Abstract
Kidney stones are a major public health concern with continuously increasing worldwide prevalence. Shock wave lithotripsy (SWL) is the first line treatment choice for upper urinary tract calculi with ureteroscopy and has advantages of safety and noninvasiveness, but the treatment success rate of SWL is lower than that of other therapies. It is therefore important to identify predictive factors for SWL outcome and select a suitable treatment choice for patients with upper urinary tract calculi. In recent years, computed tomography (CT) has become the gold standard for diagnosis of upper urinary tract calculi. Several factors based on CT images, including skin-to-stone distance, mean stone density, stone heterogeneity index, and variation coefficient of stone density, have been reported to be useful for predicting SWL outcome. In addition, a new method of analysis, CT texture analysis, is reportedly useful for predicting SWL outcomes. This review aims to summarize CT parameters for predicting the outcome of shock wave lithotripsy in stone cases in the upper urinary tract.
1. Introduction
Kidney stones are a major public health concern with continuously increasing prevalence [1]. In developed countries, the prevalence has increased from 5% in 1994 to approximately 10% in the 2000s [2].
The first line treatment choice for upper urinary tract calculi is currently shock wave lithotripsy (SWL). While it has advantages of safety and low-invasiveness, its treatment success rate is lower than that of other therapies [3]. Predictive factors for SWL outcome must be identified and suitable treatment choice for patients with upper urinary tract calculi must be selected.
In recent years, noncontrast computed tomography (CT) has become the gold standard for diagnosis of upper urinary tract calculi, and several factors based on CT images have been reported to be useful for prediction of SWL outcome in addition to stone size and location. Here, we review the usefulness of these predictive factors.
2. Stone Size/Volume
Although previous studies have shown that stone size is important factor for predicting SWL outcome and stones over 2 cm are associated with an inferior outcome [4–7], the imaging modality used for evaluating stone size varies among studies [8]. The difference of imaging modalities can lead to the discrepancies in the measurement of the stone dimensions [8]. A plain abdominal film (KUB) is generally viewed only in the coronal plane. In addition, a magnification error with KUB can lead to an increase in stone size by 20% [9]. Ultrasonography (US) makes it possible to measure the stone dimensions in any plane; however, the reproducibility of stone size measurements can be low because US does not offer the fixed planes like KUB or CT. US has also been shown to overestimate the stone size compared with CT, especially for small stones ≤ 5 mm [10].
Compared with KUB or US, the stone size measurements for CT images have been reported to be more accurate and reproducible with no magnification error and less observer bias [8]. Using coronal reconstruction, CT images can provide the measurement of cephalocaudal dimensions in addition to axial stone images. It has been reported that coronal CT images provide a different impression of stone size and should also be used to measure stone size more accurately [11]. Moreover, the previous study has shown that magnified bone windows constitute more accurate method of stone measurements in vitro and in vivo than standard soft tissue windows [12]. Therefore, the routine use of bone windows and the measurement of stone dimensions in the axial and coronal dimensions are recommended to accurately access the stone size [8].
Using three-dimensional analyzing software, CT images can provide information about stone volume. It has also been reported that stone volume is a better predictor of SWL outcome than stone length or width [13]. Future large-scale studies are required to decide the optimal cutoff value for stone volume.
3. Stone Location
Stone location is also an important factor for predicting SWL outcome. The previous large-scale study has reported that the treatment success rate in ureteral stone cases is higher than that in renal stone cases [6]. In addition, it has also shown that the stone-free rate in lower pole stone cases is lower than that in renal pelvic, upper pole and ureteropelvic junction cases [14–16]. We can obtain the information about stone location from CT images.
Especially in patients with lower pole kidney stones, renal collecting system anatomy should be considered for predicting SWL outcome. Although several studies have reported the effect of infundibular length and width and infundibulopelvic angle on kidney stone clearance, there was no definitive evidence until recently because those studies had limitations including retrospective design and small patient numbers [17–19]. However, the recent, well controlled, prospective study has shown that an infundibular length ≥ 25 mm is the negative predictor for SWL outcome [20]. CT images can provide the information about renal collecting system anatomy without using contrast medium.
4. Skin-to-Stone Distance
Representative studies on the relationship between skin-to-stone distance (SSD) and SWL outcomes are summarized in Table 1.
Table 1.
Review of the literature on the relationship between skin-to-stone distance and shock wave lithotripsy outcomes.
| Reference | Year | Country | Number of patients | Stone location | Predictive power |
|---|---|---|---|---|---|
| Pareek et al. [4] | 2005 | USA | 64 | Lower pole | Yes |
| El-Nahas et al. [9] | 2007 | Egypt | 120 | Kidney | No |
| Weld et al. [10] | 2007 | USA | 200 | Kidney | No |
| Perks et al. [5] | 2008 | Canada | 111 | Kidney | Yes |
| Jacobs et al. [11] | 2008 | USA | 85 | Kidney and ureter | No |
| Bandi et al. [12] | 2008 | USA | 94 | Kidney and ureter | No |
| Ng et al. [6] | 2009 | Hong Kong | 94 | Proximal ureter | Yes |
| Patel et al. [7] | 2009 | USA | 83 | Kidney | Yes |
| Wiesenthal et al. [8] | 2010 | Canada | 422 | Kidney and ureter | Yes |
| Choi et al. [13] | 2012 | Korea | 153 | Ureter | No |
| Tanaka et al. [14] | 2013 | Japan | 75 | Kidney and ureter | No |
| Lee et al. [15] | 2016 | Korea | 604 | Ureter | No |
| Yamashita et al. [16] | 2017 | Japan | 239 | Kidney and ureter | No |
SSD was first reported to be a useful predictor of SWL outcome by Pareek et al. (2005) [21]. In their retrospective study, which targeted 64 patients with lower pole kidney stones, SSD was calculated by measuring three distances from the center of the stone to the skin (0°, 45°, and 90° angles) on noncontrast CT. They showed that SWL for patients with an SSD > 10 cm is likely to fail. Since then, it has been reported that greater SSD is a significant predictor of SWL failure not only in patients with lower pole kidney stones, but also in patients with kidney stones or ureteral stones [22–25]. On the contrary, several studies have reported no association between SSD and SWL outcome [13, 15, 26, 27]. In their retrospective study of patients with renal stones, Weld et al. (2007) reported that stone location impacted SWL success more than SSD, and SSD could not be applied to all renal stones [15]. Jacobs et al. (2008) reported that the impact of SSD on SWL outcome varied by the type of lithotripter used [27]. Recent studies from Asian countries have also shown no association between SSD and SWL success [28–31]. This might be because the number of morbidly obese patients is relatively small in Asian countries.
Future prospective large-scale studies are required to further evaluate the significance of SSD on SWL outcome and examine whether this variable has different predictive powers based on the stone location, the type of lithotripter, and the degree of obesity.
5. Mean Stone Density
Mean stone density (MSD) is the mean CT attenuation value of stones and can represent the stone hardness. El-Nahas et al. (2007) reported that MSD > 1000 Hounsfield units (HU) was a significant independent predictor of SWL failure in their prospective study of patients with renal stones [26]. Perks et al. (2008) showed, in their retrospective study of patients with renal stones, that MSD < 900 HU could predict SWL success [22]. On the basis of these results, patients with MSD > 900-1,000 HU have reportedly less successful SWL results in American Urological Association Guidelines [32, 33]. MSD has also been reported to be important in determining the efficacy of SWL treatment by other studies and is widely recognized as a significant predictor of SWL outcome in clinical practice [13, 25, 34–36].
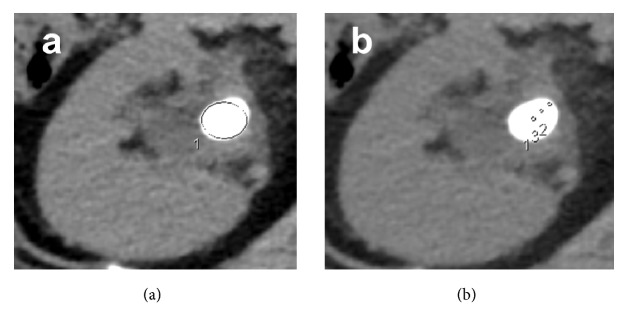
However, the cutoff value of MSD is different between the studies, ranging between 593 HU and 1200 HU. One reason may be that the methods for measuring MSD differ between studies. The various methods of measurement of MSD in previous studies are summarized in Table 2. CT image vision depends on the CT window setting, i.e., abdominal windows or bone windows. The measurement of MSD could also vary depending on the method of placement of the region of interest (ROI). In previous studies, MSD has been measured by two main techniques. In one, the elliptical ROI incorporates the stone as a treatment object without including adjacent soft tissue (Figure 1(a)). In the other method, MSD is calculated from three consistent, small, nonoverlapping ROIs chosen for each stone (Figure 1(b)).
Table 2.
Measuring method of mean stone density in previous studies.
| Reference | Year | Number of patients | Measuring method | |||
|---|---|---|---|---|---|---|
| CT windows | ROI placement | |||||
| Abdominal | Bone | Elliptical ROI | Three ROIs | |||
| El-Nahas et al. [9] | 2007 | 120 | ○ | ○ | ||
| Perks et al. [19] | 2007 | 76 | N/A | ○ | ||
| Perks et al. [5] | 2008 | 111 | ○ | ○ | ○ | |
| Kacker et al. [20] | 2008 | 325 | ○ | ○ | ||
| Bandi et al. [12] | 2009 | 94 | ○ | N/A | ||
| El-Gamal et al. [21] | 2009 | 76 | ○ | N/A | ||
| Wiesenthal et al. [8] | 2010 | 422 | ○ | ○ | ||
Figure 1.
Two techniques used to measure MSD (abdominal window). (a) Elliptical ROI. (b) Average of three ROIs.
As shown in Table 2, MSD measuring methods are different between studies. The recent study has reported that MSD values measured by the various measuring methods were different and the establishment of an accurate and reproducible method for measuring MSD is necessary [37]. To utilize MSD more efficiently, large-scale prospective studies are required. After an appropriate method of measuring of MSD has been ascertained, the optimal cutoff value must be decided.
6. Stone Heterogeneity Index/Variation Coefficient of Stone Density
Zarse et al. (2007) reported that the internal structure of calcium oxalate monohydrate stones on CT images could predict lithotripsy fragility in vitro [38]. In addition, Kim et al. (2007) reported a correlation between stone structure and morphology of cystine stones on CT images, and fragility by SWL [39]. The results indicate that stone heterogeneity can affect SWL outcome.
Recently, stone heterogeneity index (SHI), i.e., deviation of stone density, has been reported to be an independent predictor of SWL outcome in patients with ureteral calculi in a large retrospective study (Lee et al., 2016) [30]. Standard deviation is generally used to quantify the amount of variation or dispersion of data values. They reviewed 604 patients with radiopaque ureteral calculi and investigated whether SHI affects the treatment outcome. Two weeks after a single SWL session, treatment success was defined as either stone-free or clinically insignificant, with asymptomatic, residual fragments ≤ 3 mm in the largest stone diameter. Multivariate logistic regression analyses revealed that higher SHI was an independent predictor of treatment success. SHI was concluded to be a useful clinical parameter for stone fragility.
We reported (2017) variation coefficient of stone density (VCSD) as a new predictive parameter associated with stone heterogeneity [31]. Variation coefficient is the standard deviation divided by the mean value. It is generally used to compare dispersion between multiple groups with different average values. We reviewed 245 patients with upper urinary tract calculi who had undergone SWL and compared the predictive powers of MSD, SHI, and VCSD for SWL success. We defined treatment success as stone-free or clinically insignificant residual fragments < 4 mm at maximum diameter within three months following a single SWL session. On receiver operating characteristic curves for treatment success, area under curve of VCSD was larger than that of MSD and SHI. Multivariate logistic regression analysis additionally revealed that VCSD was an independent significant predictor of SWL success in both kidney and ureteral calculi.
Future large-scale prospective studies are required to ascertain the usefulness of SHI and VCSD for predicting SWL outcome.
7. CT Texture Analysis
Texture analysis (TA) is a new method of image analysis. This method refers to the characterization of regions in an image by their texture content and attempts to quantify intuitive qualities described by terms such as entropy, kurtosis, and skewness as a function of the spatial variation in pixel intensities. In their ex vivo study, Cui et al. (2017) showed that CT TA metrics entropy and kurtosis could strongly predict fragmentation by SWL [40]. Moreover, TA features identified by machine learning provide incremental accuracy to predict SWL outcomes, according to Mannil et al. (2018) in their preliminary retrospective study targeting 224 patients with untreated kidney stones. [41]. If TA software becomes widely used in the future, it might be useful in clinical practice for prediction of SWL outcome.
8. Conclusion
With the advancement in CT technology, various factors for predicting SWL outcome have been reported, including SSD, MSD, SHI, and VCSD. In addition, a new method of image analysis, CT TA, has been developed. Information from CT images could be used effectively to make a suitable treatment plan for patients with upper urinary tract calculi.
Acknowledgments
This document was proofread and edited by the Clinical Research Center, Wakayama Medical University.
Abbreviations
- CT:
Computed tomography
- KUB:
Plain abdominal film
- US:
Ultrasonography
- HU:
Hounsfield units
- MSD:
Mean stone density
- ROI:
Region of interest
- SHI:
Stone heterogeneity index
- SSD:
Skin-to-stone distance
- SWL:
Shock wave lithotripsy
- TA:
Texture analysis
- VCSD:
Variation coefficient of stone density.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Pearle M. S. Shock-wave lithotripsy for renal calculi. The New England Journal of Medicine. 2012;367(1):50–57. doi: 10.1056/NEJMct1103074. [DOI] [PubMed] [Google Scholar]
- 2.Scales C. D., Smith A. C., Hanley J. M., Saigal C. S. Prevalence of kidney stones in the United States. European Urology. 2012;62(3):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf J. S., Jr. Treatment selection and outcomes: ureteral calculi. Urologic Clinics of North America. 2007;34(3):421–430. doi: 10.1016/j.ucl.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Lingeman J. E., Coury T. A., Newman D. M., et al. Comparison of results and morbidity of percutaneous nephrostolithotomy and extracorporeal shock wave lithotripsy. The Journal of Urology. 1987;138(3):485–490. doi: 10.1016/S0022-5347(17)43236-8. [DOI] [PubMed] [Google Scholar]
- 5.Galvin D. J., Pearle M. S. The contemporary management of renal and ureteric calculi. BJU International. 2006;98(6):1283–1288. doi: 10.1111/j.1464-410X.2006.06514.x. [DOI] [PubMed] [Google Scholar]
- 6.Abe T., Akakura K., Kawaguchi M., et al. Outcomes of shockwave lithotripsy for upper urinary-tract stones: a large-scale study at a single institution. Journal of Endourology. 2005;19(7):768–773. doi: 10.1089/end.2005.19.768. [DOI] [PubMed] [Google Scholar]
- 7.Egilmez T., Tekin M. I., Gonen M., Kilinc F., Goren R., Ozkardes H. Efficacy and safety of a new-generation shockwave lithotripsy machine in the treatment of single renal or ureteral stones: Experience with 2670 patients. Journal of Endourology. 2007;21(1):23–27. doi: 10.1089/end.2006.0174. [DOI] [PubMed] [Google Scholar]
- 8.Patel S. R., Nakada S. Y. Quantification of preoperative stone burden for ureteroscopy and shock wave lithotripsy: Current state and future recommendations. Urology. 2011;78(2):282–285. doi: 10.1016/j.urology.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Olcott E. W., Sommer F. G., Napel S. Accuracy of detection and measurement of renal calculi: In vitro comparison of three-dimensional spiral CT, radiography, and nephrotomography. Radiology. 1997;204(1):19–25. doi: 10.1148/radiology.204.1.9205217. [DOI] [PubMed] [Google Scholar]
- 10.Ray A. A., Ghiculete D., Pace K. T., Honey R. J. D. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76(2):295–300. doi: 10.1016/j.urology.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Nadler R. B., Stern J. A., Kimm S., Hoff F., Rademaker A. W. Coronal imaging to assess urinary tract stone size. The Journal of Urology. 2004;172(3):962–964. doi: 10.1097/01.ju.0000134885.08558.88. [DOI] [PubMed] [Google Scholar]
- 12.Eisner B. H., Kambadakone A., Monga M., et al. Computerized tomography magnified bone windows are superior to standard soft tissue windows for accurate measurement of stone size: an in vitro and clinical study. The Journal of Urology. 2009;181(4):1710–1715. doi: 10.1016/j.juro.2008.11.116. [DOI] [PubMed] [Google Scholar]
- 13.Bandi G., Meiners R. J., Pickhardt P. J., Nakada S. Y. Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU International. 2009;103(4):524–528. doi: 10.1111/j.1464-410X.2008.08069.x. [DOI] [PubMed] [Google Scholar]
- 14.Albala D. M., Assimos D. G., Clayman R. V., et al. Lower pole I: A prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis - Initial results. The Journal of Urology. 2001;166(6):2072–2080. doi: 10.1016/S0022-5347(05)65508-5. [DOI] [PubMed] [Google Scholar]
- 15.Weld K. J., Montiglio C., Morris M. S., Bush A. C., Cespedes R. D. Shock Wave Lithotripsy Success for Renal Stones Based on Patient and Stone Computed Tomography Characteristics. Urology. 2007;70(6):1043–1046. doi: 10.1016/j.urology.2007.07.074. [DOI] [PubMed] [Google Scholar]
- 16.Pearle M. S., Lingeman J. E., Leveillee R., et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. The Journal of Urology. 2005;173(6):2005–2009. doi: 10.1097/01.ju.0000158458.51706.56. [DOI] [PubMed] [Google Scholar]
- 17.Sampaio F. J. B., Aragao A. H. M. Limitations of Extracorporeal Shockwave Lithotripsy for Lower Caliceal Stones: Anatomic Insight. Journal of Endourology. 1994;8(4):241–247. doi: 10.1089/end.1994.8.241. [DOI] [PubMed] [Google Scholar]
- 18.Elbahnasy A. M., Clayman R. V., Shalhav A. L., et al. Lower-pole caliceal stone clearance after shockwave lithotripsy, percutaneous nephrolithotomy, and flexible ureteroscopy: Impact of radiographic spatial anatomy. Journal of Endourology. 1998;12(2):113–119. doi: 10.1089/end.1998.12.113. [DOI] [PubMed] [Google Scholar]
- 19.Ghoneim I. A., Ziada A. M., ElKatib S. E. Predictive factors of lower calyceal stone clearance after extracorporeal shockwave lithotripsy (ESWL): A focus on the infundibulopelvic anatomy. European Urology. 2005;48(2):296–302. doi: 10.1016/j.eururo.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Torricelli F. C. M., Marchini G. S., Yamauchi F. I., et al. Impact of renal anatomy on shock wave lithotripsy outcomes for lower pole kidney stones: Results of a prospective multifactorial analysis controlled by computerized tomography. The Journal of Urology. 2015;193(6):2002–2007. doi: 10.1016/j.juro.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Pareek G., Hedican S. P., Lee F. T., Jr., Nakada S. Y. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology. 2005;66(5):941–944. doi: 10.1016/j.urology.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Perks A. E., Schuler T. D., Lee J., et al. Stone attenuation and skin-to-stone distance on computed tomography predicts for stone fragmentation by shock wave lithotripsy. Urology. 2008;72(4):765–769. doi: 10.1016/j.urology.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 23.Ng C.-F., Siu D. Y.-W., Wong A., Goggins W., Chan E. S., Wong K.-T. Development of a scoring system from noncontrast computerized tomography measurements to improve the selection of upper ureteral stone for extracorporeal shock wave lithotripsy. The Journal of Urology. 2009;181(3):1151–1157. doi: 10.1016/j.juro.2008.10.161. [DOI] [PubMed] [Google Scholar]
- 24.Patel T., Kozakowski K., Hruby G., Gupta M. Skin to stone distance is an independent predictor of stone-free status following shockwave lithotripsy. Journal of Endourology. 2009;23(9):1383–1385. doi: 10.1089/end.2009.0394. [DOI] [PubMed] [Google Scholar]
- 25.Wiesenthal J. D., Ghiculete D., John D'A Honey R., Pace K. T. Evaluating the importance of mean stone density and skin-to-stone distance in predicting successful shock wave lithotripsy of renal and ureteric calculi. Urolithiasis. 2010;38(4):307–313. doi: 10.1007/s00240-010-0295-0. [DOI] [PubMed] [Google Scholar]
- 26.El-Nahas A. R., El-Assmy A. M., Mansour O., Sheir K. Z. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. European Urology. 2007;51(6):1688–1694. doi: 10.1016/j.eururo.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs B. L., Smaldone M. C., Smaldone A. M., Ricchiuti D. J., Averch T. D. Effect of skin-to-stone distance on shockwave lithotripsy success. Journal of Endourology. 2008;22(8):1623–1627. doi: 10.1089/end.2008.0169. [DOI] [PubMed] [Google Scholar]
- 28.Choi J. W., Song P. H., Kim H. T. Predictive factors of the outcome of extracorporeal shockwave lithotripsy for ureteral stones. Korean Journal of Urology. 2012;53(6):424–430. doi: 10.4111/kju.2012.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M., Yokota E., Toyonaga Y., et al. Stone attenuation value and cross-sectional area on computed tomography predict the success of shock wave lithotripsy. Korean Journal of Urology. 2013;54(7):454–459. doi: 10.4111/kju.2013.54.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J. Y., Kim J. H., Kang D. H., et al. Stone heterogeneity index as the standard deviation of Hounsfield units: A novel predictor for shock-wave lithotripsy outcomes in ureter calculi. Scientific Reports. 2016;6(1) doi: 10.1038/srep23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita S., Kohjimoto Y., Iguchi T., et al. Variation coefficient of stone density: A novel predictor of the outcome of extracorporeal shockwave lithotripsy. Journal of Endourology. 2017;31(4):384–390. doi: 10.1089/end.2016.0719. [DOI] [PubMed] [Google Scholar]
- 32.Assimos D., Krambeck A., Miller N. L., et al. Surgical management of stones: american urological association/endourological society guideline, Part I. The Journal of Urology. 2016 doi: 10.1016/j.juro.2016.05.090. [DOI] [PubMed] [Google Scholar]
- 33.Assimos D., Krambeck A., Miller N. L., et al. Surgical management of stones: american urological association/endourological society guideline, Part II. The Journal of Urology. 2016;196(4):1153–1160. doi: 10.1016/j.juro.2016.05.090. [DOI] [PubMed] [Google Scholar]
- 34.Perks A. E., Gotto G., Teichman J. M. H. Shock Wave Lithotripsy Correlates With Stone Density on Preoperative Computerized Tomography. The Journal of Urology. 2007;178(3):912–915. doi: 10.1016/j.juro.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 35.Kacker R., Zhao L., Macejko A., et al. Radiographic parameters on noncontrast computerized tomography predictive of shock wave lithotripsy success. The Journal of Urology. 2008;179(5):1866–1871. doi: 10.1016/j.juro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 36.El-Gamal O., El-Badry A. A simple objective method to assess the radiopacity of urinary calculi and its use to predict extracorporeal shock wave lithotripsy outcomes. The Journal of Urology. 2009;182(1):343–347. doi: 10.1016/j.juro.2009.02.111. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita S., Kohjimoto Y., Iwahashi Y., et al. Three-dimensional mean stone density measurement is superior for predicting extracorporeal shock wave lithotripsy success. International Journal of Urology. 2018 doi: 10.1111/iju.13827. [DOI] [PubMed] [Google Scholar]
- 38.Zarse C. A., Hameed T. A., Jackson M. E., et al. CT visible internal stone structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urolithiasis. 2007;35(4):201–206. doi: 10.1007/s00240-007-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S. C., Burns E. K., Lingeman J. E., Paterson R. F., McAteer J. A., Williams J. C., Jr. Cystine calculi: Correlation of CT-visible structure, CT number, and stone morphology with fragmentation by shock wave lithotripsy. Urolithiasis. 2007;35(6):319–324. doi: 10.1007/s00240-007-0117-1. [DOI] [PubMed] [Google Scholar]
- 40.Cui H. W., Devlies W., Ravenscroft S., et al. CT texture analysis of ex vivo renal stones predicts ease of fragmentation with shockwave lithotripsy. Journal of Endourology. 2017;31(7):694–700. doi: 10.1089/end.2017.0084. [DOI] [PubMed] [Google Scholar]
- 41.Mannil M., von Spiczak J., Hermanns T., Poyet C., Alkadhi H., Fankhauser C. D. Three-dimensional texture analysis with machine learning provides incremental predictive information for successful shock wave lithotripsy in patients with kidney stones. The Journal of Urology. 2018;200(4):829–836. doi: 10.1016/j.juro.2018.04.059. [DOI] [PubMed] [Google Scholar]