Abstract
Nucleic acids are a rapidly emerging therapeutic modality with the potential to become the third major drug modality alongside antibodies and small molecules. Owing to the unfavourable physico-chemical characteristics of nucleic acids, such as large size and negative charge, intracellular delivery remains a fundamental challenge to realizing this potential. Delivery technologies such as lipids, polymers and peptides have been used to facilitate delivery, with many of the most successful technologies using macropinocytosis to gain cellular entry; mostly by default rather than design. Fundamental knowledge of macropinocytosis is rapidly growing, presenting opportunities to better tailor design strategies to target this pathway. Furthermore, certain types of tumour cells have been observed to have high levels of macropinocytic activity and traffic cargo to favourable destinations within the cell for endosomal release, providing unique opportunities to further use this entry route for drug delivery. In this article, we review the delivery systems reported to be taken up by macropinocytosis and what is known about the mechanisms for regulating macropinocytosis in tumour cells. From this analysis, we identify new opportunities for exploiting this pathway for the intracellular delivery of nucleic acids to tumour cells.
This article is part of the Theo Murphy meeting issue ‘Macropinocytosis’.
Keywords: macropinocytosis, intracellular drug delivery, nucleic acids, nanoparticle, oncology
1. Introduction
(a). Nucleic acids are an emerging therapeutic modality
Nucleic acid-based therapeutics are an emerging class of drug modalities that have great potential to deliver benefits to patients over currently established modalities. The major classes of approved drugs are small molecules and antibodies, both of which elicit their function by binding to specific regions of a protein. For small molecules, this approach relies on the presence of an accessible binding pocket on the protein. However, it is estimated that only 2–5% of the proteins are accessible to small molecule drugs [1]. Antibodies, by contrast, can be engineered to bind more targets, but they are predominantly limited to cell surface or circulating proteins. Furthermore, pharmaceutical development of proteins is relatively complex. By contrast, nucleic acid therapeutics can act by manipulating gene expression using a mechanism dependent on binding to RNA/DNA via highly specific Watson–Crick base pairing or translation/transcription of the genetic code, circumventing the extensive optimization required to achieve specific binding using small molecules or protein-based modalities. More significantly, this offers the potential to target any disease-causing gene, greatly expanding the druggable target space. There are many different types of nucleic acids that have been used in therapeutic applications, ranging from relatively small molecules less than 10 kDa in size to larger molecules of several hundred kDa. The most clinically advanced of these are antisense oligonucleotides, DNA, short interfering RNA (siRNA) and modified messenger RNA (modRNA), with the greatest number of clinical trials reported for the treatment of cancer [2].
(b). Intracellular delivery is a key bottleneck
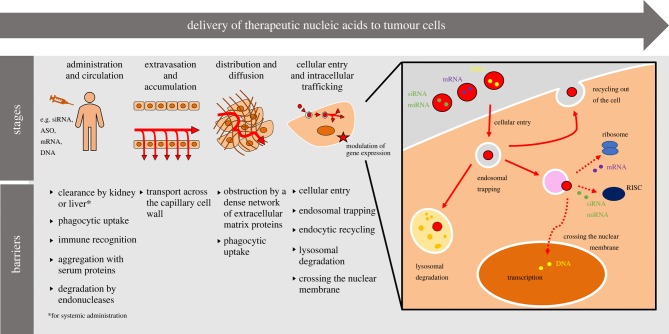
Functional delivery of nucleic acids can be defined as the delivery of a nucleic acid to a specific location where it can exert its intended pharmacological effect such as gene expression or inhibition. This can be outside of the cell, but for most nucleic acid-based therapeutics this will be a subcellular location where the nucleic acid can interact with molecular machinery such as ribosomes or enzymatic complexes. There are several obstacles to overcome on the journey from the point of administration to the correct subcellular destination, as summarized in figure 1. First, foreign nucleic acids need to enter the circulation or target tissue by crossing physiological barriers, the nature of which depends on the administration route. Second, they need to avoid being removed or degraded before reaching the target cell. This requires evasion of excretion, immune recognition and nuclease-mediated degradation. Third, nucleic acids must cross the plasma membrane and enter the cell. Many nucleic acids will enter cells by endocytosis, which results in further entrapment into membrane-bound vesicles such as endosomes and lysosomes, from which cargo must escape—this represents the final and most significant barrier to intracellular delivery. In contrast to small molecules that can enter cells by passive diffusion, nucleic acids are too large and negatively charged to cross cellular membranes efficiently, hence additional methods are required to facilitate delivery.
Figure 1.
Stages and barriers for delivery of nucleic acids to tumour cells. Delivery technologies for nucleic acids are designed to overcome several barriers dependent on the route of administration. For systemic administration, the delivery technology must protect the nucleic acid from clearance or degradation to permit extravasation and accumulation into the target tissue. It must then facilitate distribution through the tissue to the target cell type. Finally, the delivery technology must enable efficient intracellular delivery by crossing the cellular membrane and escaping endosomes, while avoiding endo-lysosomal trapping and expulsion from the cell by recycling. For nucleic acid modalities requiring access to the nucleus, there is the additional barrier of the nuclear membrane to overcome. ASO, antisense oligonucleotide; RISC, RNA-induced silencing complex; siRNA, short interfering RNA; miRNA, microRNA; mRNA, messenger RNA.
(c). Non-viral delivery systems for nucleic acid delivery
Non-viral systems are an attractive option for overcoming these barriers owing to their ease of manufacture and relatively low risk of toxicity. The materials that have been investigated for nucleic acid delivery cover different shapes, sizes and surface chemistries [3]. Successful classes of materials include lipids, polymers, inorganic particles, peptides and proteins, as well as biologically inspired materials such as exosomes. These systems must address all the biological barriers to delivery as well as having properties suitable for pharmaceutical development (e.g. non-toxic, stable and scalable). Despite significant progress in the development of these systems, intracellular delivery and specifically endosomal release remain major bottlenecks. Less than 2% of internalized material is estimated to reach productive compartments [4]. As a result, there has been a concerted effort to investigate cellular entry and trafficking mechanisms. Distinct endocytic routes of entry into the cell that have been implicated in the internalization of delivery systems are phagocytosis, macropinocytosis (MP), clathrin-mediated (CME) and caveolae-mediated endocytosis (CVME). In this review, we focus on MP as a mechanism for non-viral drug delivery of nucleic acids specifically to tumour cells. MP was initially defined as the actin-driven non-specific bulk uptake of extracellular fluid, the hallmark of which is actin-driven membrane ruffling leading to the formation of enclosed vesicles of diameter greater than 0.2 µm, called macropinosomes [5]. This definition has been since refined by increasing knowledge about the molecular regulators governing this process, which are utilised to identify a process as MP. Known MP regulators are found at the plasma membrane (Ras, phosphoinositide 3′-kinase (PI3 K), SGEF, ARF6), associated with macropinosomes (PIKfyve, Rabankyrin-5, SWAP-70, SNX1, SNX9, SNX18) or both (Rac, Src, Rab34, Rab5, PAK1, SNX5). A comprehensive analysis of these regulators can be found in several excellent reviews [5–7]. Once a molecule has been internalized into macropinosomes, it may enter the classical late endosomal/lysosomal pathway from which it can be trafficked to a specific intracellular location to carry out a metabolic function or be marked for degradation. Alternatively, molecules can remain separate from this pathway and be recycled back to the plasma membrane [6]. This is indicative of a highly complex and regulated pathway with a series of important biological functions in the cell, including nutrient scavenging and immune signalling. Macropinocytic activity is found in almost all cell types but importantly the activity of this pathway varies based on the biological function of the cell as well as environmental factors. MP has been implicated in the cellular uptake of non-viral delivery systems for nucleic acids along with other endocytic portals. However, it is unclear what the relative productivity of the different cellular entry and trafficking portals are and how we can best exploit them for intracellular delivery. In this review article, we evaluate reports of MP-mediated cellular entry of delivery systems for nucleic acids. We also focus on tumour cells as a potential target for MP-based delivery and finally we discuss opportunities for exploiting MP for the intracellular delivery of nucleic acids.
2. Delivery systems using macropinocytosis for cellular entry
Delivery systems where MP has been reported as a mode of entry are summarized in table 1. This encompasses a range of different sizes, shapes and charge. In the following section, we will consider the experimental approaches used to determine the mechanisms of cellular entry and what is known about different types of delivery systems, focusing on the most clinically developed systems.
Table 1.
Nucleic acid delivery technologies reported to enter cells by macropinocytosis. EIPA, 5-(N-ethyl-N-isopropyl) amiloride.
| experimental approach for identification of MP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| delivery technology | cargo | mode of internalization | cell type | size in nm | shape | charge (at physiological ph) | small molecule inhibition | fluorescence colocalization marker | genetic manipulation | in vivo analysis | reference |
| lipid and lipid-like nanoparticles | |||||||||||
| CkkE-12 | siRNA | MP | HeLa | 70 | spherical | neutral | EIPA | 70 kDa dextran | Dong et al. [8] | ||
| Dlin-MC3-DMA | siRNA | MP, CME | HeLa | 70–90 | spherical | neutral | EIPA | 70 kDa dextran | siRNA knockdown of CTBP1, Rac1, Rabnkyrin-5 | electron microscopy of siRNA-gold | Gilleron et al. [4] |
| C12–200 | siRNA | MP | HeLa | 80 | spherical | neutral | EIPA, cytochalasin D | 70 kDa dextran, ovalbumin, membrane ruffling, Cdc42 | siRNA knockdown of Cdc42, Rac1 | Love et al. [9], Sahay et al. [10] | |
| DOTAP/DOPC and DC-Chol/DOPE | DNA | MP | CHO | 190–202 | spherical | +48.9 to + 50.2 | wortmannin | 70 kDa Dextran | Cardarelli et al. [11] | ||
| charge reversing lipoplex | DNA | MP | CHO | 277–374 | spherical | +27.7 to + 50.2 | amiloride, wortmannin | Zhang et al. [12] | |||
| polymeric nanoparticles | |||||||||||
| His-pLK | DNA | MP, CME | HepG2 | 110 | spherical | +18 | PMA, wortmannin, DMA | Gonclaves [13] | |||
| PLL-PEG | DNA | MP | COS-7 | rods: l 100–200, w 20 Toroids: 30–60 | rods and Toroids | neutral | amiloride | Walsh et al. [14] | |||
| PLL-g-PEG | DNA | MP, CME | COS-7 | 80–90 | spherical | ND | wortmannin | Luhmann et al. [15] | |||
| cell-penetrating peptides | |||||||||||
| R8-DOPE/CHEMS or R8-EPC/Chol liposomes | DNA | MP | NIH 3T3 | 102–149 | spherical | +35 to + 40 | amiloride | Khalil et al. [16] | |||
| DOPE/DOTAP + CPP | siRNA | MP | B16F10, HT1080 | 462 | spherical | neutral | amiloride | Asai et al. [17] | |||
| Other | |||||||||||
| ApoE lipoprotein + calcium phosphate | siRNA | MP | glioblastoma | 20–40 | spherical | negative | amiloride, EIPA | 70 kDa dextran | fluorescence uptake in presence of EIPA | Huang et al. [18] | |
| PC-12 derived exosomes | miRNA | MP, CME | BMSCs | 40–150 | spherical | negative | EIPA, LY294002 | 70 kDa dextran | Tian et al. [19] | ||
| BJ fibroblast derived exosomes with CD47 | siRNA | MP | PANC-1 | 40–150 | spherical | negative | EIPA | Kamerkar et al. [20] | |||
(a). Limitations of experimental approaches for the classification of macropinocytosis
Identification of the role of MP in the internalization of nanoparticles is fraught with pitfalls for several reasons. To name a few, there is a lack of specific inhibitors, constitutive rates of MP vary between cell types, MP can be activated by external stimuli, nanoparticles use multiple entry routes into a cell and MP activity is highly sensitive to serum conditions. This means there is no single method that enables classification of MP with high confidence, rather a series of orthogonal methods are required. The experimental approaches that have been used to determine MP uptake of delivery systems are presented in table 1.
The most basic evidence for MP is observation of membrane ruffling. Such observations can be made by microscopy but this is limited to qualitative assessments, and alone is not sufficient to classify a process as MP. The most commonly cited method for classification of macropinocytosis in the drug delivery literature has been the use of chemical or pharmacological inhibitors, which target specific proteins or biochemical processes known to be critical for MP. This is coupled with subsequent measurement of the reduction in nanoparticle uptake or impaired functional delivery. These experiments are often done in conjunction with markers known to traffic by certain endocytic routes (70 kDa dextran, MP; transferrin, clathrin-dependent endocytosis; lactosylceramide, caveolae-mediated endocytosis; cholera toxin B, clathrin-dependent endocytosis; see [21] for a comprehensive list), which are useful as controls to assess the function of inhibitors. Commonly used small molecule inhibitors of MP are pH modifiers (amiloride or its derivative 5-(N-ethyl-N-isopropyl)amiloride (EIPA) [22,23]), actin inhibitors (jasplakinolide [24], blebbstatin [25], cytochalasin D) and PI 3-kinase inhibitors (Wortmannin, LY294002 [26]). These may be used in conjunction with inhibitors for other endocytic entry routes (commonly used examples: chlorpromazine, clathrin-mediated endocytosis; methyl-β-cyclodextrin, lipid rafts; genistein, caveolae-mediated endocytosis; filipin, clathrin-independent endocytosis; see [27] for a comprehensive list). The use of inhibitors is significantly limited by the degree of specificity and off-target effects. A detailed analysis of the use of chemical inhibitors in the context of studying gene delivery systems by Vercauteren et al. [28] demonstrated that commonly used endocytic inhibitors (chlorpromazine, genistein, methyl-β-cyclodextrin and potassium depletion) had poor specificity and significantly reduced cellular viability across commonly used cell types; furthermore, it was found that inhibitory effects were highly cell-type dependent. Although none of these are inhibitors of MP, they feature heavily in studies seeking to assess mechanisms of nanoparticle delivery. In addition to the use of inhibitors, information can be gained by stimulation of MP by growth factors such as epidermal growth factor (EGF) [29] and phorbol-12-myristate-13-acetate (PMA).
Fluorescence colocalization microscopy is another widely used technique for the study of MP in drug delivery. The simplest approach is to image fixed cells following exposure to labelled nanoparticles and labelling by antibodies. The need for fixation is a major disadvantage of this approach because it can create artefacts and cause redistribution of endocytic organelles [21]. The most pertinent example of this in the literature is regarding cell-penetrating peptides (CPPs), where the original mode of entry was thought to be non-endocytic but later studies found that this was a result of fixation protocols [30]. More reliable information can be gained from live-cell experiments using co-treatment experiments. This involves exposure to the marker and labelled nanoparticle to be tracked, followed by measurement of colocalization. Fluorescence colocalization experiments are dependent on the capabilities of the microscopy technique used, combined with robust statistical image analysis. This approach has been aided by advances in automated confocal fluorescence microscopy, which have been used in combination with sophisticated image analysis algorithms to gain more dynamic quantitative information [31,32].
It is also well established that endocytic trafficking activity is impacted by the cellular environment. This is especially relevant in the context of MP, which can be both a constitutive and stimulated process. Consequently, culture conditions such as serum composition and starvation will affect the rate of MP and thus internalization of a nanoparticle. For example, it has been demonstrated that MP is required for the supply of key amino acids such as glutamine to proliferating Ras-transformed cancer cells [33]. This seminal study showed that the rate of MP is tightly linked to the concentration of glutamine in the culture medium, but many studies in the context of drug delivery are done at glutamine concentrations well above the physiological range. Studies have shown that the cellular response to inhibitors and stimulators commonly used in drug delivery studies is strongly effected by serum content [34]. It was demonstrated that certain cell types lose sensitivity to EGF in the presence of serum, highlighting this as an important consideration when using EGF to study drug delivery systems. Many fundamental studies of MP use serum-starved cells to delineate between constitutive and stimulated MP, however, in the context of drug delivery, it is necessary to perform experiments in physiological conditions to translate findings to a therapeutic context. Importantly, a deeper understanding of the link between growth factors, activation of corresponding signalling pathways and MP activity in vivo, where multiple pathways could be activated in a cell- and context-specific manner, are required in the future. Yet, in vivo studies are limited by the technical challenge of achieving cellular-resolution imaging in tissues, and the additional toxicities of inhibitors in a complex physiological context. Nevertheless, this has been achieved by some groups delivering EIPA by local injection or by osmotic pumps [18,35].
The drawbacks discussed here represent a major limitation in much of the literature exploring mechanisms of cellular delivery with nanoparticles. The most reliable studies are where conclusions are drawn from studies including both small molecule inhibitors and endocytic marker colocalization studies, corroborated by genetic methods that specifically deplete or enrich a protein with consideration given to the in vivo environment. It is apparent that many of the studies cited in table 1 are reliant on a single method to classify cellular entry as MP, hence caution must be taken in interpreting these results. This is perhaps because the focus of many of these studies has been on formulation design rather than investigating cellular uptake mechanisms. Moreover, the experimental techniques to study MP are becoming more sophisticated as the field develops, particularly through technical progress in microscopy and more advanced in vitro cellular models. Several excellent recent reviews propose new methods to study intracellular delivery [36–39], which are sure to yield more mechanistic insights. The ensuing discussion will place more emphasis on where multiple methods have been used to study delivery.
(b). Lipid and lipid-like delivery systems
Lipid-based delivery systems are currently the most clinically advanced vectors for nucleic acid delivery [40]. The most successful of these are Dlin-X-DMA-based ionizable lipid-based lipid nanoparticles (LNPs). These comprise four different lipids: an ionizable lipid (e.g. dilinoleylmethyl-4-dimethylaminobutyrate (Dlin-MC3-DMA) [41], 2,2-dilinoleyl-4-(2-dimethylaminoethyl)-[1,3]-dioxolane (DLin-KC2-DMA) [42]) helper lipids (1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol) and a PEG-based lipid (DMG-PEG). This system was originally developed for intravenous siRNA delivery to hepatocytes but has since been applied to deliver multiple types of nucleic acids (DNA [43], mRNA [44]) using multiple administration routes [45]. Dlin-X-DMA-based LNPs are now in late-stage clinical development and approaching commercialization [46]. Owing to their success in clinical translation, Dlin-X-DMA-based LNPs are perhaps the most intensively studied LNPs in terms of mechanism, with most of these investigations having taken place in the context of intravenous delivery of siRNA to hepatocytes. In this context, uptake of KC2-based LNPs was shown to be dependent on opsonization by apolipoproteins (ApoE3) in the blood [47]. ApoE3 bound to the surface of LNPs engage low-density lipoprotein receptors for uptake, implicating a CME mechanism for delivery. An in-depth in vitro analysis of MC3-based LNPs (a lipid structurally related to KC2) containing siRNA by Gilleron et al. [4] in HeLa cells revealed that both CME and MP are involved in LNP uptake. It was demonstrated that partial inhibition of each pathway by siRNA separately reduced LNP uptake by 50–70%, but simultaneous inhibition did not result in any additional reduction, suggesting a co-dependent process. Interestingly, macropinocytic activity was increased in the presence of LNP and inhibited by blocking CME, suggesting that CME-mediated entry may be triggering MP in a similar way to what has been observed in adenoviral cellular entry [48]. Examining the kinetics of uptake showed that the bulk of functional delivery, in this case, gene silencing, occurred after 2 h when MP would be most active. This study indicates that MP is critical for internalization, however endosomal escape is required to achieve functional delivery. The ionizable lipid (MC3) is the key component responsible for promoting endosomal release in this system. At neutral pH the ionizable lipid is primarily uncharged, permitting association with ApoE proteins and reducing toxicity in the systemic circulation, whereas at low pH found in endosomes, the ionizable lipid is primarily positively charged. The charged lipid interacts with anionic lipids on the inner leaflet of the endosomal membrane, inducing a change in lipid structure from a lamellar phase to hexagonal phase and resulting in membrane disruption [42]. The extent to which the lipid is ionized depends on its pKa. The specific pKa of MC3 is 6.4, meaning that approximately 80% is uncharged at physiological pH 7.4, whereas 80% is charged at endosomal pH 5.5. The pKa is a critical determinant of the biological performance of this type of LNP [41]. From an understanding of this system, it becomes apparent that the dynamic pH changes that occur during the endosomal/lysosomal pathway are critical to the endosomal release process. For example, it might be expected that limited release occurs at early stages of the pathway where pH is relatively high, whereas most release may occur at latter stages of the pathway. It follows that in addition to the pH of the vesicles, the rate at which the transition occurs will also be important. It is well established that there is wide range of transfectability between cell types both in vitro and in vivo. A possible explanation for this could be differences in the dynamics of pH changes occurring through macropinosome formation and subsequent intracellular trafficking.
Other promising lipid-based systems identified for nucleic acid delivery have come from a high-throughput screening of lipid libraries pioneered by Anderson et al. [49] These efforts have yielded new materials that are highly effective primarily in the context of intravenous delivery of siRNA to hepatocytes. Lipidoids are one such class of materials developed from this. C12–200-based LNPs are one of the early lipidoids discovered by this approach [9]. C12–200 is a polyamine-core lipidoid formulated with helper lipids and PEG lipids by a similar process to the previously discussed MC3-based LNPs. A mechanistic investigation in HeLa cells identified MP to be the main mechanism of uptake for this system, MP was confirmed through a combination of depletion of key MP regulators (Cdc42 and Rac1) and colocalization with genetically labelled CdC42 and labelled ovalbumin, both of which are markers for MP. However, in contrast to Dlin-X-DMA-based LNPs there was no dependence on clathrin-based mechanisms or ApoE [10]. One of the main findings of this study was that 70% of siRNA was recycled out of the cell in a process dependent on Niemann-Pick intracellular cholesterol transporter 1 (NPC1). This is not unique to lipidoid formulations, as blockade of NPC1 during Dlin-X-DMA-based LNP-mediated transfection of siRNA also resulted in enhanced potency [50]. It is unclear if there is a link between enhanced endocytic recycling and entry by MP, but blocking recycling or routing LNPs away from recycling pathways is a potential strategy for enhancing functional delivery. Other lipidoid formulations based on polyamine cores have been developed by this approach and have been demonstrated to result in functional delivery of siRNA and/or mRNA. Examples of materials are 304O13 [51], OF-02 [52], TarN3C10 [53] and 7C1 [54]. There is limited information about the specific delivery mechanism for these materials, however these systems have tropism for different cell and tissue types suggesting mechanistic differences. A class of LNPs developed by high-throughput screening where some mechanistic investigation has been undertaken is lipopeptides, which comprise a lipopeptide formulated with helper and PEG lipids. CkkE-12 LNPs, an optimized lipopeptide formulation, was found to have approximately 100-fold greater potency than C12–200 LNPs for siRNA delivery [8]. CkkE-12 LNPs were found to also use MP as the main mechanism of uptake by HeLa cells, identified by use of EIPA and observation of colocalization with 70 kDa dextran. Interestingly, as found for ionizable LNPs, the mechanism of uptake was ApoE-dependent, indicating that MP is not the sole mechanism of uptake.
Additional examples of lipid-based systems reported to use MP are cationic lipid-based liposomes, DOTAP–DOPC and DC-Chol–DOPE [11], which were investigated in the context of DNA delivery to Chinese hamster ovary (CHO) cells. Both these liposomes were approximately 200 nm in size, which is relatively large compared to the previously discussed lipidoid formulations that are typically 100 nm or less. There was little dependence on CME or CVME for uptake, most probably owing to size restrictions of non-macropinocytic entry routes, which are between 60 and 120 nm [55].
Relative to Dlin-X-DMA-based LNPs, the mechanism of endosomal release of cationic lipid-based liposomes is not well understood, but considering their structural similarities it is likely the mechanism of release is dependent on pH. Notably, the relationship between pKa and efficient intracellular delivery is dependent on the type of lipid. The pKas of 7C1 and C12–200 are 5.0 [54] and 7.25 [56], respectively; however DLin-X-DMA-based LNPs with these pKa values were found to have low levels of functional delivery. A potential explanation for this is that different LNPs may escape from different stages of the endo-lysosomal pathway or the specific mode of endosomal escape of different LNPs may favour different uptake/trafficking routes.
(c). Polymeric delivery systems
Polymers are a major class of drug delivery system that have a history of use for nucleic acid delivery stretching back over 50 years [57]. Early systems were based on cationic polymers complexed to a nucleic acid, examples being polyethyleneimine (PEI), polyamidoamine-based dendrimers, polyaspartamides, poly(lactic-co-glycolic acid) (PLGA), poly [2-dimethylaminoethyl methacrylate] (PDMAEMA), poly-l-lysine (PLL), poly (beta-amino ester) (PBAE) and chitosan. As the field has developed, modifications to these polymers have been introduced to specifically address delivery barriers. Strategies have included attaching targeting ligands to promote cellular uptake, functional groups to facilitate endosomal escape, groups to increase stealth and manipulating charge to avoid toxicity. This has led to systems with an assortment of sizes, shapes and charges. MP has been reported to be involved in uptake in several cases; however, many of these studies have been reliant on pharmacological inhibition alone, which is not as specific as other methods such as genetic manipulation [28]. An exception to this is a study on PEGylated PLGA particles in HeLa cells [58]. This study used genetic manipulation to label endocytic compartments and manipulate trafficking mechanisms. Although these particles were not found to use MP as the major entry route into the cell, it was found that blocking Dynamin 1, a protein essential for CME, resulted in stimulation of MP and an overall higher uptake of the nanoparticles. This is evidence that MP could be used to increase the level of uptake of delivery systems into a cell, but the primary bottleneck for many systems is avoiding lysosomal degradation. There have been reports that it is possible to direct polymeric particles through a non-lysosomal pathway. An investigation of a PEGylated form of PLL complexed with DNA in COS-7 cells has shown that entry by MP results in deposition of particles to vesicles that were not endosomes or lysosomes [14]. The shapes of particles studied here were distinct from other reports that primarily use spherical particles. The particles were a mixture of rods (100–200 nm with a width of 20 nm) and toroids (30–60 nm). The diversion to a different intracellular fate could be a product of the shape of the particle, which has also been reported for rod-shaped materials such as carbon nanotubes [59]. Interestingly, there does not appear to be a general correlation between particle size and MP; uptake has been reported for particles as small as 10 nm [60]. Taken together, this demonstrates that macropinocytic uptake is not restricted to a certain particle size or particle property. Contrary to these reports where macropinocytic uptake appears to be beneficial for uptake, it does not always result in enhanced functional delivery. An investigation of 110 nm PLL-based polyplexes in HepG2 cells showed that both CME and MP were involved in uptake, but additional stimulation of MP resulted in reduced functional delivery of DNA [13].
As with all nucleic acid delivery systems, there is a need for polymers to promote endosomal release to be effective. Initially, cationic polymers were thought to promote endosomal escape by the proton sponge effect. This involves protonation of the polyplex at low pH accompanied by an influx of counterions resulting in an osmotic imbalance within the endosome that eventually leads to endosomal rupture [61]. However, this has been refuted by some recent studies demonstrating that osmotic force alone would not be able to rupture an endosome [62]. It is more likely that polyplexes induce endosomal escape by a combination of factors in addition to proton sponge, specifically an initial membrane destabilization caused by the cationic polymer interacting with the cell membrane, and secondly repulsion between charged polymers causing expansion within the endosome referred to as the ‘umbrella effect’ [63]. It is logical that the mechanism of uptake and trafficking will affect the functioning of this mechanism. For example, it may be easier for polyplexes to break out of intracellular vesicles with less stable membrane structures and, as with LNPs, certain endo-lysosomal pH conditions will be favourable depending on the design of the delivery system.
(d). Cell-penetrating peptide-based delivery systems
Cellular uptake mechanisms have been studied relatively intensively in the field of CPPs, partly because they were originally thought to have a unique mechanism of entry that could bypass lysosomal degradation [64]. CPPs can broadly be defined as peptides that show a high degree of transport across a cell membrane. The first CPP to gain attention was the transactivator of transcription (TAT) protein present in HIV-1. This was followed closely by a homeoprotein discovered in Drosophila melanogaster. Based on these findings, peptides have been derivatized to identify hundreds of amino acid sequences with cell-penetrating properties, including cationic, amphipathic and hydrophobic peptides. CPPs have been exploited for drug delivery by complexing or conjugating molecules directly to the CPP and attaching CPPs to nanoparticles. There have been conflicting reports on the mechanism of uptake of CPPs, with several routes including MP reported to be implicated in uptake [30,65]. This is most probably owing to methodological limitations that have been highlighted in a study, which demonstrated that cell type, serum starvation and inhibitor specificity all strongly influence the entry route for CPPs [66]. In contrast to the lipidic and polymeric delivery systems discussed up to this point, a relatively strong molecular basis has been identified for the link between macropinocytosis and CPPs. Arginine-rich peptides have been shown to interact with heparan sulfate proteoglycans resulting in the activation of Rac, which induces actin polymerization and subsequent lamellipodia formation, indicative of MP [67]. Importantly, it has also been observed that there is a concentration dependence on the mechanism employed for uptake [30,68]. In the context of HeLa cells, MP is active at low concentrations of CPPs but a high concentration of CPPs induces a non-endocytic mode of entry into the cytosol or nucleus of the cell [30]. Additionally, inhibition of MP promoted uptake of CPPs through this non-endocytic route, implying in this case that MP is counter-productive for functional delivery. The CPP systems discussed so far have involved the peptide alone but there have also been reports of CPPs attached to nanoparticles, resulting in entry by MP. In this case, the mechanism of uptake can be dependent on the attachment topology of the CPPs as well as the type of particle. An investigation of arginine peptides (R8) attached to a liposome loaded with DNA in 3T3 cells showed cellular entry by MP only when there was a high peptide density on the liposome surface [16]. There was an overall increase in uptake compared to a low-density configuration and, interestingly, there was a disproportionally large increase in functional delivery of DNA, suggesting the trafficking route taken by macropinocytic entry was more productive than the CME observed at low peptide density.
In addition to lipids, polymers and peptides, MP-based internalization has also been reported for other classes of delivery systems, such as extracellular vesicles (EVs) [20,69,70] and inorganic nanoparticles [18]. These are generally less well developed than the lipidic and polymeric systems but are showing great promise, moreover these reports demonstrate the variety of particles that can use MP as an entry pathway.
3. Tumour cells are targets for MP-based drug delivery
The examples discussed in the previous section demonstrate that macropinocytic uptake can be exploited for a broad range of cell types and cargos. It is also important to appreciate that certain cell types offer additional opportunities to target MP for delivery. It is well known that a high rate of fluid-phase uptake via phagocytosis and MP is a hallmark of cells such as macrophages and dendritic cells, and as a result, it is possible to deliver nucleic acids to these types of cells without a delivery system [71,72]. This approach is relatively inefficient and therefore limited to therapies where the barriers to delivery are low, typically targets requiring low levels of transfection to achieve a pharmacodynamic effect or where local administration is possible. Outside of these cell types certain disease states result in cells with elevated levels of MP, offering opportunities for drug delivery—cancer is one such example. One of the first reports of high MP activity in tumour cells was obtained by bright field microscopy by Warren Lewis in 1937 [73]. This seminal observation has since been confirmed by multiple studies that have elucidated the molecular basis of this phenomenon using state-of-the-art high-resolution microscopy combined with genetic manipulation. It is now known that MP plays a key role in the proliferation of cancer cells by functioning as a nutrient supply route [33]. In this section, we will discuss what factors drive MP in tumour cells and specific features of the associated intracellular trafficking routes.
(a). Activation of cancer-related pathways drive MP
MP is initiated by activation of the Ras superfamily of small GTPases and activation of the PI3 K pathway by growth factors. The process starts with the generation of sheet-like extensions of the cell membrane, often referred to as ruffles, which are formed by polymerized cortical actin beneath the plasma membrane [26,74–76]. In tumour cells, membrane ruffling results in macropinosome formation by forming O-shaped cups that can be eventually closed, entrapping extracellular fluid into the macropinosome [71]. Importantly, Veltman et al. resolved a spatio-temporal link between Ras activation and actin-rich ruffle formation [77]. This study applied lattice light sheet microscopy to follow MP cup formation in Dictyostelium. Patches of activated Ras/PI(3,4,5)P3/Rac1, delineated by a narrow ring of active SCAR (suppressor of cAMP receptor), were observed to stimulate polymerization of cortical actin resulting in the formation of a cup-shaped ruffle.
Activation of Ras/PI3 K signalling cascades by oncogenic mutations in Ras, chemokines and/or growth factors has been found to activate MP in tumour cells. Specific factors are listed in table 2. One of the first links between Ras mutations and MP was found in quiescent rat embryo fibroblasts, where the introduction of oncogenic human H-Ras proteins via microinjection increased the incidence of membrane ruffles and MP. This lasted more than 15 h after injection [85]. Interestingly, comparison of K-Ras and H-Ras revealed that K-Ras is a more potent inducer of membrane ruffling and MP [86]. This has also been observed in pancreatic adenocarcinoma-derived human MIA PaCa-2 cells with an activating K-Ras mutation, G12C [78].
Table 2.
Growth factors and tumour oncogenes/suppressors modulating macropinocytosis.
| protein | activity in macropinocytosis | associated cancer | reference |
|---|---|---|---|
| Ras | membrane ruffling, macropinosome formation and trafficking | pancreas, biliary tract, large intestine, small intestine, lung, ovary | reviewed in Prior et al. [78] |
| Rac1 | membrane ruffling | skin melanomas | Krauthammer et al. [79] |
| PI3 K | membrane ruffle extension, macropinosome formation | large intestine, central nervous system, gastric, breast, endometrium, lung, head and neck cancers |
Samuels et al. [80] Bauer et al. [81] |
| PTEN | PI3 signalling pathway antagonist | endometrium, brain, skin, prostate | reviewed in Bauer et al. [81] |
| neurofibromin (NF1) | RasGAP/Ras inhibitor | neurofibromatosis type 1 | Bloomfield et al. [82] |
| EGF/EGFR | MP initiation | lung, colon, breast, ovary, brain, head and neck | reviewed in Normanno et al. [83] |
| CXCL12/CXCR4 | MP initiation | pituitary tumour, familial chronic lymphocytic leukaemia, breast, gastric, pancreas, ovary, colon | reviewed in Guo et al. [84] |
In addition to Ras, links have been found between oncogenic aberrations in the regulation of phosphoinositides and MP. Membrane phosphoinositides act as markers of membrane identity and are carefully controlled throughout the MP process by a range of kinases and phosphatases. PI(4,5)P2 is converted to PI(3,4,5)P3 by class I PI3 K on membrane ruffles, with PI(3,4,5)P3 levels peaking at cup closure, then degraded to PI(3)P, which is the predominant phosphoinositide of the early macropinosomal membrane. Activating mutations in the class I PI3 K gene PIK3CA, and decreased expression of PTEN, which dephosphorylates PI(3,4,5)P3 to PI(4,5)P2, are both frequently found in cancer cells [87–89]. These mutations cause the net accumulation of PI(3,4,5)P3 in cancer cells and thus an increase in MP activity [90]. Together, these frequently found mutations in Ras, PI3 K and PTEN in cancer cells demonstrate that upregulation of MP is important for a broad range of cancers.
MP also plays an important role in the regulation of metabolic processes, with cancer cells using MP as a method to take up nutrients. MP in MIA PaCa-2 cells resulted in protein uptake and subsequent degradation into amino acids, including glutamine, which was found to be critical for cellular proliferation [33]. This has also been highlighted by the finding that the growth factor M-CSF and chemokine CXCL12 (also known as stromal-derived factor 1α) activate MP, which provides an amino acid supply route for macrophages [91]. The CXCL12 receptor, CXCR4, is expressed in multiple tumour cells and its stimulation triggers actin-dependent membrane ruffling and activation of MP-associated signalling pathways including PI3 K/Akt [84,92,93]. Moreover, stimulation of CXCR4 with CXCL12 or 12-mer oligoarginine CPPs triggered MP and uptake of these molecules into HeLa cells [94]. Notably, the CXCR4 receptor is internalized via MP in Hep3B cells [95] and CXCR4 intracellular localization has been linked to clinical outcomes in lung adenocarcinoma patients. High levels of CXCR4 localization in the plasma membrane and endo-lysosomal system were associated with distant metastasis and decreased disease-free survival [96]. In fact, heterogeneity in CXCR4 expression has been observed, with only a proportion (between 1% and 20%) of the tumour cells in the ovarian cancer biopsies expressing CXCR4 mRNA, indicating the high variability of MP-inducing pathways even in one individual [97]. Interestingly, M-CSF and CXCL12 also promote MP in tumour cells in vivo via cross-talk with tumour-associated macrophages. M-CSF and CXCL12 secreted by tumour cells stimulate tumor-associated macrophages, which in turn promote tumour cell growth and metastatic behaviour by secreting EGF, which is known to induce MP [29,98,99].
Increased rates of MP in tumour cells result in the transport of amino acids into the cell, which has been linked with activation of the mechanistic target of rapamycin complex-1 (mTORC1), a key regulator of cellular metabolic processes such as protein translation [91]. Additionally, mTORC1 is activated through Akt recruitment to macropinosomes and the associated inhibition of the TSC1/2 protein complex activity [91,100]. Notably, it has been demonstrated that activation of mTORC1 by blockade of TSC2 enhances ionizable lipid-based LNP-mediated functional delivery of mRNA in HeLa cells, although it is important to make the distinction that this is achieved by enhancing the protein translation rate of the mRNA, not by enhancing endosomal release. Therefore, it is unlikely to be applicable to other types of nucleic acids [101]. The association of abnormalities in Ras signalling, phosphoinositide regulation, chemokine and growth factor production with elevated MP in cancer provides opportunities to exploit for specific targeting of nucleic acids, which will be discussed in more depth in §4.
(b). MP entry favours cargo delivery to late endosomes and multivesicular bodies in tumour cells
Although certain types of tumour cells exhibit enhanced MP, it is critically important that nucleic acids are trafficked to a productive intracellular location. Therefore, it is important to examine how macropinocytic uptake relates to intracellular trafficking. MP by macrophages results in maturation of macropinosomes, which acquire sequentially early and late endosomal markers, Rab5 and Rab7, respectively. Macropinosomes then fuse with a stable resident lysosomal compartment positive for LAMP1 and RagC [91,102]. Macropinosomes may also recycle their contents out of the cell, as has been shown for M-CSF-stimulated macrophages [103]. Lysosomal degradation is a barrier to intracellular delivery common across most cell types and endocytic entry portals. Significantly, there are exceptions: newly formed macropinosomes in EGF-stimulated A431 epidermoid carcinoma cells show only weak staining for EEA1, indicating the lack of fusion with early endosomes. Macropinosome self-fusion was also observed within 5 min after uptake. After a 60 min chase, the fluid-phase marker, FITC-dextran was either exocytosed via recycling compartments or remained in the perinuclear compartment, which was negative for cathepsin D, further indicating the lack of fusion with lysosomes [104]. These data corroborated an earlier study showing lack of colocalization between the fluid-phase marker, Texas Red-labelled dextran, and the early/late endo-lysosomal markers, transferrin and LDL, respectively [105]. Additionally, EM analysis of SAOS osteosarcoma cells revealed that α2β1 integrins internalized via MP were detected in multivesicular body-like organelles [106]. Alongside these findings, it is also important to consider that MP is a nutrient supply route that functions by degrading proteins in lysosomes, which apparently contradicts these reports. A possible explanation for this is that the fusion with lysosomes is cargo-specific. Alternatively, it may be that certain macropinocytic trafficking pathways retain cargo in non-lysosomal compartments for time periods longer than the scope of these studies. In either scenario, this is likely to be of benefit for intracellular delivery. Recent work has shown that the majority of endosomal escape occurs in late endosomes/multivesicular bodies [107], suggesting that trapping at this stage of the pathway or increasing retention time before lysosomal degradation would be beneficial.
In addition to avoiding lysosomal degradation, recycling is another limiting factor of nucleic acid delivery. Recycling has been observed in EGF-stimulated HEK293-GFP-SNX5 cells where macropinosome cargo was partially recycled via highly dynamic tubular extensions associated with early endosomes [108]. In this study, the remaining macropinosomes were shown to recruit phosphoinositol-binding sorting nexin 5 (SNX5), Rab5 and EEA1 but were LAMP1-negative. This was followed by accumulation of the late endosomal marker, Rab7, but later time points were not included in the study so ultimate fusion with lysosomes cannot be excluded. These studies demonstrate the variety of intracellular itineraries that are possible following macropinocytic internalization.
4. Opportunities to exploit MP for enhancing intracellular delivery
By combining knowledge of the design of delivery systems with mechanisms of MP in tumour cells, it becomes apparent that there are many opportunities to exploit MP to enhance intracellular delivery of nucleic acids. Key opportunities are discussed in the following section.
(a). Targeting specific cell types, diseases and environments
Perhaps the simplest approach to targeting MP for drug delivery would be to focus nucleic acid delivery strategies on tumours that have an intrinsically high level of MP activity. Oncogenic mutations associated with high MP activity are summarized in table 2. Some of these have been exploited already, for example groups have successfully shown that exosomal and lipoprotein-based delivery systems are preferentially taken up in Ras mutant tumours by MP [18,20]. Considering the role of MP in nutrient supply, tumours in particularly nutrient-deficient environments offer a potential opportunity for exploitation. In terms of intracellular trafficking, it has been shown that macropinosome fusion to lysosomes does not occur in some cell types, hence such cell types may be attractive targets for drug delivery. Another approach is to match the acidification profiles of trafficking routes with the intended action of a given delivery system. The majority of lipidic and polymeric delivery systems rely on a pH shift from the physiological pH to endosomal pH to enable endosomal membrane disruption. The precise ionization behaviour of the material in relation to pH changes in the endo-lysosomal pathway is critical for successful function. If the kinetics, pH and physiology of trafficking by the MP pathway are favourable to the transitions occurring in the delivery system, then MP uptake will be more productive than other pathways. Conversely, if they are not particularly well-matched then other pathways may be more productive. An example of this has been shown for CPPs whereby blocking MP using EIPA resulted in entry via direct translocation into the cytosol, although this was also observed in the presence of MβCD, an inhibitor of caveolae/lipid-raft-mediated endocytosis [30]. This is a possible explanation for why CPP-based studies have shown that increasing the rate of macropinocytic entry can be both positive and negative for functional delivery depending on the format of the delivery system. Extending these concepts into a clinical setting, patient selection strategies could be employed by screening tumour types with markers for MP and/or favourable trafficking profiles. Potential markers are CXCR4 expression and endo-lysosomal pH profiles. Measuring pH through the endocytic pathway has been attempted, however, the accuracy of measurements is low owing to proteins interfering with the behaviour of pH-sensitive fluorophores [109]. Nevertheless, broad knowledge of these pH changes could be useful in selecting or designing delivery systems for specific patients. Furthermore, identification of novel MP regulators, e.g. regulation by short mircoRNA [72], will enhance the precision with which patients can be targeted.
(b). Manipulating MP-related uptake and trafficking routes
To realize the full potential of nucleic acid-based therapeutics, ideally, it would be possible to target any cell type. The issue in this regard is that not all cells have high MP activity or favourable trafficking pathways. In this case, it may be possible to manipulate the MP pathway to favour intracellular delivery.
Studies have demonstrated that it is possible to increase intracellular delivery successfully by enhancing MP activity [67]. Nakase et al. investigated drug delivery using exosomes in the presence of MP-stimulating compounds [110]. Stimulation of A431 epidermoid carcinoma cells with EGF or CXCL12 enhanced MP and exosome delivery 27-fold and 2.3-fold, respectively. Moreover, EGF treatment significantly enhanced the cytotoxicity of saporin-encapsulated exosomes, suggesting that induction of MP improved saporin bioactivity. In addition, loading of EGF into exosomes improved uptake and improved saporin cytotoxic effects [110]. Another approach to enhance MP included the use of the arginine-rich CPP R8, which has been previously implicated in syndecan-4 proteoglycan clustering on plasma membranes, resulting in an induction of MP [111]. In this case, the conjugation of arginine-rich CPPs to EVs induced membrane ruffling and MP, resulting in higher cellular uptake of peptide-EV conjugates.
Outside of synthetic delivery systems, various pathogens including viruses and bacterial cells have been found to target the MP pathway to enter host cells. Viral particles have been shown to associate with filopodia—thin plasma membrane extensions—and ‘surf’ along towards endocytic ‘hot spots’, being internalized in a process sensitive to MP inhibitors [112,113]. Vaccinia virus, a prototype poxvirus, was shown to associate with filopodia and stimulate p21-activated kinase 1 (PAK1), causing ruffle formation thus promoting MP. Experimental evidence suggests that vaccinia virus mimics the apoptotic ‘eat me’ signal and binds to cells via exposed phosphatidylserine in the viral membrane. Chlamydia trachomatis also associates with filopodia and stimulates Rac1 to induce membrane ruffle formation. It is thought that targeting MP enables uptake of large pathogens and prevents their recognition by the immune system. Pathogen-based delivery systems are efficient delivery vehicles but have a higher risk of toxicity and a more complex development path compared to non-viral systems; however, mimicking the action of pathogens in the design of non-viral delivery systems could be used to enhance delivery.
Although there are clear examples of how MP-mediated cellular entry may be manipulated, the modulation of intracellular trafficking is less obvious, although this is perhaps more important as endosomal release is the primary bottleneck for intracellular delivery. A possible approach to enhance endosomal release is to intervene in the MP pathway by blocking fusion of late endosomes/multivesicular bodies with lysosomes, holding delivery systems in the stage of the endosomal pathway where escape is most probably to occur. One way this could be done is by modulating macropinosome size through modulation of guanine nucleotide exchange factors' (GEFs) and GTPase activating proteins' (GAPs) activity [77]. Another potential family of targets are the phosphoinositides, and their associated kinases and phosphatases, which have roles in controlling ruffle formation and act as markers of membrane identify throughout the stages of the macropinocytic pathway [76,114]. Likewise, blocking endocytic recycling can be used to retain cargo in the cell and enhance functional delivery, as has been demonstrated for LNP-mediated delivery of siRNA [10,50].
5. Conclusion
MP is undoubtedly a broadly applicable mechanism that can be exploited to address the first barrier for intracellular delivery, which is to gain cellular entry. This is exemplified by the breadth of particles, cell types and cargos that have been reported to successfully deliver cargo by MP. Although multiple endocytic portals such as CME and caveolae-mediated endocytosis (CVE) can effectively internalize particles, they have specific size and charge limitations [115]. Furthermore, MP is efficient enough to support the development of therapeutic strategies, as has been demonstrated by the clinical progress of systems using this mode of entry. In the short term, an attractive strategy is to target cell types and environments with features known to be correlated with high macropinocytic activity, such as Ras mutated tumours or nutrient-deficient environments. In the longer term, precisely manipulating MP to enhance intracellular delivery is an exciting possibility. Opportunities to do this, driven by advancements in fundamental understanding of MP, are already emerging. Stimulating macropinocytic uptake, blocking macropinocytic recycling and preventing fusion of macropinosomes with lysosomes are all interesting possibilities. The success of such strategies will be dependent on how well significant gaps can be addressed. While most knowledge of MP comes from in vitro studies; the in vivo situation is more complex. There is interplay between other cells in the tumour microenvironment as well as environmental factors that are difficult to account for in vitro. MP also plays a critical role in governing cellular metabolism, hence there is a high risk that modulating this pathway will result in toxicity. These factors make identifying suitable targets for manipulation challenging, but plenty of inspiration can be drawn from how nature has designed pathogens that can successfully exploit MP for intracellular delivery.
Data accessibility
This article has no additional data.
Authors' contributions
A.S.D., M.R.H. and A.N.K. contributed equally.
Competing interests
All authors are employees of AstraZeneca who are actively investigating drug delivery systems for nucleic acids for commercial applications.
Funding
We received no funding for this study.
References
- 1.Overington JP, Al-Lazikani B, Hopkins AL. 2006. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996. ( 10.1038/nrd2199) [DOI] [PubMed] [Google Scholar]
- 2.Kaczmarek JC, Kowalski PS, Anderson DG. 2017. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 9, 60 ( 10.1186/s13073-017-0450-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin H, Kanasty RL, Eltoukhy A, Vegas AJ, Dorkin JR, Anderson DG. 2014. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555. ( 10.1038/nrg3763) [DOI] [PubMed] [Google Scholar]
- 4.Gilleron J, et al. 2013. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646. ( 10.1038/nbt.2612) [DOI] [PubMed] [Google Scholar]
- 5.Kerr MC, Teasdale RD. 2009. Defining macropinocytosis. Traffic 10, 364–371. ( 10.1111/j.1600-0854.2009.00878.x) [DOI] [PubMed] [Google Scholar]
- 6.Lim JP, Gleeson PA. 2011. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol. Cell Biol. 89, 836–843. ( 10.1038/icb.2011.20) [DOI] [PubMed] [Google Scholar]
- 7.Drive K. 2013. NIH Public Access. 7, 1895–1906. [Google Scholar]
- 8.Dong Y, et al. 2014. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA 111, 3955–3960. ( 10.1073/pnas.1322937111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love KT, et al. 2010. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 9915 ( 10.1073/pnas.1005136107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahay G, et al. 2013. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31, 653–658. ( 10.1038/nbt.2614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardarelli F, Pozzi D, Bifone A, Marchini C, Caracciolo G. 2012. Cholesterol-dependent macropinocytosis and endosomal escape control the transfection efficiency of lipoplexes in CHO living cells. Mol. Pharm. 9, 334–340. ( 10.1021/mp200374e) [DOI] [PubMed] [Google Scholar]
- 12.Zhang X-X, Allen PG, Grinstaff M. 2011. Macropinocytosis is the major pathway responsible for DNA transfection in CHO cells by a charge-reversal amphiphile. Mol. Pharm. 8, 758–766. ( 10.1021/mp100366h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GonçalvesGon C, Mennesson E, Fuchs R, Gorvel JP, Midoux P, Pichon C. 2004. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol. Ther. 10, 373–385. ( 10.1016/j.ymthe.2004.05.023) [DOI] [PubMed] [Google Scholar]
- 14.Walsh M, et al. 2006. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-l-lysine compacted DNA: implications for cancer gene therapy. Mol. Pharm. 3, 644–653. ( 10.1021/mp0600034) [DOI] [PubMed] [Google Scholar]
- 15.Lühmann T, Rimann M, Bittermann AG, Hall H. 2008. Cellular uptake and intracellular pathways of PLL-g-PEG-DNA nanoparticles. Bioconjug. Chem. 19, 1907–1916. ( 10.1021/bc800206r) [DOI] [PubMed] [Google Scholar]
- 16.Khalil IA, Kogure K, Futaki S, Harashima H. 2006. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 281, 3544–3551. ( 10.1074/jbc.M503202200) [DOI] [PubMed] [Google Scholar]
- 17.Asai T, et al. 2014. Cell-penetrating peptide-conjugated lipid nanoparticles for siRNA delivery. Biochem. Biophys. Res. Commun. 444, 599–604. ( 10.1016/j.bbrc.2014.01.107) [DOI] [PubMed] [Google Scholar]
- 18.Huang J-L, et al. 2017. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat. Commun. 8, 15144 ( 10.1038/ncomms15144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian T, et al. 2014. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 289, 22 258–22 267. ( 10.1074/jbc.M114.588046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamerkar S, et al. 2017. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503. ( 10.1038/nature22341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson P, Jones AT, Stephens DJ. 2005. Intracellular trafficking pathways and drug delivery: fluorescence imaging of living and fixed cells. Adv. Drug Deliv. Rev. 57, 43–61. ( 10.1016/j.addr.2004.05.003) [DOI] [PubMed] [Google Scholar]
- 22.West MA, Bretscher MS, Watts C. 1989. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109, 2731–2739. ( 10.1083/jcb.109.6.2731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T. 2010. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188, 547–563. ( 10.1083/jcb.200908086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269, 14 869–14 871. [PubMed] [Google Scholar]
- 25.Straight AF, et al. 2003. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299, 1743–1747. ( 10.1126/science.1081412) [DOI] [PubMed] [Google Scholar]
- 26.Araki N, Johnson MT, Swanson JA. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260. ( 10.1083/jcb.135.5.1249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta D, Donaldson JG. 2012. Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell Logist. 2, 203–208. ( 10.4161/cl.23967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC. 2010. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol. Ther. 18, 561–569. ( 10.1038/mt.2009.281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haigler HT, McKanna JA, Cohen S. 1979. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J. Cell Biol. 83, 82–90. ( 10.1083/jcb.83.1.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. 2007. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 8, 848–866. ( 10.1111/j.1600-0854.2007.00572.x) [DOI] [PubMed] [Google Scholar]
- 31.Vercauteren D, Deschout H, Remaut K, Engbersen JFJ, Jones AT, Demeester J, De Smedt SC, Braeckmans K. 2011. Dynamic colocalization microscopy to characterize intracellular trafficking of nanomedicines. ACS Nano 5, 7874–7884. ( 10.1021/nn2020858) [DOI] [PubMed] [Google Scholar]
- 32.Collinet C, et al. 2010. Systems survey of endocytosis by multiparametric image analysis. Nature 464, 243–249. ( 10.1038/nature08779) [DOI] [PubMed] [Google Scholar]
- 33.Commisso C, et al. 2013. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637. ( 10.1038/nature12138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Soraj M, He L, Peynshaert K, Cousaert J, Vercauteren D, Braeckmans K, De Smedt SC, Jones AT. 2012. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J. Control Release. 161, 132–141. ( 10.1016/j.jconrel.2012.03.015) [DOI] [PubMed] [Google Scholar]
- 35.Commisso C, Flinn RJ, Bar-Sagi D. 2014. Determining the macropinocytic index of cells through a quantitative image-based assay. Nat. Protoc. 9, 182–192. ( 10.1038/nprot.2014.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermeulen LMP, Brans T, De Smedt SC, Remaut K, Braeckmans K. 2018. Methodologies to investigate intracellular barriers for nucleic acid delivery in non-viral gene therapy. Nano Today 21, 74–90. ( 10.1016/j.nantod.2018.06.007) [DOI] [Google Scholar]
- 37.Xu Y, et al. 2014. Quantitation of physiological and biochemical barriers to siRNA liver delivery via lipid nanoparticle platform. Mol. Pharm. 11, 1424–1434. ( 10.1021/mp400584h) [DOI] [PubMed] [Google Scholar]
- 38.Martens TF, Remaut K, Demeester J, De Smedt SC, Braeckmans K. 2014. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today 9, 344–364. ( 10.1016/j.nantod.2014.04.011) [DOI] [Google Scholar]
- 39.Stewart MP, Langer R, Jensen KF. 2018. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem. Rev. 118, 7409–7531. ( 10.1021/acs.chemrev.7b00678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cullis PR, Hope MJ. 2017. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475. ( 10.1016/j.ymthe.2017.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayaraman M, et al. 2012. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 51, 8529–8533. ( 10.1002/anie.201203263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semple SC, et al. 2010. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176. ( 10.1038/nbt.1602) [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni JA, et al. 2017. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomed. Nanotechnol. Biol Med. 13, 1377–1387. ( 10.1016/j.nano.2016.12.014) [DOI] [PubMed] [Google Scholar]
- 44.Yanez AM, et al. 2018. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl Acad. Sci. USA 115, E3351–E3360. ( 10.1073/pnas.1720542115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardi N, et al. 2015. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control Release 217, 345–351. ( 10.1016/j.jconrel.2015.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni JA, Cullis PR, van der Meel R. 2018. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 28, nat.2018.0721 ( 10.1089/nat.2018.0721) [DOI] [PubMed] [Google Scholar]
- 47.Akinc A, et al. 2010. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364. ( 10.1038/mt.2010.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, Greber UF. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158, 1119–1131. ( 10.1083/jcb.200112067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaczmarek JC, Kowalski PS, Anderson DG. 2017. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 9, 60 ( 10.1186/s13073-017-0450-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, et al. 2016. The Niemann-Pick C1 inhibitor NP3.47 enhances gene silencing potency of lipid nanoparticles containing siRNA. Mol. Ther. 24, 2100–2108. ( 10.1038/mt.2016.179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitehead KA, et al. 2014. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 5, 4277 ( 10.1038/ncomms5277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenton OS, et al. 2017. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 29, 1–7. ( 10.1002/adma.201606944) [DOI] [PubMed] [Google Scholar]
- 53.Dong Y, et al. 2016. Poly(glycoamidoamine) brushes formulated nanomaterials for systemic siRNA and mRNA delivery in vivo. Nano Lett. 16, 842–848. ( 10.1021/acs.nanolett.5b02428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahlman JE, et al. 2014. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 9, 648 ( 10.1038/nnano.2014.84) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conner SD, Schmid SL. 2003. Regulated portals of entry into the cell. Nature 422, 37–44. ( 10.1038/nature01451) [DOI] [PubMed] [Google Scholar]
- 56.Chen D, et al. 2012. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled micro fluidic formulation. J. Amer. Chem. Soc. 134, 6948–6951. ( 10.1021/ja301621z) [DOI] [PubMed] [Google Scholar]
- 57.Lächelt U, Wagner E. 2015. Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem. Rev. 115, 11 043–11 078. ( 10.1021/cr5006793) [DOI] [PubMed] [Google Scholar]
- 58.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. 2012. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 9, 1–14. ( 10.1016/j.nano.2012.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa PM, Bourgognon M, Wang JT-W, Al-Jamal KT. 2016. Functionalised carbon nanotubes: from intracellular uptake and cell-related toxicity to systemic brain delivery. J. Control Release 241, 200–219. ( 10.1016/j.jconrel.2016.09.033) [DOI] [PubMed] [Google Scholar]
- 60.Van Der Aa MAEM, et al. 2007. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm. Res. 24, 1590–1598. ( 10.1007/s11095-007-9287-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behr J. 1997. The proton sponge: a trick to enter cells the viruses did not exploit. Int. J. Chem. 2, 34–36. [Google Scholar]
- 62.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. 2012. The possible ‘proton sponge’ effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 21, 149–157. ( 10.1038/mt.2012.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen J, Szoka FC. 2012. Nucleic acid delivery: the missing pieces of the puzzle? Acc. Chem. Res. 45, 1153–1162. ( 10.1021/ar3000162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bechara C, Sagan S. 2013. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 587, 1693–1702. ( 10.1016/j.febslet.2013.04.031) [DOI] [PubMed] [Google Scholar]
- 65.Jones AT. 2007. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J. Cell. Mol. Med. 11, 670–684. ( 10.1111/j.1582-4934.2007.00062.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Soraj MH, Watkins CL, Vercauteren D, De Smedt SC, Braeckmans K, Jones AT.. 2010. siRNA versus pharmacological inhibition of endocytic pathways for studying cellular uptake of cell penetrating peptides. J Control Release 148, e86–e87. ( 10.1016/j.jconrel.2010.07.062) [DOI] [PubMed] [Google Scholar]
- 67.Nakase I, et al. 2006. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry 46, 492–501. ( 10.1021/bi0612824) [DOI] [PubMed] [Google Scholar]
- 68.Fretz MM, Penning NA, Al-Taei S, Futaki S, Takeuchi T, Nakase I. 2007. Temperature-, concentration-and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. Biochem. J. 403, 335–342. ( 10.1042/BJ20061808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakase I, Noguchi K, Aoki A, Takatani-Nakase T, Fujii I, Futaki S. 2017. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 7, 1991 ( 10.1038/s41598-017-02014-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. 2017. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control Release 266, 100–108. ( 10.1016/j.jconrel.2017.09.019) [DOI] [PubMed] [Google Scholar]
- 71.Swanson JA. 2008. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 9, 639–649. ( 10.1038/nrm2447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park JK, Peng H, Katsnelson J, Yang W, Kaplan N, Dong Y, Rappoport JZ, He CC, Lavker RM. 2016. MicroRNAs-103/107 coordinately regulate macropinocytosis and autophagy. J. Cell Biol. 215, 667–685. ( 10.1083/jcb.201604032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewis WH. 1937. Pinocytosis by malignant cells. Am. J. Cancer 29, 666–679. ( 10.1158/ajc.1937.510) [DOI] [Google Scholar]
- 74.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. ( 10.1016/0092-8674(92)90164-8) [DOI] [PubMed] [Google Scholar]
- 75.Hoeller O, et al. 2013. Two distinct functions for PI3-kinases in macropinocytosis. J. Cell Sci. 126, 4296–4307. ( 10.1242/jcs.134015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida S, Hoppe AD, Araki N, Swanson JA. 2009. Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages. J. Cell Sci. 122, 3250–3261. ( 10.1242/jcs.053207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Veltman DM, Williams TD, Bloomfield G, Chen B-C, Betzig E, Insall RH. 2016. A plasma membrane template for macropinocytic cups. Elife 5, e20085 ( 10.7554/eLife.20085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prior IA, Lewis PD, Mattos C. 2012. A comprehensive survey of Ras mutations in cancer. Cancer Res. 72, 2457–2467. ( 10.1158/0008-5472.CAN-11-2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krauthammer M, et al. 2012. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 44, 1006–1014. ( 10.1038/ng.2359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samuels Y, et al. 2004. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 ( 10.1126/science.1096502) [DOI] [PubMed] [Google Scholar]
- 81.Bauer TM, Patel MR, Infante JR. 2015. Targeting PI3 kinase in cancer. Pharmacol. Ther. 146, 53–60. ( 10.1016/j.pharmthera.2014.09.006) [DOI] [PubMed] [Google Scholar]
- 82.Bloomfield G, Traynor D, Sander SP, Veltman DM, Pachebat JA, Kay RR. 2015. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. Elife 4, e04940 ( 10.7554/eLife.04940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Normanno N, et al. 2006. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366, 2–16. ( 10.1016/j.gene.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 84.Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. 2016. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35, 816–826. ( 10.1038/onc.2015.139) [DOI] [PubMed] [Google Scholar]
- 85.Bar-Sagi D, Feramisco JR. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068. ( 10.1126/science.3090687) [DOI] [PubMed] [Google Scholar]
- 86.Walsh AB, Bar-Sagi D. 2001. Differential activation of the Rac pathway by Ha-Ras and K-Ras. J. Biol. Chem. 276, 15 609–15 615. ( 10.1074/jbc.M010573200) [DOI] [PubMed] [Google Scholar]
- 87.Lawrence MS, et al. 2014. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 505, 495–501. ( 10.1038/nature12912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samuels Y, et al. 2005. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 7, 561–573. ( 10.1016/j.ccr.2005.05.014) [DOI] [PubMed] [Google Scholar]
- 89.Palm W, Araki J, King B, DeMatteo RG, Thompson CB. 2017. Critical role for PI3-kinase in regulating the use of proteins as an amino acid source. Proc. Natl Acad. Sci. USA 114, E8628–E8636. ( 10.1073/pnas.1712726114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. 2017. The PI3 K pathway in human disease. Cell 170, 605–635. ( 10.1016/j.cell.2017.07.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshida S, Pacitto R, Yao Y, Inoki K, Swanson JA. 2015. Growth factor signaling to mTORC1 by amino acid-laden macropinosomes. J. Cell Biol. 211, 159–172. ( 10.1083/jcb.201504097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duda DG, Kozin S V, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. 2011. CXCL12 (SDF1α)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin. Cancer Res. 17, 2074–2080. ( 10.1158/1078-0432.CCR-10-2636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scotton CJ, et al. 2002. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 62, 5930–5938. [PubMed] [Google Scholar]
- 94.Tanaka G, et al. 2012. CXCR4 stimulates macropinocytosis: implications for cellular uptake of arginine-rich cell-penetrating peptides and HIV. Chem. Biol. 19, 1437–1446. ( 10.1016/j.chembiol.2012.09.011) [DOI] [PubMed] [Google Scholar]
- 95.Cepeda EB, et al. 2015. Mechanisms regulating cell membrane localization of the chemokine receptor CXCR4 in human hepatocarcinoma cells. Biochim. Biophys. Acta 1853, 1205–1218. ( 10.1016/j.bbamcr.2015.02.012) [DOI] [PubMed] [Google Scholar]
- 96.Wagner PL, et al. 2009. CXCL12 and CXCR4 in adenocarcinoma of the lung: association with metastasis and survival. J. Thorac. Cardiovasc. Surg. 137, 615–621. ( 10.1016/j.jtcvs.2008.07.039) [DOI] [PubMed] [Google Scholar]
- 97.Muller A, et al. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56. ( 10.1038/35065016) [DOI] [PubMed] [Google Scholar]
- 98.Rigo A, et al. 2010. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol. Cancer 9, 273 ( 10.1186/1476-4598-9-273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noy R, Pollard JW. 2014. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61. ( 10.1016/j.immuni.2014.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pacitto R, Gaeta I, Swanson JA, Yoshida S. 2017. CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages. J. Leukoc. Biol. 101, 683–692. ( 10.1189/jlb.2A0316-141RR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel S, et al. 2017. boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 17, 5711–5718. ( 10.1021/acs.nanolett.7b02664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Racoosin EL, Swanson JA. 1993. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 121, 1011–1020. ( 10.1083/jcb.121.5.1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Racoosin EL, Swanson JA. 1989. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J. Exp. Med. 170, 1635–1648. ( 10.1084/jem.170.5.1635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamasaki M, Araki N, Hatae T. 2004. Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 277, 298–306. ( 10.1002/ar.a.20027) [DOI] [PubMed] [Google Scholar]
- 105.Hewlett LJ, Prescott AR, Watts C. 1994. The coated pit and macropinocytic pathways serve distinct endosome populations. J. Cell Biol. 124, 689–703. ( 10.1083/jcb.124.5.689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liberali P, et al. 2008. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 27, 970–981. ( 10.1038/emboj.2008.59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Juliano RL. 2018. Intracellular trafficking and endosomal release of oligonucleotides: what we know and what we don't. Nucleic Acid Ther. 28, 166–177. ( 10.1089/nat.2018.0727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerr MC, et al. 2006. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J. Cell Sci. 119, 3967–3980. ( 10.1242/jcs.03167) [DOI] [PubMed] [Google Scholar]
- 109.Desai AS, Chauhan VM, Johnston APR, Esler T, Aylott JW. 2013. Fluorescent nanosensors for intracellular measurements: synthesis, characterisation, calibration and measurement. Front. Physiol. 4, 401 ( 10.3389/fphys.2013.00156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. 2015. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 5, 10300 ( 10.1038/srep10300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakase I, Osaki K, Tanaka G, Utani A, Futaki S. 2014. Molecular interplays involved in the cellular uptake of octaarginine on cell surfaces and the importance of syndecan-4 cytoplasmic V domain for the activation of protein kinase Cα. Biochem. Biophys. Res. Commun. 446, 857–862. ( 10.1016/j.bbrc.2014.03.018) [DOI] [PubMed] [Google Scholar]
- 112.Mercer J, Helenius A. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320, 531–535. ( 10.1126/science.1155164) [DOI] [PubMed] [Google Scholar]
- 113.Ford C, Nans A, Boucrot E, Hayward RD. 2018. Chlamydia exploits filopodial capture and a macropinocytosis-like pathway for host cell entry. PLoS Pathog. 14, e1007051 ( 10.1371/journal.ppat.1007051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levin R, Grinstein S, Schlam D. 2015. Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1851, 805–823. ( 10.1016/j.bbalip.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 115.Doherty GJ, McMahon HT. 2009. Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902. ( 10.1146/annurev.biochem.78.081307.110540) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.