Abstract
Introduction:
This experimental study compares myocardial function after prolonged arrest by St. Thomas’ Hospital polarizing cardioplegic solution (esmolol, adenosine, Mg2+) with depolarizing (hyperkalaemic) St. Thomas’ Hospital No 2, both administered as cold oxygenated blood cardioplegia.
Methods:
Twenty anaesthetized pigs on tepid (34°C) cardiopulmonary bypass (CPB) were randomised to cardioplegic arrest for 120 min with antegrade, repeated, cold, oxygenated, polarizing (STH-POL) or depolarizing (STH-2) blood cardioplegia every 20 min. Cardiac function was evaluated at Baseline and 60, 150 and 240 min after weaning from CPB, using a pressure-conductance catheter and epicardial echocardiography. Regional tissue blood flow, cleaved caspase-3 activity and levels of malondialdehyde were evaluated in myocardial tissue samples.
Results:
Preload recruitable stroke work (PRSW) was increased after polarizing compared to depolarizing cardioplegia 150 min after declamping (73.0±3.2 vs. 64.3±2.4 mmHg, p=0.047). Myocardial tissue blood flow rate was high in both groups compared to the Baseline levels and decreased significantly in the STH-POL group only, from 60 min to 150 min after declamping (p<0.005). Blood flow was significantly reduced in the STH-POL compared to the STH-2 group 240 min after declamping (p<0.05). Left ventricular mechanical efficiency, the ratio between total pressure-volume area and blood flow rate, gradually decreased after STH-2 cardioplegia and was significantly reduced compared to STH-POL cardioplegia after 150 and 240 min (p<0.05 for both).
Conclusion:
Myocardial protection for two hours of polarizing cardioplegic arrest with STH-POL in oxygenated blood is non-inferior compared to STH-2 blood cardioplegia. STH-POL cardioplegia alleviates the mismatch between myocardial function and perfusion after weaning from CPB
Keywords: adenosine, esmolol, potassium, cardioplegic arrest, cardiac function, ventricular dysfunction
Introduction
Depolarized cardioplegic arrest with repeated administration of hyperkalaemic solutions has been used for decades in cardiothoracic surgery.1 Hyperkalaemic crystalloid solutions with intracellular (low sodium) or extracellular (high sodium) composition administered once are also established routines in clinical practice.2,3 The most widely used cardioplegia worldwide is hyperkalaemic solutions given intermittently as cold crystalloid or oxygenated blood cardioplegia.4,5 Various normokalaemic, substrate-enriched cardioplegic solutions have been evaluated after continuous, intermittent or single-dose administration. This alternative concept, polarized arrest, has several potential advantages, proven in both experimental and clinical studies.6–9 Still, the use of polarized cardioplegic arrest is not widely established clinically.
In a porcine study, the normokalaemic St. Thomas’ Hospital polarizing cardioplegic solution (STH-POL), formulated with a mixture of the short-acting β-adrenergic blocker esmolol, adenosine and Mg2+, improves myocardial contractility after 60 min of cardioplegic arrest compared to the standard depolarizing St. Thomas’ Hospital cardioplegic solution No 2 (STH-2).10 Esmolol, adenosine and Mg2+ each target ion channels in the myocyte cellular membrane, thus, preventing rapid depolarization and triggering of the action potential.11 Furthermore, STH-POL blood cardioplegia improves myocardial energy status compared to STH-2 blood cardioplegia just before and early after aortic declamping following 60 min of cardioplegic arrest.12 The aim of the present study was to compare STH-POL and STH-2 oxygenated blood cardioplegia in an experimental setting with prolonged (120 min) myocyte membrane polarization or depolarization, with clear differences in total potassium load. In this clinically relevant animal study, left ventricular function was evaluated and compared up to 4 hours after 120 min of cardioplegic arrest. We hypothesize that the experimental STH-POL cardioplegic solution is non-inferior compared to STH-2 with respect to myocardial function in the early hours after aortic declamping.
Methods
The experiments were conducted in accordance with the European Communities Council Directive of 2010 (63/EU) and approved by the Norwegian State Commission for Laboratory Animals (Project 20135835). Twenty-eight anaesthetized young pigs (Norwegian Landrace) of either gender, weighing 42 ± 3 (SD) kg were used. The protocol for premedication and anaesthesia has been evaluated (for details see Supplementary Material).13 Prophylactic antibiotic therapy with cefalotin 1.0 g IV followed by 0.5 g during CPB and 1.0 g after weaning was administered. Fluid substitution, Ringer’s acetate 15 mL/kg/h with 20 mmol/L KCl added, was given throughout the experiment. Additional Ringer’s acetate 5 mL/kg/h was administered after weaning from CPB.
Instrumentation and evaluation
The right femoral artery and vein were cannulated for blood sampling and infusion. An early arterial blood gas analysis determined the need for ventilator adjustments. Rectal temperature was monitored and an open suprapubic cystotomy with insertion of a catheter measured diuresis. After midline sternotomy and pericardiotomy, heparin (125 IU/kg) was given IV to prevent catheter clotting. A continuous cardiac output catheter (CCO/EDV 177HF 75, Edwards Lifesciences Inc., Irvine, CA, USA), advanced from the left internal mammary vein into the pulmonary artery, monitored cardiac output, right ventricular end-diastolic volume (EDV), central venous pressure (CVP) and pulmonary artery pressure (PAP) (Vigilance II® and TruWave® transducers, Edwards Lifescience Inc.). A microtip pressure catheter (Millar MPC-500, Houston, TX, USA) was inserted into the proximal aorta through the left internal mammary artery. The haemodynamic parameters were digitised and later analysed (Ponemah ACQ-7700 and Ponemah Physiology Platform v. 5.2, Data Sciences International, St. Paul, MN, USA). A dual-field pressure-conductance catheter (CA71083-PL, CDLeycom, Hengelo, the Netherlands) connected to a Sigma-M signal conditioner (CDLeycom) was inserted through the apex and into the left ventricle (LV). The distal part was positioned above the aortic valve, verified by epicardial echocardiography (Vivid E9, GE Vingmed Ultrasound, Horten, Norway). An infant feeding tube inserted into the left atrium was used for microsphere injections and a tourniquet placed around the inferior vena cava allowed brief intermittent dynamic preload reductions.
Baseline variables were obtained: arterial blood gases (OPTI CCA-TS2, OPTI Medical Systems, Atlanta, GA), serum troponin-T (Troponin-T hs, Roche Diagnostics GmbH, Mannheim, Germany), haemodynamic variables and the first injection of 15 µm fluorescent microspheres (Dye-Trak “F”; Triton Technology Inc., San Diego, CA, USA). After a short period of pre-oxygenation and respirator shut-off in the end expirium, LV pressure-volume loops were registered before and during a brief period of inferior vena cava occlusion. During a bolus of 5 mL hypertonic saline (10%) injected into the pulmonary artery, pressure-volume loops were recorded three times for the estimation of parallel conductance.14,15 Echocardiographic recordings were obtained for evaluation of strain and strain rate during brief periods of respiratory shut-off. Pulsed-wave Doppler signals in the aortic outflow tract, speckle tracking echocardiography (STE) in the 4-chamber and the short-axis views and tissue Doppler imaging (TDI) of the anterior left ventricular wall in short-axis view were recorded (for details see Supplementary Material).
Experimental protocol
The animals were block-randomised to either the STH-POL or the STH-2 group (10 per group). The heart-lung machine was primed with 1200 mL Ringer’s acetate. Systemic heparinization (500 IU/kg) was followed by cannulation of the brachiocephalic artery (EOPA 18 Fr, Medtronic Inc., Minneapolis, MN, USA) and the right atrial appendage (MC2 28/36 Fr, Medtronic Inc.). Cardiopulmonary bypass (CPB) was established with a flow of 90 mL/min/kg and a water temperature of 32°C in the heat exchanger. After aortic cross-clamping, cardioplegia was induced, either with normokalaemic STH-POL or hyperkalaemic STH-2 cardioplegia. Both solutions were pre-prepared as concentrate, modified with procaine and administered as cold (12°C), oxygenated, blood cardioplegia, freshly mixed by a dual-head pump with separate cooling. The cardioplegia was given into the aortic root, with the flow set to 7% of CPB flow, following a standardised protocol with an initial ‘high-dose’ for 3 min and 2 min of ‘low-dose’ at 20, 40, 60, 80 and 100 min after cross-clamping. The final concentrations of key components in the cardioplegic solutions are presented in Table 1. The aortic cross-clamp time was 120 min. A left heart vent catheter (DLP 13, Medtronic Inc.), temporarily replacing the pressure-conductance catheter, was placed through the apex and allowed passive drainage of the heart during the arrest. The body temperature drifted towards 34°C and CPB flow was reduced to 72 mL/min/kg when the rectal temperature reached 35°C or after 20 min. After 100 min of cross-clamping, systemic rewarming was initiated, with CPB flow reset to 90 mL/min/kg and the water temperature at 40°C. Arterial blood gases were obtained before cross-clamping, after 60 min and prior to declamping after 120 min. During CPB, the ventilator volume was reduced to 50%. Additional heparin (250 IU/kg) was given after 60 min of cross-clamping. Defibrillation was the only allowed anti-arrhythmic intervention if ventricular fibrillation occurred at declamping. After 10 min of reperfusion, the animals were weaned from CPB and the residual blood in the circuit infused followed by decannulation. Protamine sulphate 2 mg/kg was given for heparin reversal.
Table 1.
Final molar concentrations in oxygenated blood cardioplegia.
| STH-POL |
STH-2 |
|||
|---|---|---|---|---|
| High-dose 3 min |
Low-dose 2 min |
High-dose 3 min |
Low-dose 2 min |
|
| Esmolol (mM) | 1.35 | 0.68 | − | − |
| Adenosine (mM) | 0.50 | 0.25 | − | − |
| Mg2+ (mM) | 20 | 10 | 16 | 9 |
| K+ (mM) | 4.3 | 4.3 | 22 | 14 |
| Cl− (mM) | 106 | 106 | 134 | 120 |
| Procaine-HCl (mM) | 0.8 | 0.4 | 0.8 | 0.4 |
STH-POL: Modified St.Thomas’ Hospital polarizing cardioplegic solution; STH-2: Modified St.Thomas’ Hospital cardioplegic solution No 2.
After weaning from CPB, general haemodynamics were continuously recorded for 240 min. As for Baseline, measurements of LV pressures, volumes, systolic and diastolic function, regional tissue blood flow and echocardiographic recordings were repeated at 60, 150 and 240 min after reperfusion. Finally, the animal was euthanized with an intracardiac injection of high-dose potassium chloride, the heart was explanted and samples were obtained for regional tissue blood flow measurements, tissue water content, caspase-3 activity and levels of malondialdehyde (MDA).
Global and regional left ventricular function
Associated data files obtained by the pressure-conductance catheter (1 for evaluation of function, 3 for parallel conductance) were coded and analysed as 80 independent entities with custom-made software. The mean of 5-8 cardiac cycles during the stable situations and load-independent variables obtained during dynamic preload reductions were calculated. Absolute volumes were estimated by correcting for parallel conductance and cardiac output. Volumes were indexed by body surface area calculated as BSA (m2) = (BW2/3 x k)/100, where BW is body weight in kg and k for pigs is 9 m2/kg−2/3.16 The LV mechanical efficiency (Joules/mL/g) was calculated as a ratio between the total pressure-volume area (PVA) and the blood flow rate by the formula; Mechanical efficiency = (PE+SW) x HR/BFR, where PE and SW are pressure-volume areas for the LV potential energy and stroke work in Joules, HR is heart rate and BFR is LV blood flow rate per gramme of tissue.
Regional myocardial strain and strain rate were recorded with STE and TDI. Peak systolic strain in the circumferential, radial and longitudinal direction and radial peak ejection strain rate were measured with appropriate software (EchoPAC BT12, GE Vingmed Ultrasound).
Myocardial tissue samples and analyses
Multiple tissue samples were obtained from the LV endo-, mid- and epicardium and the right ventricle (RV). Myocardium, snap-frozen and stored in liquid nitrogen, was analysed for apoptosis by caspase-3 activity (K105-400, BioVision Inc., Milpitas, CA, USA) and for oxidative stress by MDA (K739-100, BioVision Inc.). Samples were homogenised, lysed and analysed according to the manufacturer’s instructions. Samples from the left and right ventricles and reference blood were weighed, hydrolysed, microspheres isolated, colours dissolved and quantified by fluorospectrophotometry. Blood flow rate was calculated as earlier described.17 Tissue water content was calculated as a fraction of the wet weight after drying samples for three weeks at 60°C.
Statistical analysis
Data analyses were performed by SPSS v. 23 (IBM Corp., Armonk, NY) and the values given as mean±SEM or median (25% percentile; 75% percentile) unless otherwise noted. Groups were compared at Baseline by two-sample Student t-test and Wilcoxon-Mann-Whitney test on ranks, whenever appropriate. Haemodynamic variables during and after CPB were compared with two-way analysis of variance for repeated measures (RM-ANOVA). For details see Supplementary Material.
Results
Characteristics at Baseline and during CPB
Eight animals were excluded due to reasons other than technical failure during surgery or instrumentation. Of these, seven animals, two in the STH-POL and five in the STH-2 group, developed severe pulmonary hypertension and right heart failure soon after declamping and weaning from CPB. The concomitant reduction of LV filling and systemic hypotension ended in myocardial ischemia and LV failure. One animal in the STH-POL group developed severe tachyarrhythmia at reperfusion, making further evaluation futile. Excluded animals were replaced by the subsequent experiment and results are given for 10 animals in each group.
At Baseline, the STH-POL and STH-2 groups were similar regarding variables describing general haemodynamics, left ventricular and myocardial function, tissue blood flow rate, temperature, blood gases and serum troponin-T levels (Supplement Table A, Figures 2-4). Mean arterial pressure (MAP) was significantly lower in the STH-POL at 60, 80 and 100 min of cardioplegic arrest compared to the STH-2 group (Figure 1). Serum potassium did not differ before cross-clamping while gradually increasing over time in both groups, but was more pronounced with STH-2 cardioplegia. At declamping, the serum potassium averaged 4.4±0.1 mmol/L in the STH-POL vs. 5.5±0.2 mmol/L in the STH-2 group (p < 0.001). Arterial blood gases, haemoglobin and other electrolytes did not differ between the groups during cross-clamping (Supplement Table B). Electrical cardioversion of ventricular fibrillation at declamping was needed in 2 out of 10 animals in the STH-POL vs. 8 out of 10 in the STH-2 group (p=0.025).
Figure 2.
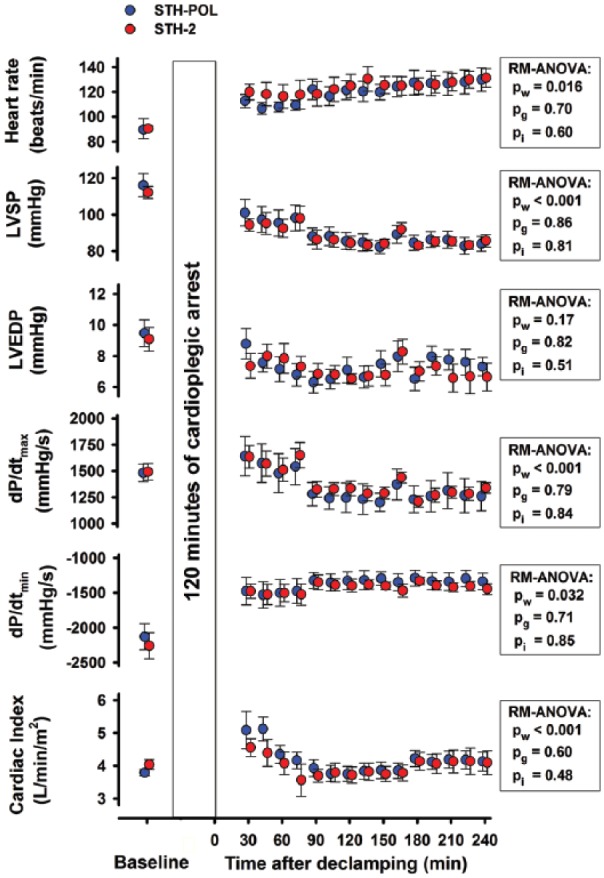
Haemodynamic variables, mean (SEM) or median (25%; 75%), at Baseline and every 15 min of reperfusion after CPB and 120 min of cardiac arrest with polarizing (STH-POL; n=10) and depolarizing (STH-2; n=10) cardioplegia. LVSP and LVEDP: left ventricular peak systolic- and end-diastolic pressures; dP/dtmax and dP/dtmin: peak positive and peak negative of the first derivate of the left ventricular pressure; RM-ANOVA: analysis of variance for repeated measurements; pw, pg and pi: p-values for within subjects, between groups and interaction from two-way RM-ANOVA, respectively.
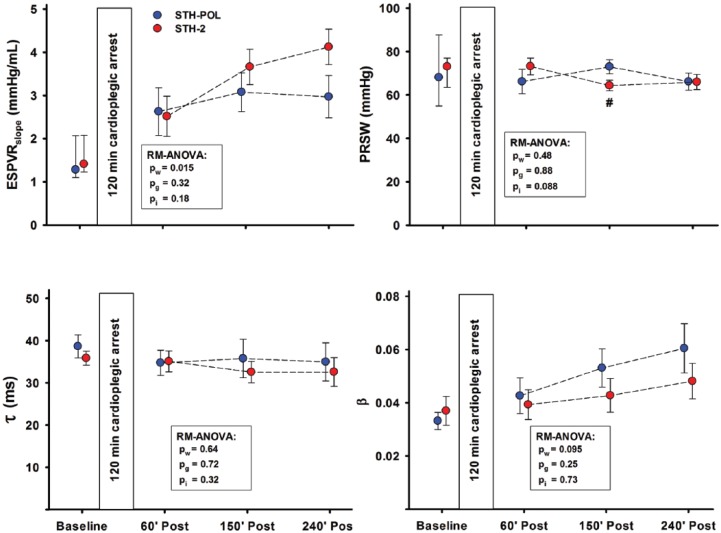
Figure 3.
Left ventricle functional variables mean (SEM) or median (25%; 75%) at Baseline and 60, 150 and 240 min after CPB and aortic declamping following 120 min of cardiac arrest with polarizing (STH-POL) and depolarizing (STH-2) cardioplegia, n=10 in both groups. Statistics as in Figure 2. ESPVRslope: slope of the end-systolic pressure volume relationship; PRSW: slope of preload recruitable stroke work; Tau (τ): isovolumic relaxation constant; Beta (β): the logarithmic end-diastolic pressure-volume relationship. #: significantly different from STH-POL at 150 min of reperfusion.
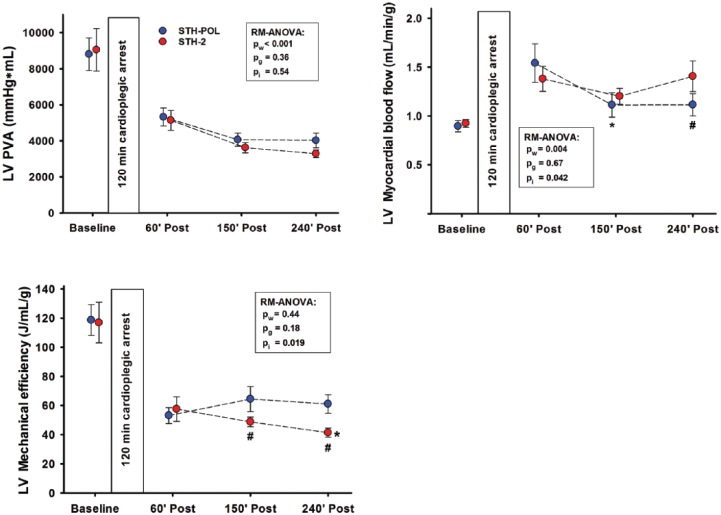
Figure 4.
Left ventricular (LV) pressure-volume area (PVA), myocardial blood flow rate and mechanical efficiency at Baseline and 60, 150 and 240 min after CPB and aortic declamping following 120 min of cardioplegic arrest, n=10 in both groups. Statistics as in Figure 2. *: significantly different from 60 to 150 or 240 min of reperfusion within group; #: significantly different between groups at corresponding point of time.
Figure 1.
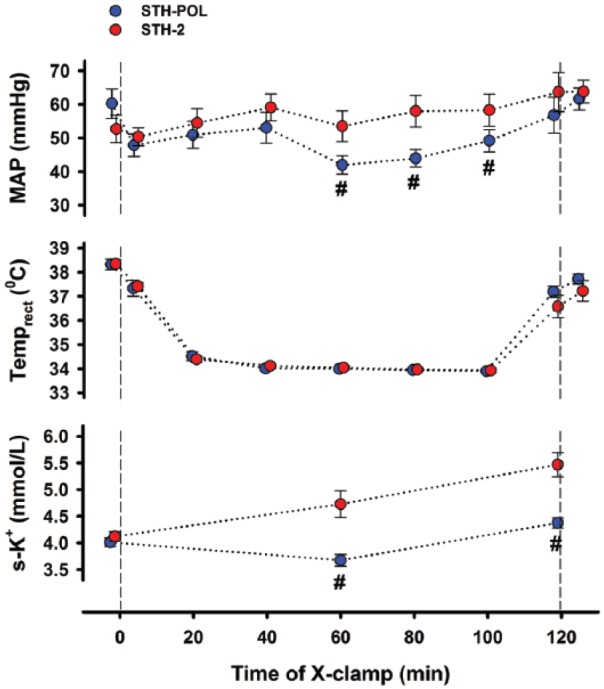
Mean arterial pressure (MAP), temperature and serum potassium (mean±SEM) during 120 min of cardioplegic arrest with polarizing (STH-POL; n=10) and depolarizing (STH-2; n=10) cardioplegia. #: p<0.05 vs. STH-2 at the corresponding point in time.
Haemodynamic and cardiac variables after CPB
The heart rate (HR) gradually increased in both groups over time while the left ventricular systolic pressure (LVSP) decreased after weaning from CPB (Figure 2). The left ventricular end-diastolic pressure (LVEDP) was unchanged. The peak positive derivative of LV pressure, dP/dtmax, decreased from 90 min and onwards, while peak negative dP/dtmin increased. Cardiac index (CI) decreased gradually in the first 90 min after declamping, levelled out around 175 min and then stabilised at levels comparable to the Baseline in both groups. The slope of the LV end-systolic pressure-volume relation (ESPVRslope) increased in both groups during reperfusion (Figure 3). At 240 min after declamping, there was a borderline difference between the groups (p=0.088). PRSW was significantly increased in the STH-POL compared to the STH-2 group 150 min after declamping (73.0±3.2 vs. 64.3±2.4 mmHg, p=0.047), but did not differ after 240 min. The LV isovolumic relaxation time constant τ and the logarithmic end-diastolic pressure-volume relation β did not differ over time or between groups. Both peak systolic strain in the longitudinal direction (STE) and radial peak ejection strain rate (TDI) decreased over time in both groups (Supplement Figure A and B).
The LV end-diastolic volume (LVEDV), stroke volume and stroke work decreased in both groups from 60 min to 150 min after aortic declamping and reperfusion, paralleled by a borderline significant (pw=0.052) decrease in the RV end-diastolic volume (Supplement Table C).
Myocardial blood flow and mechanical efficiency
Regional tissue blood flow rate in the RV wall did not differ between the groups after declamping, decreased from 60 min to 150 min after declamping (p = 0.001) and did not change further (Supplement Table C). LV tissue blood flow rate was high in both groups compared to levels seen at Baseline (Figure 4). Blood flow decreased significantly in the STH-POL group only (p<0.005) from 60 min to 150 min after declamping and was essentially unchanged in the STH-2 group. At 240 min after declamping, LV blood flow was significantly reduced in the STH-POL compared to the STH-2 group (p<0.05). The ratio between LV mechanical energy gain and blood flow rate was reduced by approximately 50% 60 min after aortic declamping compared to Baseline (Figure 4). Left ventricular mechanical efficiency gradually decreased in the STH-2 group only and was significantly lower than in the STH-POL group after 150 and 240 min.
Myocardial oxidative stress, apoptosis and ischemic injury
Apoptotic activation evaluated by caspase-3 activity was more pronounced in the RV and subepicardial LV tissue compared to the myocardium in the mid- and subendocardial left ventricle, but with no differences between the treatment groups (Figure 5). Oxidative stress evaluated by MDA did not differ between different regions of the left and the right ventricle. Serum troponin-T levels accumulated over time from 60 to 240 min after declamping, but did not differ between groups (Supplement Table C). The levels of serum potassium were increased throughout the reperfusion period in the STH-2 compared to the STH-POL group (Supplement Table D).
Figure 5.
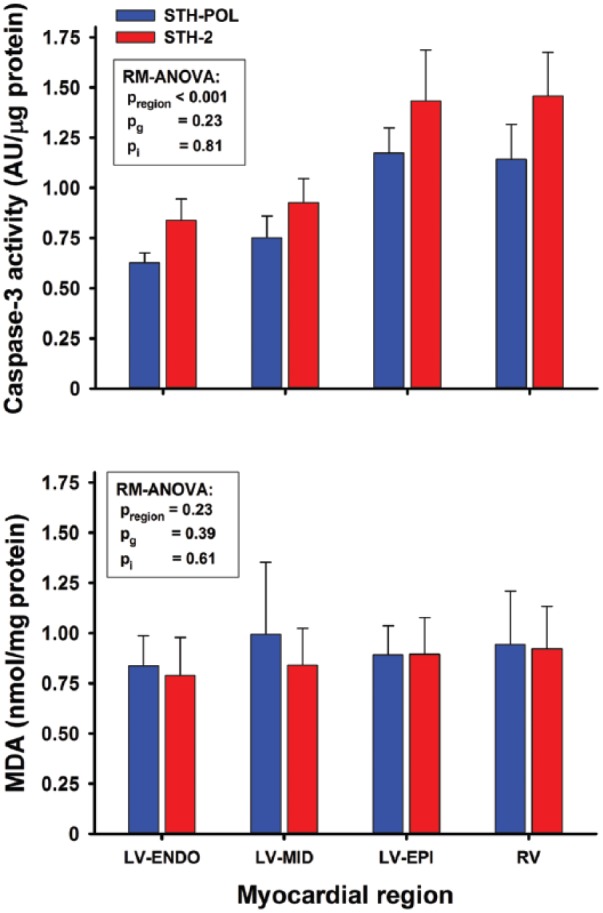
Caspase-3 activity and levels of MDA in myocardial tissue samples obtained at 240 min after reperfusion following CPB and 120 min of cardioplegic arrest with polarizing (STH-POL) or depolarizing (STH-2) cardioplegia, n=10 in both groups. MDA: malondialdehyde; LV: left ventricle; RV: right ventricle. pregion, pg and pi: p-values for regions within hearts, between groups and interaction from two-way RM-ANOVA, respectively.
Discussion
This porcine study indicates better myocardial contractile efficiency, demonstrated as a reduced mismatch between myocardial function and flow after weaning from CPB following 120 min of cardioplegic arrest with St.Thomas’ Hospital polarizing solution compared to standard blood cardioplegia. The pressure-volume loop area of the LV potential energy and mechanical performance strongly correlates with myocardial oxygen consumption and, hence, with myocardial blood flow rate.18–21 In the present study, both cardioplegic protocols demonstrate a mismatch between performance and myocardial blood flow rate compared to Baseline after 120 min of cardiac arrest (Figure 4). However, cardiac function did not differ between the groups evaluated by variables like CI and ejection fraction after reperfusion and weaning (Figure 2, Supplement Table C). Myocardial blood flow rate was reduced in the STH-POL compared to the STH-2 group at 240 min after declamping. This could be interpreted as a reduction of myocardial stunning following polarizing compared to depolarizing cardioplegia.22,23 Depolarizing cardioplegia exposes the myocardium to high levels of potassium during cardioplegic arrest. Several studies have demonstrated that hyperkalaemic cardiac arrest contributes to myocardial stunning.6,24,25
In a previous study, creatine phosphate and adenosine triphosphate (ATP) levels were temporary increased just before and 20 min after declamping following 60 min of cardioplegic arrest with STH-POL compared to STH-2 cardioplegia.12 Creatine phosphate exerts both anti-oxidant and anti-apoptotic effects.26 One could speculate if these factors might contribute to the improved mechanical efficiency (Figure 4) also seen after 120 min of cardioplegic arrest.
Myocardial blood flow rate is influenced by factors such as preload, afterload, heart rate and resistance in myocardial vessels.27 Neither LVEDP, LVEDV, HR nor MAP differed between the groups after weaning from CPB (Figure 2, Supplement Table C). Both adenosine and esmolol are short-acting and probably do not influence myocardial vascular resistance after reperfusion and weaning.11,28,29 In the STH-2 group, hyperkalaemia was present at 60, 150 and 240 min after declamping compared to the STH-POL group (Supplement Table D). Hyperkalaemic cardioplegic solutions may induce vasoconstriction by direct depolarization of vascular smooth muscle or the underlying endothelium in coronary arteries.30,31 Conversely, hyperkalaemia does not directly reduce myocardial vascular resistance following dilatation and, hence, relative hyperaemia.32
In the STH-POL group, two of 10 animals required defibrillation for ventricular fibrillation after declamping compared to eight out of 10 in the STH-2 group, probably related to increased potassium levels in the latter (Figure 1). Evidence of increased myocardial damage and apoptotic activity in the STH-2 group compared to the STH-POL group was not found in the present study, based on activated caspase-3 or s-troponin-T release (Figure 5, Supplement Table C). There is no evidence of myocardial damage caused by elective external cardioversion in patients, evaluated by the release of s-troponin-T.33,34
Myocardial levels of xanthine oxidase are found to be low in both pigs and in humans.35 Temporary high levels of hypoxanthine are to be expected in the STH-POL group due to degradation of the adenosine component.12 However, no difference in tissue levels of MDA was found, demonstrating that degradation of hypoxanthine to uric acid by xanthine oxidase did not cause an extra myocardial oxidative stress in the present study.36
Contractility, evaluated as PRSW, was increased 150 min after declamping with STH-POL cardioplegia, but did not differ from the STH-2 group after 240 min (Figure 3). In a previous study comparing STH-POL and STH-2 cardioplegia, LV contractility and myocardial strain rate were better maintained 180 min after weaning following 60 min of cardioplegic arrest.10 With an ischemic period of 120 min of cardioplegic arrest in this present study, group differences with regard to contractility faded after 240 min.
Limitations
The present study was performed in young, porcine hearts without clinically relevant pathology. Both the ischemic time and the observation period after declamping were limited. In our experience, 120 min of cardioplegic arrest following weaning from CPB and 240 min of reperfusion is close to the limit of what is feasible and reproducible for a translational animal study design in pigs. The replacement of three vs. five animals in the STH-POL and the STH-2 groups could introduce a selection bias. However, irrespective of cardioplegic solution, the frequency of arrhythmias and pulmonary hypertension after myocardial ischaemia/reperfusion and CPB is high in this model. For the purpose of standardisation, treatment interventions in this protocol were limited, which is reflected in a high mortality rate.
Clinical implications
Results from the present study demonstrate polarizing cardioplegia with esmolol, adenosine and magnesium to be non-inferior to standard depolarizing, potassium-based, repeated, oxygenated, blood cardioplegia. Clinical trials determining safety should be conducted before implementation into a new clinical routine. In theory, the use of STH-POL could be beneficial, especially in patients with preoperative left ventricular dysfunction and/or in patients requiring a long period of cardioplegic arrest.
Conclusion
Two hours of cardioplegic arrest with St.Thomas’ Hospital polarizing solution in oxygenated blood alleviates mismatch between myocardial function and perfusion after weaning from CPB compared to St.Thomas’ Hospital No 2 blood cardioplegia.
Supplemental Material
Supplemental material, Supplementary_File_NEW_12.04.18 for Left ventricular dysfunction after two hours of polarizing or depolarizing cardioplegic arrest in a porcine model by Terje Aass, Lodve Stangeland, Christian Arvei Moen, Atle Solholm, Geir Olav Dahle, David J. Chambers, Malte Urban, Knut Nesheim, Rune Haaverstad, Knut Matre and Ketil Grong in Perfusion
Acknowledgments
The technical assistance from Cato Johnsen, Kjersti Milde, Gry-Hilde Nilsen and the staff at the Vivarium, University of Bergen is greatly appreciated.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Co-author David J. Chambers declares a conflict of interest in that he is co-inventor of a novel cardioplegic solution in which esmolol and adenosine are essential components. A European patent has been granted for this cardioplegia; a US patent application is filed. All other authors declare no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was received from the Western Norway Regional Health Authority, the University of Bergen Heart Fund, the Norwegian Health Association and the Grieg Foundation.
ORCID iD: Terje Aass  https://orcid.org/0000-0003-4207-3454
https://orcid.org/0000-0003-4207-3454
References
- 1. Yamamoto H, Yamamoto F. Myocardial protection in cardiac surgery: a historical review from the beginning to the current topics. Gen Thorac Cardiovasc Surg 2013; 61: 485–496. [DOI] [PubMed] [Google Scholar]
- 2. Braathen B, Jeppsson A, Schersten H, et al. One single dose of histidine-tryptophan-ketoglutarate solution gives equally good myocardial protection in elective mitral valve surgery as repetitive cold blood cardioplegia: a prospective randomized study. J Thorac Cardiovasc Surg 2011; 141: 995–1001. [DOI] [PubMed] [Google Scholar]
- 3. Ramanathan R, Parrish DW, Armour TK, et al. Use of del Nido cardioplegia in adult cardiac surgery. Thorac Cardiovasc Surg 2015; 63: 624–627. [DOI] [PubMed] [Google Scholar]
- 4. Ferguson ZG, Yarborough DE, Jarvis BL, et al. Evidence-based medicine and myocardial protection–where is the evidence? Perfusion 2015; 30: 415–422. [DOI] [PubMed] [Google Scholar]
- 5. Zeng J, He W, Qu Z, et al. Cold blood versus crystalloid cardioplegia for myocardial protection in adult cardiac surgery: a meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth 2014; 28: 674–681. [DOI] [PubMed] [Google Scholar]
- 6. Dobson GP, Faggian G, Onorati F, et al. Hyperkalemic cardioplegia for adult and pediatric surgery: end of an era? Front Physiol 2013; 4: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehlhorn U, Sauer H, Kuhn-Regnier F, et al. Myocardial beta-blockade as an alternative to cardioplegic arrest during coronary artery surgery. Cardiovasc Surg 1999; 7: 549–557. [DOI] [PubMed] [Google Scholar]
- 8. Dobson GP, Letson HL. Adenosine, lidocaine, and Mg2+ (ALM): from cardiac surgery to combat casualty care–teaching old drugs new tricks. J Trauma Acute Care Surg 2016; 80: 135–145. [DOI] [PubMed] [Google Scholar]
- 9. Malhotra A, Wadhawa V, Ramani J, et al. Normokalemic nondepolarizing long-acting blood cardioplegia. Asian Cardiovasc Thorac Ann 2017; 25: 495–501. [DOI] [PubMed] [Google Scholar]
- 10. Aass T, Stangeland L, Moen CA, et al. Myocardial function after polarizing versus depolarizing cardiac arrest with blood cardioplegia in a porcine model of cardiopulmonary bypass. Eur J Cardiothorac Surg 2016; 50: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chambers DJ, Fallouh HB. Cardioplegia and cardiac surgery: pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol Ther 2010; 127: 41–52. [DOI] [PubMed] [Google Scholar]
- 12. Aass T, Stangeland L, Chambers DJ, et al. Myocardial energy metabolism and ultrastructure with polarizing and depolarizing cardioplegia in a porcine model. Eur J Cardiothorac Surg 2017; 52: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fannelop T, Dahle GO, Matre K, et al. An anaesthetic protocol in the young domestic pig allowing neuromuscular blockade for studies of cardiac function following cardioplegic arrest and cardiopulmonary bypass. Acta Anaesthesiol Scand 2004; 48: 1144–1154. [DOI] [PubMed] [Google Scholar]
- 14. Steendijk P, Staal E, Jukema JW, et al. Hypertonic saline method accurately determines parallel conductance for dual-field conductance catheter. Am J Physiol Heart Circ Physiol 2001; 281: H755–763. [DOI] [PubMed] [Google Scholar]
- 15. Szwarc RS, Mickleborough LL, Mizuno S, et al. Conductance catheter measurements of left ventricular volume in the intact dog: parallel conductance is independent of left ventricular size. Cardiovasc Res 1994; 28: 252–258. [DOI] [PubMed] [Google Scholar]
- 16. Hawk C, Leary SL, Morris TH. Formulary for laboratory animals. 3rd Edition. Ames: Blackwell Publishing Professional, 2005, p.203. [Google Scholar]
- 17. Kowallik P, Schulz R, Guth BD, et al. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation 1991; 83: 974–982. [DOI] [PubMed] [Google Scholar]
- 18. Gibbs CL. Mechanical determinants of myocardial oxygen consumption. Clin Exp Pharmacol Physiol 1995; 22: 1–9. [DOI] [PubMed] [Google Scholar]
- 19. Knaapen P, Germans T, Knuuti J, et al. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation 2007; 115: 918–927. [DOI] [PubMed] [Google Scholar]
- 20. Suga H. Ventricular energetics. Physiol Rev 1990; 70: 247–277. [DOI] [PubMed] [Google Scholar]
- 21. Vinten-Johansen J, Duncan HW, Finkenberg JG, et al. Prediction of myocardial O2 requirements by indirect indices. Am J Physiol 1982; 243: H862–868. [DOI] [PubMed] [Google Scholar]
- 22. Heusch G, Schulz R. Perfusion-contraction match and mismatch. Basic Res Cardiol 2001; 96: 1–10. [DOI] [PubMed] [Google Scholar]
- 23. Kassiotis C, Rajabi M, Taegtmeyer H. Metabolic reserve of the heart: the forgotten link between contraction and coronary flow. Prog Cardiovasc Dis 2008; 51: 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damiano RJ, Jr., Cohen NM. Hyperpolarized arrest attenuates myocardial stunning following global surgical ischemia: an alternative to traditional hyperkalemic cardioplegia? J Card Surg 1994; 9: 517–525. [DOI] [PubMed] [Google Scholar]
- 25. Anselmi A, Abbate A, Girola F, et al. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg 2004; 25: 304–311. [DOI] [PubMed] [Google Scholar]
- 26. Gaddi AV, Galuppo P, Yang J. Creatine phosphate administration in cell energy impairment conditions: a summary of past and present research. Heart Lung Circ 2017; 26: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 27. Schremmer B, Dhainaut JF. Regulation of myocardial oxygen delivery. Intensive Care Med 1990; 16 Suppl 2: S157–163. [DOI] [PubMed] [Google Scholar]
- 28. Belardinelli L, Shryock JC, Song Y, et al. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J 1995; 9: 359–365. [DOI] [PubMed] [Google Scholar]
- 29. Sum CY, Yacobi A, Kartzinel R, et al. Kinetics of esmolol, an ultra-short-acting beta blocker, and of its major metabolite. Clin Pharmacol Ther 1983; 34: 427–434. [DOI] [PubMed] [Google Scholar]
- 30. Han JG, Yang Q, Yao XQ, et al. Role of large-conductance calcium-activated potassium channels of coronary arteries in heart preservation. J Heart Lung Transplant 2009; 28: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 31. He GW. Endothelial function related to vascular tone in cardiac surgery. Heart Lung Circ 2005; 14: 13–18. [DOI] [PubMed] [Google Scholar]
- 32. Goodwill AG, Dick GM, Kiel AM, et al. Regulation of coronary blood flow. Compr Physiol 2017; 7: 321–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cemin R, Rauhe W, Marini M, et al. Serum troponin I level after external electrical direct current synchronized cardioversion in patients with normal or reduced ejection fraction: no evidence of myocytes injury. Clin Cardiol 2005; 28: 467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lund M, French JK, Johnson RN, et al. Serum troponins T and I after elective cardioversion. Eur Heart J 2000; 21: 245–253. [DOI] [PubMed] [Google Scholar]
- 35. Muxfeldt M, Schaper W. The activity of xanthine oxidase in heart of pigs, guinea pigs, rabbits, rats, and humans. Basic Res Cardiol 1987; 82: 486–492. [DOI] [PubMed] [Google Scholar]
- 36. Kim YJ, Ryu HM, Choi JY, et al. Hypoxanthine causes endothelial dysfunction through oxidative stress-induced apoptosis. Biochem Biophys Res Commun 2017; 482: 821–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_File_NEW_12.04.18 for Left ventricular dysfunction after two hours of polarizing or depolarizing cardioplegic arrest in a porcine model by Terje Aass, Lodve Stangeland, Christian Arvei Moen, Atle Solholm, Geir Olav Dahle, David J. Chambers, Malte Urban, Knut Nesheim, Rune Haaverstad, Knut Matre and Ketil Grong in Perfusion