Abstract
Macrophages respond to several stimuli by forming florid membrane ruffles that lead to fluid uptake by macropinocytosis. This type of induced macropinocytosis, executed by a variety of non-malignant and malignant cells, is initiated by transmembrane receptors and is involved in nutrient acquisition and mTOR signalling. However, macrophages also perform a unique type of constitutive ruffling and macropinocytosis that is dependent on the presence of extracellular calcium. Calcium-sensing receptors are responsible for this activity. This distinct form of macropinocytosis enables macrophages to continuously sample their microenvironment for antigenic molecules and for pathogen- and danger-associated molecular patterns, as part of their immune surveillance functions. Interestingly, even within the monocyte lineage, there are differences in macropinocytic ability that reflect the polarized functional roles of distinct macrophage subsets. This review discusses the shared and distinct features of both induced and constitutive macropinocytosis displayed by the macrophage lineage and their roles in physiology, immunity and pathophysiology. In particular, we analyse the role of macropinocytosis in the uptake of modified low-density lipoprotein (LDL) and its contribution to foam cell and atherosclerotic plaque formation. We propose a combined role of scavenger receptors and constitutive macropinocytosis in oxidized LDL uptake, a process we have termed ‘receptor-assisted macropinocytosis'.
This article is part of the Theo Murphy meeting issue ‘Macropinocytosis’.
Keywords: macropinocytosis, macrophage, calcium-sensing receptor, atherosclerosis, cd36, oxLDL
1. Introduction
Macrophages are key components of the innate immune system that play important roles in homeostasis and in the control of the disease. They are professional phagocytes, exceptionally specialized in fighting infection, bridging innate and adaptive immunity, and supporting tissue development, maintenance and remodelling. Central to the diverse functions of macrophages is their ability to survey, sample and recognize microbial and apoptotic targets for engulfment. While recent advances have identified various subsets of macrophages based on their characteristics and observed abilities in distinct tissue environments, these subsets are ultimately unified by their fundamental ability to efficiently internalize, degrade and process extracellular material.
Small molecules and ligands can be internalized through the endocytic pathway by most cells. However professional phagocytes, like macrophages, can additionally internalize larger material through the receptor-guided process of phagocytosis, or the uptake of large volumes of the fluid phase via macropinocytosis. Macrophages continuously sample their environment through actin-dependent ruffling and membrane extension [1], which are associated with the formation of large sealed vacuoles or macropinosomes. As a result, phagocytes have the remarkable ability to internalize the equivalent of their entire surface area approximately every 30 min [2].
The formation of macropinosomes shares important features with phagocytosis. Both processes depend on tightly regulated phosphoinositide signalling at the plasma membrane [3–5]. Plasmalemmal phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) is converted by phosphatidylinositol 3-kinase (PI3K) to phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) and these inositides jointly contribute to recruit RhoG, Rac1, Cdc42 and actin-nucleating factors to form and extend lamellipodia and ruffles during both processes [6–12]. PtdIns(3,4,5)P3 also plays a role in the termination of Rho-GTPase activity and, together with the subsequent disappearance of PtdIns(4,5)P2, fosters the breakdown of actin that facilitates ruffle closure and macropinosome/phagosome internalization [13–17].
2. Constitutive versus inducible macropinocytosis
Macropinocytosis has been studied in a variety of cell types following stimulation by growth factors. Phagocytes are also stimulated to perform macropinocytosis by growth factors, and additionally by chemokines like CXCR4 [18–20]. Such ‘induced’ macropinocytosis plays an important role in the nutrient acquisition, delivering extracellular solutes to the endosomal system. Induced macropinocytosis transports proteins and amino acids to lysosomes [21] and is therefore intimately tied to intracellular metabolic pathways controlled by the mTOR complex 1 (mTORC1). Peak levels of PtdIns(3,4,5)P3 and the related inositide PtdIns(3,4)P2 can recruit proteins such as Ras and Akt that, combined with luminal amino acids, activate mTORC1 [22]. This complex promotes cell growth and proliferation through the upregulation of protein, lipid and nucleic acid biosynthesis and the downregulation of catabolism by autophagy [23]. mTORC1 activation has been reported to occur in conjunction with platelet-derived growth factor (PDGF), macrophage colony stimulating factor (M-CSF) and CXCR4-induced macropinocytosis [19,24]. Cancerous cells, which often bypass the normal checkpoints in growth factor signalling, dysregulate both macropinocytosis and mTOR activity [22,23]. For example, cancer cells use the CXCL12/CXCR4/mTOR axis to stimulate nutrient uptake by macropinocytosis, while promoting angiogenesis, tumour progression and metastasis [25–29].
In many transformed cells, the process of macropinocytosis is ongoing, owing to the expression of oncogenes. Oncogenic mutations in H-Ras, v-Src and K-Ras all result in ‘constitutive’ macropinocytosis [30–35]. Such constitutive macropinocytosis associated with cancerous reprogramming represents an adaptation strategy to fuel unrestricted cellular growth [36]. In particular, oncogenic forms of Ras and Src are associated with ongoing macropinocytosis and simultaneously hyperactivate mTORC1, promoting unchecked proliferation [22]. Since macropinocytosis stimulated by oncogenic Ras mutations promotes tumour cell feeding [32,37], inhibition by the amiloride analogue EIPA blocks the growth of the transformed cells on albumin [32].
Moreover, the growth of cells in nutrient-poor regions of pancreatic tumours is dependent on the scavenging of extracellular proteins by macropinocytosis [38]. Thus, constitutive macropinocytosis can act as a cancer survival mechanism to promote the acquisition of macromolecules present in the extracellular fluid. As such, enhanced macropinocytosis is now considered a hallmark of cancer metabolism [23].
However, unlike transformed cells, stimulation is necessary for the induction of macropinocytosis in most non-malignant cells. Macrophages and immature dendritic cells are a rather unique exception, as they exhibit ‘constitutive’ macropinocytosis, in addition to the induced macropinocytic response [1,39–44]. Macrophages and dendritic cells depend on their ability to avidly sample their environment for antigens to present to lymphocytes, and constitutive macropinocytosis fulfils this function [45]. It delivers antigen to both MHC Class I and Class II [43,46,47]. In addition, constitutive macropinocytosis also results in the internalization of solutes bearing pathogen- and danger-associated molecular patterns that can be recognized by innate immune sensors in endomembranes or in the cytosol [48,49] (discussed in §4). Clearly, constitutive macropinocytosis contributes to the sentinel functions of these professional phagocytes [2,43]. Another previously unappreciated function of macrophage also depends on constitutive macropinocytosis. Macrophages are able to take up low-density lipoproteins by macropinocytosis, including native and modified LDL [39,50–54]; these are implicated in foam cell formation and atherosclerotic pathology in coronary heart disease (discussed in §5).
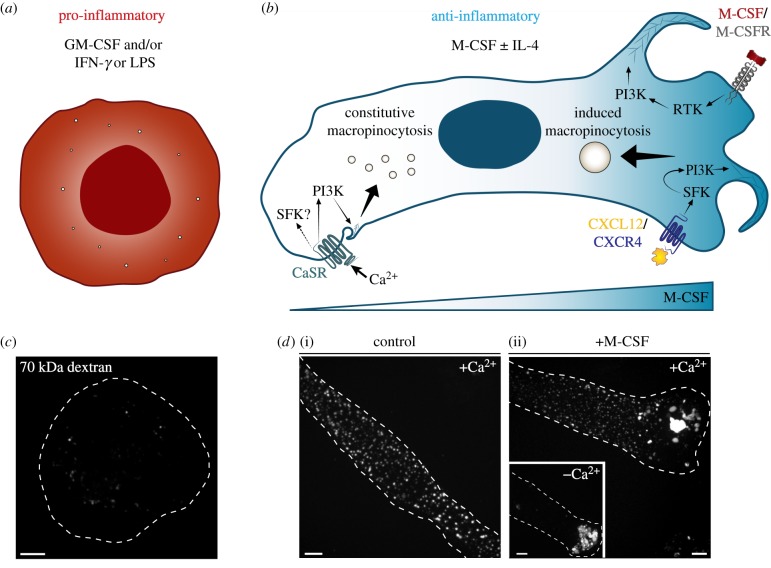
Recent work has investigated the signalling mechanisms involved in induced versus constitutive macropinocytosis. Both induced and constitutive forms of macropinocytosis are dependent on PI3K, Rho-GTPases and actin rearrangements (figure 1b and [7–9,14,57,58]), although the size of the resulting vacuoles differs: the macropinosomes formed constitutively are notably smaller than those induced by growth promoters (figure 1d and [48]). Regarding PI3K, the particular isoforms expressed can vary by cell type, but in macrophages, pharmacological studies have suggested the involvement of the Class I p110 δ and/or γ catalytic subunit isoforms in macropinocytosis [19] or at least in the M-CSF receptor signalling that is associated with macropinosome generation [59,60], although another study disputes the involvment of Class I PI3Ks [40]. Nonetheless, wortmannin and LY294002, used at concentrations applied routinely to inhibit Class I kinases, block both induced and constitutive macropinocytosis [14,48,61]. In addition, as expected, both forms of macropinocytosis are sensitive to actin inhibitors such as latrunculin [48]. However, upstream of the initiation of PI3K signalling, the mechanisms of induced and constitutive macropinocytosis differ. The constitutive macropinocytosis of macrophages and dendritic cells is associated with the ongoing generation of high levels of phosphatidic acid at the plasma membrane, which—by means that are poorly understood, and have been extrapolated from cell line studies—stimulates ruffling by activation of Rac1 and Cdc42 [1,40]. Moreover, constitutive macropinocytosis is considerably less sensitive to amiloride and its analogues [38], which are prototypical inhibitors of stimulated macropinocytosis.
Figure 1.
Regulation of induced and constitutive forms of macropinocytosis and their modulation by macrophage polarization. Pro-inflammatory macrophages (a), commonly differentiated by incubation with GM-CSF and/or IFN-γ or LPS, do not perform appreciable constitutive macropinocytosis. Note the paucity of macropinosomes. However, anti-inflammatory macrophages (b), which can be generated by incubation in M-CSF ± IL-4, are capable of both induced and constitutive forms of macropinocytosis. Induced macropinocytosis commonly occurs downstream of receptor stimulation by growth factors (e.g. M-CSF) or chemokines (e.g. CXCL12). In the case of the M-CSF receptor, traffic to and from the macropinosome, and downstream signalling pathways activated by M-CSF stimulation have been studied in some detail [55,56]. After such receptors activate tyrosine kinase activity, PI3K is recruited to stimulate the production of PtdIns(3,4,5)P3 from plasmalemmal PtdIns(4,5)P2, which promotes the recruitment of cytosolic factors (i.e. Rho-GTPases, SCAR/WAVE, WASP, Arp2/3 complex) that promote ruffling and remodel the actin cytoskeleton leading to macropinosome formation. Constitutive macropinocytosis occurs in the absence of growth factor stimulation, but is dependent on extracellular calcium, which stimulates calcium-sensing receptors (CaSR). There is preliminary evidence that tyrosine kinases are involved in CaSR-mediated signalling, but their role in constitutive macropinocytosis has not been fully characterized. Like induced macropinocytosis, PI3K is required for the ruffling and actin polymerization dynamics involved in constitutive macropinocytosis. (c) Isolated human peripheral blood mononuclear cells were incubated for 5 days in GM-CSF (25 ng ml−1), followed by 2 days in LPS (500 ng ml−1) plus IFN-γ (10 ng ml−1), to generate pro-inflammatory macrophages. These cells were then incubated with fluorescently labelled 70 kDa dextran (25 µg ml−1) for 15 min at 37°C, and the number of dextran-positive macropinosomes was visualized by confocal microscopy. Cell outline is shown with a dotted line. Scale bar, 5 µm. (d) Isolated human peripheral blood mononuclear cells were incubated for 7 days in M-CSF (25 ng ml−1) to generate anti-inflammatory macrophages. These cells were then incubated with fluorescently labelled 70 kDa dextran (25 µg ml−1) in the presence (i) or absence (ii) of M-CSF (200 ng ml−1) for 15 min at 37°C, in medium with (i,ii) or without calcium ((ii) inset). Dextran-positive macropinosomes were visualized as in (c). Cell outlines are shown with dotted lines. Scale bars, 5 µm. CSFR, colony stimulating factor receptor; IL-4, interleukin 4; RTK, receptor tyrosine kinase; SFK, Src family kinases.
While induced macropinocytosis is initiated by growth factors or chemokines that bind to receptors or G protein-coupled receptors (GPCR; figure 1b), constitutive macropinocytosis is maintained by the ongoing activity of the calcium-sensing receptor (CaSR). As such, it is exquisitely dependent on the presence of extracellular calcium and pharmacological inhibition of CaSR arrests constitutive ruffling and macropinocytosis in macrophages [48]. On the other hand, induced macropinocytosis is seemingly calcium independent (figure 1d(ii), inset). The types of receptors capable of eliciting inducible macropinocytosis are more varied: they include tyrosine kinase receptors and also GPCRs. Non-receptor tyrosine kinases, mainly Src family kinases (SFK), are often involved [14,41,62–64], leading to the recruitment and activation of PI3K and the stimulation of Rac1 and Cdc42, [9,57,58]. Preliminary experiments suggest that SFKs may also be involved in constitutive macropinocytosis [65,66], but definitive evidence is still lacking.
3. Macropinocytosis and macrophage polarization
Not all macrophages have comparable macropinocytic activity. Macrophage polarization dictates macropinocytic ability and the observed differences are related to the diverging function of the specific cell types in the host. Macrophage polarization into distinct populations occurs in the tissue microenvironment as a result of exposure to select cytokines, chemokines and other stimuli including bacterial lipopolysaccharides. Though now appreciated to be a continuum [67], polarization is best studied analysing extreme phenotypes such as the markedly pro-inflammatory (M1) or predominantly anti-inflammatory (M2) macrophages (figure 1a,b). Anti-inflammatory macrophages are generated by the exposure to M-CSF ± interleukin 4 (IL-4) (figure 1b) and have a variety of immunoregulatory functions: they dampen inflammation, prime type II adaptive responses and promote angiogenesis and tissue remodelling. Owing to their anti-inflammatory predisposition, this subset of macrophages is poised to survey, sample and discriminate a variety of host- or pathogen-associated molecules, without eliciting unneeded inflammation, in a manner similar to immature dendritic cells [45]. To this end, anti-inflammatory macrophages readily perform constitutive as well as induced macropinocytosis (figure 1d and [48,61]). As discussed in more detail in §6, these two types of macropinocytosis differ in their mode of induction and signalling (figure 1b). Pro-inflammatory macrophages are generated by stimulation with granulocyte-macrophage colony stimulating factor (GM-CSF) and/or interferon gamma (IFN-γ) or lipopolysaccharide (LPS) (figure 1a) and are characterized by robust microbicidal activity (via generation and delivery to phagosomes of reactive oxygen and nitrogen species), high capacity to present antigens and the secretion of pro-inflammatory cytokines like IL-12, all key to priming type I adaptive immunity. These macrophages no longer perform sentinel functions like antigen sampling and, as such, human pro-inflammatory macrophages do not perform constitutive macropinocytosis (as measured by fluid-phase uptake of 70 kDa dextran; figure 1c and [48,61]). Both human [61] and murine [68] pro-inflammatory macrophages have been shown to exhibit induced macropinocytosis under some conditions.
It is noteworthy, however, that macrophages are plastic and can interconvert between polarization states, or even exhibit mixed identities, depending on the tissue microenvironment [69]. While macrophage polarization was once seen as a linear continuum, it is now perceived as a modular spectrum of activation, to account for this plasticity and versatility [70]. Therefore, an individual macrophage can display a blend of pro-inflammatory, regulatory and wound-healing functionality that fluctuates as environmental signals change [70]. How this plasticity affects macropinocytic ability remains to be illuminated, but likely will depend on the local requirements for macrophage-regulated inflammation versus surveillance.
Anti-inflammatory (M2) macrophages exhibit active constitutive macropinocytosis and can be further stimulated by agents like M-CSF. By comparison, pro-inflammatory (M1) macrophages are rather quiescent. Their much-reduced constitutive macropinocytic activity has been attributed to the lower abundance and activation of PI3K, which in turn regulate Ras-related C3 botulinum toxin substrate 1 (Rac1) and Ras homology growth-related (RhoG) activity [61]. However, the machinery required for macropinocytosis is present and capable of activation in M1 cells. Indeed, stimulation of pro-inflammatory cells with LPS or CC chemokine ligand 19 (CCL19) results in macropinosomes formation [61].
4. Macropinocytosis and innate immunity
As mentioned above, constitutive macropinocytosis is of particular interest because it has a unique role in macrophage function. The constant environmental sampling that results from constitutive macropinocytosis contributes to antigen presentation and to priming the adaptive immune response. Interestingly, this form of macropinocytosis is also able to modulate macrophage innate function by providing ligands to pattern recognition receptors like cytosolic nucleotide-binding oligomerization domain-containing protein 2 (NOD2) [48] and endosomal/lysosomal Toll-like receptors 3, 7, 8 and 9 (TLR3, 7, 8 and 9) [49], which can initiate nuclear factor (NF)-κB-mediated inflammatory and microbicidal responses [45]. Induced macropinocytosis can be initiated through TLR4 [39,41] and may boost the uptake of antigens and of pathogen- or danger-associated signals. It may also contribute to the nutrient acquisition, as is thought to be the case for growth factors. While the complete sphere of macrophage responses influenced by macropinocytosis remains to be characterized, these initial studies nonetheless provide unique perspectives into the role of macropinocytosis in normal physiology.
5. Macropinocytosis, modified lipoprotein uptake and atherosclerosis
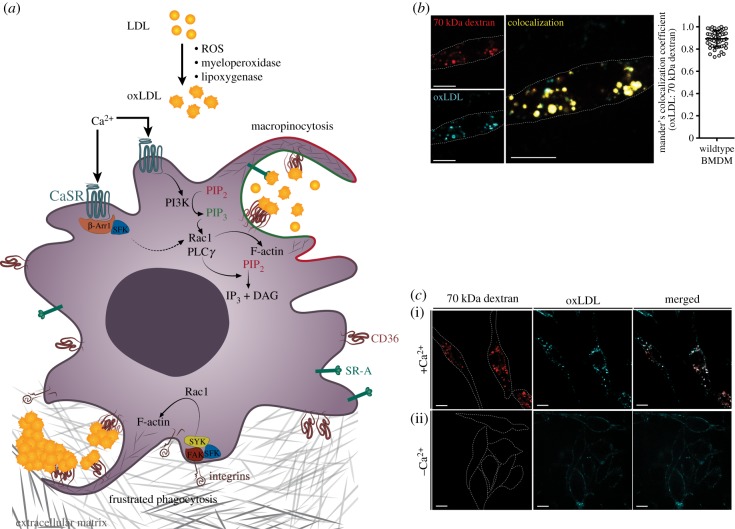
The inordinate amounts of extracellular fluid taken up by macropinocytosis, while essential for the normal function of macrophages, can also contribute to disease. Indeed, macropinocytosis had been proposed to mediate the uptake of lipoproteins by phagocytes, thereby contributing to plaque development during atherosclerosis [71]. Atherosclerosis, a major health problem in developed countries, is a consequence of endothelial barrier dysfunction leading to sub-endothelial retention of low-density lipoprotein (LDL) in the intimal space of the vasculature. Athero-prone endothelium displays impaired barrier function in areas that experience disturbed laminar flow, such as branched arteries, allowing for the entry of LDL into the arterial intima where lipoproteins are subjected to oxidative modification through exposure to lipoxygenase, myeloperoxidase and reactive oxygen species [72–74]. The resulting oxidized LDL (oxLDL) can be internalized by macrophages, in part by macropinocytosis. Additionally, excess LDL that reaches the sub-endothelial space can be retained by anchoring to the extracellular matrix through its interaction with glycosaminoglycans, facilitating its subsequent modification to oxLDL within the arterial wall [75]. Anchorage to the extracellular matrix can lead to aggregation of oxLDL; such bound aggregates are thought to be targets for phagocytosis by the macrophages. Both modes of internalization are illustrated in figure 2a.
Figure 2.
The role of constitutive macropinocytosis in the uptake of modified lipoproteins. (a) In vivo, low-density lipoprotein (LDL) can become oxidized by reactive oxygen species (ROS), myeloperoxidase and/or lipoxygenase within the inflammatory environment of the arterial intima. Scavenger receptors, such as CD36 or SR-A, bind and anchor the resulting oxidized LDL (oxLDL) to the membrane, facilitating internalization by constitutive macropinocytosis stimulated by CaSR. PI3K is activated downstream of CaSR ligation, resulting in phosphorylation of PtdIns(4,5)P2 to PtdIns(3,4,5)P3. This leads to recruitment and activation of Rho-GTPases (i.e. Rac1) that facilitate Arp2/3-mediated F-actin polymerization. Phospholipase C gamma (PLCγ) activation, either through CaSR signalling via active G alpha subunit (Gα) or through interaction with PtdIns(3,4,5)P3, mediates the breakdown of PtdIns(4,5)P2 into Ins(3,4,5)P3 and diacylglycerol (DAG). Formation and modification of DAG leads to the recruitment of Rac guanine nucleotide exchange factors (GEFs), facilitating F-actin polymerization and constitutive membrane ruffling. Ligation of CaSR can also lead to recruitment of β-arrestin1, which provides a docking site for SFKs, enabling their activation. Active SFKs can further promote constitutive macropinocytosis through activation of Rac1. Within the arterial intima, LDL and oxLDL have been found to bind and aggregate on the extracellular matrix. Such aggregates are thought to induce phagocytosis that may be frustrated by the inability of the macrophage to engulf the adherent aggregate. This type of phagocytosis likely requires activation of integrins, recruitment of non-receptor tyrosine kinases (e.g. FAK, SFK and SYK) and activation of Rho-family GTPases, resulting in large-scale F-actin polymerization. (b) Mouse bone marrow-derived macrophages (BMDMs) were generated by a 7-day treatment with M-CSF (10 ng ml−1). These cells were then incubated with fluorescently labelled 70 kDa dextran (25 µg ml−1) and oxLDL (2 µg mL−1) in a calcium-containing medium for 15 min at 37°C. Positive overlap of oxLDL and dextran channels is shown in yellow. Scale bar, 10 µm. The significance of the co-localization between oxLDL-647 and 70 kDa-TMR-dextran was quantified as the Manders overlap coefficient, which averaged 0.892 ± 0.073 and is highly significant (p < 0.001). (c) BMDMs were generated as in (b). These cells were then incubated with fluorescently labelled 70 kDa dextran (25 µg ml−1) and oxLDL (2 µg ml−1) in medium containing calcium (i) or in nominally calcium-free medium (ii) for 15 min at 37°C. The spatial localization of oxLDL and presence of dextran-positive macropinosomes were visualized by confocal microscopy. Merged images are shown in the rightmost panels. Cell outlines are shown with dotted lines. Scale bar, 10 µm. PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate; IP3, inositol 1,4,5-trisphosphate.
Of the various myeloid cells involved in plaque development, monocyte-derived macrophages contribute importantly to lesion formation, accounting for roughly 50% of the immune cells present in the atherosclerotic lesions [76]. Such macrophages identify and respond to oxLDL through scavenger receptors (discussed below). Chronic exposure to lipoproteins within an inflammatory environment prompts macrophages to internalize them and process the associated lipids. Subsequent cholesterol accumulation within these macrophages prompts their conversion to lipid-laden ‘foam’ cells. When the lipid burden becomes excessive, foam cells experience apoptosis [77]; moreover, because the lipid-laden macrophages have reduced phagocytic activity, a failure in efferocytosis of the apoptotic foam cells causes them to undergo necrosis [78]. The accumulation of inflammatory cells around the resulting necrotic core, combined with thinning of the lesional fibrous cap, can cause the plaque to become larger and less stable, making it increasingly prone to rupture, thereby initiating acute thrombotic vascular events (i.e. heart attacks or strokes).
Scavenger receptors, first described by Brown and Goldstein [79,80], were first associated with the ability to bind modified lipoproteins [81]. Since then, researchers have gone on to identify a handful of scavenger receptors able to mediate binding and internalization of oxLDL. These include CD36, scavenger receptor A (SR-A), lectin-like oxidized LDL receptor 1 (LOX-1) and macrophage receptor with collagenous structure (MARCO). CD36 has been shown to mediate ≈60% of oxLDL uptake by macrophages in vitro [82], while in vivo CD36 and SR-A reportedly mediate about 90% of foam cell formation, with CD36 responsible for ≈70% of this phenotype [83,84]. Accordingly, foam cell formation is notably decreased in CD36-null macrophages [85]. It warrants recognition that in the absence of CD36 and SR-A, mild plaque burden can still establish in vivo, but is unable to progress to complex, advanced stage lesions [86,87]. This mild plaque development may be owing to macrophage-mediated fluid-phase pinocytosis of LDL in vivo [52,88]. Nonetheless, a plethora of literature exists outlining the importance of scavenger receptors in the binding of modified LDL and in foam cell formation.
Although structurally unrelated, the various types of scavenger receptors share the presence of conserved clusters of cationic patches in their ligand-binding domain, which allow for binding of negatively charged targets such as oxLDL and apoptotic cells that expose phosphatidylserine. Remarkably, while the binding event is envisaged to result from an electrostatic association, it remains unclear how scavenger receptors convey signals to mediate internalization of their ligand. Indeed, in the case of CD36, the N- and C-terminal cytosolic domains are extremely short (only 7 and 13 residues long, respectively [89]) and lack obvious intracellular signalling domains or motifs. Thus, the mode of internalization of oxLDL remains a mystery.
The signalling pathways activated in macrophages in response to oxLDL treatment have been studied extensively in an effort to understand how internalization of this important ligand occurs. SFK-dependent activation downstream of focal adhesion kinase (FAK) has been shown to occur upon oxLDL treatment, which is lost in CD36-null peritoneal macrophages [90]. This likely reflects an interaction between CD36 and integrins, which is thought to contribute to macrophage trapping in the arterial intima. Additionally, CD36 engagement by oxLDL triggers activation of the SFK member Lyn, the mitogen-activated protein kinases (MAPK) family member MEKK4 and JNK1/2. Furthermore, SFK-mediated activation of Rho-family guanine nucleotide-exchange factors (RhoGEFs) Vav1, Vav2 and Vav3 was reported and interference with this process disrupted oxLDL uptake and foam cell formation in vitro and in vivo [91,92]. Despite these observations, the mode of entry of oxLDL and the upstream signalling events leading to the internalization remain poorly understood.
Although conflicting data exist with regard to the mode of modified LDL uptake, there is a consensus on a crucial step needed for this process to occur: actin cytoskeletal reorganization is required for oxLDL internalization. Pharmacological inhibition of F-actin formation using latrunculin B or cytochalasin D had been shown to cause a marked (≈80%) reduction in oxLDL internalization [52,93]. Additionally, activation of Rac1 and Cdc42 that leads to actin reorganization promoted uptake of CD36 into vesicles that were smaller than conventional induced macropinosomes [42]. Although we initially concluded that oxLDL uptake was part of an actin-dependent process separate from macropinocytosis [52], we were not aware at the time that macrophages exhibited constitutive macropinocytic behaviour in the absence of exogenous stimulants. The realization that an alternative form of macropinocytosis exists constitutively in macrophages and gives rise to smaller vacuoles offers us a reason to revisit the possibility that macropinocytosis may be an important mode of entry for oxLDL in macrophages.
It is worth considering that macropinocytosis is not merely a means to engulf bulk fluid and soluble ligands but is a very effective means of internalizing vast areas of the plasma membrane. This distinction is particularly relevant for constitutive macropinocytosis, where smaller vesicles with a greater surface-to-volume ratio are formed. We therefore propose that cells expressing a sizable density of scavenger receptors like CD36 and SR-A and undergoing constitutive macropinocytosis would thereby internalize significant amounts of ligands, particularly oxLDL. We propose to designate this process as ‘receptor-assisted macropinocytosis', which implies that the uptake is not simply the internalization of ligands suspended in the bulk fluid but is aided by the concentrative effect of the receptors that bind and immobilize additional ligands on the cell surface prior to vacuole sealing (figure 2a). This concept aligns well with the observation that oxLDL uptake occurs largely through an actin-dependent process. The ruffling behaviour that is necessary for constitutive macropinocytosis can also aid oxLDL binding by creating zones that allow for increased CD36 clustering at the cell membrane [94].
Recent observations in our laboratory, using improved imaging methods, support the notion that much of the oxLDL enters macrophages via constitutive macropinocytosis. When added to bone marrow-derived murine macrophages, labelled oxLDL was found in the same compartment where 70 kDa dextran was located (figure 2b). Owing to its large hydrodynamic radius, this type of dextran is felt to be excluded from clathrin-coated pits and caveolae and is preferentially internalized via macropinocytosis. More importantly, oxLDL uptake was markedly depressed when constitutive macropinocytosis was inhibited by the removal of extracellular calcium, as was the uptake of dextran (figure 2c). In the latter case, oxLDL was observed lining the surface of the cells, confirming its ability to bind to scavenger receptors and the requirement for macropinocytosis for its entry. The removal of extracellular calcium does not significantly affect the endocytic uptake [48]. Together, these findings support the notion of receptor-assisted macropinocytosis.
6. Macrophage polarization and oxLDL uptake
The relative importance of pro-inflammatory (M1) and anti-inflammatory (M2) macrophages to the formation of foam cells and the development of atherosclerotic plaque has been an ongoing topic of discussion. Various authors have sought to determine whether pro- or anti-inflammatory macrophages are involved in the exacerbation or in the resolution/stabilization of the plaque. For the development of atherosclerosis, pro-inflammatory macrophages are thought to contribute to the inflammatory milieu of the arterial intima. Secretion of their pro-inflammatory mediators (i.e. TNF-α, iNOS, IL-1β, MCP-1, proteolytic enzymes) primes surrounding myeloid cells, upregulates adhesion molecules on endothelial cells and aids in extracellular matrix breakdown, resulting in disease progression [95,96]. On the other hand, anti-inflammatory macrophages aid in tissue remodelling, increasing collagen production and secretion of anti-inflammatory mediators (i.e. TGF-β, IL-10) [96]. It would therefore seem easy to glean that pro- and anti-inflammatory macrophages would exacerbate and resolve arterial lesions, respectively. However, the presence and abundance of these macrophages are heterogeneous within the lesion, with their spatial and temporal distribution changing as the plaque progresses. Anti-inflammatory macrophages were found to predominate during early lesion development, while pro-inflammatory macrophages were found largely in advanced lesions [97]. As a result, a fierce debate as to whether a macrophage phenotypic switching occurs within the lesion has developed over the past decade [97–100]. In this context, it is interesting that anti-inflammatory macrophages express higher levels of CD36 and SR-A compared to pro-inflammatory macrophages, an observation validated by both RNA-seq and protein quantification [101]. This informs the observation that anti-inflammatory macrophages internalize significantly more oxLDL than pro-inflammatory macrophages [102], consistent with the notion that these cells are important during the early stages of lesion development.
In accordance with the receptor-assisted macropinocytosis idea, anti-inflammatory macrophages were shown to have an eight-fold increase in constitutive macropinocytosis compared to pro-inflammatory cells [61]. This could be owing to a greater accumulation of PtdIns(3,4,5)P3 at the plasma membrane, downstream of CaSR activation [48], which would in turn allow for the recruitment/activation of Rho GEFs and stimulation of Rho-GTPase activity and actin polymerization (figure 2a). Alternatively, more active SFKs could account for the enhanced macropinocytosis [52,103]; SFK activity has been linked to GPCR signalling, through the adaptor protein β-arrestin. β-arrestin, which binds to active GPCRs, provides a docking site for SFKs by interacting with their SH3 and catalytic domains [66,104], enabling kinase activation of downstream effectors. Of note, activation of CaSR has been shown to recruit β-arrestin1 to the plasma membrane, facilitating membrane ruffling through a cytoskeletal-signalling module [65,105]. As such, CaSR is capable of recruitment and activation of SFK to promote the actin-dependent ruffling necessary for macropinocytosis.
7. Concluding remarks
We believe that the emerging evidence that oxLDL is able to enter macrophages through constitutive macropinocytosis sheds light on the process of foam cell and plaque formation. Although uptake of modified lipoproteins through fluid-phase uptake had been suggested previously, the receptor-assisted macropinocytosis model serves to account for the high efficiency of the process, without the need to invoke scavenger receptor-initiated signalling.
Acknowledgements
We sincerely thank Dr Johnathan Canton for providing the confocal images used in figure 1 of this manuscript. S.A.D. is the recipient of the Queen Elizabeth II Graduate Scholarship in Science and Technology. M.E.M. was the recipient of a Heart and Stroke Pfizer Research Fellowship.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the conception and drafting of text and figures and have read and approved the final manuscript.
Competing interests
We declare no competing interests.
Funding
Work in the authors' laboratories is supported by Canadian Institutes of Health Research grant nos FDN-143202 and FDN-143230.
References
- 1.Bohdanowicz M, et al. 2013. Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes. Mol. Biol. Cell. 24, 1700–1712. ( 10.1091/mbc.e12-11-0789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Brodie SE, Cohn ZA. 1976. Membrane flow during pinocytosis. A stereologic analysis. J. Cell Biol. 68, 665–687. ( 10.1083/jcb.68.3.665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin R, Grinstein S, Schlam D. 2014. Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta 1851, 805–823. ( 10.1016/j.bbalip.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 4.Marques PE, Grinstein S, Freeman SA. 2017. SnapShot: macropinocytosis. Cell 169, 766 ( 10.1016/j.cell.2017.04.031) [DOI] [PubMed] [Google Scholar]
- 5.Botelho RJ, Scott CC, Grinstein S. 2004. Phosphoinositide involvement in phagocytosis and phagosome maturation. Curr. Top. Microbiol. Immunol. 282, 1–30. ( 10.1007/978-3-642-18805-3_1) [DOI] [PubMed] [Google Scholar]
- 6.Araki N, Hamasaki M, Egami Y, Hatae T. 2006. Effect of 3-methyladenine on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell Struct. Funct. 31, 145–157. ( 10.1247/csf.06029) [DOI] [PubMed] [Google Scholar]
- 7.Araki N, Egami Y, Watanabe Y, Hatae T. 2007. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell Res. 313, 1496–1507. ( 10.1016/j.yexcr.2007.02.012) [DOI] [PubMed] [Google Scholar]
- 8.Yoshida S, Gaeta I, Pacitto R, Krienke L, Alge O, Gregorka B, Swanson JA. 2015. Differential signaling during macropinocytosis in response to M-CSF and PMA in macrophages. Front. Physiol. 6, 8 ( 10.3389/fphys.2015.00008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welliver TP, Swanson JA. 2012. A growth factor signaling cascade confined to circular ruffles in macrophages. Biol. Open. 1, 754–760. ( 10.1242/bio.20121784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoppe AD, Swanson JA. 2004. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell 15, 3509–3519. ( 10.1091/mbc.e03-11-0847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira OV. et al. 2001. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19–25. ( 10.1083/jcb.200107069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewitt S, Tian W, Hallett MB. 2006. Localised PtdIns(3,4,5)P3 or PtdIns(3,4)P2 at the phagocytic cup is required for both phagosome closure and Ca2+ signalling in HL60 neutrophils. J. Cell Sci. 119, 443–451. ( 10.1242/jcs.02756) [DOI] [PubMed] [Google Scholar]
- 13.Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. 2000. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368. ( 10.1083/jcb.151.7.1353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki N, Johnson MT, Swanson JA. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260. ( 10.1083/jcb.135.5.1249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, Heath C, Stahl P, Grinstein S. 2005. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J. Cell Biol. 169, 139–149. ( 10.1083/jcb.200412162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta ZB, Pietka G, Lowe M. 2014. The cellular and physiological functions of the Lowe syndrome protein OCRL1. Traffic Cph Den. 15, 471–487. ( 10.1111/tra.12160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohdanowicz M, Balkin DM, De Camilli P, Grinstein S. 2011. Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol. Biol. Cell 23, 176–187. ( 10.1091/mbc.e11-06-0489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. 2015. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 5, 10300 ( 10.1038/srep10300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacitto R, Gaeta I, Swanson JA, Yoshida S. 2017. CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages. J. Leukoc. Biol. 101, 683–692. ( 10.1189/jlb.2A0316-141RR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka G. et al. 2012. CXCR4 stimulates macropinocytosis: implications for cellular uptake of arginine-rich cell-penetrating peptides and HIV. Chem. Biol. 19, 1437–1446. ( 10.1016/j.chembiol.2012.09.011) [DOI] [PubMed] [Google Scholar]
- 21.Racoosin EL, Swanson JA. 1992. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J. Cell Sci. 102, 867–880. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida S, Pacitto R, Inoki K, Swanson J. 2017. Macropinocytosis, mTORC1 and cellular growth control. Cell Mol. Life Sci. 75, 1227–1239. ( 10.1007/s00018-017-2710-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell JS, Massi D, Teng MWL, Mandala M. 2018. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin. Cancer Biol. 48, 91–103. ( 10.1016/j.semcancer.2017.04.015) [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Martín C, Escribano C, Pablos JL, Riol-Blanco L, Rodríguez-Fernández JL. 2011. Chemokine CXCL12 uses CXCR4 and a signaling core formed by bifunctional Akt, extracellular signal-regulated kinase (ERK)1/2, and mammalian target of rapamycin complex 1 (mTORC1) proteins to control chemotaxis and survival simultaneously in mature dendritic cells. J. Biol. Chem. 286, 37 222–37 236. ( 10.1074/jbc.M111.294116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G, Chen S-M, Wang X, Ding X-F, Ding J, Meng L-H. 2012. Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells. J. Biol. Chem. 287, 12 132–12 141. ( 10.1074/jbc.M111.302299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto I. et al. 2008. Blocking on the CXCR4/mTOR signalling pathway induces the anti-metastatic properties and autophagic cell death in peritoneal disseminated gastric cancer cells. Eur. J. Cancer Oxf. Engl. 44, 1022–1029. ( 10.1016/j.ejca.2008.02.043) [DOI] [PubMed] [Google Scholar]
- 27.Ieranò C. et al. 2014. CXCR4 and CXCR7 transduce through mTOR in human renal cancer cells. Cell Death Dis. 5, e1310 ( 10.1038/cddis.2014.269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weekes CD. et al. 2012. Stromal cell-derived factor 1α mediates resistance to mTOR-directed therapy in pancreatic cancer. Neoplasia N. Y. N. 14, 690–701. ( 10.1593/neo.111810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler ME, Hatch MMS, Wu N, Muawad SA, Hughes CCW. 2016. mTORC2 mediates CXCL12-induced angiogenesis. Angiogenesis 19, 359–371. ( 10.1007/s10456-016-9509-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. 2000. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell. 11, 3453–3467. ( 10.1091/mbc.11.10.3453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Sagi D, Feramisco JR. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068. ( 10.1126/science.3090687) [DOI] [PubMed] [Google Scholar]
- 32.Commisso C. et al. 2013. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637. ( 10.1038/nature12138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. ( 10.1016/0092-8674(92)90164-8) [DOI] [PubMed] [Google Scholar]
- 34.Schmees C, Villaseñor R, Zheng W, Ma H, Zerial M, Heldin C-H, Hellberg C, Margolis B. 2012. Macropinocytosis of the PDGF β-receptor promotes fibroblast transformation by H-RasG12 V. Mol. Biol. Cell 23, 2571–2582. ( 10.1091/mbc.e11-04-0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veithen A, Cupers P, Baudhuin P, Courtoy PJ. 1996. v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci. 109, 2005–2012. [DOI] [PubMed] [Google Scholar]
- 36.Recouvreux MV, Commisso C. 2017. Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front. Endocrinol. 8, 261 ( 10.3389/fendo.2017.00261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung S, Choi J, Cheong H. 2015. Catabolic pathways regulated by mTORC1 are pivotal for survival and growth of cancer cells expressing mutant Ras. Oncotarget 6, 40 405–40 417. ( 10.18632/oncotarget.6334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamphorst JJ. et al. 2015. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553. ( 10.1158/0008-5472.CAN-14-2211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S-H, et al. 2009. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ. Res. 104, 1355–1363. ( 10.1161/CIRCRESAHA.108.192880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galán JE, Mellman I. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325–334. ( 10.1016/S0092-8674(00)00038-6) [DOI] [PubMed] [Google Scholar]
- 41.Lim JP, Wang JTH, Kerr MC, Teasdale RD, Gleeson PA. 2008. A role for SNX5 in the regulation of macropinocytosis. BMC Cell Biol. 9, 58 ( 10.1186/1471-2121-9-58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27, 280–288. ( 10.1002/eji.1830270141) [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Cella M, Danieli C, Lanzavecchia A. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400. ( 10.1084/jem.182.2.389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao W, Li K, Liao K. 2009. Macropinocytosis contributes to the macrophage foam cell formation in RAW264.7 cells. Acta Biochim. Biophys. Sin. 41, 773–780. ( 10.1093/abbs/gmp066) [DOI] [PubMed] [Google Scholar]
- 45.Schlam D, Canton J. 2016. Every day I'm rufflin’: calcium sensing and actin dynamics in the growth factor-independent membrane ruffling of professional phagocytes. Small GTPases 8, 65–70. ( 10.1080/21541248.2016.1197873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. 1995. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 3, 783–791. ( 10.1016/1074-7613(95)90067-5) [DOI] [PubMed] [Google Scholar]
- 47.Peppelenbosch MP, DeSmedt M, Pynaert G, van Deventer SJH, Grooten J. 2000. Macrophages present pinocytosed exogenous antigen via MHC class I whereas antigen ingested by receptor-mediated endocytosis is presented via MHC class II. J. Immunol. 165, 1984–1991. ( 10.4049/jimmunol.165.4.1984) [DOI] [PubMed] [Google Scholar]
- 48.Canton J, Schlam D, Breuer C, Gütschow M, Glogauer M, Grinstein S. 2016. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat. Commun. 7, 11284 ( 10.1038/ncomms11284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wall AA, Luo L, Hung Y, Tong SJ, Condon ND, Blumenthal A, Sweet MJ, Stow JL. 2017. Small GTPase Rab8a-recruited phosphatidylinositol 3-kinase γ regulates signaling and cytokine outputs from endosomal toll-like receptors. J. Biol. Chem. 292, 4411–4422. ( 10.1074/jbc.M116.766337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barthwal MK, Anzinger JJ, Xu Q, Bohnacker T, Wymann MP, Kruth HS. 2013. Fluid-phase pinocytosis of native low density lipoprotein promotes murine M-CSF differentiated macrophage foam cell formation. PLoS One 8, e58054 ( 10.1371/journal.pone.0058054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chellan B, Reardon CA, Getz GS, Bowman MAH. 2016. Enzymatically modified low-density lipoprotein promotes foam cell formation in smooth muscle cells via macropinocytosis and enhances receptor-mediated uptake of oxidized low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 36, 1101–1113. ( 10.1161/ATVBAHA.116.307306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins RF, Touret N, Kuwata H, Tandon NN, Grinstein S, Trimble WS. 2009. Uptake of oxidized low density lipoprotein by CD36 occurs by an actin-dependent pathway distinct from macropinocytosis. J. Biol. Chem. 284, 30 288–30 297. ( 10.1074/jbc.M109.045104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Csányi G. et al. 2017. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid. Redox Signal. 26, 886–901. ( 10.1089/ars.2016.6834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruth HS. et al. 2005. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J. Biol. Chem. 280, 2352–2360. ( 10.1074/jbc.M407167200) [DOI] [PubMed] [Google Scholar]
- 55.Huynh J, Kwa MQ, Cook AD, Hamilton JA, Scholz GM. 2012. CSF-1 receptor signalling from endosomes mediates the sustained activation of Erk1/2 and Akt in macrophages. Cell Signal. 24, 1753–1761. ( 10.1016/j.cellsig.2012.04.022) [DOI] [PubMed] [Google Scholar]
- 56.Lou J, Low-Nam ST, Kerkvliet JG, Hoppe AD. 2014. Delivery of CSF-1R to the lumen of macropinosomes promotes its destruction in macrophages. J. Cell Sci. 127, 5228–5239. ( 10.1242/jcs.154393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ard R. et al. 2015. Regulation of macropinocytosis by diacylglycerol kinase ζ. PLoS One 10, e0144942 ( 10.1371/journal.pone.0144942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. 2010. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188, 547–563. ( 10.1083/jcb.200908086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houslay DM. et al. 2016. Coincident signals from GPCRs and receptor tyrosine kinases are uniquely transduced by PI3Kβ in myeloid cells. Sci. Signal. 9, ra82 ( 10.1126/scisignal.aae0453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouchemore KA, Sampaio NG, Murrey MW, Stanley ER, Lannutti BJ, Pixley FJ. 2013. Specific inhibition of PI3K p110δ inhibits CSF-1-induced macrophage spreading and invasive capacity. FEBS J. 280, 5228–5236. ( 10.1111/febs.12316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redka DS, Gütschow M, Grinstein S, Canton J. 2018. Differential ability of proinflammatory and anti-inflammatory macrophages to perform macropinocytosis. Mol. Biol. Cell 29, 53–65. ( 10.1091/mbc.E17-06-0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abella JV, Parachoniak CA, Sangwan V, Park M. 2010. Dorsal ruffle microdomains potentiate Met receptor tyrosine kinase signaling and down-regulation. J. Biol. Chem. 285, 24 956–24 967. ( 10.1074/jbc.M110.127985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng M, et al. 2015. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J. Mol. Cell Cardiol. 81, 49–53. ( 10.1016/j.yjmcc.2015.01.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. 2001. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J. Biol. Chem. 276, 49 236–49 243. ( 10.1074/jbc.M108523200) [DOI] [PubMed] [Google Scholar]
- 65.Bouschet T, Martin S, Kanamarlapudi V, Mundell S, Henley JM. 2007. The calcium-sensing receptor changes cell shape via a β-arrestin-1–ARNO–ARF6–ELMO protein network. J. Cell Sci. 120, 2489–2497. ( 10.1242/jcs.03469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luttrell LM. et al. 1999. β-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661. ( 10.1126/science.283.5402.655) [DOI] [PubMed] [Google Scholar]
- 67.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. ( 10.1016/j.it.2004.09.015) [DOI] [PubMed] [Google Scholar]
- 68.Anzinger JJ, Chang J, Xu Q, Barthwal MK, Bohnacker T, Wymann MP, Kruth HS. 2012. Murine bone marrow-derived macrophages differentiated with GM-CSF become foam cells by PI3Kγ-dependent fluid-phase pinocytosis of native LDL. J Lipid Res. 53, 34–42. ( 10.1194/jlr.M018887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murray PJ. et al. 2014. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. ( 10.1016/j.immuni.2014.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. ( 10.1038/nri2448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anzinger JJ, Chang J, Xu Q, Buono C, Li Y, Leyva FJ, Park B-C, Greene LE, Kruth HS. 2010. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler. Thromb. Vasc. Biol. 30, 2022–2031. ( 10.1161/ATVBAHA.110.210849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiu J-J, Chien S. 2011. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387. ( 10.1152/physrev.00047.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morel DW, DiCorleto PE, Chisolm GM. 1984. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arterioscler. Thromb. Vasc. Biol. 4, 357–364. [DOI] [PubMed] [Google Scholar]
- 74.Williams KJ, Tabas I. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15, 551–561. ( 10.1161/01.ATV.15.5.551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sambandam T, Baker JR, Christner JE, Ekborg SL. 1991. Specificity of the low density lipoprotein-glycosaminoglycan interaction. Arterioscler. Thromb. Vasc. Biol. 11, 561–568. ( 10.1161/01.ATV.11.3.561) [DOI] [PubMed] [Google Scholar]
- 76.Tacke F, et al. 2007. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194. ( 10.1172/JCI28549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tabas I. 2010. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46. ( 10.1038/nri2675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lusis AJ. 2000. Atherosclerosis. Nature 407, 233–241. ( 10.1038/35025203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown MS, Goldstein JL, Krieger M, Ho YK, Anderson RG. 1979. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 82, 597–613. ( 10.1083/jcb.82.3.597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldstein JL, Ho YK, Basu SK, Brown MS. 1979. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl Acad. Sci USA 76, 333–337. ( 10.1073/pnas.76.1.333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basu SK, Brown MS, Ho YK, Goldstein JL. 1979. Degradation of low density lipoprotein. Dextran sulfate complexes associated with deposition of cholesteryl esters in mouse macrophages. J. Biol. Chem. 254, 7141–7146. [PubMed] [Google Scholar]
- 82.Heit B. et al. 2013. Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization. Dev. Cell. 24, 372–383. ( 10.1016/j.devcel.2013.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Febbraio M. et al. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105, 1049–1056. ( 10.1172/JCI9259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kunjathoor VV. et al. 2002. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277, 49982–49988. ( 10.1074/jbc.M209649200) [DOI] [PubMed] [Google Scholar]
- 85.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4, 211–221. ( 10.1016/j.cmet.2006.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. 2005. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115, 2192–2201. ( 10.1172/JCI24061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manning-Tobin JJ. et al. 2008. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29, 19–26. ( 10.1161/ATVBAHA.108.176644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buono C, Anzinger JJ, Amar M, Kruth HS. 2009. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Invest. 119, 1373–1381. ( 10.1172/JCI35548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silverstein RL, Li W, Park YM, Rahaman SO. 2010. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans. Am. Clin. Climatol. Assoc. 121, 206–220. [PMC free article] [PubMed] [Google Scholar]
- 90.Park YM, Febbraio M, Silverstein RL. 2009. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145. ( 10.1172/JCI35535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rahaman SO, Swat W, Febbraio M, Silverstein RL. 2011. Vav family Rho guanine nucleotide exchange factors regulate CD36-mediated macrophage foam cell formation. J. Biol. Chem. 286, 7010–7017. ( 10.1074/jbc.M110.192450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahaman SO, Li W, Silverstein RL. 2013. Vav Guanine nucleotide exchange factors regulate atherosclerotic lesion development in mice. Arterioscler. Thromb. Vasc. Biol. 33, 2053–2057. ( 10.1161/ATVBAHA.113.301414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vance DT. et al. 2016. A phagocytosis assay for oxidized low-density lipoprotein versus immunoglobulin G-coated microbeads in human U937 macrophages. Anal. Biochem. 500, 24–34. ( 10.1016/j.ab.2016.01.007) [DOI] [PubMed] [Google Scholar]
- 94.Wong HS, et al. 2016. Chemokine signaling enhances CD36 responsiveness toward oxidized low-density lipoproteins and accelerates foam cell formation. Cell Rep. 14, 2859–2871. ( 10.1016/j.celrep.2016.02.071) [DOI] [PubMed] [Google Scholar]
- 95.Bisgaard LS. et al. 2016. Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression – implications for atherosclerosis research. Sci. Rep. 6, 35234 ( 10.1038/srep35234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanna RN. et al. 2012. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis novelty and significance. Circ. Res. 110, 416–427. ( 10.1161/CIRCRESAHA.111.253377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khallou-Laschet J, et al. 2010. Macrophage plasticity in experimental atherosclerosis. PLoS ONE 5, e8852 ( 10.1371/journal.pone.0008852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. 2011. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998. ( 10.1161/CIRCULATIONAHA.110.984146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. 2008. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606. ( 10.1096/fj.08-112201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin S, Moon S, Park Y, Kwon J, Lee S, Lee C-K, Cho K, Ha N-J, Kim K. 2009. Role of cordycepin and adenosine on the phenotypic switch of macrophages via induced anti-inflammatory cytokines. Immune Netw. 9, 255–264. ( 10.4110/in.2009.9.6.255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang SC-C. et al. 2014. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855. ( 10.1038/ni.2956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Tits LJH, Stienstra R, van Lent PL, Netea MG, Joosten LAB, Stalenhoef AFH. 2011. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis 214, 345–349. ( 10.1016/j.atherosclerosis.2010.11.018) [DOI] [PubMed] [Google Scholar]
- 103.Veithen A, Amyere M, Van Der Smissen P, Cupers P, Courtoy PJ. 1998. Regulation of macropinocytosis in v-Src-transformed fibroblasts: cyclic AMP selectively promotes regurgitation of macropinosomes. J. Cell Sci. 111, 2329–2335. [DOI] [PubMed] [Google Scholar]
- 104.Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. 2000. β-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC role of β-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J. Biol. Chem. 275, 11 312–11 319. ( 10.1074/jbc.275.15.11312) [DOI] [PubMed] [Google Scholar]
- 105.Bhattacharya M. et al. 2002. β-Arrestins regulate a Ral-GDS–Ral effector pathway that mediates cytoskeletal reorganization. Nat. Cell Biol. 4, 547–555. ( 10.1038/ncb821) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.