Abstract
Schistosomiasis is a leading cause of pulmonary hypertension (PH) worldwide. Recent studies reveal that the type-2 immune cytokines IL-4 and IL-13, as well as consequent activation of TGF-β, are key factors in the pathogenesis of Schistosoma-PH. Paclitaxel has been reported to act as an adjuvant for Th2 inflammation while downregulating TGF-β activation. Moreover, paclitaxel blocks PH in monocrotaline and SU5416-hypoxia models. We hypothesized that paclitaxel would augment Th2 inflammation while blocking TGF-β activation and PH after schistosomiasis exposure. Wild-type mice (C57BL6/J; 6/group) were intraperitoneally (IP) sensitized and then intravenously (IV) challenged with Schistosoma mansoni eggs. One day after IV egg challenge, the mice were treated with a single IP dose of 25 mg/kg paclitaxel or vehicle. Right ventricular (RV) catheterization was performed and granuloma volumes and vascular remodeling were quantified. Lung cytokines were quantified by ELISA and reverse transcription polymerase chain reaction, and the quantity of active TGF-β was determined using a cell reporter line. We also investigated hypoxia-induced PH. Paclitaxel treatment significantly protected mice from Schistosoma-PH, with decreased RV systolic pressure (P = 0.005) and pulmonary vascular media thickness. Inflammation was significantly suppressed, contrary to our hypothesis, with decreased IL-4 and IL-13 levels, smaller granulomas, and less active TGF-β following paclitaxel treatment. There was no change in IFN-γ or FoxO1 or FoxO3 expression. Paclitaxel did not suppress chronic hypoxia-induced PH, which is also TGF-β-driven but independent of type-2 immunity. Paclitaxel protects against Schistosoma-induced PH in mice, although by blocking proximate Th2 inflammation rather than suppressing distal TGF-β activation.
Keywords: parasitic infections, pulmonary hypertension experimental, inflammation
Introduction
Schistosomiasis is a disease caused by infection of a snail-born parasite from the Schistosoma genus of trematodes. The common species that cause schistosomiasis are Schistosoma mansoni, japonicum, and haematobium. Schistosoma is highly endemic in sub-Saharan African nations as well as Brazil, the Middle East, and Southeast Asia.1 Over 200 million people are chronically affected with schistosomiasis, of which close to 6% develop World Health Organization (WHO) Group 1 pulmonary arterial hypertension (PAH) after chronic and recurrent Schistosoma infection.2 PAH is thought to predominantly occur in those infected with the species S. mansoni compared to the other endemic species.3 Despite the serious impact of schistosomiasis worldwide, it remains massively undertreated relative to the impact of the disease worldwide and thus is considered as one of the six “neglected tropical diseases.”4
Recent findings indicate that the pathophysiology of experimental Schistosoma-pulmonary hypertension (PH), and likely human Schistosoma-PAH, is significantly related to the inflammatory responses arising from host immune system.5 The primary response to the early larval antigens is type-1 immunity,6 which is characterized by the secretion of IL-1, IL-12, and INF-γ, cytokines also characteristic of Th1-polarized CD4 T cells. Adult worms do not trigger a substantial immune response themselves.7 However, once the worms start laying eggs in chronic infection, the immune response against the now released egg antigens is dominated by type-2 immunity,3,8–10 which is characterized by the production of cytokines IL-4, IL-5, IL-10, and IL-13: cytokines also characteristic of Th2-polarized CD4 T cells. Furthermore, the downstream effect of type-2 cytokines triggers the activation of transforming growth factor β (TGF-β) which is a key driver of smooth muscle and endothelial cell proliferation and vascular remodeling, which results in the PH phenotype.11 TGF-β is also associated with a Th17 immune response. We recently reported that in Schistosoma-induced PH, the protein thrombospondin-1 activates TGF-β and that blocking thrombospondin-1 was protective against the PH phenotype.12 Thus, a therapeutic approach that specifically blocks TGF-β activation could be a novel approach to prevent or reverse Schistosoma-induced PH. It would be ideal to have this TGF-β inhibiting effect independent of the Th2 response, which could occur by modulating a Th1 or Th17 immune phenotype or directly inhibiting TGF-β, as blocking type-2 immunity in individuals who live in areas endemic for schistosomiasis could leave the host potentially susceptible to recurrent or poorly controlled infection.
One potentially attractive medication is paclitaxel, an FDA-approved drug that is a widely used chemotherapeutic. It inhibits cell division by binding to tubulin, which prevents the disassembly of microtubules.13 Paclitaxel is commonly used in breast cancer, ovarian cancer, lung cancer, and AIDS-related Kaposi sarcoma. A recent study suggested that paclitaxel can augment both type-1 and type-2 immunity in a model of ovalbumin sensitization and challenge.14 Paclitaxel has also been shown to suppress TGF-β signaling in skin grafts in a scleroderma model and in hepatic stellate cells.15,16 Moreover, paclitaxel has recently been shown to augment the FoxO1 transcription factor, which blocks pulmonary vascular smooth muscle cell proliferation in monocrotaline and SU5416-hypoxia-exposed rats with experimental PH.17 Therefore, based on these data, we hypothesized that paclitaxel would augment type-2 immunity while blocking TGF-β activation and PH following S. mansoni exposure.
Methods
Animal models
Six-week-old C57BL6/J background wild-type (WT) mice were purchased from Jackson Laboratories. All animal studies and protocols were approved by the University of Colorado Institutional Animal Care and Use Committee.
S. mansoni-induced PH
We used our well-established mouse model of S. mansoni-induced PH, as described previously.8,11,12 Briefly, we harvested eggs from cercaria-infected Swiss Webster mice provided by NIAID Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD, USA). The experimental mice were intraperitoneally sensitized with 240 S. mansoni eggs per gram of mice weight and then intravenously challenged with 175 S. mansoni eggs per gram of mice two weeks later (experiment outline in Fig. 1a).
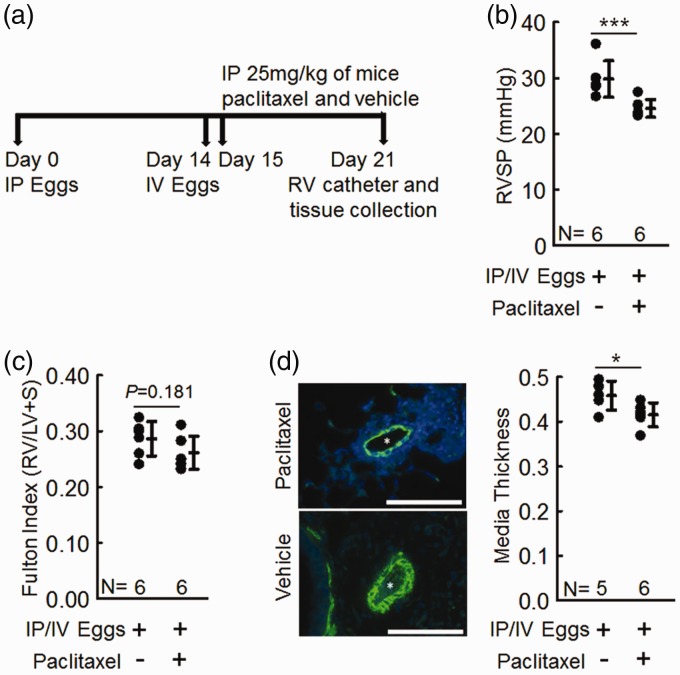
Fig. 1.
Paclitaxel-treated mice are protected from Schistosoma-induced PH compared to vehicle-treated mice. (a) Experimental time line for testing the effect of paclitaxel in Schistosoma-induced PH. (b) RVSP of intraperitoneal and intravenous (IP/IV) egg-injected mice; n = 6 per group; t-test. (c) RV hypertrophy (Fulton index = (RV/LV+Septum)); n = 6 per group; t-test. (d) Vascular media thickness of paclitaxel-treated and vehicle-treated mice; n = 5 in vehicle-treated mice, n = 6 in paclitaxel-treated mice; t-test; graphs show mean ± SD; scale bars = 50 µm.
Right ventricular systolic pressure (RVSP) and right ventricular hypertrophy measurement
To measure RVSP, the mice were anesthetized with IP ketamine-xylazine and a tracheostomy placed for mechanical ventilation. The abdomen and diaphragm were surgically opened, and a 1-Fr pressure–volume catheter (PVR-1035, Millar AD Instruments, Houston, TX, USA) was placed directly into the right ventricle (RV) and then the left ventricle (LV) chambers through the free walls. The lungs were then flushed with phosphate buffered solution (PBS), the right lobes were sutured, and the left lung was inflated with 1% low melt agarose for formalin fixation and paraffin embedding (FFPE) for histology assessment. We divided the right lung lobes for protein by snap freezing quantification or placed in RNAlater (Life Technologies, Carlsbad, CA, USA) for RNA quantification. RV hypertrophy (Fulton Index) was measured by dividing RV mass by LV plus septum mass.
Hypoxia-induced model of PH
As described previously,12,18 WT mice were placed into hypoxia chamber with maintained 10% FiO2 at Denver altitude for two weeks. The partial pressure of oxygen was regulated by a flow of nitrogen gas into hypoxia chamber under the control of ProOx 110 (Biospherix) oxygen sensor.
Paclitaxel treatment
Paclitaxel (LC Laboratories, Woburn, MA, USA) was reconstituted in PBS and a dose of 25 mg/kg of mice was IP administered one day after intravenous augmentation of the mice (challenge) with S. mansoni eggs. The dose was selected as per the prior report.17 Control mice were given PBS only. In the chronic hypoxia model, paclitaxel was IP administered at a dose of 25 mg/kg of mice, at days 1 and 8 .
Vascular remodeling assessment
Formalin-fixed and paraffin-embedded lung tissue was immunostained for α-smooth muscle actin (antibody from Dako, Agilent, Santa Clara, CA, USA) as previously reported.8,11,12 Images from stained slides were captured using Nikon Eclipse E800 microscope (Nikon, Melville, NY, USA) and CCD camera (Photometrics, Tucson, AZ, USA). The vascular media thickness was quantified using image processing software (Image Pro Plus v4.5.1, Media Cybernetics, Bethesda, MD, USA).
Estimated granuloma volume assessment
The optical rotator stereological method was used to estimate the peri-egg granuloma volume.19 Images of hematoxylin and eosin (H&E)-stained granulomas that surround a single egg were captured. Peri-egg granuloma volumes were measured using image processing software (Image Pro Plus v4.5.1, Media Cybernetics, Bethesda, MD, USA) by using the egg as the center point.
Protein assessment and ELISA
Snap-frozen whole-lung tissue was macerated and sonicated in RIPA buffer containing anti-proteases. Bradford assay (5000201, BioRad, Hercules, CA, USA) was used to measure protein concentration. IL-4, IL-13, and IFN-γ protein concentrations in mouse lung lysates were quantified by ELISA using kits (M4000B, M1300CB, and MIF00, respectively) from R&D Systems (Minneapolis, MN, USA).
Messenger (mRNA) assessment
Whole-lung tissue banked in RNAlater was used to obtain RNA using Qiagen RNAeasy kit (Hilden, Germany). Reverse transcription polymerase chain reaction (RT-PCR) for IL-23, FoxO1, FoxO3, and β-actin was performed using commercially available primers (Mm00518984_m1 Il23a, Mm00490671_m1 Foxo1, Mm011185722_m1 Foxo3, and Mm02619580_g1 Actb, respectively) from Applied Biosystems (Foster City, CA, USA). The 2-ΔCt method was used for analyzing the results.
TGF-β assay and GRP-Rho A quantification
The concentrations of active TGF-β and GTP-RhoA in the whole lung was assessed as described previously.8,12 Briefly, whole-lung tissue lysates were added to a cellular assay using mink lung epithelial cells (MLEC) transfected with a human plasminogen activator inducer (PAI)-1 promoter having firefly luciferase reporter gene to detect TGF-β activity (MLECs were kindly provided by Dr. Daniel Rifkin, NYU).20 To measure total TGF-β concentration, lysates were heated for 20 min at 100 ℃. The luciferase activity was recorded as relative light units (RLU). RLU values were converted to TGF-β activity (pg/mL) using a standard curve generated using serial dilution of recombinant TGF-β1. To determine the concentration of active GTP Rho A, GLISA was performed using freshly made lung lysates. The activity was recorded at optical density (OD) of 490 nm using the GTP-RhoA GELISA kit (Cytoskeleton Inc. Cat. no. BK124).
Flow cytometry assessment
Lungs from the experimental mice were digested for flow cytometry assessment to identify IL-4 and IL-13 producing Th2 CD4+ T cells as described previously.8,12 In summary, experimental mice lungs were perfused with PBS, macerated and suspended in 1 mL of 0.4 mg/mL of liberase in RPMI, and put in a 37 ℃ incubator for 30 min. The digested lungs were resuspended in plain 1 mL RMPI plus 100 µM EDTA and passed through an 18-gauge needle and then through a 16-gauge needle five times. The suspension was then filtered with a 100-µm filter and centrifuged at 1200 rpm for 10 min. The dispersed cells were resuspended in flow wash buffer (Invitrogen). Before staining extracellularly, cells were blocked for 20 min in ice using anti-CD16/CD32 antibody (1/50 dilution). The cells were incubated at 4 ℃ for 30 min with mouse AF700 anti-CD3 and mouse BV510 anti-CD4 antibodies, followed by fixation with IC fixation buffer for 20 min. Intracellular antibodies (mouse anti-INF-γ, anti-IL-4, anti-IL-17A, and anti-IL-17F labeled with PerCp-Cy5.5, AF488, APC, and PE, respectively) were diluted in permeabilization buffer (eBioscience, ThermoFisher Scientific) and incubated for 30 min at 4℃. The cells were then washed and ready for analysis. Acquisition was performed with a BD celesta flow cytometer.
Statistical analysis
SigmaPlot (version 13, Systat Software, San Jose, CA, USA) and ProStat (version 6, Poly Software International, Pearl River, NY, USA) were used to perform statistical analyses and presenting graphs. Statistical differences between the two groups were assessed by t-test. ANOVA was used to assess differences for ≥ 3 groups followed by post-hoc Tukey test. Non-normally distributed data was analyzed by non-parametric analysis. P values < 0.05 were considered statistically significant.
Results
Paclitaxel treatment protects from Schistosoma-induced PH
As shown in Fig. 1a, we sensitized female C57Bl/6 mice with S. mansoni eggs intraperitoneally, followed by intravenous challenge two weeks later. Previous utilization of this model demonstrates that the mice develop PH one week after challenge.3 One day after intravenous challenge, we treated the mice with 25 mg/kg IP paclitaxel or vehicle one day after intravenous challenge.
We found by RV catheterization that the paclitaxel-treated Schistosoma-exposed mice had significantly lower right ventricular systolic pressure (RVSP; Fig. 1b) and less RV hypertrophy (Fig. 1c) compared to vehicle-treated Schistosoma-exposed mice. Further, quantification of the vascular remodeling by immunostaining for α-smooth muscle actin showed significant reduction in the vascular media thickness in paclitaxel treated Schistosoma-exposed mice compared to vehicle-treated Schistosoma-exposed mice (Fig. 1d).
Paclitaxel treatment blocks the type-2 immune response induced by Schistosoma
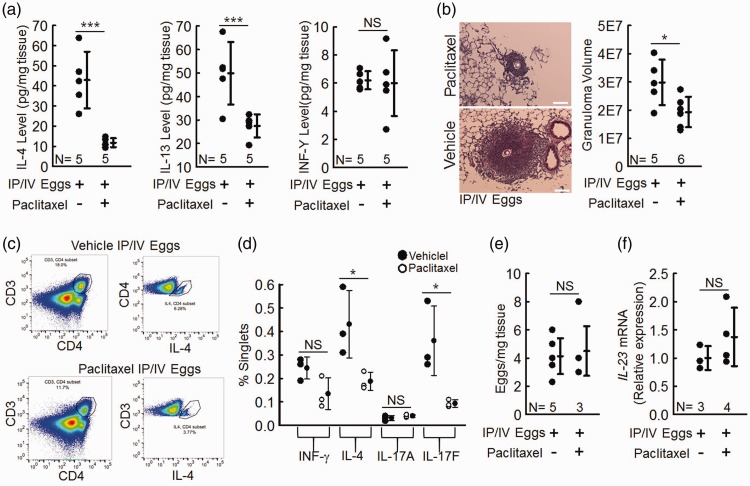
Inflammation is critical to the pathogenesis of Schistosoma-PH. The immune response in chronic Schistosoma-induced PH is type 2, characterized by the cytokines IL-4 and IL-13.8 We assessed type-2 immunity by performing ELISA on whole-lung lysates to determine the concentration of IL-4 and IL-13. Paclitaxel-treated Schistosoma-exposed mice had considerably lower IL-4 and IL-13 concentrations (Fig. 2a). The size of the peri-egg granulomas is another indicator of the severity of type-2 inflammation around S. mansoni eggs in the lung.3 We quantified the estimated granuloma volume and found that paclitaxel-treated Schistosoma-exposed mice had a significantly lower granuloma volume than vehicle-treated Schistosoma-exposed mice (Fig. 2b), also consistent with a decrease in type-2 immunity.
Fig. 2.
Schistosoma-exposed mice have a significantly lower Th-2 immune response and no significant difference in Th-1 and Th-17 immune response when treated with paclitaxel. (a) IL-4, IL-13, and INF-γ protein concentrations in paclitaxel-treated and vehicle treated mice; t-test. (b) Estimated granuloma volumes; t-test. (c) Flow sorting of CD4+ and Th2 CD4+ (intracellular IL-4+) T cells (representative of n = 3 per group). (d) Percentage of all cells which are Th-1 (IFN-γ+), Th-2 (IL-4+), and Th-17 (IL-17A+ or IL-17F+) T cells; n = 3 per group; t-test. (e) IL-23 mRNA expression (as a marker of Th-17 inflammation); t-test. (f) Residual intrapulmonary Schistosoma egg burden in paclitaxel-treated and vehicle-treated mice; t-test. Graphs show mean ± SD.
It is also possible that paclitaxel could modulate type-1 immunity, although this is not a characteristic of Schistosoma egg-induced PH.3 We assayed for the quantity of IFN-γ, the characteristic cytokine of type-1 immunity, and found it to be unchanged by paclitaxel treatment (Fig. 2a).
Paclitaxel treatment decreases CD4 Th2 cells
Th2 CD4+ T cells are major producers of the type-2 immune cytokines IL-4 and IL-13.8 We analyzed the density and phenotype of CD4+ T cells in Schistosoma IP/IV egg exposed mice using flow cytometry. We found that paclitaxel-treated Schistosoma-exposed mice had fewer CD4+ T cells compared to vehicle-treated Schistosoma-exposed mice (Fig. 2c). We then identified Th-1, Th-2, or Th-17 phenotypes of CD4+ T cells by intracellularly staining for key markers of each type, specifically INF-γ (Th-1), IL-4 (Th-2), and IL-17A or IL-17F (Th-17). There was no change in INF-γ+ CD4+ T cells between paclitaxel and vehicle treated Schistosoma-exposed mice, which is indicative of no type-1 immune activation (Fig. 2d). In contrast, we found the type-2 immune response was blocked by paclitaxel, as there were significantly fewer IL-4+CD4+ T cells in paclitaxel-treated Schistosoma-exposed mice compared to vehicle treatment. Our assessment of the type-17 immune response had a mixed result, in which IL-17A+ CD4+ T cells were unchanged while IL-17F+ CD4+ T cells were decreased in paclitaxel-treated Schistosoma-exposed mice compared to vehicle treatment. To clarify whether paclitaxel affected the type-17 immune response, we analyzed the whole-lung mRNA concentration of IL-23, which is a marker for the differentiation and activation of Th17 cells.21 We found no significant difference in IL-23 mRNA concentration in paclitaxel-treated compared to vehicle-treated Schistosoma-exposed mice (Fig. 2f), indicating there was no major change in Th17 immunity.
One possible reason why the RVSPs could be lower in paclitaxel-treated Schistosoma-exposed mice is that they could clear the Schistosoma eggs faster. We tested for this by quantifying the number of eggs left in the lungs at the time of RV catheterization. We found no difference in the residual egg density between paclitaxel-treated and vehicle-treated Schistosoma-exposed mice (Fig. 2e).
Paclitaxel treatment blocks the downstream activation of TGF-β
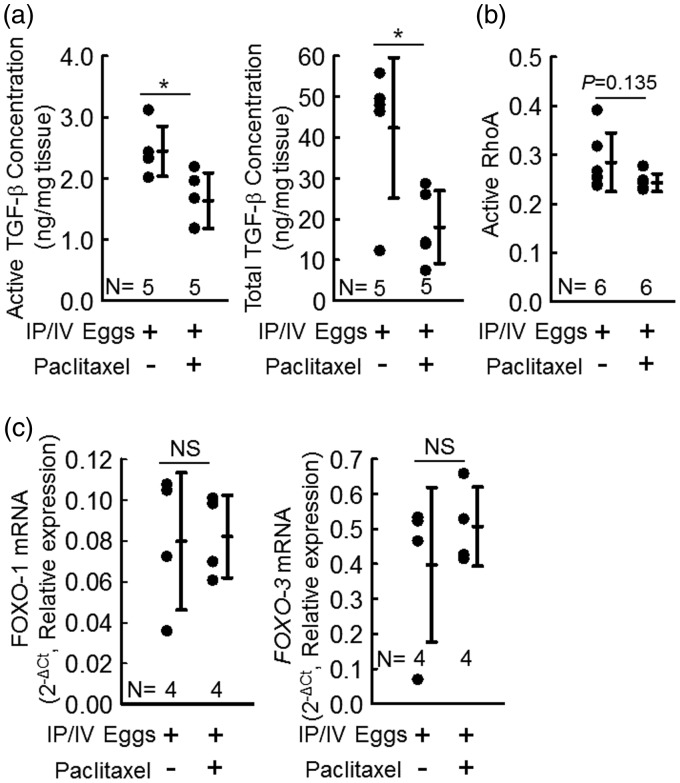
Activation of TGF-β by the Th-2 immune response is a critical step in developing Schistosoma-PH.11 We measured the concentration of active TGF-β in whole-lung lysates and found that paclitaxel-treated Schistosoma-exposed mice had significantly lower concentrations of active TGF-β compared to vehicle-treated Schistosoma-exposed mice (Fig. 3a). TGF-β expression is regulated by positive feedback loops. We assessed the total TGF-β concentration by heating the samples to activate all TGF-β present and quantified the concentration of total TGF-β. Interestingly, we found the concentration of total TGF-β was also significantly decreased in the paclitaxel-treated Schistosoma-exposed mice compared to the vehicle-treated Schistosoma-exposed mice. Next, we assessed the concentration of GTP-bound (active) RhoA concentration, a non-canonical signaling target of TGF-β, which contributes to the vasoconstriction of pulmonary vessels by activating Rho kinase.22 We found a mild trend towards decreased GTP-RhoA in paclitaxel-treated Schistosoma-exposed mice compare to vehicle-treated Schistosoma-exposed mice (P = 0.135; Fig. 3b). This result indicates that there could be a modest suppression of vasoconstriction, in addition to the significant decrease of fixed vascular remodeling, primarily due to Smad-mediated TGF-β signaling,11 that we identified above as a result of paclitaxel treatment. An alternative target which could be modulated by paclitaxel is the FoxO transcription factors. We quantified the expression of FoxO1 and FoxO3 by RT-PCR and found no changes by paclitaxel versus vehicle treatments (Fig. 3c).
Fig. 3.
Schistosoma-exposed mice have shown significantly lower active and total TGF-β protein concentration when treated with paclitaxel. (a) Active and total TGF-β concentrations in paclitaxel-treated and vehicle-treated mice; t-test. (b) Active (GTP-bound) RhoA concentration paclitaxel-treated and vehicle-treated mice; t-test. (c) Foxo1 and Foxo3 mRNA relative expression in paclitaxel-treated and vehicle-treated mice; n = 4 per group; t-test. Graphs show mean ± SD.
Paclitaxel treatment did not protect from non-Th-2 mediated (hypoxia-induced) PH
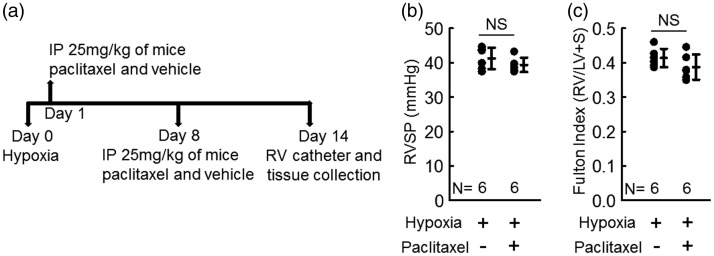
The decrease in active TGF-β in Schistosoma-exposed mice that we observed following paclitaxel treatment could be due to either a direct effect on TGF-β such as inhibiting its expression or activation or at the more proximate level of blocking the upstream type-2 immunity. In order to determine whether paclitaxel has a direct effect on the activation of TGF-β, we tested the effect of paclitaxel in the chronic hypoxia mouse model, which is also TGF-β dependent.12,23 Chronic hypoxia does not trigger a prototypic type-2 immunity, however, nor is the chronic hypoxia phenotype dependent on type-2 immune signaling.12 We exposed C57Bl/6 mice to normobaric hypoxia for two weeks (Fig. 4a) while we treated the mice with 25 mg/kg IP paclitaxel or vehicle weekly. We found by RV catheterization at the end of the experiment that there was no difference in PH severity between paclitaxel-treated and vehicle-treated hypoxic mice by RVSP (Fig. 4b) or RV hypertrophy (Fig. 4c), indicating that paclitaxel is unlikely to have a direct effect on TGF-β itself.
Fig. 4.
Paclitaxel treatment has no effect on chronic hypoxia-induced PH. (a) Experimental time line for testing the effect of paclitaxel in hypoxia-induced PH. (b) RVSP of hypoxic mice; t-test. (c) RV hypertrophy (Fulton index = (RV/LV + Septum)) of hypoxic mice; t-test. Graphs show mean ± SD.
Discussion
Previous studies have shown that type-2 inflammation and activation of TGF-β are key steps to the pathogenesis of multiple forms of PH, including Schistosoma-induced PH.3,8,12 Here, we evaluated the effect of paclitaxel on these two critical steps that lead to development of PH. We found that paclitaxel overall protected against the RVSP, RV hypertrophy, and vascular media thickness induced by Schistosoma exposure.
Surprisingly, and in contrast to our hypothesis, we found treatment with paclitaxel blocked the type-2 immune response in Schistosoma-exposed mice, despite the previously report that paclitaxel can augment type-2 inflammation in ovalbumin sensitized and challenged mice.14 The evidence we observed that type-2 immunity was suppressed following Schistosoma exposure includes lower IL-4 and IL-13 concentrations in whole-lung lysates, fewer CD4+ T cells and Th2-specific CD4+ T cells (which are a key source of the IL-4 and IL-13 cytokines8), and smaller peri-egg granulomas. The mechanism by which paclitaxel blocked the type-2 immunity is not clear, but it may have suppressed the recruitment, activation, and/or proliferation of the CD4+ T cells by its anti-proliferative mechanism of action. It is also unclear why the prior report of ovalbumin exposed mice found augmented type-2 immunity with paclitaxel in contrast to our findings; causes for the differences between these two models may be related to the specific antigen, route of administration, or compartment studied: the ovalbumin study used subcutaneous exposure and measured circulating immunoglobulins and splenocyte cytokine expression, as opposed to intravenous Schistosoma eggs and lung-specific immunity here. Another possibility is we used a higher dose of paclitaxel than the prior report (about a 500-µg dose for a 20-mg mouse here, versus a 200-µg fixed dose per mouse in the prior study12), potentially accounting for a different impact of Th2 inflammation.
We observed that treatment with paclitaxel lowered the concentration of active TGF-β after Schistosoma exposure. This suppression could result from either a direct effect of paclitaxel on TGF-β or its activation (as was reported with skin grafts in a scleroderma mouse model15 and in hepatic stellate cells16), or via an indirect effect through suppression of the proximate type-2 immunity upstream of TGF-β activation. We distinguished between these two possibilities using the chronic hypoxia mouse model, which is TGF-β dependent but type-2 immunity-independent,12,18,24 to determine if paclitaxel has a direct effect on TGF-β activation. We found that chronic hypoxia-exposed mice were not protected from PH by paclitaxel treatment. A possible limitation of these data is that although the paclitaxel was given one day after the start of the hypoxia exposure, this could have been too late to stop an immediate activation of TGF-β in this model. This result suggests that the suppression of TGF-β activation we observed in the Schistosoma-PH model was likely due to the suppression of proximate type-2 immunity blocking TGF-β activation, rather than a direct effect of paclitaxel on TGF-β activation itself. Increased TGF-β signaling can also alter the liver fibrosis phenotype in mice infected with S. mansoni cercariae, with increased fibrosis observed in BMPR2+/- mice.25
Other mechanisms that could mediate how paclitaxel impacts Schistosoma-induced PH is by altering Th1 or Th17 immunity, by decreasing FoxO transcription factors, or by altering the clearance of Schistosoma eggs. Schistosoma worm antigens (not used here) characteristically trigger type-1 immunity and egg antigens trigger type-2 immunity. We did not identify any change in IFN-γ expression by paclitaxel treatment in this model; indeed, we previously found that IFN-γ is not increased in Schistosoma egg challenged mice.3 We also did not observe a change in the density of Th1 CD4+ T cells. Type-17 immunity has been reported to play a key role in the development of chronic inflammatory diseases;21 however, we found no significant change in the protein concentrations of IL-17 or IL-23 with paclitaxel. We also quantified the mRNA expression of FoxO isoforms 1 and 3, which have been previously implicated in other etiologies of PH,17,26,27 but we did not find any change in either isoform following paclitaxel treatment. Our assessment of FoxO expression could be limited, however, in that FoxO activity can also be regulated at the level of phosphorylation which we did not assess. We also quantified the number of eggs left in the lungs after one week and found that paclitaxel treatment did not appear to block egg clearance in these mice.
In summary, we found that paclitaxel suppresses type-2 immunity, which subsequently downregulates activation of TGF-β and decreases the PH severity following Schistosoma exposure. Paclitaxel may be worthy of further study to prevent or reverse this or other etiologies of PH. However, the use of paclitaxel for patients with schistosomiasis-associated PAH who live in schistosomiasis-endemic areas may not be not an optimal therapeutic option, as blocking the type-2 immunity would be potentially harmful in those with ongoing parasite infection or are susceptible to reinfection due to their environmental exposures.
Acknowledgments
Schistosome-infected mice were provided by the NIAID Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD, USA) through NIH-NIAID Contract HHSN272201000005I for distribution through BEI Resources.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
Grant funding was provided by the NIH Grants 3R01HL135872-01S1 (BK and BBG), P01HL014985 (RMT and BBG), R03HL133306 (BBG), and R01HL135872 (BBG); and American Heart Association Grant 17POST33670045 (RK).
References
- 1.Butrous G. Saudi guidelines on the diagnosis and treatment of pulmonary hypertension: schistosomiasis and pulmonary arterial hypertension. Ann Thorac Med 2014; 9: S38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham BB, Kumar R. Schistosomiasis and the pulmonary vasculature (2013 Grover Conference series). Pulm Circ 2014; 4: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham BB, Mentink-Kane MM, El-Haddad H, et al. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. Am J Pathol 2010; 177: 1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol 2004; 2: 12–13. [DOI] [PubMed] [Google Scholar]
- 5.Papamatheakis DG, Mocumbi AOH, Kim NH, et al. Schistosomiasis-associated pulmonary hypertension. Pulm Circ 2014; 4: 596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maizels RM, Balic A, Gomez-Escobar N, et al. Helminth parasites–masters of regulation. Immunol Rev 2004; 201: 89–116. [DOI] [PubMed] [Google Scholar]
- 7.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 2011; 11: 375–388. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Mickael C, Chabon J, et al. The causal role of IL-4 and IL-13 in Schistosoma mansoni pulmonary hypertension. Am J Respir Crit Care Med 2015; 192: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosby A, Jones FM, Kolosionek E, et al. Praziquantel reverses pulmonary hypertension and vascular remodeling in murine schistosomiasis. Am J Respir Crit Care Med 2011; 184: 467–473. [DOI] [PubMed] [Google Scholar]
- 10.Crosby A, Jones FM, Southwood M, et al. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am J Respir Crit Care Med 2010; 181: 279–288. [DOI] [PubMed] [Google Scholar]
- 11.Graham BB, Chabon J, Gebreab L, et al. Transforming growth factor-β signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation 2013; 128: 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R, Mickael C, Kassa B, et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun 2017; 8: 15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 2014; 25: 2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan L, Wu L, Chen J, et al. Paclitaxel acts as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Vaccine 2010; 28: 4402–4410. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhu S, Wang T, et al. Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med 2005; 2: e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Zhong D-W, Wang Q-W, et al. Paclitaxel ameliorates fibrosis in hepatic stellate cells via inhibition of TGF-beta/Smad activity. World J Gastroenterol 2010; 16: 3330–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savai R, Al-Tamari HM, Sedding D, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med 2014; 20: 1289–1300. [DOI] [PubMed] [Google Scholar]
- 18.Cahill E, Rowan SC, Sands M, et al. The pathophysiological basis of chronic hypoxic pulmonary hypertension in the mouse: vasoconstrictor and structural mechanisms contribute equally. Exp Physiol 2012; 97: 796–806. [DOI] [PubMed] [Google Scholar]
- 19.Tandrup T, Gundersen HJ, Jensen EB. The optical rotator. J Microsc 1997; 186: 108–120. [DOI] [PubMed] [Google Scholar]
- 20.Abe M, Harpel JG, Metz CN, et al. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 1994; 216: 276–284. [DOI] [PubMed] [Google Scholar]
- 21.Toussirot E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets 2012; 11: 159–168. [DOI] [PubMed] [Google Scholar]
- 22.Liu R-M, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol 2015; 6: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y-F, Feng J-A, Li P, et al. Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 2006; 100: 564–571. [DOI] [PubMed] [Google Scholar]
- 24.Ambalavanan N, Nicola T, Hagood J, et al. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 2008; 295: L86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crosby A, Soon E, Jones FM, et al. Hepatic shunting of eggs and pulmonary vascular remodeling in Bmpr2(+/-) mice with schistosomiasis. Am J Respir Crit Care Med 2015; 192: 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng C, Zhong Z, Wu D, et al. Role of FoxO1 and apoptosis in pulmonary vascular remolding in a rat model of chronic thromboembolic pulmonary hypertension. Sci Rep 2017; 7: 2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Zhang H, Li M, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res 2014; 114: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]