
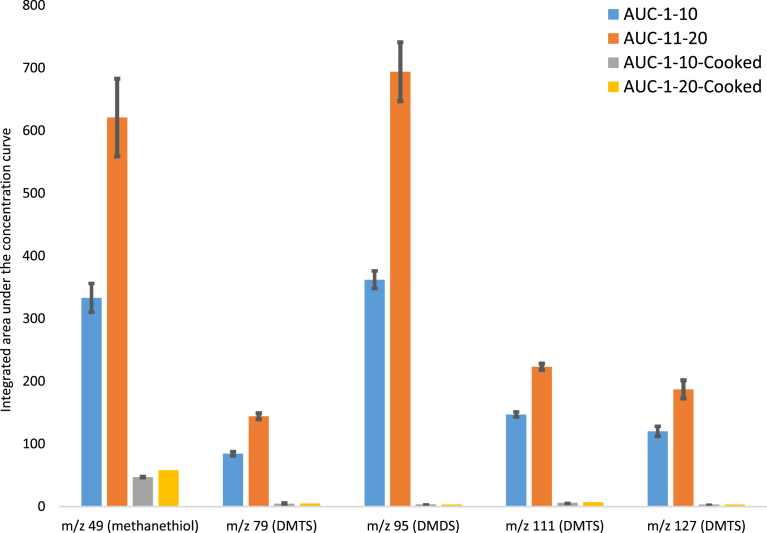
The average in vivo release for the last ten mouthfuls (AUC-11-20) compared to the first ten mouthfuls (AUC-1-10) for raw and cooked cabbage are shown in Fig. 4. It should be emphasized, that the cabbage samples were swallowed and that the volatiles measured were mainly due to residual cabbage juice and pieces present on the surfaces of the oral cavity in contact with saliva. All sulfur volatiles increased in the raw cabbage over the twenty mouthfuls and were significantly higher (p < 0.001) for the latter ten mouthfuls. There was no significant increase or build up over time of any volatile in the cooked cabbage samples. These data demonstrated that SMSCO-derived sulfur volatiles were generated within the mouth during the typical timescales of an eating event (8 min period). The amount of SMSCO in the fresh cabbage powder was estimated to be 932 mg/100g and 875 mg/100 g after steaming for 5 minutes. In a previous study, cysteine S-methyl–sulfoxide conjugates were mostly retained (85.7%) in Allium vegetables after steaming for 4 minutes, but were substantially lost with longer cooking times (<15 minutes) [35]. The enzyme co-factor pyridoxal-5′-phosphate (P5P, vitamin B-6) and associated pyridoxal kinase enzyme activity are required for CBL activity [12]. We speculate that P5P was not available in the cooked cabbage samples, preventing CBL activity.
Fig. 4.
Mean (n = 6) area under curve for 1–10 mouthfuls (AUC-1-10) and AUC for 11–20 mouthfuls (AUC-11-20) of raw and cooked cabbage (AUC-1-10-Cooked, AUC-11-20-cooked), for methanethiol (m/z 49), dimethyl disulfide (m/z 95) and dimethyl trisulfide (m/z 79, m/z 127, bottom) and unidentified ion (m/z 111).
4. Conclusions
An ex vivo PTR-MS method was developed for real-time measurement of SMSCO breakdown in red cabbage by CBL activity in bacteria present in saliva and quantitative differences between individuals were characterized for the first time. Significant buildup of SMSCO-derived volatiles in vivo over an eight minute repeated mouthful eating session was also demonstrated. Relationships between the degree of volatile production and the perception of raw cabbage aroma in ex vivo saliva samples was determined using human subjects (n = 21). This study clearly showed for the first time in raw plant tissue (cabbage) almost instantaneous production of MT, before the formation DMDS, DMTS and TTH. The PTR-MS data from ex vivo experiments showed that there was up to a 10-fold difference in the concentration of MT between the lowest and highest producing individuals, affecting sensory properties. The rates of formation of higher molecular weight sulfur volatiles also differed widely between individuals. The presence of sulfur volatiles generated in mouth from SMSCO by bacterial enzymes may be part of the unique flavor experience of eating raw or minimally processed cabbage and other brassica vegetables. In contrast, there was little evidence that the breakdown of aroma glycosides by salivary α-amylase enzyme played any role in the sensory differences of the ex vivo cabbage samples.
Apart from differences in individual aroma release in the oral cavity and perception as shown in this study, the breakdown of SMSCO during the oral phase of digestion may have wider implications for individual digestion, the gut microbiome and health [11, 12]. Future research using a larger cohort will be required to confirm the results described in this study and better ascertain whether the degree of in mouth volatile production from SMSCO is related to brassica vegetable liking and/or consumption frequency. Better characterization of the variation in different bacterial species in individual oral microflora, the level of CBL enzyme produced and the degree of sulfur volatile production may be warranted in future investigations. Additionally, the effects of bacterial enzymes resulting in differences in odor release and flavor perception should be further explored to better understand whether this is a common feature of other plant foods.
Declarations
Author contribution statement
Damian Frank: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Udayasika Piyasiri: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Nicholas Archer, Ingrid Appelqvist: Conceived and designed the experiments; Analyzed and interpreted the data.
Jenifer Jenifer: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Mateljan G. George Mateljan Foundation; 2007. The World's Healthiest Foods: Essential Guide for the Healthiest Way of Eating. [Google Scholar]
- 2.Lešková E., Kubíková J., Kováčiková E., Košická M., Porubská J., Holčíková K. Vitamin losses: retention during heat treatment and continual changes expressed by mathematical models. J. Food Compos. Anal. 2006;19:252–276. [Google Scholar]
- 3.Bate N.J., Rothstein S.J. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998;16:561–569. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 4.ul Hassan M.N., Zainal Z., Ismail I. Green leaf volatiles: biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol J. 2015;13:727–739. doi: 10.1111/pbi.12368. [DOI] [PubMed] [Google Scholar]
- 5.Ployon S., Morzel M., Canon F. The role of saliva in aroma release and perception. Food Chem. 2017;226:212–220. doi: 10.1016/j.foodchem.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 6.Hanschen F.S., Schreiner M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in brassica oleracea varieties. Front. Plant Sci. 2017;8:1–17. doi: 10.3389/fpls.2017.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmands W.B.M., Gooderham N.J., Holmes E., Mitchell S.C. S-Methyl-L-cysteine sulphoxide: the Cinderella phytochemical? Toxicol Res. 2013;2:11–22. [Google Scholar]
- 8.Starkenmann C., Le Calve B., Niclass Y., Cayeux I., Beccucci S., Troccaz M. Olfactory perception of cysteine-S-conjugates from fruits and vegetables. J. Agric. Food Chem. 2008;56:9575–9580. doi: 10.1021/jf801873h. [DOI] [PubMed] [Google Scholar]
- 9.Tulio A.Z., Yamanaka H., Ueda Y., Imahori Y. Formation of methanethiol and dimethyl disulfide in crushed tissues of broccoli florets and their inhibition by freeze-thawing. J. Agric. Food Chem. 2002;50:1502–1507. doi: 10.1021/jf010673g. [DOI] [PubMed] [Google Scholar]
- 10.Ukai K., Sekiya J. Rapid purification and characterization of cystine lyase b from broccoli inflorescence. Phytochemistry. 1999;51:853–859. doi: 10.1271/bbb.61.1890. [DOI] [PubMed] [Google Scholar]
- 11.Cooper A.J.L., Pinto J.T. Cysteine S-conjugate beta-lyases. Amino Acids. 2006;30:1–15. doi: 10.1007/s00726-005-0243-4. [DOI] [PubMed] [Google Scholar]
- 12.Cooper A.J.L., Krasnikov B.F., Niatsetskaya Z.V., Pinto J.T., Callery P.S., Villar M.T., Artigues A., Bruschi S.A. Cysteine S-conjugate β-lyases: important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids. 2011;41:7–27. doi: 10.1007/s00726-010-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washio J., Shimada Y., Yamada M., Sakamaki R., Takahashi N. Effects of pH and lactate on hydrogen sulfide production by oral Veillonella spp. Appl. Environ. Microbiol. 2014;80:4184–4188. doi: 10.1128/AEM.00606-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin H.W., Lindsay R.C. Mechanisms of formation of volatile-sulfur compounds following the action of cysteine sulfoxide lyases. J. Agric. Food Chem. 1994;42:1529–1536. [Google Scholar]
- 15.Chin H.W., Lindsay R.C. Modulations of volatile sulfur-volatile sulfur-compounds in cruciferous vegetables. In: Mussinan C.J., Keelan M.E., editors. Sulfur Compounds in Foods. vol. 564. 1994. pp. 90–104. [Google Scholar]
- 16.Anufrieva N.V., Morozova E.A., Kulikova V.V., Bazhulina N.P., Manukhov I.V., Degtev D.I., Gnuchikh E.Y., Rodionov A.N., Zavilgelsky G.B., Demidkina T.V. Sulfoxides, analogues of L-methionine and L-cysteine as pro-drugs against gram-positive and gram-negative bacteria. Acta Naturae. 2015;7:128–135. [PMC free article] [PubMed] [Google Scholar]
- 17.Chu C.C., Wu W.S., Shieh J.P., Chu H.L., Lee C.P., Duh P.D. The anti-inflammatory and vasodilating effects of three selected dietary organic sulfur compounds from Allium species. J. Funct. Biomater. 2017;8:5. doi: 10.3390/jfb8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aas J.A., Paster B.J., Stokes L.N., Olsen I., Dewhirst F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Ruth S.M., Grossmann I., Geary M., Delahunty C.M. Interactions between artificial saliva and 20 aroma compounds in water and oil model systems. J. Agric. Food Chem. 2001;49:2409–2413. doi: 10.1021/jf001510f. [DOI] [PubMed] [Google Scholar]
- 20.Kejriwal S., Bhandary R., Thomas B., Kumari S. Estimation of levels of salivary mucin, amylase and total protein in gingivitis and chronic periodontitis patients. J. Clin. Diagn. Res. 2014;8:56–60. doi: 10.7860/JCDR/2014/8239.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.M Van Ruth S., Roozen J.P., Cozijnsen J.L. Changes in flavour release from rehydrated: diced bell peppers (Capsicum annuum) by artificial saliva components in three mouth model systems. J. Sci. Food Agric. 1995;67:189–196. [Google Scholar]
- 22.Pääkkönen K., Plit L. Equilibrium water content and the state of water in dehydrated white cabbage. J. Food Sci. 1991;56:1597–1599. [Google Scholar]
- 23.Frank D., Appelqvist I., Piyasiri U., Delahunty C. In vitro measurement of volatile release in model lipid emulsions using proton transfer reaction mass spectrometry. J. Agric. Food Chem. 2012;60:2264–2273. doi: 10.1021/jf204120h. [DOI] [PubMed] [Google Scholar]
- 24.Perraud V., Meinardi S., Blake D.R., Finlayson-Pitts B.J. Challenges associated with the sampling and analysis of organosulfur compounds in air using real-time PTR-ToF-MS and offline GC-FID. Atmos. Meas. Tech. 2016;9:1325–1340. [Google Scholar]
- 25.Frank D., Kaczmarska K., Paterson J., Piyasiri U., Warner R. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat Sci. 2017;133:61–68. doi: 10.1016/j.meatsci.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Morales-Lopez J., Centeno-Alvarez M., Nieto-Camacho A., Lopez M.G., Perez-Hernandez E., Perez-Hernandez N., Fernandez-Martinez E. Evaluation of antioxidant and hepatoprotective effects of white cabbage essential oil. Pharmaceut. Biol. 2017;55:233–241. doi: 10.1080/13880209.2016.1258424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buttery R.G., Guadagni D.G., Ling L.C., Seifert R.M., Lipton W. Additional volatile components of cabbage, broccoli, and cauliflower. J. Agric. Food Chem. 1976;24:829–832. [Google Scholar]
- 28.Spadone J.C., Matthey-Doret W., Blank I. Formation of methyl(methylthio)-methyl disulfide in broccoli (Brassica oleracea (L.) var. italica) In: Bredie W.L.P., Petersen M.A., editors. Developments in Food Science. vol. 43. Elsevier; 2006. pp. 309–314. [Google Scholar]
- 29.Belitz H.D., Grosch W., Schieberle P. Springer Berlin Heidelberg; 2009. Food Chemistry. [Google Scholar]
- 30.Hemingway K.M., Alston M.J., Chappell C.G., Taylor A.J. Carbohydrate-flavour conjugates in wine. Carbohydr. Polym. 1999;38:283–286. [Google Scholar]
- 31.Pages-Helary S., Andriot I., Guichard E., Canon F. Retention effect of human saliva on aroma release and respective contribution of salivary mucin and alpha-amylase. Food Res. Int. 2014;64:424–431. doi: 10.1016/j.foodres.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Munoz-Gonzalez C., Cueva C., Angeles Pozo-Bayon M., Victoria Moreno-Arribas M. Ability of human oral microbiota to produce wine odorant aglycones from odourless grape glycosidic aroma precursors. Food Chem. 2015;187:112–119. doi: 10.1016/j.foodchem.2015.04.068. [DOI] [PubMed] [Google Scholar]
- 33.Block E. vol. 1068. American Chemical Society; 2011. Challenges and artifact concerns in analysis of volatile sulfur compounds; pp. 35–63. (Volatile Sulfur Compounds in Food). [Google Scholar]
- 34.de Laeter J.R., Böhlke J.K., De Bièvre P., Hidaka H., Peiser H.S., Rosman K.J.R., Taylor P.D.P. Atomic weights of the elements. Review 2000 (IUPAC technical report) Pure and Applied Chemistry. 2003;75:683. [Google Scholar]
- 35.Kubec R., Drhová V., Velíšek J. Thermal degradation of S-methylcysteine and its sulfoxide. Important flavor precursors of brassica and Allium vegetables. J. Agric. Food Chem. 1998;46:4334–4340. [Google Scholar]