Abstract
Uterine fibroids have been described as an associate to acute venous thromboembolism (VTE), with case reports showing an association between large uterine fibroids, acute deep venous thrombosis (DVT), and acute pulmonary embolism (PE). However, there is little known about the association or causation between uterine fibroids, chronic thromboembolic disease (CTED), and chronic thromboembolic pulmonary hypertension (CTEPH). We report on six women with uterine fibroids and CTEPH, as well as one woman with CTED, all of whom presented with exertional dyspnea, lower extremity swelling, and in the cases of CTEPH, clinical, echocardiographic, and hemodynamic evidence of pulmonary hypertension and right heart failure. Compression of the pelvic veins by fibroids was directly observed with invasive venography or contrast-enhanced computed tomography in five cases. All seven women underwent pulmonary thromboendarterectomy (PTE) followed by marked improvement in functional, clinical, and hemodynamic status.
Keywords: chronic thromboembolic pulmonary hypertension, pulmonary thromboendarterectomy, right ventricle function and dysfunction, uterine fibroid, venous thromboembolism
Introduction
Uterine fibroids are a common diagnosis in women of reproductive age, ranging from a prevalence approaching 70% in Caucasian women to as high as 80% in African American women.1 It is well-known that women with uterine fibroids are at increased risk of uterine bleeding. However, prior studies have also demonstrated an association between uterine fibroids and acute venous thromboembolism (VTE). Moreover, rare case studies have reported on the association between large uterine fibroids, acute deep venous thrombosis (DVT), and acute pulmonary embolism (PE).2–5 The link between uterine fibroids and VTE likely arises from the mass compressing effect of large fibroids, leading to venous stasis in the pelvis and lower extremities. Other potential links include polycythemia and reactive thrombocytosis secondary to menorrhagia, leading to a higher risk of developing VTE.6,7 Menorrhagia may also lead to periods of interrupted anticoagulation. If indeed uterine fibroids increase the risk of acute VTE, and namely acute PE, then there is a potential that uterine fibroids consequently increase the risk of developing chronic thromboembolic disease (CTED)/chronic thromboembolic pulmonary hypertension (CTEPH). Up to 4% of patients following an acute PE may develop CTEPH, with progressive increases in pulmonary vascular resistance (PVR) and pulmonary hypertension (PH) leading to right ventricular (RV) dysfunction, clinical right heart failure, and death.8–13 Known risk factors for VTE and CTED/CTEPH include thrombophilic disorders (antiphospholipid antibody syndrome and mutations in factor V Leiden, protein C, protein S, antithrombin III, and prothrombin gene) and other medical conditions such as ventriculo-atrial shunts, infected pacemaker wires, absence of a spleen, history of malignancy, chronic inflammatory disorders, and myeloproliferative syndromes.14–16 However, extrinsic compression of the iliocaval venous system by large uterine fibroids is not a recognized risk factor for CTED/CTEPH.
CTEPH is the only curable cause of PH5 and pulmonary thromboendarderectomy (PTE) is the treatment of choice for eligible patients in experienced CTEPH and PTE centers.12–21 Herein, we report a case series of seven women who were diagnosed with CTED or CTEPH, and in the course of their evaluation, were found to have a history of large uterine fibroids compressing the pelvic venous system or inferior vena cava (IVC). The main purpose of this case series is to demonstrate that large uterine fibroids are a potential risk factor for CTED/CTEPH.
Methods
Data were collected from the Temple University Hospital Pulmonary Hypertension, Right Heart Failure and CTEPH/PTE Program from June 2013 (program inception) to December 2016. This review was conducted among a cohort of the first 71 patients who were evaluated for CTEPH and underwent PTE surgery within our program. There were 38 women within this CTEPH cohort and seven were diagnosed with having large uterine fibroids. Each patient referred into the Temple University Hospital CTEPH/PTE Program was presented at our weekly multidisciplinary CTEPH meeting that includes medical PH experts, a PTE surgeon, radiologist, interventional cardiologist, and nursing staff. Following diagnostic studies, which included computed tomography angiogram (CTA), ventilation/perfusion (VQ) scan, right heart catheterization (RHC), and pulmonary angiogram, our multidisciplinary CTEPH team established candidacy for surgical treatment with PTE for all seven women.
All seven women had a negative hypercoagulable workup, which included protein C and S antigen, protein C and protein S activity, prothrombin gene mutation, factor V Leiden, anticardiolipin antibodies, beta 2 glycoprotein antibodies, antithrombin III activity, activated partial thromboplastin time, prothrombin time, and homocysteine level. The PTE was performed by median sternotomy, under cardiopulmonary bypass, with periods of circulatory arrest using the accepted surgical approach, as has been previously reported by our group.22
Case descriptions
Case 1
Patient 1 is a 48-year-old Caucasian woman with a past medical history (PMH) significant for deep vein thrombosis (DVT) and PE status post (s/p) IVC filter placement two years before presentation. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2.
Table 1.
Clinical parameters of seven patients before and after PTE.
| Parameters | Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | |
| Age (years) | 48 | 53 | 35 | 57 | 40 | 52 | 55 | |||||||
| JVP (cmH2O) | 25 | 6 | 12 | 5 | 5 | 6 | 20 | 5 | 15 | 5 | 10 | 3 | 20 | 7 |
| Increased P2 intensity | + | − | + | − | − | − | + | − | + | − | − | − | − | − |
| Weight (kg) | 100.3 | 73 | 102.5 | 97.1 | 110.7 | 106.4 | 69 | 59 | 112 | 103.4 | 59.9 | 54.9 | 75.6 | 57.2 |
| SpO2 (%) | 94 | 95 | 94 | 94 | 97 | 99 | 99 | 98 | 93 | 92 | 100 | 96 | 94 | 100 |
| Supplemental oxygen | + | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 6MWD (m) | − | 165 | 439 | − | 238 | 311 | − | 270 | 128 | 311 | 414 | 396 | 176 | 274 |
| ECG – RVH | + | + | − | − | − | − | − | − | + | − | − | − | + | − |
| ECG – RVS | + | − | − | − | + | − | − | + | + | − | + | − | + | − |
| BNP (pg/mL) | 1980 | 10 | 52 | − | 69 | 80 | − | − | 510 | 90 | 129 | 49 | − | 746 |
PTE, pulmonary thromboendarterectomy; NYHA, New York Heart Association; PH, pulmonary hypertension; JVP, jugular venous pressure; SpO2, oxygen saturation; 6MWD, 6-min walk distance; ECG, electrocardiogram; RVH, right ventricular hypertrophy; RVS, right ventricular strain; BNP, brain natriuretic peptide.
Table 2.
Hemodynamic and echocardiographic parameters of seven patients before and after PTE.
| Parameters | Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | Pre PTE | Post PTE | |
| Hemodynamics | ||||||||||||||
| RAP (mmHg) | 10 | 6 | 12 | 4 | 8 | 8 | 18 | 5 | 18 | 9 | 13 | 3 | 24 | 8 |
| mPAP (mmHg) | 66 | 30 | 36 | 19 | 23 | 18 | 40 | 29 | 43 | 44 | 60 | 25 | 52 | 34 |
| CI (L/min/m2) | 1.2 | 3.2 | 2.9 | 2.7 | 3.6 | 2.9 | 2.0 | 3.4 | 2.8 | 2.3 | 1.4 | 3.1 | 1.7 | 3.7 |
| PVR (Wood units) | 23.0 | 3.0 | 3.9 | 1.8 | 1.3 | 1.2 | 10 | 3.3 | 6.6 | 8.4 | 18.5 | 2.2 | 12.1 | 3.7 |
| Echocardiographic | ||||||||||||||
| RVIDd (mm) | 46 | 37 | 35 | − | 24 | 20 | − | 39 | 54 | 48 | 39 | 36 | 51 | 35 |
| RV:LV | 1.6 | 1.1 | 1.0 | − | 0.6 | 0.5 | − | 0.9 | 1.8 | 1.5 | 1.1 | 1.3 | 1.6 | 0.9 |
| PASP (mmHg) | 87 | 13 | 49 | 41 | 30 | 18 | 74 | 39 | 92 | 79 | 77 | 13 | 145 | 72 |
PTE, pulmonary thromboendarterectomy; HR, heart rate; mPAP, mean pulmonary arterial pressure; RAP, right atrial pressure; CI, cardiac index; PVR, pulmonary vascular resistance; RVIDd, right ventricular internal diameter at end-diastole; RV, right ventricle; LV, left ventricle; PASP, pulmonary artery systolic pressure.
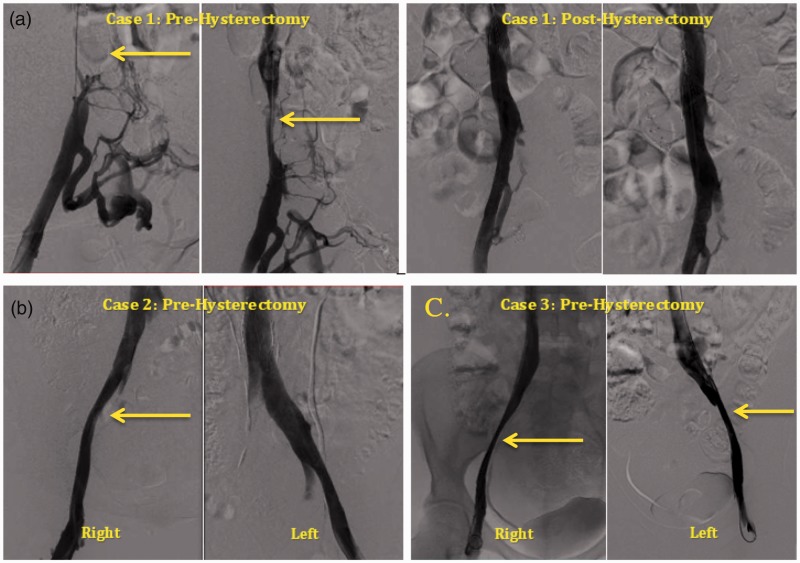
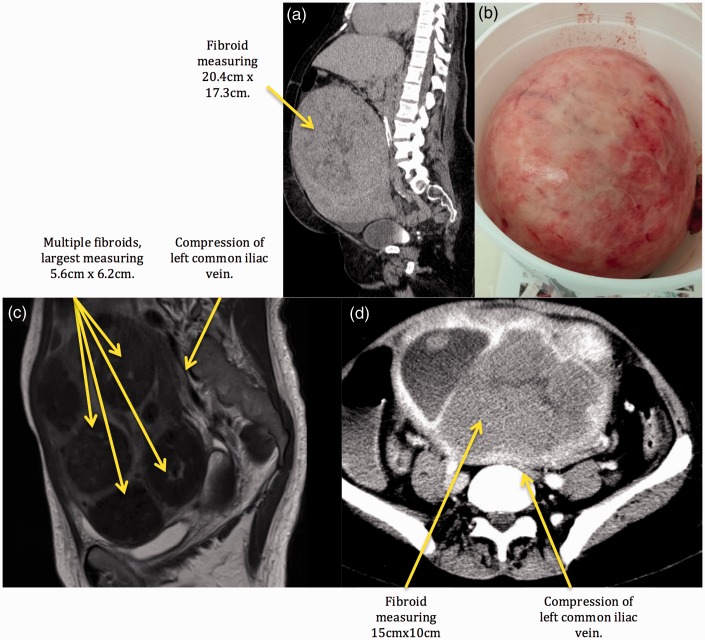
The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography. In addition, a CT of the abdomen and pelvis with IV contrast demonstrated one large uterine fibroid measuring 20.4 × 17.3 × 20.2 cm with compression of surrounding structures, including the IVC, both common iliac veins, and infra-renal IVC filter. In addition, invasive venogram of lower extremities revealed an obstructed IVC with surrounding collateral veins and a thrombus inside the compressed IVC filter (Fig. 1a). The right common femoral vein, the right external iliac vein, and the right common iliac vein were patent. A large uterine fibroid, as described above, was found to be the cause of the patient's compressed IVC (Fig. 2). Based on these studies, diagnosis of type 1 CTEPH23,24 was made and PTE was performed on hospital day 10.
Fig. 1.
Invasive venograms of iliocaval venous system. (a) Pre- and post-hysterectomy venogram for case 1, obstruction of the IVC. (b) Pre-hysterectomy venogram for case 2, compression of right external iliac vein. (c) Pre-hysterectomy venogram for case 3, compression of right and left external iliac veins. *Arrows identifying pelvic venous compression.
Fig. 2.
Radiologic evidence and surgical specimen. (a) Case 1, CT of the abdomen and pelvis. (b) Case 1, surgical fibroid specimen. (c) Case 4, MRI of the pelvis, compression of the left common iliac vein. (d) Case 5, CT of the abdomen and pelvis, compression of the left common iliac vein.
Following PTE, the patient had marked immediate improvement in hemodynamics on postoperative day 1 (Table 2). Sixteen days after PTE, a supra-cervical abdominal hysterectomy was performed to alleviate iliocaval compression. Thereafter, the patient underwent an invasive lower extremity venogram, which revealed patent iliac veins and a widely patent IVC with complete resolution of the filter-related thrombosis (Fig. 1a). The IVC filter was then fully expanded. Importantly, she has had no recurrence of DVT in the last 2.5 years since her hysterectomy and PTE.
Outpatient clinical follow-up revealed significant improvements in physical examination and laboratory studies (Table 1). Echocardiogram follow-up revealed mildly dilated RV, normal RV function, and complete resolution of RVOT notch.
Case 2
Patient 2 is a 53-year-old African American woman with a PMH significant for DVT and PE s/p IVC filter placement. She presented to our outpatient PH and CTEPH clinic with a three-year history of exertional dyspnea, bilateral lower extremity swelling, and multiple episodes of exertional syncope. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2.
The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography.
CT of the abdomen and pelvis with IV contrast demonstrated a heterogeneous enlarged uterus measuring 10.2 × 7.7cm with several large calcified fibroids. Pelvic ultrasound showed a large intramural fibroid measuring 8.0 × 6.5 × 5.4 cm. Invasive venogram of the lower extremities revealed compression of the right external iliac vein by a calcified uterine fibroid (Fig. 1b). Based on these studies, diagnosis of type 2 CTEPH23,24 was made and candidacy for PTE was established.
Following PTE, the patient had a significant improvement in hemodynamics (Table 2). Outpatient clinical follow-up (39 days after PTE) revealed significant improvements in physical examination and laboratory studies (Table 1). Twelve-lead ECG revealed normal sinus rhythm without RVH or RV strain. Echocardiogram follow-up revealed normal RV size and mild RV dysfunction.
The patient is actively being followed by obstetrics and gynecology who are planning a total hysterectomy in view of compression of the pelvic veins by a large calcified fibroid.
Case 3
Patient 3 is a 35-year-old woman with a PMH significant for DVT and PE s/p IVC filter placement five months before presentation. She presented to our outpatient PH and CTEPH clinic with a five-month history of progressive dyspnea. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2. The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography.
Invasive venogram of lower extremities revealed severe compression of the right and left external iliac veins from large uterine fibroids (Fig. 1c).
Following PTE, there were improvements noted in New York Heart Association (NYHA) class, as well as 6-min walking distance (6MWD). Echocardiogram follow-up was notable for a normal RV size and function, without any evidence of RVOT notch.
The patient underwent a hysterectomy 203 days after PTE. The pathology report revealed a distorted enlarged uterus with multiple fragments of fibroids, the largest measuring 5.6 cm in dimension. She has had no recurrence of DVT or PE in the nine months following her hysterectomy and PTE.
Case 4
Patient 4 is a 52-year-old woman with a PMH significant for DVT s/p IVC filter placement. The patient presented to our outpatient PH and CTEPH clinic with a one-year history of exertional chest pain and dyspnea. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2. The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography.
Magnetic resonance imaging (MRI) of the pelvis with contrast was notable for an enlarged fibroid uterus measuring 15 × 10 cm and extending into the lower abdomen. There was moderate compression of the IVC at the bifurcation of the right common iliac vein and there was significant compression of the left common iliac vein by three fibroids, the largest measuring 5.8 × 6.2 cm (Fig. 2).
Following PTE, the patient had marked immediate improvement in hemodynamics on postoperative day 3 (Table 2) and outpatient clinical follow-up revealed significant improvements in physical examination and laboratory data (Table 1). Echocardiogram follow-up revealed normal RV size, low normal RV systolic function, and a late systolic RVOT Doppler notch.
The patient underwent total abdominal hysterectomy 198 days after PTE and has had no subsequent DVT or PE for two years since PTE and hysterectomy.
Case 5
Patient 5 is a 57-year-old Caucasian woman with a PMH significant for DVT and PE s/p IVC filter placed ten years ago. She presented with exertional dyspnea and bilateral lower extremity swelling, progressive over ten years with acute deterioration. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2. The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography.
A CT of the abdomen and pelvis with IV contrast revealed an enlarged uterus measuring 19 × 13 cm with multiple calcified and non-calcified fibroids. The largest fibroid was massive, at 15 × 10 cm, and was compressing the left common iliac vein (Fig. 2).
This patient had relatively distal CTEPH and did not have significant hemodynamic improvement post PTE. The second outpatient clinical follow-up (70 days after PTE) revealed significant improvements in physical examination and laboratory data (Table 1). Echocardiogram follow-up revealed moderately dilated RV, mild to moderate RV dysfunction, and complete resolution of RVOT notch.
Case 6
Patient 6 is a 40-year-old African American woman with a PMH significant for DVT and PE s/p IVC filter placed four years before presentation and total abdominal hysterectomy performed two years before presentation for treatment of uterine fibroids and endometriosis leading to menorrhagia. The operative report of the patient's total abdominal hysterectomy was notable for a large fibroid and extensive endometriosis in the pelvis. The myometrium was noted to be trabeculated and measured up to 5.5 cm in thickness at the site of the largest fibroid. She presented to our outpatient PH and CTEPH clinic with a five-year history of exertional dyspnea and bilateral lower extremity swelling. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2. The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography.
Following PTE, the patient had marked improvement in hemodynamics on postoperative day 3 (Table 2). Outpatient clinical follow-up revealed significant improvements in physical examination and laboratory data (Table 1). Echocardiogram follow-up revealed mild to moderately dilated RV size, normal RV function along with resolution of RVOT notch.
During the two years from her hysterectomy to PTE, and in the last 20 months, there have been no further diagnosed episodes of recurrent acute DVT or PE. In the absence of an alternative explanation such as a provoked risk for VTE or a hematologic abnormality on hypercoagulable workup, we have considered the possibility of venous compression from her fibroid uterus as seen in our previous cases.
Case 7
Patient 7 is a 55-year-old woman with a PMH significant for PE and DVT s/p IVC filter placement 11 years before presentation to our center. Following IVC filter placement, she was diagnosed with uterine fibroids and underwent a hysterectomy 10 years before presentation. She had no subsequent acute DVT or PE following her hysterectomy. She presented to our hospital with a history of progressive dyspnea on exertion and bilateral lower extremity swelling, progressive over the last 12 years, with acute deterioration. Clinical, echocardiographic, and hemodynamic characteristics are outlined in Tables 1 and 2.
The diagnosis of CTEPH was made from lung VQ scan, CTA, and invasive pulmonary angiography.
Following PTE, the patient had marked immediate improvement in hemodynamics on postoperative day 2 (Table 2). Outpatient clinical follow-up revealed significant improvement in physical examination (Table 1). Patient follow-up revealed significantly improved functional capacity and near normalization of right heart function. The patient has had no subsequent acute DVT or PE diagnosed since hysterectomy and PTE. In the absence of an alternative explanation such as a provoked risk for VTE or a hematologic abnormality on hypercoagulable workup, her history is also concerning for compression of the pelvic veins by large uterine fibroids.
Discussion
Herein, we demonstrate the association between the presence of large uterine fibroids and the debilitating, life-threatening condition of CTED/CTEPH. This case series reports the clinical, hemodynamic, and echocardiographic findings of seven patients diagnosed with either CTED or CTEPH and large uterine fibroids with visualization of compression of the pelvic veins or IVC in five out of seven cases. All patients were treated with PTE after our CTEPH team established candidacy, and all patients had significant clinical improvement after PTE representative of the typical outcomes reported from our PTE program previously.22 These cases, although not suggesting a cellular mechanism for the formation of chronic thrombus in the lungs, do reveal a potential association between the common condition of large uterine fibroids and VTE through mechanical iliocaval venous compression and venous stasis.
Importantly, in our CTED/CTEPH cohort, all patients lacked typical risk factors for a provoked VTE and a negative hypercoagulable workup. Of note, these patients had evidence of acute DVT/PE before IVC filter placement. In addition, all seven women had their IVC filter placed before the diagnosis of CTED/CTEPH. Postoperatively, the IVC filter remained in place in all patients and none have had recurrence of DVT/PE after PTE. These observations significantly decrease the possibility that the IVC filter itself was a contributor to their initial DVT/PE event or was causal to the development of CTEPH.
In cases 1, 2, and 3, compression of the pelvic veins and/or IVC by large uterine fibroids was directly observed on invasive venogram before PTE. Our other patients had evidence of large fibroids and compression of the pelvic venous system either on imaging, which included ultrasound of the pelvis, CT of the abdomen and pelvis or MRI (cases 1, 2, 3, 4, and 5), or on hysterectomy operative report (cases 3 and 6). In fact, five patients in our case series underwent a hysterectomy (cases 1, 3, 4, 6, and 7) with no subsequent VTE, and one is awaiting hysterectomy per gynecology recommendations (case 2). Hence, compression of the iliocaval venous system by large uterine fibroids, predisposing to VTE and thereby increasing the risk for CTED/CTEPH, was found or strongly suggested by invasive venogram in three cases, abdominopelvic imaging in two cases and prior history of hysterectomy in two cases.
The previous literature has reported on a few cases of uterine fibroids related to VTE, but none related to CTED/CTEPH.25–32 The mechanism of pelvic venous compression as an etiology for thrombosis has been described in patients diagnosed with May–Thurner syndrome31–35 and was first recognized in 1957.34 In more recent years, other venous vascular compression syndromes have been described in the literature as possible etiologies of flow stasis (or turbulence), leading to thrombosis.35 Some examples include thoracic outlet syndrome, Paget–Schroetter syndrome (effort thrombosis), quadrilateral space syndrome, celiac artery compression syndrome, and renal vein entrapment syndrome.35 These observations of external venous and arterial compression predisposing to thrombosis are consistent with Virchow's triad and are consistent with the observations herein that large uterine fibroids compressing the pelvic venous system may also be a cause of recurrent thrombosis.
The prevalence of uterine fibroids within PH populations is not known. In our CTEPH/PTE cohort, seven out of 38 women (18%) had large uterine fibroids. Our findings do not establish an incidence or prevalence of fibroids in CTEPH. However, the relatively high percentage of women with large uterine fibroids within our CTEPH/PTE cohort, combined with imaging evidence of pelvic vein compression in these women, raises the question as to whether fibroids may pose a risk factor for VTE and eventual CTEPH. Approximately 25% of patients diagnosed with CTEPH did not have a prior history of DVT or PE at the time of diagnosis.18 Further work is needed to determine the prevalence of uterine fibroids in general PH populations as well as large CTEPH cohorts in order to completely explore these associations.
Conclusion
Herein, we report a case series of seven women who were diagnosed with CTED or CTEPH, and in the course of their evaluation, were found to have a history of large uterine fibroids compressing the pelvic venous system or IVC. Given that vascular compression is a known cause of thrombosis, our findings suggest that large uterine fibroids leading to pelvic venous compression may represent a risk factor for VTE and eventual development of CTEPH.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Stewart EA. Uterine fibroids. N Engl J Med 2015; 372(17): 1646–1655. [DOI] [PubMed] [Google Scholar]
- 2.Shiota M, Kotani Y, Umemoto M, et al. Deep-vein thrombosis is associated with large uterine fibroids. Tohoku J Exp Med 2011; 224(2): 87–89. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld H, Byard RW. Lower extremity deep venous thrombosis with fatal pulmonary thromboembolism caused by benign pelvic space-occupying lesions: an overview. J Forensic Sci 2012; 57(3): 665–668. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa H, Ideishi M, Nishimura T, et al. Deep venous thrombosis and pulmonary thromboembolism associated with a huge uterinemyoma: a case report. Angiology 2000; 51(2): 161–166. [DOI] [PubMed] [Google Scholar]
- 5.Riat R, Chowdary P, Mavrides E, et al. Is there an association between thrombosis and fibroids? A single centre experience and literature review. Int J Lab Hematol 2013; 35(1): e13–e16. [DOI] [PubMed] [Google Scholar]
- 6.Ramanan S, Chapman-Wardy J, Watson R. Bleeding versus clotting: a complex case of a large fibroid uterus causing menorrhagia and a DVT. Case Rep Obstet Gynecol 2016; 2016: 4169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher H, Wharfe G, Williams NP, et al. Venous thromboembolism as a complication of uterine fibroids: a retrospective descriptive study. J Obstet Gynaecol 2009; 29(8): 732–736. [DOI] [PubMed] [Google Scholar]
- 8.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998. Arch Intern Med 2003; 163: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 9.Raza F, Alkhouli M, Rogers F, et al. Case series of 5 patients with end-stage renal disease with reversible dyspnea, heart failure, and pulmonary hypertension related to arteriovenous dialysis access. Pulm Circ 2015; 5(2): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raza F, Dillane C, Mirza A, et al. Differences in right ventricular morphology, not function, indicate the nature of increased afterload in pulmonary hypertensive subjects with normal left ventricular function. Echocardiography 2017; 34: 1584–1592. [DOI] [PubMed] [Google Scholar]
- 11.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States. Arch Intern Med 2011; 171(9): 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saouti N, Man F, Westerhof N, et al. Predictors of mortality in inoperable chronic thromboembolic pulmonary hypertension. Respir Med 2009; 103: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 13.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49(2): 1601792. [DOI] [PubMed] [Google Scholar]
- 14.Pengo V, Lensing AWA, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350: 2257–2264. [DOI] [PubMed] [Google Scholar]
- 15.Poch DS, Auger WR. Chronic thromboembolic pulmonary hypertension: detection, medical and surgical treatment approach, and current outcomes. Heart Fail Rev 2016; 21: 309–322. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Mayer E, Simonneau G, et al. Chronic thromboembolic pulmonary hypertension. Circulation 2006; 113: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 17.Auger WR, Kim NH, Kerr KM, et al. Chronic thromboembolic pulmonary hypertension. Clin Chest Med 2007; 28: 255–269. [DOI] [PubMed] [Google Scholar]
- 18.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH) results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 19.Archibald CJ, Auger WR, Fedullo PF, et al. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med 1999; 160(2): 523–528. [DOI] [PubMed] [Google Scholar]
- 20.Iwase T, Nagaya N, Ando M, et al. Acute and chronic effects of surgical thromboendarterectomy on exercise capacity and ventilatory efficiency in patients with chronic thromboembolic pulmonary hypertension. Heart 2001; 86: 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. [DOI] [PubMed] [Google Scholar]
- 22.Raza F, Vaidya A, Lacharite-Roberge AS, et al. Initial clinical and hemodynamic results of a regional pulmonary thromboendarterectomy program. J Cardiovasc Surg 2018; 59(3): 428–437. [DOI] [PubMed] [Google Scholar]
- 23.Thistlethwaite PA, Kemp A, Du L, et al. Outcomes of pulmonary endarterectomy for treatment of extreme thromboembolic pulmonary hypertension. J Thorac Cariovasc Surg 2006; 131(2): 307–313. [DOI] [PubMed] [Google Scholar]
- 24.Thistlethwaite PA, Kaneko K, Madani MM, et al. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg 2008; 14: 274–282. [PubMed] [Google Scholar]
- 25.Ogawa N, Hayashi Y, Maehara T, et al. A surgically treated case of acute pulmonary embolism owing to deep vein thrombosis of the leg mainly caused by uterine myoma. Kyobu Geka 1992; 45(7): 631–634. [PubMed] [Google Scholar]
- 26.Stanko CM, Severson MA, Molpus KL. Deep venous thrombosis associated with large leiomyomata uteri: a case report. J Reprod Med 2001; 46(4): 405–407. [PubMed] [Google Scholar]
- 27.Podduturi V, Armstrong-Briley DR, Guileyardo JM. Sudden death by pulmonary thromboembolism due to a large uterine leiomyoma with a parasitic vein to the mesentery. Case Rep Obstet Gynecol 2014; 2014: 181265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcone M, Serra P. Massive pulmonary embolism in a woman with leiomyomatous uterus causing pelvic deep venous thrombosis. Ann Ital Med Int 2005; 20(2): 104–107. [PubMed] [Google Scholar]
- 29.Hawes J, Lohr J, Blum B, et al. Large uterine fibroids causing mechanical obstruction of the inferior vena cava and subsequent thrombosis: a case report. Vasc Endovascular Surg 2006; 40(5): 425–427. [DOI] [PubMed] [Google Scholar]
- 30.Khademvatani K, Rezaei Y, Kerachian A, et al. Acute pulmonary embolism caused by enlarged uterine leiomyoma: a rare presentation. Am J Case Rep 2014; 15: 300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May R, Thurner J. The cause of the predominately sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957; 8: 419–427. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2000; 11(7): 823–836. [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Choi D, Guk Ko Y, et al. Percutaneous treatment of deep vein thrombosis in May-Thurner syndrome. Cardiovasc Intervent Radiol 2006; 29(4): 571–575. [DOI] [PubMed] [Google Scholar]
- 34.Peters M, Syed RK, Katz M, et al. May-Thurner syndrome: a not so uncommon cause of a common condition. Proc (Bayl Univ Med Cent) 2012; 25(3): 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eliahou R, Sosna J, Bloom AI. Between a rock and a hard place: clinical and imaging features of vascular compression syndromes. Radiographics 2012; 32: E33–E49. [DOI] [PubMed] [Google Scholar]