Abstract
The circumcision of males is emphatically linked to numerous sexual dysfunctions. Many of the purported benefits do not hold up to the scrutiny of extensive literature surveys. Involuntary circumcision, particularly when not medically warranted, is also associated with many psychological and emotional traumas. Current methods to reconstruct the ablated tissue have significant drawbacks and produce a simple substitute that merely imitates the natural foreskin. Extracellular matrix–based scaffolds have been shown to be highly effective in the repair and regeneration of soft tissues; however, due to the unique nature of the foreskin tissue, commercially available biomaterial scaffolds would yield poor results. Therefore, this study discusses the development and evaluation of a tissue engineering scaffold derived from decellularized human foreskin extracellular matrix for foreskin reconstruction. A chemicophysical decellularization method was applied to human foreskin samples, sourced from consenting adult donors. The resulting foreskin dermal matrices were analyzed for their suitability for tissue engineering purposes, by biological, histological, and mechanical assessment; fresh frozen foreskin was used as a negative control. Sterility of samples at all stages was ensured by microbiological analysis. MTT assay was used to evaluate the absence of viable cells, and histological analysis was used to confirm the maintenance of the extracellular matrix structure and presence/integrity of collagen fibers. Bioactivity was determined by submitting tissue extracts to enzyme-linked immunosorbent assay and quantifying basic fibroblast growth factor content. Mechanical properties of the samples were determined using tensile stress tests. Results found foreskin dermal matrices were devoid of viable cells (p < 0.0001) and the matrix of foreskin dermal matrices was maintained. Basic fibroblast growth factor content doubled within after decellularization (p < 0.0001). Tensile stress tests found no statistically significant differences in the mechanical properties (p < 0.05). These results indicate that the derived foreskin dermal matrix may be suitable in a regenerative approach in the reconstruction of the human foreskin.
Keywords: Foreskin dermal matrix, extracellular matrix, fresh frozen foreskin
Introduction
Few parts of the human anatomy can compare to the incredibly multifaceted nature of the human foreskin. At times dismissed as “just skin,” the adult foreskin is, in fact, a highly vascularized and densely innervated bilayer tissue, with a surface area of up to 90 cm2, and potentially larger.1 On average, the foreskin accounts for 51% of the total length of the penile shaft skin2,3 and serves a multitude of functions. The tissue is highly dynamic and biomechanically functions like a roller bearing; during intercourse, the foreskin “unfolds” and glides as abrasive friction is reduced and lubricating fluids are retained.3,4 The sensitive foreskin is considered to be the primary erogenous zone of the male penis2,3 and is divided into four subsections: inner mucosa, ridged band, frenulum, and outer foreskin (Figure 1(a)); each section contributes to a vast spectrum of sensory pleasure through the gliding action of the foreskin, which mechanically stretches and stimulates the densely packed corpuscular receptors.3,5 Specialized immunological properties should be noted by the presence of Langerhans cells and other lytic materials,3,6 which defend against common microbes, and there is robust evidence supporting HIV protection.7–9 The glans and inner mucosa are physically protected against external irritation and contaminants while maintaining a healthy, moist surface.6 The foreskin is also immensely vascularized and acts as a conduit for essential blood vessels within the penis, such as supplying the glans via the frenular artery.2,10
Figure 1.
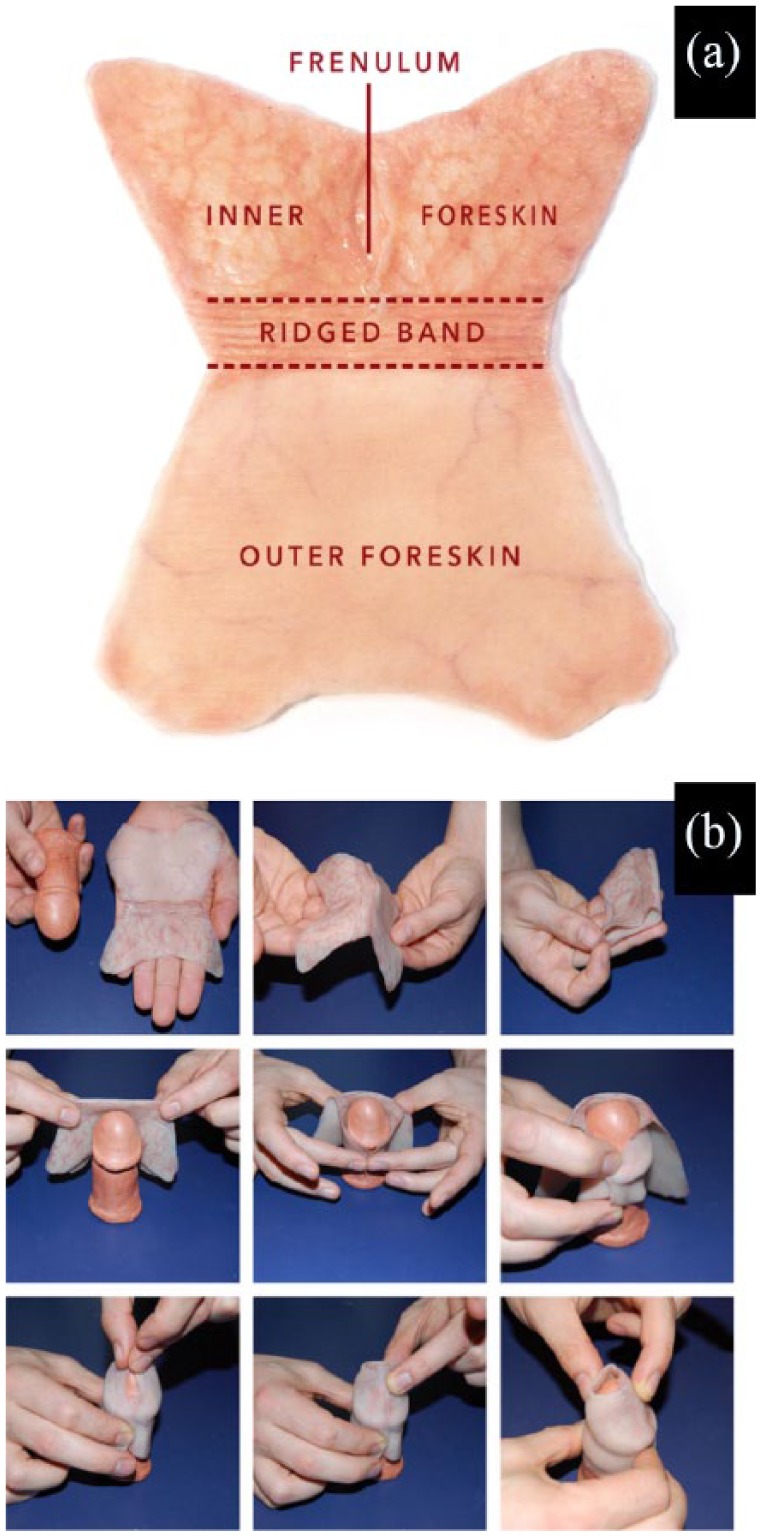
The human foreskin shape after surgical removal: (a) schematic representation of human foreskin after surgical removal via circumcision and (b) representation of the sequential steps performed to identify the shape of the human foreskin after surgical removal via circumcision.
The practice of foreskin amputation, circumcision (Figure 1(b)), has ancient origins, but its modern incarnation can easily be traced to the late 19th century, where many Western practitioners introduced into standard medical practice various forms of medicalized genital injury for both males and females.11,12 A prevailing belief was that many physical and mental afflictions were rooted in masturbation and sexual promiscuity.11,12 Males were prescribed circumcision as a preventive, as it intentionally cripples natural function and dulls sexual pleasure.11–13 Despite the explicit nature of these origins, the practice has persisted. It is difficult to determine precisely how many living males have been subjected to circumcision, voluntarily or otherwise, but a conservative estimate suggests that about 650 million males worldwide are circumcised (about 23% of the world’s male population).14 Moreover, there is a prodigious amount of ethical and moral issues regarding the practice of circumcising non-consenting minors when it is not medically warranted, as it infringes on one’s right to bodily autonomy,15 and many international medical and ethical institutions have lambasted the practice16–18 and have refuted the modern medical justifications and supposed benefits of male circumcision.19–23
Understandably, circumcision is associated with a plethora of complications. Most notably, males report a substantial decrease in sensitivity due to keratinization of the exposed glans and inner mucosa24 and the general loss of the densely innervated and reflexogenic tissue; circumcised males are far less sensitive than their intact counterparts.13,25 This state with diminished neuroreceptors, sensitivity, and vascularity, wrought by circumcision, is heavily associated with erectile dysfunction.25–27 Ejacul-ation and orgasm are complex physiological responses to physical, emotional, and social processes and are not well-understood. What is known is that ejaculation is primarily dependent on afferent signals which originate in the encapsulated nerve endings of the glans, foreskin, and penile shaft skin, and the response is controlled heavily by the autonomic nervous system.28 The loss of the mechanical gliding and stretch receptors of the sensitive foreskin and frenulum is associated with delayed ejaculation or the inability to ejaculate.27,29,30 In addition, circumcised males suffer from premature ejaculation at higher rates than their intact counterparts.31–33 This is believed to be due to the full exposure of the sensitive corona of the glans, which is more directly stimulated during intercourse in circumcised males.34
Circumcision is also associated with an unsurprising reduction in sexual pleasure.29,35 One survey of males circumcised as adults found 22 out of 38 said they regret their decision, as intercourse worsened.36 During intercourse, the immobile shaft skin of the circumcised member contributes to vaginal dryness and abrasion,37 leading to painful intercourse for female partners.31,37 The immobilized shaft skin can also lead to tight, painful erections35 and is susceptible to injury and pain during intercourse and masturbation.32 Loss of the foreskin’s gliding action causes circumcised males more difficulty in penetrating their respective partners,27 as the force required for penetration is increased 10-fold.38 Literature surveys show that there is no evidence that circumcision reduces the transmission of HIV or other sexually transmitted diseases (STDs).20,21 Despite this, circumcised males are far less likely to use condoms,39,40 likely due to decreased sensitivity. Males who have been subjected to involuntary circumcision have long reported emotional trauma, feelings of violation, and many other types of circumcision-related psychological distress.40–42 This indicates that circumcision is not only an issue of ethics and morals but also one that pertains to public health, both physical and mental. More severe consequences of circumcision involve botched procedures and death, which are virtually always avoidable and caused by practitioner negligence.43–47
Aggrieved with their circumcision status, some males seek to restore their ablated foreskins. Foreskin reconstruction methods have been in practice since at least the second century BCE, with the two methods, surgical and nonsurgical, changing very little since their inception.48 Surgical reconstruction methods traditionally involve autografts of skin from elsewhere on the body or manipulation of remaining penile shaft skin to reconstruct a pseudo-foreskin.49,50 Nonsurgical methods make use of tissue expansion: mechanical stress is applied to the residual shaft skin and over time the skin tube is lengthened, also resulting in a pseudo-foreskin.48 Currently, surgical methods have fallen out of popularity, as autografts are always fundamentally different from the natural foreskin, leading to poor cosmetic appearance and functionality, especially when compared to procedural costs.48,51 In addition, due to the difficulty of reconstructing the specialized foreskin tissue using currently available surgical methods, there are several instances of botched procedures, leaving patients with grim results.52 The current consensus is that nonsurgical tissue expansion methods are state of the art, as they produce a pseudo-foreskin with much higher cosmetic appearance and functionality than surgical methods, are far less expensive, and without the associated risks of surgical methods.53 Unfortunately, there is a significant learning curve associated with nonsurgical methods, is very time intensive, and it can take many years for one to complete their restoration efforts.48
Despite this, men who have completed restoration report increased sensitivity, improved sexual satisfaction, and the lessening or even resolution of their circumcision-related psychological distresses.53–55 While some men highly regard the results of nonsurgical restoration, it is far from a perfect reconstruction. Circumcision always ablates the ridged band, and in some cases the frenulum as well.56 The other densely innervated portions of the foreskin are also lost, leaving only residual nervous tissue of the shaft skin.5 Tissue expansion cannot restore these specialized structures, and it is unclear whether the process promotes any nerve regeneration.5 Touch-up surgeries can improve cosmetic appearance and functionality.48 However, results echo the natural form of the foreskin. Under this context, Foregen Onlus Association—an international, donor-funded non-profit company—is devoted to providing a solution to circumcised males who desire complete restoration of sexual sensation, mobility, lubrication, and other properties intrinsic to the foreskin, utilizing principles of Tissue Engineering & Regenerative Medicine, as well as building on previous work in surgical reconstruction. This approach intends to regenerate the ablated tissue as opposed to merely replacing it with a foreskin “substitute.” One-third of the tissue engineering triad is biomaterial scaffolding, and to that effect, the purpose of this study is to develop an extracellular matrix (ECM)-based biomaterial scaffold, derived from the human foreskin, on which a neoforeskin can be engineered. To accomplish this, a novel decellularization method,57 designed and realized at Emilia Romagna Regional Skin Bank, will be applied to donor foreskin tissue, and the mechanical, biological, and structural characteristics will be assessed for its prospective use as a tissue engineering scaffold.
Materials and methods
Ethics statement
All human tissue in this work was provided by adult, male donors following their written informed consent. Procurement of the donor foreskin tissue was approved by Ministère de l’Enseignement supérieur, de la Recherche et de l’Innovation (France). Foreskin samples were packed in accordance with IATA and international rules, regulations, and guidelines while being transported to Emilia Romagna Regional Skin Bank. The work in this study and its use of human tissue was reviewed and approved by Comitato Etico IRST IRCCS-AVR (Italy).
Procurement of human foreskin samples and decellularization
Human foreskin tissue was surgically harvested from 15 living, adult donors for therapeutic purposes, after consenting to the donation of the respective tissue for scientific research. After excision, the samples were dipped in a freezing solution composed of RPMI 1640 medium (Biowest, Riverside, MO, USA) plus antibiotics and 10% cryoprotectant (CRYO·ON DMSO; Alchimia) and subsequently frozen for storage at −80°C. Samples were packaged and shipped to Emilia Romagna Regional Skin Bank while being maintained at storage temperature.
Upon arrival, the human foreskin samples were processed under sterile conditions. Initially, the dermal and epidermal layers of the tissue were physically separated using 2.5% trypsin, diluted to 1× (Life Technology, Monza, Italy) with 0.9% NaCl saline solution (Fresenius Kabi AG, Bad Homburg, Germany). The isolated dermal layers were sectioned into two pieces and maintained in isotonic saline solution. One half of the sectioned dermal tissue was then submitted to an enzymatic/physical process of decellularization developed by our laboratory,57 after partial modification. Half of the sectioned samples were transported to a sterile, laminar flow biosafety cabinet. Inside the biosafety cabinet, the foreskin sections were placed inside cell culture flasks, such that the upper portion of the dermal sections adhered to the inside surface of the flask. The adhered sections were then covered with 2.5% trypsin, diluted to 4× (Life Technology) with 0.9% NaCl saline solution. The foreskin sections were left in the enzymatic solution for 24 h. During the enzymatic decellularization, the foreskin dermis–solution complex was placed in an incubator with a controlled atmosphere and temperature (5% CO2/air and 37°C). Following the residence time, foreskin sections were washed in sterile 0.9% NaCl saline. The sections were left in the sterile saline for 10 min to ensure the removal of any potential enzymatic residue.
The resulting foreskin dermal matrices (FDMs) were dipped in RPMI medium (containing 10,000 IU/mL penicillin, 10 mg/mL streptomycin, 25 μg/mL amphotericin B) for 15 min and sealed in sterile cryofreezing bags (Agricons Ricerche, Padova, Italy), without the addition of cryoprotectant. A programmed, gradual drop of the temperature was performed on samples for their final storage in tanks of liquid nitrogen vapor at −195°C. To evaluate the effect of the decellularization process on the human foreskin samples, as well as the viability of the resulting FDMs as a tissue engineering scaffold, the samples were analyzed through a variety of assays, with fresh frozen sections used a control.
Microbiological analysis
Microbiological analysis was performed on the samples to ensure the maintenance of sterility at all steps throughout the experimental process. Immediately after the tissue sectioning, fresh frozen halves were incubated at 34°C on culture plates with growth media that is selective for either bacteria (COS Columbia agar + 5% sheep blood; BioMerieux, Bagno a Ripoli (FI), Italy) or fungi (Sabouraud dextrose agar + CAF; Biolife, Milano, Italy) for 3 and 14 days, respectively. The other halves were decellularized, frozen, and then incubated on selective growth media as described above.
Histological processing and analysis
Sectioned foreskin samples were processed under histological techniques, to guarantee the structural integrity of the tissue and quantify the content of collagen and elastic fibers. Fresh frozen foreskin (FFF) sections were fixed with a 10% formalin solution (Kaltek, Padova, Italy) and paraffin embedded. After processing, histological sections (5 μm in thickness) were stained with hematoxylin and eosin (H&E), Weigert’s elastic stain, or Masson’s Trichrome (Diapath, Martinengo (BG), Italy). Following staining, the presence, structure, and integrity of the collagen and elastic fibers of the matrix were analyzed. FDM samples were processed in the same manner, to evaluate the maintenance of the matrices’ structural integrity after the decellularization process. Histological processing additionally enabled for the qualitative evaluation of cellular removal in FDM samples.
Image analysis
Following histological processing, additional analysis was performed using image processing techniques, which enabled the measurement of collagen and elastic fiber content of the samples. This was accomplished by employing the software ImageJ (National Institutes of Health, Bethesda, MD, USA) and the method of color deconvolution.58 This method separates the pixels of an RGB image into three separate channels, based on three predetermined colors. ImageJ provides measured pixel intensity, as well as pixel count. Higher pixel intensity values are proportional to higher amounts of stain, which correspond to larger quantities of collagen or elastic fibers. Utilizing the integrated density and sample area of the processed images, collagen and elastic fiber content of both FFF and FDM samples was measured and is expressed as a mean quantitative fraction ± standard deviation (SD).
Cell viability analysis (MTT assay)
MTT assay was performed on both FFF and FDM sections to evaluate tissue viability before and after the application of the decellularization process. In this instance, six uniform samples were excised using a biopsy punch (0.5 mm in diameter) from FFF and FDM sections each. Afterward, all 12 samples were weighed and incubated with MTT (Acros Organics, Morris, NJ, USA) solution (0.5 mg/mL) for 3 h at 37°C in 5% CO2/air. Afterward, all samples were placed in dimethyl sulfoxide (CRYO·ON DMSO; Alchimia, Padova, Italy) for 10 min. By the Beer–Lambert law, the absorbance of the resulting colored solutions is directly proportional to cell viability and was measured using a spectrophotometer, set to a wavelength of 570 nm. From the collected measurements, viability rates were calculated as the ratio of optical density (OD) at 570 nm and the mass in grams (g). Quantitative analysis was performed to assess the viability rates of all samples, both of fresh frozen tissue and FDM.
Measurement of basic fibroblast growth factor via extract
To evaluate foreskin tissue bioactivity and the effects of the decellularization method, fibroblast growth factor (FGFb) content was measured using an extract derived from both FFF and FDM sections, using methods from previously published literature.59–61 FGFb was chosen as the growth factor of interest due to its involvement in the regeneration of a wide variety of tissue types: skin, blood vessel, muscle, adipose, tendon/ligament, cartilage, bone, tooth, and nerve tissues.60 Samples were incubated at 4°C for 72 h, in RPMI 1640 serum-free culture medium, and used as an extraction vehicle, in a ratio sample/extraction vehicle volume of 25 cm2/100 mL. Extracts were assessed for the presence of FGFb using Quantikine® enzyme-linked immunosorbent assay (ELISA) human FGF basic immunoassay kits (R&D Systems, Minneapolis, MN, USA). The measured OD is proportional to protein content (pg/mL) of the samples. Quantitative analysis was performed assessing the absorbances of both FFF and FDM samples.
Mechanical properties
The mechanical properties of human foreskin were analyzed to evaluate their maintenance after the application of the decellularization method. Five FDM and five FFF samples were evaluated in air using an MTS apparatus (Sintech-1/M; MTS Adamel Lhomargy, Ivry sur Seine, France) and TestWorksTM v.4 software (MTS Systems, Eden Prairie, MN, USA). Samples were kept at −80°C and thawed for 24 h at 4°C before testing. Dimensions of the samples were measured using a digital caliper, with each sample measuring 3 cm ×1 cm in area and 0.8–1.5 mm in thickness. Cross-sectional area was estimated as the product of the nominal specimen width and the average membrane thickness. Samples were pulled to failure (tensile failure) using a 1 kN load cell at a rate of 12.7 mm/min. To maintain their moisture, samples were kept in their storage medium. The tensile tests were performed by clamping the bottom and top of the 3 cm ×1 cm specimens to the stationary lower grip and mobile upper grip of the tensile testing apparatus, respectively. The maximum load, tensile strength, modulus of elasticity (Young’s modulus), and stiffness were measured for each sample.
Statistical analysis
Statistical analysis was performed using XLMiner Analysis ToolPak (Frontline Systems Inc., Incline Village, NV, USA) software. All results are reported as mean ± SD, with a statistical significance of p < 0.05. To compare FFF and FDM samples with respect to each parameter, a Student’s t test was employed to analyze cell viability, FGFb, collagen, and elastic fiber content, and mechanical data.
Results
Prior to decellularization of the human foreskin samples, a macroscopic evaluation of the complete, intact samples displays a pinkish appearance and a distinctive “butterfly” shape, which has never been shown before and was initially proposed and identified by us in preliminary work to ascertain the correct and expected shape of the foreskin following circumcision (Figure 1). The epidermal layer appeared darker in the outer areas compared to that of the inner foreskin (Figure 2(a)). Following decellularization, the foreskin tissue exhibited the white coloration of decellularized tissue, which suggests the removal of the epidermal layer of the processed tissue, while ideally maintaining a compact matrix with the bioactive components preserved (Figure 2(b)).
Figure 2.
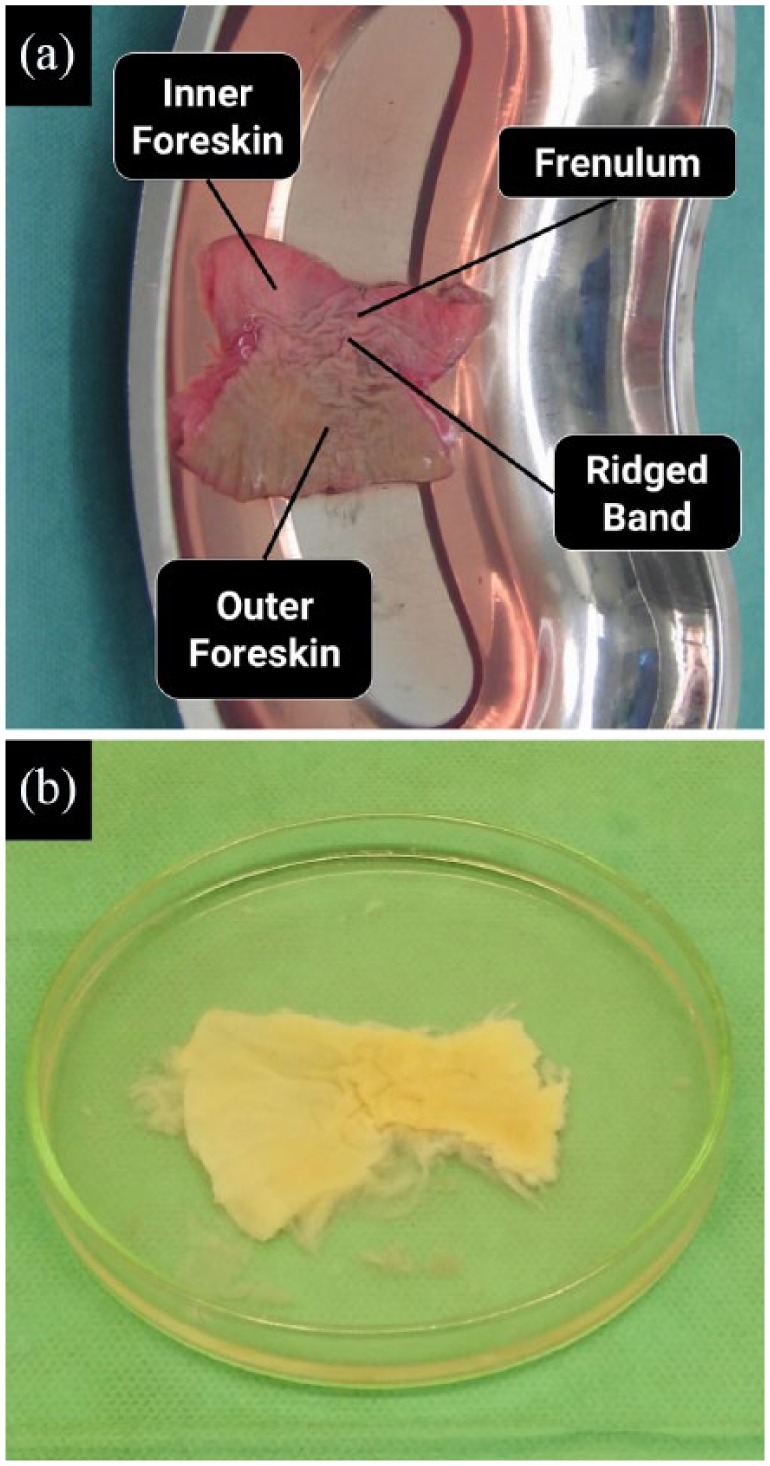
Macroscale appearance of the human foreskin tissue: (a) fresh frozen foreskin tissue prior to the decellularization process and analysis and (b) foreskin dermal matrix, post-processing and decellularization.
Microbiological analysis confirmed that sterile conditions were maintained at all stages: procurement, storage, decellularization, and analysis, for both FFF and decellularized foreskin samples. No bacteria or fungi were identified on the selective growth media after incubation for the respective time durations (data not shown). Prior to the application of the decellularization process, the cell viability of FFF samples was found to be 13.6 ± 1.6 OD/g. After decellularization, the intense purple color of tissue after incubation with tetrazolium salts, which corresponds to cellular viability, was absent in the uncolored, decellularized tissue, and cell viability was significantly reduced to 1.1 ± 0.75 OD/g in all FDM samples (Figure 3). In addition, the drastic reduction in cells was evident during qualitative histological analysis of the FDM samples stained with H&E (Figure 4(c)), although the primary focus was to analyze the structure of the decellularized matrices.
Figure 3.
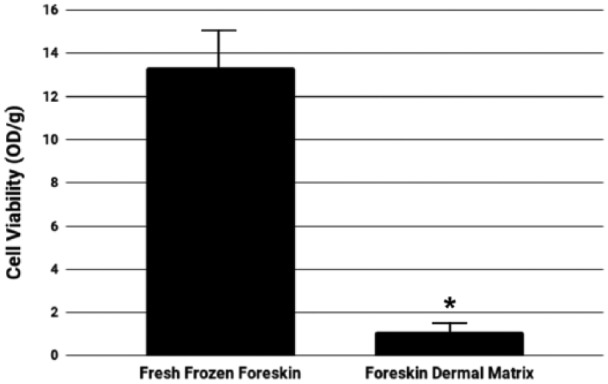
Cellular viability of fresh frozen foreskin and foreskin dermal matrices. Graphical representation of the visible reduction of viable cells prior to and after decellularization of the human foreskin samples (*p < 0.0001).
Figure 4.
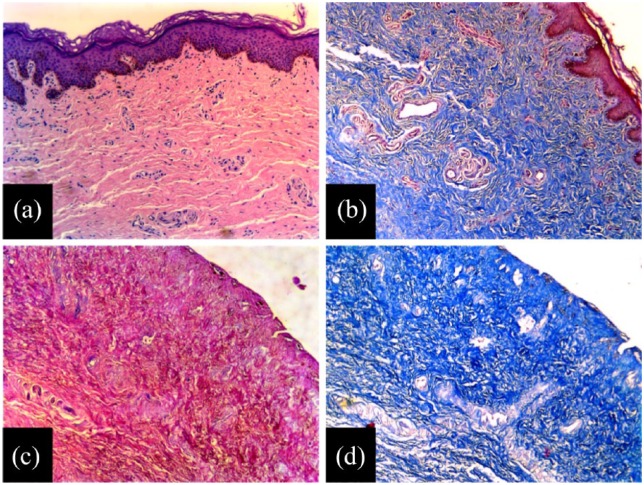
Structural analysis of fresh frozen foreskin tissue and foreskin dermal matrices. Before decellularization, (a) H&E staining shows that fresh frozen foreskin samples display normal structure, and (b) with Masson’s Trichrome, the samples show normal collagen fibers. Following decellularization, foreskin dermal matrix samples exhibit a well-maintained structure with (c) H&E staining and (d) maintenance of collagen fibers with Masson’s Trichrome. The drastic removal of cellular components in the foreskin dermal matrix is also evident through H&E and Masson’s Trichrome.
Through histological analysis, sections of the decellularized samples stained with H&E demonstrated maintenance in the architecture and structural integrity of the dermal layer; decellularized samples exhibited a compact and well-preserved ECM (Figure 4(c)), when compared against the fresh frozen control (Figure 4(a)). Moreover, analysis with Masson’s Trichrome staining further confirmed the structural integrity of the decellularized extracellular matrices (Figure 4(d)), by the presence of well-maintained collagen fibers, when compared to that of the control (Figure 4(b)). Histological analysis also made it evident that the epidermal layers were indeed entirely removed by the decellularization process, as established in our protocol. Imaging of the samples stained with Masson’s Trichrome and Weigert’s elastic stain (Figure 5) found no sizable difference between FFF and FDM samples with regard to collagen or elastic fiber content, demonstrating overall conservation of these biopolymers in the matrix (Table 1).
Figure 5.
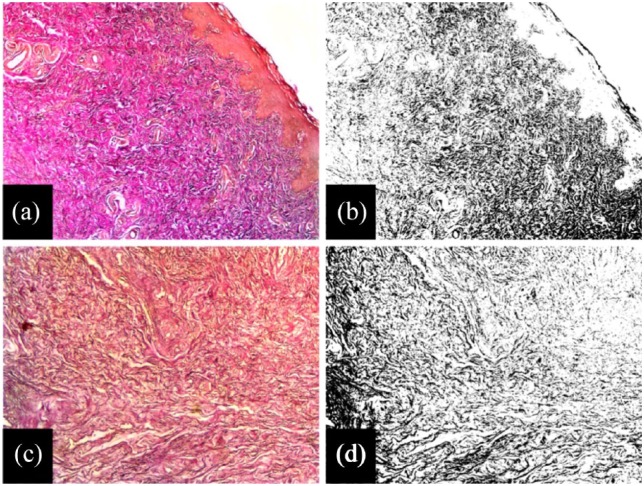
Elastic fiber quantification of fresh frozen foreskin tissue and foreskin dermal matrices by image analysis. (a) Prior to decellularization, Weigert’s elastic stain shows a relatively high elastic fiber density in fresh frozen foreskin tissue. (b) Using color deconvolution, the pixels representing the stained elastic fibers can be isolated and quantified. Following decellularization, (c) foreskin dermal matrix samples exhibit a maintenance in their elastic fiber content, which can also be quantified using (d) color deconvolution. This same technique can be applied to collagen fibers.
Table 1.
Mean quantitative fraction of collagen and elastic fiber quantities of fresh frozen foreskin and foreskin dermal matrix (mean ± SD, n = 5).
| % Collagen fibers | % Elastic fibers | |
|---|---|---|
| Fresh frozen foreskin | 40.9 ± 4.7 | 30.3 ± 1.2 |
| Foreskin dermal matrix | 43.7 ± 2.2 | 30.7 ± 2.3 |
SD: standard deviation.
Student’s t test; fresh frozen foreskin versus foreskin dermal matrix (*p < 0.05).
Using ELISA assay on tissue extracts derived from both FFF and FDM samples, FGFb content was measured, which corresponds to tissue bioactivity. Before decellularization, foreskin samples have an FGFb content of 455.4 ± 70.1 pg/mL; however, after decellularization, FGFb nearly doubles in content, as the growth factor was found in FDM samples at 770.5 ± 36.6 pg/mL (Figure 6). All FDM samples were found to have this twofold increase in FGFb content, compared to the fresh frozen control. Mechanical tensile testing of the samples found no differences between FFF and FDM samples in any of the measured parameters (maximum load, tensile strength, Young’s modulus, or stiffness; Table 2).
Figure 6.
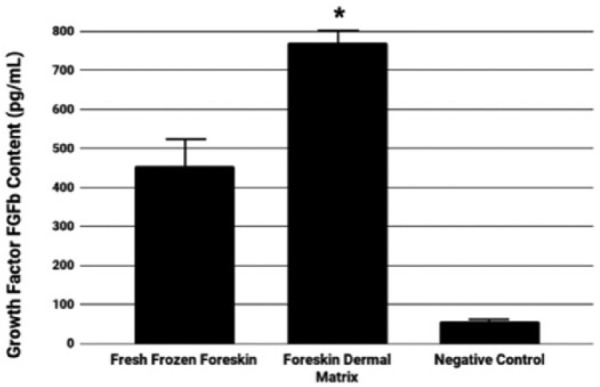
Basic fibroblast growth factor content of fresh frozen foreskin and foreskin dermal matrices. Graphical representation of FGFb content quantified from extract of human foreskin samples before and after decellularization (*p < 0.0001).
Table 2.
Tensile test results of fresh frozen foreskin and foreskin dermal matrix (mean ± SD, n = 5).
| Fresh frozen foreskin | Foreskin dermal matrix | |
|---|---|---|
| Maximum load (N) | 17.66 ± 5.88 | 11.98 ± 7.43 |
| Tensile strength (MPa) | 1.25 ± 0.92 | 1.07 ± 0.68 |
| Young’s modulus of elasticity (MPa) | 2.84 ± 0.25 | 3.01 ± 1.26 |
| Stiffness (N/mm) | 2.65 ± 1.34 | 2.94 ± 3.58 |
SD: standard deviation.
Student’s t test; fresh frozen foreskin versus foreskin dermal matrix (*p < 0.05).
Discussion
The study described here intends to be the first step in the overall process of developing an innovative, regenerative therapy to reconstruct the ablated foreskin tissue of circumcised males faithfully. To this aim, we developed a functional tissue engineering scaffold, derived from the decellularized ECMs of the human foreskin dermis. The value of utilizing the biological scaffold described here is due to the presence of the same intrinsic anatomic and structural components that are biologically inherent in the foreskin tissue. This makes the scaffold more than comparable to the natural foreskin and therefore second to none regarding biomaterial choice for the proposed aim. Several biological/synthetic biomaterial matrices are already commercially available for the reconstruction of damaged tissue, including the penis,62–64 but the origin of the biomaterials is different from that of the tissue being treated, so the natural anatomical/structural characteristics are poorly retained.
After submitting the procured foreskin tissue to the decellularization process developed by our lab, the resulting matrices maintained their natural, well-ordered morphology. Collagen and elastic fiber content and biomechanical properties of the matrices were conserved as well. The decellularized matrices were also found to have a significant, twofold increase in FGFb content, increasing the overall bioactivity of the scaffold. This drastic increase in bioactivity is crucial in for regenerative processes, as FGFb plays a role in promoting vascularization and blood vessel growth, as well as the regeneration of several other tissues relevant to the foreskin.60 The observed increase in bioactivity is likely related to the method of decellularization, as previously published literature describes how different methods of decellularization can affect growth factor release, and consequently, matrix bioactivity.65,66
Human-derived dermal matrices have been used as biomaterial scaffolds for tissue engineering purposes in a wide variety of applications. Our group, in particular, has achieved excellent results through using decellularized dermal matrices in the treatment of several clinical conditions, such as abdominal wall defect, breast, and pelvic reconstruction, as well as rotator cuff repair.67–72 While dermal matrices are a very popular naturally derived biomaterial, few groups have shown interest in developing scaffolds by decellularizing foreskin tissue in a comparable manner to this work. One group had promising results using their foreskin-derived matrix in urethral tissue engineering.73 Another group engineered an acellular AlloDerm foreskin tissue, to be used in tympanoplasty, and the AlloDerm facilitated rapid healing rates.74 A significant difference between these two group’s works and our approach is the form of the scaffold. In our work, retention of the foreskin’s distinctive butterfly shape is vital to engineer our proposed neoforeskin; however, in these other applications, the scaffold’s form is relatively less pertinent. Two other groups have additionally used acellular dermal matrices in the reconstruction of penile shaft skin.77,78 In both cases, the matrices were derived from tissue elsewhere on the patient’s body, but both groups reported good cosmetic and functional results of the reconstructed shaft skin. Although neither group went so far as to reconstruct the foreskin, the promising findings from these other groups are encouraging to our aim.
An unfortunate drawback and limitation to our approach, however, is the low availability of foreskin tissue from adult donors. Neonatal human foreskin tissue and its derivatives, on the other hand, are quite accessible and sold by many laboratory supply vendors. Although pursuing that alternative approach is arguably more efficient, the use of neonatal tissue in biomedical science and engineering is a highly unethical practice.75 Due to this low availability of adult foreskin donor tissue, there is regretfully only a finite amount of experimental protocols that our group was able to perform to characterize our FDMs effectively. To characterize the effectiveness of the decellularization method to a further degree, direct measurements of residual DNA content could be made, as well as a more thorough histological investigation using DAPI staining. Measurement of the glycosaminoglycans, proteoglycans, and other biomolecules, and a more comprehensive study of the ECM organization through total hydroxyproline measurements, would additionally be beneficial.
An additional, albeit necessary, limitation to our approach is the need for the donor foreskin to retain its distinctive butterfly shape, seen in Figure 2, as opposed to further processing which is seen in other pieces of literature detailing foreskin decellularization. This ensures the complex network of channels within the matrix are preserved, which will easily facilitate vascularization and invasion of other cell types. Due to the geometries of the penis and the foreskin, we believe this shape also facilitates the most effective approach at implantation of the proposed neoforeskin, which can be visualized by following Figure 1(b) in reverse order. As biofabrication techniques improve, our group intends to transition away from a method requiring donor tissue and toward utilizing bioprinting methods. However, as it stands today, current biofabrication technologies are unable to replicate functioning microvasculature (<10 μm in diameter), something which the human foreskin is abundant in.76 For the time being, our group is working to secure additional sources of adult donor foreskin tissue.
To develop an innovative, regenerative therapy to repair the damage caused by circumcision, we take advantage of our expertise in the development of biological, acellular scaffolds, through the use of our patented decellularization method (PTC/IB2008/002753).58 Similar to the results of our previous work, the decellularized FDM developed in this study was able to maintain a balance of cellular removal and the maintenance of structural, mechanical, and biological properties of foreskin tissue. Preclinical experiments with in vivo animal models yet need to be performed and will comprise future work. The results of this study indicate that this method of decellularization, when applied to the human foreskin, yields an acellular biological matrix with characteristics and properties necessary to be used as a functional tissue engineering scaffold for the purpose of foreskin reconstruction and in the clinical treatment of circumcised males.
Acknowledgments
The authors thank their colleagues Milena Fini, Matilde Tschon, and Gianluca Giavaresi from Codivilla-Putti Research Institute (Rizzoli Orthopaedic Institute, Bologna, Italy) who assisted this research by providing their expertise in mechanical analysis.
Footnotes
Declaration of conflicting interests: E. B and D. M are inventors of the patent on the decellularization method that was applied to the human foreskin samples. V. P and E. B are science advisory board members of Foregen Onlus Association.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was funded by Foregen Onlus Association.
ORCID iD: Eric J Cunningham  https://orcid.org/0000-0002-7159-8594
https://orcid.org/0000-0002-7159-8594
References
- 1. Werker PM, Terng AS, Kon M. The prepuce free flap: dissection feasibility study and clinical application of a super-thin new flap. Plast Reconstr Surg 1998; 102: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 2. Taylor JR, Lockwood AP, Taylor AJ. The prepuce: specialized mucosa of the penis and its loss to circumcision. Br J Urol 1996; 77: 291–295. [DOI] [PubMed] [Google Scholar]
- 3. Cold CJ, Taylor JR. The prepuce. BJU Int 1999; 83(Suppl. 1): 34–44. [DOI] [PubMed] [Google Scholar]
- 4. Morgan WKC. Penile plunder. Med J Aust 1967; 1: 1102–1103. [DOI] [PubMed] [Google Scholar]
- 5. Hill G. Foreskin motion generates Meissner corpuscle stimulation. BMJ 2003; 309: 679. [Google Scholar]
- 6. Parkash S, Raghuram R, Venkatesan K, et al. Sub-preputial wetness—its nature. Ann Natl Acad Med 1982; 18: 109–112. [Google Scholar]
- 7. Dezzutti CS. Mechanisms of HIV Transmission through Epithelial Cell Barriers. Presented at the 12th World AIDS Conference Geneva, June/July 1998 [abstract 278/32124], http://www.cirp.org/library/disease/HIV/dezzutti/ (accessed 3 December 2018). [Google Scholar]
- 8. Schwartz O. Langerhans cells lap up HIV-1. Nat Med 2007; 13: 245–246. [DOI] [PubMed] [Google Scholar]
- 9. De Witte L, Nabatov A, Pion M, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med 2007; 13: 367–371. [DOI] [PubMed] [Google Scholar]
- 10. Persad R, Sharma S, McTavish J, et al. Clinical presentation and pathophysiology of meatal stenosis following circumcision. Br J Urol 1995; 75: 91–93. [DOI] [PubMed] [Google Scholar]
- 11. Remondino PC. History of circumcision from the earliest times to the present: moral and physical reasons for its performance. Philadelphia, PA and London: F.A. Davis, 1891. [Google Scholar]
- 12. Kellogg JH. Treatment for self-abuse, and its effects. In: Plain Facts for Old and Young. Battle Creek, MI: Good Health Publishing Company, 1910, pp. 320–364. [Google Scholar]
- 13. Sorrells ML, Snyder JL, Reiss MD, et al. Fine-touch pressure thresholds in the adult penis. BJU Int 2007; 99: 864–869. [DOI] [PubMed] [Google Scholar]
- 14. Statistics on Human Genital Mutilation. NOHARMM, http://www.noharmm.org/HGMstats.htm (2011).
- 15. Svoboda JS, Van Howe RS, Dwyer JG. Informed consent for neonatal circumcision: an ethical and legal conundrum. J Contemp Health Law Policy 2000; 17: 61–133. [PubMed] [Google Scholar]
- 16.Non-therapeutic circumcision of male minors. The Netherlands: Royal Dutch Medical Society, 2010. [Google Scholar]
- 17. Lindboe A, Malmberg F, Aula MK, et al. Let the boys decide on circumcision. Oslo: Nordic Ombudsmen, 2013. [Google Scholar]
- 18. Sorokan ST, Finlay JC, Jefferies AL, et al. Newborn male circumcision. Paediatr Child Health 2015; 20: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chessare JB. Circumcision: is the risk of urinary tract infection really the pivotal issue? Clin Pediatr 1992; 31: 100–104. [DOI] [PubMed] [Google Scholar]
- 20. Van Howe RS. Circumcision and HIV infection: review of the literature and meta-analysis. Int J STD AIDS 1999; 10: 8–16. [DOI] [PubMed] [Google Scholar]
- 21. Van Howe RS. Does circumcision influence sexually transmitted diseases? A literature review. BJU Int 1999; 83(Suppl. 1): 52–62. [DOI] [PubMed] [Google Scholar]
- 22. Van Howe RS. A cost-utility analysis of neonatal circumcision. Med Decis Making 2004; 24: 584–601. [DOI] [PubMed] [Google Scholar]
- 23. Macneily AE. Routine circumcision: the opposing view. Can Urol Assoc J 2007; 1: 395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foley JM. The unkindest cut of all. Fact 1966; 3(4): 2–9. [Google Scholar]
- 25. Fink KS, Carson CC, DeVellis RS. Adult circumcision outcomes study: effect on erectile function, penile sensitivity, sexual activity and satisfaction. J Urol 2002; 167: 2113–2116. [PubMed] [Google Scholar]
- 26. Stinson JM. Importance and adult circumcision. J Natl Med Assoc 1973; 65: 161. [PMC free article] [PubMed] [Google Scholar]
- 27. Shen Z, Chen S, Zhu C, et al. Erectile function evaluation after adult circumcision. Zhonghua Nan Ke Xue 2004; 10: 18–19. [PubMed] [Google Scholar]
- 28. Alwaal A, Breyer BN, Lue TF. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril 2015; 104: 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim DS, Pang M-G. The effect of male circumcision on sexuality. BJU Int 2007; 99: 619–622. [DOI] [PubMed] [Google Scholar]
- 30. Solinis I, Yiannaki A. Does circumcision improve couple’s sex life? J Mens Health Gend 2007; 4: 361. [Google Scholar]
- 31. O’Hara K, O’Hara J. The effect of male circumcision on the sexual enjoyment of the female partner. BJU Int 1999; 83(Suppl. 1): 79–84. [DOI] [PubMed] [Google Scholar]
- 32. Zwang G. Functional and erotic consequences of sexual mutilations. In: Denniston GC, Milos MF. (eds) Sexual mutilations: a human tragedy. Boston: Springer, 1997, pp. 67–76. [Google Scholar]
- 33. Masood S, Patel HRH, Himpson RC, et al. Penile sensitivity and sexual satisfaction after circumcision: are we informing men correctly? Urol Int 2005; 75: 62–66. [DOI] [PubMed] [Google Scholar]
- 34. Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res 1986; 371: 205–230. [DOI] [PubMed] [Google Scholar]
- 35. Kim DS, Pang M-G. Extraordinarily high rates of male circumcision in South Korea: history and underlying causes. BJU Int 2002; 89: 48–54. [PubMed] [Google Scholar]
- 36. Denniston GC. Circumcision and sexual pleasure. In: Denniston GC, Hodges FM, Milos MF. (eds) Flesh and blood: perspectives on the problem of circumcision in contemporary society. Boston: Springer, 2004, pp. 45–53. [Google Scholar]
- 37. Boyle GJ. Circumcision in adults: effect on sexual function. Urology 2004; 64: 1167–1168. [DOI] [PubMed] [Google Scholar]
- 38. Taves D. The intromission function of the foreskin. Med Hypotheses 2002; 59: 180–182. [DOI] [PubMed] [Google Scholar]
- 39. Michael RT, Wadsworth J, Feinleib J, et al. Private sexual behavior, public opinion, and public health policy related to sexually transmitted diseases: a US-British comparison. Am J Public Health 1998; 88: 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyle GJ, Goldman R, Svoboda JS, et al. Male circumcision: pain, trauma and psychosexual sequelae. J Health Psychol 2002; 7: 329–343. [DOI] [PubMed] [Google Scholar]
- 41. Rhinehart J. Neonatal circumcision reconsidered. Trans Anal J 1999; 29: 215–221. [Google Scholar]
- 42. Goldman R. The psychological impact of circumcision. BJU Int 2002; 83: 93–102. [DOI] [PubMed] [Google Scholar]
- 43. Stanley D. Shane’s circumcision nightmare: I wish I’d never been born. Sydney, NSW, Australia: Women’s Day, 2000, pp. 24–25. [Google Scholar]
- 44. Black D. Sex, lies and a quest for identity: The boy raised as a girl suffered for social experiment. Toronto, Ontario: Toronto Star, May 11, 2004, A3. [Google Scholar]
- 45. Jiang J, Zhu F-Q, Luo J, et al. Severe burn of penis caused by excessive short-wave diathermy. Asian J Androl 2004; 6: 377–378. [PubMed] [Google Scholar]
- 46. Bode CO, Ikhisemojie S, Ademuyiwa AO. Penile injuries from proximal migration of the Plastibell circumcision ring. J Pediatr Urol 2010; 6: 23–27. [DOI] [PubMed] [Google Scholar]
- 47. Bollinger D. Lost boys: an estimate of U.S. circumcision-related infant deaths. Thymos J Boyhood Stud 2010; 4: 78–90. [Google Scholar]
- 48. Schultheiss D, Truss MC, Stief CG, et al. Uncircumcision: a historical review of preputial restoration. Plast Reconstr Surg 1998; 101: 1990–1998. [DOI] [PubMed] [Google Scholar]
- 49. Greer DM, Mohl PC, Sheley KA. A technique for foreskin reconstruction and some preliminary results. J Sex Res 1982; 18: 324–330. [Google Scholar]
- 50. Brandes SB, McAninch JW. Surgical methods of restoring the prepuce: a critical review. BJU Int 1999; 83(Suppl. 1): 109–113. [DOI] [PubMed] [Google Scholar]
- 51. Collier R. Whole again: the practice of foreskin restoration. CMAJ 2011; 183: 2092–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Surgery: What about surgical foreskin restoration? National Organization of Restoring Men (NORM) website; c1997–2006. n.d, http://www.norm.org/surgery.html accessed (3 December 2018).
- 53. Non-surgical foreskin restoration. Seattle, WA: Doctors Opposing Circumcision, 2016. [Google Scholar]
- 54. Anonymous. The joy of uncircumcising. BMJ 1994; 309: 676–877. [Google Scholar]
- 55. Mohl PC, Adams R, Greer DM, et al. Prepuce restoration seekers: psychiatric aspects. Arch Sex Behav 1981; 10: 383–393. [DOI] [PubMed] [Google Scholar]
- 56. Redmond R. Circumcision variations. FQ, Issue 13, March 1990. [Google Scholar]
- 57. Melandri D, Bondioli E, Giardino R, et al. Method of treatment of connective tissues and organs and uses of said tissues and organs. 2010/0247604 A1, https://patentimages.storage.googleapis.com/8e/e4/76/f8762da28a209e/US20100247604A1.pdf (2010).
- 58. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 2001; 23: 291–299. [PubMed] [Google Scholar]
- 59. Yun Y-R, Won JE, Jeon E, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng 2010; 2010: 218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bondioli E, Fini M, Veronesi F, et al. Development and evaluation of a decellularized membrane from human dermis. J Tissue Eng Regen Med 2014; 8: 325–336. [DOI] [PubMed] [Google Scholar]
- 61. Maddaluno L, Urwyler C, Werner S. Fibroblast growth factors: key players in regeneration and tissue repair. Development 2017; 144: 4047–4060. [DOI] [PubMed] [Google Scholar]
- 62. Lue TF, El-Sakka AI. Venous patch graft for Peyronie’s disease. Part I: technique. J Urol 1998; 160: 2047–2049. [DOI] [PubMed] [Google Scholar]
- 63. Perovic SV, Sansalone S, Djinovic R, et al. Penile enhancement using autologous tissue engineering with biodegradable scaffold: a clinical and histomorphometric study. J Sex Med 2010; 7: 3206–3215. [DOI] [PubMed] [Google Scholar]
- 64. Perovic SV, Byun J-S, Scheplev P, et al. New perspectives of penile enhancement surgery: tissue engineering with biodegradable scaffolds. Eur Urol 2006; 49: 139–147. [DOI] [PubMed] [Google Scholar]
- 65. Hoganson DM, Owens GE, O’Doherty EM, et al. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials 2010; 31: 6934–6940. [DOI] [PubMed] [Google Scholar]
- 66. Hoganson DM, O’Doherty EM, Owens GE, et al. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 2010; 31: 6730–6737. [DOI] [PubMed] [Google Scholar]
- 67. Ghetti M, Bondioli E, Purpura V, et al. Decellularized human dermal matrix produced by a skin bank. A new treatment for abdominal wall defects. Ann Ital Chir 2017; 5: 443–448. [PubMed] [Google Scholar]
- 68. Ghetti M, Papa V, Deluca G, et al. Histological and ultrastructural evaluation of human decellularized matrix as a hernia repair device. Ultrastruct Pathol 2018; 42: 32–38. [DOI] [PubMed] [Google Scholar]
- 69. Folli S, Curcio A, Melandri D, et al. A new human-derived acellular dermal matrix for breast reconstruction available for the European market: preliminary results. Aesthetic Plast Surg 2018; 42: 434–441. [DOI] [PubMed] [Google Scholar]
- 70. Perrone AM, Livi A, Fini M, et al. A surgical multi-layer technique for pelvic reconstruction after total exenteration using a combination of pedicled omental flap, human acellular dermal matrix and autologous adipose derived cells. Gynecol Oncol Rep 2016; 18: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fini M, Bondioli E, Castagna A, et al. Decellularized human dermis to treat massive rotator cuff tears: in vitro evaluations. Connect Tissue Res 2012; 53: 298–306. [DOI] [PubMed] [Google Scholar]
- 72. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg 2011; 95(Suppl 1): S13–S23. [DOI] [PubMed] [Google Scholar]
- 73. Jiang H, Ma LM, Zhou J, et al. Human foreskin acellular matrix graft: a good scaffold for urethral tissue engineering. Zhonghua Nan Ke Xue 2009; 15: 409–412. [PubMed] [Google Scholar]
- 74. Farahani F, Karimi Yazdi A, Ghasemi M, et al. Results of acellular dermis matrix graft used for tympanoplasty in guinea pig model. Iran J Otorhinolaryngol 2015; 27: 95–100. [PMC free article] [PubMed] [Google Scholar]
- 75. Warren JP. Ethics of using infant foreskins for bioengineering. BMJ 1999; 319: 1312. [Google Scholar]
- 76. Sarker MD, Naghieh S, Sharma NK, et al. 3D biofabrication of vascular networks for tissue regeneration: a report on recent advances. J Pharm Biomed Anal 2018; 8: 277–296.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ludolph I, Titel T, Beier JP, et al. Penile reconstruction with dermal template and vacuum therapy in severe skin and soft tissue defects caused by Fournier’s gangrene and hidradenitis suppurativa. Int Wound J 2014; 13: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Triana Junco P, Dore M, Nuñez Cerezo V, et al. Penile reconstruction with skin grafts and dermal matrices: indications and management. Eur J Pediatr Surg Rep 2017; 5: e47–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]