Abstract
Background
Several mosquito population suppression strategies based on the rearing and release of sterile males have provided promising results. However, the lack of an efficient male selection method has hampered the expansion of these approaches into large-scale operational programmes. Currently, most of these programmes targeting Aedes mosquitoes rely on sorting methods based on the sexual size dimorphism (SSD) at the pupal stage. The currently available sorting methods have not been developed based on biometric analysis, and there is therefore potential for improvement. We applied an automated pupal size estimator developed by Grupo Tragsa with laboratory samples of Anopheles arabiensis, Aedes albopictus, Ae. polynesiensis, and three strains of Ae. aegypti. The frequency distribution of the pupal size was analyzed. We propose a general model for the analysis of the frequency distribution of mosquito pupae in the context of SSD-sorting methods, which is based on a Gaussian mixture distribution functions, thus making possible the analysis of performance (% males recovery) and purity (% males on the sorted sample).
Results
For the three Aedes species, the distribution of the pupae size can be modeled by a mixture of two Gaussian distribution functions and the proposed model fitted the experimental data. For a given population, each size threshold is linked to a specific outcome of male recovery. Two dimensionless parameters that measure the suitability for SSD-based sorting of a specific batch of pupae are provided. The optimal sorting results are predicted for the highest values of SSD and lowest values of intra-batch variance. Rearing conditions have a strong influence in the performance of the SSD-sorting methods and non-standard rearing can lead to increase pupae size heterogeneity.
Conclusions
Sex sorting of pupae based on size dimorphism can be achieved with a high performance (% males recovery) and a reasonably high purity (% males on the sorted sample) for the different Aedes species and strains. The purity and performance of a sex sorting operation in the tested Aedes species are linked parameters whose relation can be modeled. The conclusions of this analysis are applicable to all the existing SSD-sorting methods. The efficiency of the SSD-sorting methods can be improved by reducing the heterogeneity of pupae size within rearing containers. The heterogeneity between batches does not strongly affect the quality of the sex sorting, as long as a specific separation threshold is not pre-set before the sorting process. For new developments, we recommend using adaptive and precise threshold selection methods applied individually to each batch or to a mix of batches. Adaptive and precise thresholds will allow the sex-sorting of mixed batches in operational conditions maintaining the target purity at the cost of a reduction in performance. We also recommend a strategy whereby an acceptable level of purity is pre-selected and remains constant across the different batches of pupae while the performance varies from batch to batch to fit with the desired purity.
Keywords: Sterile insect technique, Biometrical analysis, Morphometrics frequency distribution models, Sexual size dimorphism, Sex sorting methods, Aedes aegypti, Aedes albopictus, Aedes polynesiensis, Anopheles arabiensis
Background
There is a global renewed interest in area-wide integrated mosquito management strategies based on the mass production and release of sterile males to suppress target populations [1–4]. These techniques are usually referred to as genetic control methods and include, among others, the sterile insect technique (SIT), the incompatible insect technique (IIT) and the release of insects carrying a dominant lethal gene (RIDL) [1, 4–7]. Several small-scale projects have demonstrated the high potential of these strategies to suppress mosquito populations [6, 8–10]. The scaling-up of these projects from pilot to operational has been hampered by several problems, the most significant one being the lack of an efficient sex-sorting method [1, 11, 12]. Given that only the female mosquitoes bite and transmit the human pathogens, those methods must be capable of ensuring a predefined acceptable level of female contamination while maximizing the male pupae recovery.
The successful use of genetic sexing strains (GSS) for the sex sorting of Ceratitis capitata and other fruit fly species [13–17] has encouraged researchers to develop similar GSS strains for mosquitoes. GSS strains that can be sex-sorted at early developmental stages (eggs or L1) are generally accepted to be the optimal solution for mass-scale SIT and related techniques [11, 12]. A genetic sexing strain based on the tolerance to dieldrin has been developed for Anopheles arabiensis; however, this strain presents several problems and has limited potential for SIT applications [18, 19]. In addition, several transgenic genetic sexing strains developed for different mosquito vector species are also of limited applied potential due to either lack of stability, low male performance or subject to extensive regulation [11, 12].
The current lack of a functional GSS has led to the mosquito population suppression projects to use alternative ways in the sex-sorting process. For the mosquito species with strong sexual size dimorphism (SSD), mainly Aedes and Culex species, mechanical methods have been generally adopted for sorting [6, 8, 20]. Although several designs and proposals for sex sorting on a mass scale have been suggested in the past [21, 22], all mosquito genetic control programmes currently use either plate separators [23] or sieves [8] for sex sorting that have been devised for small-scale rearing conditions. The development of new designs with automation capability for unattended sorting would increase the efficiency of those projects and allow their upgrade to large operational programmes [12, 24].
The efficiency of SSD-sorting methods in terms of male recovery, female contamination and speed depends on technical and biological factors. The technical features basically affect the rate of separation per time unit, and differ between methods. The main biological determinant is the size distribution between sexes and their overlap as well as the effect of rearing conditions on this characteristic. All the SSD-sorting methods rely on the same principle: the separation in two samples by a threshold size. It should be noted that an analysis of the biological determinants of the distribution of size will in principle be applicable to all SSD-sorting methods
In order to improve the performance of new designs of sex-sorting methods based on SSD, a previous biometric analysis is required, specifically dealing with the analysis of the frequency distribution of the size of sexes. However, there is scarce information regarding the distribution of size in mosquitoes. Usually, the scope of the biometric studies in mosquitoes has been to find correlations between the body size and other biological traits [25–29], providing only point and variance estimates, and only a limited number of studies have included detailed frequency distributions [30–32]. For insects, most of the frequency distributions of the size can fit to normal probability functions. When a strong SSD is present, each sex can fit to an independent normal curve [33]. SSD is generally assumed as a species-specific (or population-specific) trait with a narrow degree of variation caused by complex interactions of factors [34–37].
Several experiments have shown that variations in the mosquito larval rearing conditions can increase or reduce the average size of the resulting pupae [38–41]. The SSD is slightly influenced by intraspecific competition [40, 42], but not by food availability [38] or the pollution by conspecifics [43] as size of both sexes is equally affected by these parameters and the difference between the average size of each sex remains constant. The objective of the present study is to optimize the utilization of the SSD-sorting methods through the understanding of the frequency distribution in the pupal size of different mosquito species and strains, with the ultimate goal to: (i) understand the performance of the current sex-sorting methods in different conditions; (ii) assess the relationship between the parameters of importance for sex-sorting devices: female contamination and male recovery; (iii) evaluate the suitability of size-based sex sorting methods for different species and strains of mosquitoes; and (iv) propose features that will optimize the performance of SSD-sorting methods.
To achieve these objectives, we developed a general model for the size distribution of mosquito at the pupal stage. The frequency distribution can be modelled as a mixture of two normal probability density functions. We analyzed the frequency distribution in size of four important mosquito vector species that are currently the target of area-wide integrated vector control projects using SIT-based methods: Aedes aegypti, Ae. albopictus, Ae. polynesiensis and Anopheles arabiensis. The intraspecific variation is also evaluated for Ae. aegypti, since three different laboratory strains were included in the analysis. The use of an automated pupae size estimator system based on artificial vision developed by Grupo Tragsa, Spain, allowed the collection of a large amount of size measurements thus facilitating the achievement of our objective.
Methods
Laboratory strains
The Aedes aegypti Sri Lanka strain originates from mosquitoes collected from the Narahenpita area, District of Colombo, Western Province, Sri Lanka. This strain was kindly provided by Ms. Asha Wijegunawardana (University of Kelaniya, Sri Lanka) and has been maintained in the Insect Pest Control Laboratory of the Joint Food and Agriculture Organization and International Atomic Energy Agency (IPCL-Joint FAO/IAEA) laboratories since 2017. F28 mosquitoes from this strain were analyzed in the present study.
The Ae. aegypti GSS has been developed by classical genetic approaches and has been maintained in the IPCL-Joint FAO/IAEA since 2017. F5 mosquitoes from this strain were used in the present study.
The Ae. aegypti WB2 line was recently generated by transfer of Wolbachia wAlbB from Aedes albopictus into Aedes aegypti via embryonic microinjection at Michigan State University (personal communication, Zhiyong Xi), and has been maintained in the IPCL laboratories since 2016. This strain was introgressed into the genomic background of an Ae. aegypti strain from Brazil, provided by Professor Margareth Capurro (University of Sao Paolo, Brazil), through a series of seven backcrosses using in every generation Wolbachia-infected females mated with Ae. aegypti Brazil males. This resulted in the construction of the Ae. aegypti WB2-BRA strain used. F12 mosquitoes from this strain were analyzed in the present study.
The incompatible Ae. polynesiensis “Aito” (BC9) strain carries Wolbachia B from Ae. riversi. This strain was generated through multiple backcrosses between Aedes riversi females and Aedes polynesiensis aposymbiotic males (Hapairai, 2013). This strain which has been maintained at Institut Louis Malardè (ILM), Tahiti since 2010 was recently used in a pilot IIT field study on the atoll of Tetiaroa, French Polynesia (Bossin et al. manuscript in preparation).
The Ae. albopictus Rimini strain was originated from field collections in northern Italy. It has been maintained in the IPCL since 2010.
The An. arabiensis Dongola strain was originated from the Northern State of Sudan. It has been maintained in the IPCL since 2005. It is also available at the Malaria Research and Reference Reagent Resource Center, MR4, as MRA-856.
Mosquito rearing
Standard rearing conditions have been used for the maintenance of experimental colonies, egg collection and hatching of Ae. albopictus and Ae. aegypti [44, 45], Ae. polynesiensis [39], and An. arabiensis [46, 47] colonies.
Pupae production
For each species or strain, three larval containers were prepared for pupae production as described below. These three replicates represented a random sample of the different rearing units found in a mass rearing facility.
For Ae. aegypti and Ae. albopictus, 2000 first-instar larvae were introduced in white acrylonitrile-butadiene-styrene (ABS) plastic trays (41 × 30 × 8 cm) with 1.5 l of deionized water. Since larvae of Ae. polynesiensis must be reared under lower densities [39], 1200 first-instar larvae were introduced in 40 × 60 × 15 cm containers with 4 l of water. The larvae were fed with the standard Aedes IPCL diet [48, 49] at a concentration of 75 g per liter of diet. The diet regime ranged from 0.2 mg of dry weight per larvae on the first day to 0.8 mg on the last days.
Different batches of eggs of Ae. arabiensis were hatched in white plastic trays (41 × 30 × 8 cm). Two days after the hatching, approximately 500-1000 larvae were visually isolated in the same kind of trays with 1.5 l of deionized water. The larvae were fed with the standard Anopheles IPCL diet [46], in a concentration of 10 g/l ranging from 5ml on the first day to 20 ml on the last days.
All pupae in each container were collected on a daily basis starting at 24 hours from the beginning of the pupation. A batch of pupae was defined as all the pupae produced in 24 hours for a specific species/strain and replicate(s). The selected batches were sex-sorted under a binocular microscope. All the pupae in a batch were classified into males and females groups, and the resulting samples are referred to as batch-sex groups. The batch where the proportion of male and female was closer to 50%, usually on the 2nd or 3rd day from the beginning of pupation, was selected for the analysis.
Measurement of pupal size
The lateral profile area of the pupae was automatically measured by means of a computer vision system (Fig. 1), which comprises: (i) a translucent rigid surface with circular uniform movement, acting as conveyor on which the mosquito pupae are arranged; (ii) a uniform and high intensity led white light backlight system and (iii) a high resolution/high speed camera placed in top position. In this way, the mosquito pupae pass continuously under the camera while being backlit by the lighting system. The backlighting of the pupae allows photograph of them with a high contrast, which facilitates their subsequent extraction and isolation from the background (segmentation). In order to increase the precision and accuracy of the measurement of the areas, avoiding errors due to the effects of refraction of light by water droplets, the size of the pupae was measured in dry conditions for each session.
Fig. 1.
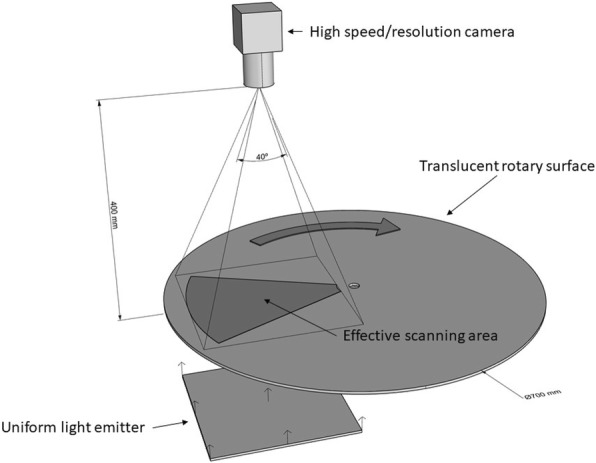
Continuous image capture size
A factor of special importance is that the backlight system must guarantee an illuminated area with a uniform intensity, at least in the interest area of capture of the camera. This is because the light intensity directly affects the size of the areas extracted in the segmentation process, and variations in the intensity could result in errors in the relative measurements. However, even with a uniform backlighting system, minor errors may occur in the measurement due to the position of each pupa with respect to the position of the camera (different projected areas) and electrical noise in the silicone sensor of the camera. To minimise these phenomena, several pictures of each pupa are recorded while in the camera capture area (around 15 shots per pupa), and then the median of all the measurements of each individual is chosen as the value of size. So, each individual has to be identified and its path has to be tracked. For this task, we have developed a predictive tracking algorithm, based on Kalman filters [50], which is able to identify and track individuals in their rotational displacement under the field of view of the camera. Additionally, the algorithm is robust enough to follow the characteristic rapid movements of the pupae in dry conditions, which are quite active.
The median size in square pixels is then transformed to a unidimensional parameter by the square root to linearize the measure of size. The measure of size presented in this study is then the square root of the pixel area. The outcome of the analysis is scale-independent, and the conclusions are valid regardless of the actual value of this magnitude. Since the pupae were sex sorted manually, we assume that a certain degree of identification error occured. Errors in the manual sex identification can affect the estimation of the statistical parameters, especially those pupae with size far larger or smaller than the corresponding sex average value. In order to minimize this effect, we considered each value that exceeded two standard deviations from the average as an error in sex identification. These values were subsequently excluded from the analysis.
Model for the frequency distribution of size
The proposed model relies on the basic assumption that the probability density function for the pupal size of each sex follows a Gaussian distribution, with the mean and standard deviation as the characteristic parameters. The mixture distribution for this situation is:
| 1 |
where N(x; μi, σi) is the normal probability density function for size (x), with mean μi and standard deviation σi. The scalars αi are the proportions of each sex in the model, being αm + αf = 1. The subscripts m and f denote males and females respectively.
Predictions from the model
One of the goals of our model is to provide a statistical tool to estimate the theoretical outcomes of male recovery and female contamination. In order to quantify them we introduce the performance (PER) and purity (PUR) functions defined as follows:
| 2 |
| 3 |
For any given size threshold (x), PER provides the male recovery percentage and PUR the percentage of males on the sorted sample. Assuming the model given by Equation 1, it is easy to show that both functions can be estimated in terms of the normal distribution function:
| 4 |
| 5 |
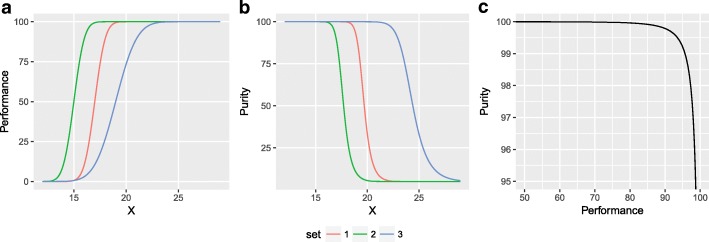
where Φ denotes the standard normal cumulative distribution function. In order to determine both functions, the parameters {αm, μi, σi} of the model must be known. Purity and performance are inversely linked. A decrease in female contamination can be achieved by reducing the value of the threshold, but this unavoidably produces a reduction in the performance (Fig. 2). The features of PER(x) and PUR(x) depend on the chosen set of parameters. However many sets give rise to functions that are related by simple symmetry transformations like translations or scaling. As long as both functions are transformed in the same way, the purity versus performance curve remains invariant (Fig. 2c). Therefore, parameters {αi, μi, σi} are not suitable to classify unequivocally the different samples as they can lead to the same purity-performance curve. In order to find a more appropriate space parameter, we introduce two new dimensionless parameters: the sexual dimorphism index (SDI) and the sexual homoscedasticity index (SHI) defined by
Fig. 2.
Depiction of the purity-performance relationship under a mixture of two Gaussian distributions applied to the analysis of sex sorting by size. The graphs consider αm = 0.5 and three different sets of parameters s = {μm, σm, μf, σf}, s1 ={10, 1, 11, 1}, s2 = {8, 1, 9, 1} and s3 = {12, 2, 14, 2}. The performance of sets 2 and 3 is obtained by translating and scaling the performance of set 1: PER2(x) = PER1(x + 2), PER3(x) = PER1(x−2/2). The same transformations are applied to purity functions. a Purity versus size (X). b Performance versus size (X). c Purity versus performance
| 6 |
| 7 |
Combining definitions (6) and (7) with Equations 4 and 5, we can rewrite performance and purity functions as
| 8 |
| 9 |
z being a dimensionless variable defined by
Equations 8 and 9 reveal that PER(z) and PUR(z) only depend on three dimensionless parameters: αm, SDI and SHI. In other words, given αm, all combinations of the original parameters {μm, μf, σm , σf} giving the same pair {SDI, SHI} have exactly the same performance and purity functions in terms of the dimensionless variable z. Consequently, the dimensionless parameters {SDI, SHI} are more suitable than classical measures of center and spread to classify unequivocally different pupae samples with regard to purity and performance.
Basic statistics and model fit
The mean and standard deviation was estimated for every batch-sex group dataset, and its deviation from the normal distribution was tested by means of the Shapiro-Wilk test. The fit of the data to the probability density function of the mixture model was tested by means of the Kolmogornov-Smirnov test. All the statistic computations were done using the base package of R [51]. The significance level was set to α = 0.05.
Partitioning of the variance
A number of pupae equal to the minimal sample size was randomly selected for each batch-sex group per species/strain. This was performed in order to get a balanced factorial design dataset. A linear model was fit for each batch by means of ordinary least squares. The model included sex and batch as fixed factors, and their interaction. The partitioning of variance was assessed through ANOVA. All statistical analyses were done using the base package of R [51].
Results
Table 1 summarizes the statistics of the pupal size of the species and strains used in the present study. In total, 7733 pupae were analyzed. The majority of the batches were not significantly different from a normal distribution, and the mixture of two Gaussian distributions fitted well to all batches of all the species/strains used when male and female pupae data are combined. Table 2 presents the significance of the goodness-of-fit between the models and the data. None of the samples was significantly different from a Gaussian mixture distribution. Only two batches separated by sex showed significant departures from normality: An. arabiensis females of the batch 3, and Ae. albopictus Rimini males of batch 3.
Table 1.
Descriptive statistics for the batches of pupae
| Batch | Sex | N | Proportion ± SE | Mean size ± SE | SD ± SE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anopheles arabiensis - Dongola | |||||||||||
| 1 | Males | 24 | 0.48 | ± | 0.10 | 13.23 | ± | 0.21 | 1.02 | ± | 0.15 |
| 1 | Females | 26 | 0.52 | ± | 0.10 | 14.40 | ± | 0.19 | 0.99 | ± | 0.14 |
| 2 | Males | 24 | 0.53 | ± | 0.10 | 13.23 | ± | 0.20 | 0.98 | ± | 0.15 |
| 2 | Females | 21 | 0.47 | ± | 0.11 | 13.41 | ± | 0.23 | 1.03 | ± | 0.16 |
| 3 | Males | 32 | 0.46 | ± | 0.09 | 13.98 | ± | 0.18 | 1.03 | ± | 0.13 |
| 3 | Females | 38 | 0.54 | ± | 0.08 | 14.16 | ± | 0.16 | 0.98 | ± | 0.11 |
| Total | Males | 80 | 0.48 | ± | 0.06 | 13.53 | ± | 0.12 | 1.07 | ± | 0.08 |
| Total | Females | 85 | 0.52 | ± | 0.05 | 14.05 | ± | 0.11 | 1.06 | ± | 0.08 |
| Aedes albopictus - Rimini | |||||||||||
| 1 | Males | 337 | 0.53 | ± | 0.03 | 18.66 | ± | 0.05 | 0.85 | ± | 0.03 |
| 1 | Females | 303 | 0.47 | ± | 0.03 | 21.9 | ± | 0.05 | 0.87 | ± | 0.04 |
| 2 | Males | 407 | 0.60 | ± | 0.02 | 17.73 | ± | 0.04 | 0.72 | ± | 0.03 |
| 2 | Females | 277 | 0.40 | ± | 0.03 | 21.26 | ± | 0.05 | 0.78 | ± | 0.03 |
| 3 | Males | 434 | 0.62 | ± | 0.02 | 18.46 | ± | 0.04 | 0.84 | ± | 0.03 |
| 3 | Females | 261 | 0.38 | ± | 0.03 | 21.62 | ± | 0.05 | 0.78 | ± | 0.03 |
| Total | Males | 1178 | 0.58 | ± | 0.01 | 18.26 | ± | 0.03 | 0.90 | ± | 0.02 |
| Total | Females | 841 | 0.42 | ± | 0.02 | 21.6 | ± | 0.03 | 0.85 | ± | 0.02 |
| Aedes polynesiensis - Aito (BC9) | |||||||||||
| 1 | Males | 164 | 0.41 | ± | 0.04 | 17.13 | ± | 0.05 | 0.66 | ± | 0.04 |
| 1 | Females | 239 | 0.59 | ± | 0.03 | 21.09 | ± | 0.05 | 0.72 | ± | 0.03 |
| 2 | Males | 202 | 0.49 | ± | 0.04 | 16.03 | ± | 0.05 | 0.72 | ± | 0.04 |
| 2 | Females | 210 | 0.51 | ± | 0.03 | 19.84 | ± | 0.05 | 0.69 | ± | 0.03 |
| 3 | Males | 195 | 0.48 | ± | 0.04 | 17.03 | ± | 0.04 | 0.56 | ± | 0.03 |
| 3 | Females | 214 | 0.52 | ± | 0.03 | 20.72 | ± | 0.05 | 0.67 | ± | 0.03 |
| Total | Males | 561 | 0.46 | ± | 0.02 | 16.70 | ± | 0.03 | 0.82 | ± | 0.02 |
| Total | Females | 663 | 0.54 | ± | 0.02 | 20.57 | ± | 0.03 | 0.87 | ± | 0.02 |
| Aedes aegypti - GSS | |||||||||||
| 1 | Males | 246 | 0.47 | ± | 0.03 | 17.34 | ± | 0.05 | 0.76 | ± | 0.03 |
| 1 | Females | 272 | 0.53 | ± | 0.03 | 20.31 | ± | 0.04 | 0.73 | ± | 0.03 |
| 2 | Males | 117 | 0.51 | ± | 0.05 | 18.20 | ± | 0.07 | 0.75 | ± | 0.05 |
| 2 | Females | 113 | 0.49 | ± | 0.05 | 22.16 | ± | 0.07 | 0.75 | ± | 0.05 |
| 3 | Males | 496 | 0.44 | ± | 0.02 | 18.04 | ± | 0.03 | 0.72 | ± | 0.02 |
| 3 | Females | 635 | 0.56 | ± | 0.02 | 22.24 | ± | 0.04 | 0.95 | ± | 0.03 |
| Total | Males | 859 | 0.46 | ± | 0.02 | 17.86 | ± | 0.03 | 0.81 | ± | 0.02 |
| Total | Females | 1020 | 0.54 | ± | 0.02 | 21.72 | ± | 0.04 | 1.22 | ± | 0.03 |
| Aedes aegypti - Sri Lanka | |||||||||||
| 1 | Males | 314 | 0.56 | ± | 0.03 | 16.41 | ± | 0.06 | 1.08 | ± | 0.04 |
| 1 | Females | 245 | 0.44 | ± | 0.03 | 19.58 | ± | 0.08 | 1.29 | ± | 0.06 |
| 2 | Males | 309 | 0.55 | ± | 0.03 | 16.36 | ± | 0.06 | 0.99 | ± | 0.04 |
| 2 | Females | 249 | 0.45 | ± | 0.03 | 20.01 | ± | 0.08 | 1.26 | ± | 0.06 |
| 3 | Males | 153 | 0.5 | ± | 0.04 | 16.36 | ± | 0.08 | 0.98 | ± | 0.06 |
| 3 | Females | 156 | 0.5 | ± | 0.04 | 19.85 | ± | 0.09 | 1.13 | ± | 0.06 |
| Total | Males | 776 | 0.54 | ± | 0.02 | 16.38 | ± | 0.04 | 1.03 | ± | 0.03 |
| Total | Females | 650 | 0.46 | ± | 0.02 | 19.81 | ± | 0.05 | 1.25 | ± | 0.03 |
| Aedes aegypti - WB2-BRA | |||||||||||
| 1 | Males | 270 | 0.68 | ± | 0.03 | 17.65 | ± | 0.04 | 0.70 | ± | 0.03 |
| 1 | Females | 129 | 0.32 | ± | 0.04 | 21.23 | ± | 0.07 | 0.83 | ± | 0.05 |
| 2 | Males | 86 | 0.44 | ± | 0.05 | 18.39 | ± | 0.07 | 0.66 | ± | 0.05 |
| 2 | Females | 111 | 0.56 | ± | 0.05 | 22.37 | ± | 0.09 | 0.99 | ± | 0.07 |
| 3 | Males | 160 | 0.38 | ± | 0.04 | 19.12 | ± | 0.06 | 0.74 | ± | 0.04 |
| 3 | Females | 264 | 0.62 | ± | 0.03 | 23.15 | ± | 0.06 | 0.95 | ± | 0.04 |
| Total | Males | 516 | 0.51 | ± | 0.02 | 18.23 | ± | 0.04 | 0.96 | ± | 0.03 |
| Total | Females | 504 | 0.49 | ± | 0.02 | 22.48 | ± | 0.05 | 1.22 | ± | 0.04 |
Batch, number of the batch, corresponding to a rearing container; N, number of pupae; Proportion, proportion of pupae; Mean size (√pixel) for the group; SD, Standard deviation; SE, standard error
Table 2.
Results for the goodness-of-fit of the data to a probability distribution function. P-values are provided for each test
| Species/Strain | Batch | S-W | K-S | |
|---|---|---|---|---|
| Males | Females | Mixture | ||
| P-value | P-value | P-value | ||
| An. arabiensis Dongola | 1 | 0.7334 | 0.7329 | 0.9094 |
| 2 | 0.7290 | 0.2108 | 0.7998 | |
| 3 | 0.9345 | 0.0102* | 0.4434 | |
| Ae. albopictus Rimini | 1 | 0.1545 | 0.3109 | 0.9730 |
| 2 | 0.0526 | 0.6420 | 0.6992 | |
| 3 | 0.0021* | 0.3856 | 0.8665 | |
| Ae. polynesiensis (Aito (BC9) | 1 | 0.3576 | 0.0488 | 0.9371 |
| 2 | 0.7452 | 0.3139 | 0.9556 | |
| 3 | 0.6154 | 0.5026 | 0.7538 | |
| Ae. aegypti GSS | 1 | 0.6916 | 0.2393 | 0.7966 |
| 2 | 0.1250 | 0.9713 | 0.9872 | |
| 3 | 0.4354 | 0.7882 | 0.9726 | |
| Ae. aegypti Sri Lanka | 1 | 0.4785 | 0.5730 | 0.9305 |
| 2 | 0.6273 | 0.8647 | 0.976 | |
| 3 | 0.7664 | 0.3403 | 0.9897 | |
| Ae. aegypti WB2-BRA | 1 | 0.1064 | 0.1099 | 0.7892 |
| 2 | 0.3019 | 0.1325 | 0.9840 | |
| 3 | 0.6914 | 0.0480 | 0.5103 | |
Abbreviations: S-W, Shapiro-Wilk test for the test of normality of the distributions of each sex; K-S, Kolmogorov-Smirnov test for the fit to a mixture of normal distributions
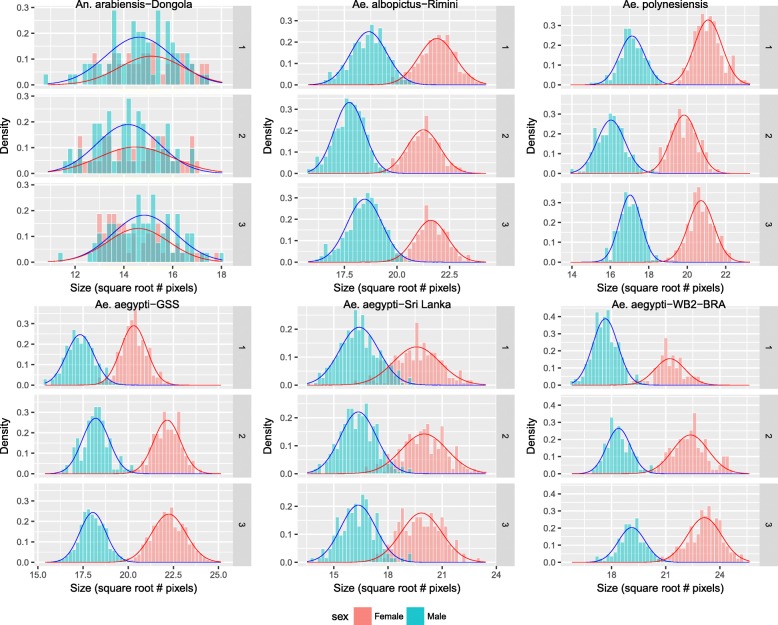
Figure 3 shows the histograms and fitted models for the three batches of each species/strain studied. After the parameters of the model have been estimated, the purity-performance characteristic curve is computed and the quality of sorting can be analyzed theoretically. Each value of pupal size is linked to a pair of purity and performance values, which are inversely related (Fig. 4). The fitted models allow simulating the output of SSD-sorting methods under different circumstances. Table 3 shows the main descriptors for the predicted output from the fitted model for each batch in the experimental data. These results should not be considered as a general prediction of how a particular strain will perform with SSD-sorting methods, since they are only applicable for the specific rearing conditions of this experiment. However, they show the potential of SSD-based sex-sorting procedures when standard rearing procedures are applied. Table 4 provides examples for one of the strains of Ae. aegypti (GSS) of how the performance-purity output varies under different simulated conditions. The simulation a describes the performance when the three batches are mixed and a threshold size is determined by keeping constant the level of purity of 99.5 %. It is shown how SDI takes lower values than any of the individual batches, and an average reduction of 17 % in performance is predicted for the same level of purity (male recovery of 74.5 % with a female contamination of 0.5 % after mixing the three batches). Simulations b and c assess the effect in the performance of the size heterogeneity and the variations in the SSD respectively. For simulation b the variance of both sexes is scaled by the same factor while the distance between means remains constant. Taking batch 1 as a reference, the standard deviation of both sexes is multiplied by 0.8 and 1.2 respectively. For simulation c, taking again batch 1 as reference, the average size of males and females is increased or decreased by 0.5 √pixels but the standard deviations are not modified in this case. It is worth mentioning that the variation of the statistical parameters in simulations b and c only affect the dimensionless parameter SDI, while SHI remains unaltered. Results show that changes in size heterogeneity and SSD have contrary effects in the performance; an increase in intra-batch variance produces a significant drop in performance whereas an increase in SSD improves the quality of sorting. Simulation d describes how the output of SSD-sorting systems vary with the election of a predefined constant threshold for all the batches.
Fig. 3.
Frequency distribution of size (√pixel) for pupae of different mosquito species and strains reared under small-scale laboratory conditions. Males are represented in blue and females in red. Three replicates are presented for each mosquito species/strain
Fig. 4.

Depiction of the SSD-sorting methods functioning simulated by a mixture of two normal distribution functions. Different threshold of sizes separates the sample in two subsamples. The subsample of smaller size has a different male proportion depending on the chosen threshold. Purity = % males on the sorted sample. Performance = % males recovery. The dotted lines depict a value of threshold. a Probability density function of male and female pupal size. b Purity versus performance
Table 3.
Predictions of the fitted models with the experimental parameters for different measures of suitability to sexual size dimorphism sorting methods. Performance (% males recovery) at different levels of purity (% males in the sorted sample)
| Species/Strain | Batch | SDI | SHI | Performance | ||
|---|---|---|---|---|---|---|
| Purity = 99.9 | Purity = 99.5 | Purity = 99.0 | ||||
| An. arabiensis Dongola | 1 | 0.58 | 1.01 | <0.01 | 0.04 | 0.23 |
| 2 | 0.09 | 0.98 | - | - | - | |
| 3 | 0.09 | 1.02 | <0.01 | <0.01 | - | |
| Total | 0.24 | 1.00 | <0.01 | <0.01 | - | |
| Ae. albopictus Rimini | 1 | 1.89 | 0.99 | 73.09 | 88.45 | 92.96 |
| 2 | 2.35 | 0.96 | 95.12 | 98.78 | 99.45 | |
| 3 | 1.95 | 1.04 | 83.92 | 93.55 | 96.19 | |
| Total | 1.91 | 1.02 | 78.97 | 91.13 | 94.62 | |
| Ae. polynesiensis Aito (BC9) | 1 | 2.87 | 0.96 | 99.41 | 99.89 | 99.96 |
| 2 | 2.69 | 1.03 | 98.93 | 99.74 | 99.88 | |
| 3 | 3.00 | 0.92 | 99.78 | 99.97 | 99.99 | |
| Total | 2.29 | 0.97 | 91.41 | 97.25 | 98.55 | |
| Ae. aegypti GSS | 1 | 2.00 | 1.02 | 80.28 | 91.51 | 94.74 |
| 2 | 2.65 | 1.00 | 98.65 | 99.68 | 99.86 | |
| 3 | 2.54 | 0.87 | 95.17 | 99.03 | 99.61 | |
| Total | 1.94 | 0.81 | 33.75 | 74.51 | 86.43 | |
| Ae. aegypti Sri Lanka | 1 | 1.35 | 0.92 | 5.54 | 31.16 | 48.47 |
| 2 | 1.63 | 0.89 | 24.63 | 60.80 | 75.26 | |
| 3 | 1.67 | 0.93 | 37.38 | 67.23 | 78.52 | |
| Total | 1.51 | 0.90 | 15.66 | 48.85 | 64.99 | |
| Ae. aegypti WB2-BRA | 1 | 2.35 | 0.92 | 95.69 | 99.15 | 99.67 |
| 2 | 2.46 | 0.82 | 89.46 | 97.94 | 99.21 | |
| 3 | 2.40 | 0.89 | 89.61 | 97.23 | 98.69 | |
| Total | 1.96 | 0.89 | 63.59 | 86.67 | 92.77 | |
Abbreviations: SDI, SHI, dimensionless parameters, characteristic of each batch
Table 4.
Predicted values for the descriptive parameters of the sex sorting of the Ae. aegypti GSS strain simulated under different conditions
| Sim. | Conditions | Group | μm | μf | σm | σf | SDI | SHI | Performance | Purity |
|---|---|---|---|---|---|---|---|---|---|---|
| - | Experimental | Batch1 | 17.34 | 20.31 | 0.76 | 0.73 | 2.00 | 1.02 | 91.51 | 99.5 |
| Batch 2 | 18.20 | 22.16 | 0.75 | 0.75 | 2.65 | 1.00 | 99.68 | 99.5 | ||
| Batch 3 | 18.04 | 22.24 | 0.95 | 0.72 | 2.54 | 0.87 | 99.03 | 99.5 | ||
| a | Combination of batches | Total | 17.86 | 21.72 | 1.22 | 0.81 | 1.94 | 0.81 | 74.51 | 99.5 |
| b | Differences in | Batch 1 | 17.34 | 20.31 | 0.76 | 0.73 | 2.00 | 1.02 | 91.51 | 99.5 |
| intra-batch variance. | sd* 1.2 | 17.34 | 20.31 | 0.91 | 0.87 | 1.66 | 1.02 | 74.38 | 99.5 | |
| SSD constant | sd* 0.8 | 17.34 | 20.31 | 0.61 | 0.58 | 2.49 | 1.02 | 99.12 | 99.5 | |
| c | Differences in SSD | Batch 1 | 17.34 | 20.31 | 0.76 | 0.73 | 2.00 | 1.02 | 91.51 | 99.5 |
| intra-batch. Variance, | SSD-0.5 | 17.59 | 20.06 | 0.76 | 0.73 | 1.66 | 1.02 | 74.14 | 99.5 | |
| Constant | SSD+0.5 | 17.09 | 20.56 | 0.76 | 0.73 | 2.33 | 1.02 | 97.99 | 99.5 | |
| d | Fixed threshold | Batch1 | 17.34 | 20.31 | 0.76 | 0.73 | 2.00 | 1.02 | 93.69 | 99.22 |
| x=18.5 √pixels | Batch 2 | 18.20 | 22.16 | 0.75 | 0.75 | 2.65 | 1.00 | 65.54 | 100.00 | |
| Batch 3 | 18.04 | 22.24 | 0.95 | 0.72 | 2.54 | 0.87 | 73.80 | 99.99 |
Abbreviations: Sim, reference for the simulation; Group, specific simulation for the group; μm, μf, σm, σf, parameters of the model; SDI, SHI, dimensionless parameters; Performance, % males recovery; Purity, % males on the sorted sample
Simulations: a, effects of sex-sorting after combining the three batches; b, effects of increase/decrease the variance; c, effects of increase/decrease the difference in means between sexes; d, effects of sex-sorting three batches with a predefined size threshold. For each simulation, the parameters that are modified from the experimental data (first 3 rows) are highlighted in bold style
The contribution of different factors to the variability in size has been assessed by means of ANOVA. The results for the partitioning of the variance are shown in Table 5. For all the Aedes species, the biggest source of variation is the SSD. For An. arabiensis, there are significant differences in size between sexes, but this factor explained only a relatively small portion of the total variance.
Table 5.
ANOVA tables for each species/strain. The factors included are batch (rearing container) and sex (male or female)
| Species/Strain | df | SS | MS | F | P | ||
|---|---|---|---|---|---|---|---|
| An. arabiensis Dongola | Batch | 2 | 9.6 | 4.798 | 5.298 | 0.006 | *** |
| Sex | 1 | 6.81 | 6.807 | 7.515 | 0.007 | *** | |
| Batch:Sex | 2 | 10.29 | 5.145 | 5.681 | 0.004 | *** | |
| Residuals | 120 | 108.69 | 0.906 | ||||
| Ae. albopictus Rimini | Batch | 2 | 174 | 87 | 133.646 | <0.001 | *** |
| Sex | 1 | 4281 | 4281 | 6565.183 | <0.001 | *** | |
| Batch:Sex | 2 | 10 | 5 | 7.864 | <0.001 | *** | |
| Residuals | 1560 | 1017 | 1 | ||||
| Ae. polynesiensis Aito (BC9) | Batch | 2 | 263 | 131 | 295.618 | <0.001 | *** |
| Sex | 1 | 3625 | 3625 | 8155.481 | <0.001 | *** | |
| Batch:Sex | 2 | 4 | 2 | 4.289 | 0.014 | * | |
| Residuals | 978 | 435 | 0 | ||||
| Ae. aegypti GSS | Batch | 2 | 278.2 | 139.1 | 223.99 | <0.001 | *** |
| Sex | 1 | 2306.3 | 2306.3 | 3713.93 | <0.001 | *** | |
| Batch:Sex | 2 | 32.9 | 16.4 | 26.45 | <0.001 | *** | |
| Residuals | 672 | 417.3 | 0.6 | ||||
| Ae. aegypti Sri Lanka | Batch | 2 | 11.8 | 5.9 | 4.587 | 0.0104 | * |
| Sex | 1 | 2764.1 | 2764.1 | 2152.107 | <0.001 | *** | |
| Batch:Sex | 2 | 10 | 5 | 3.892 | 0.020 | * | |
| Residuals | 912 | 1171.4 | 1.3 | ||||
| Ae. aegypti WB2-BRA | Batch | 2 | 260.3 | 130.2 | 208.85 | <0.001 | *** |
| Sex | 1 | 1926.1 | 1926.1 | 3090.347 | <0.001 | *** | |
| Batch:Sex | 2 | 8.3 | 4.1 | 6.627 | 0.001 | ** | |
| Residuals | 510 | 317.9 | 0.6 |
Abbreviations: df, degrees of freedom; SS, sum of squares; MS, mean sum of squares; F, F-statistics; P, P-value for the F-statistics
Discussion
For all the species and strains examined in this study, the joint frequency distribution of pupal size included an area of overlap between the individual male and female distributions. This essentially means that a complete separation of sexes according to a given threshold of size is not possible, and every threshold that separates the sample in two will leave a certain proportion of each sex in the batch of the other group: smaller females in the male group and/or bigger males in the female group. This limitation of the SSD-based sorting methods is commonly recognized, altogether with the general observation that rearing conditions have a strong effect over the performance of the methods [8, 20–22, 52]. However, the mechanisms under these observations have not been investigated in depth, which may affect the optimization of new sorting methods based on SSD.
For the four species analyzed, including the three Ae. aegypti laboratory strains, the distribution of the size of each sex considered apart followed a normal distribution, as commonly observed in insects [33]. For the three Aedes species studied here, the joint frequency distribution for both sexes is noticeably bimodal, and can be modeled through a mixture of two normal probability density functions. This model is rather simple, with only five parameters that can be easily estimated directly from a population sample. It is likely that this approach can be generalized to other Aedes species as well as to culicine mosquitoes with a marked dimorphism in size [31, 38, 53]. In addition, this method of analysis could be generally applied to all known SSD-based sorting methods, since all of them rely on separating batches of pupae in two groups through the definition of a threshold size.
The features of the distribution in sizes of the individuals determine the differences between samples/ strains/ species in the suitability for any SSD-sorting method. These differences are reflected in two main parameters: the performance (% males recovery) and sample purity (% males on the sorted sample). Both can be predicted from the probability density function. For a given set of the model parameters (αm; μi, σi), each size threshold has a pair of values of performance and purity associated. Under these model assumptions, purity and performance in each sample of pupae are unequivocally linked; each value of purity corresponds to a single value of performance. Since the evaluation of the quality of a given sorting through the predicted values of purity-performance depends on the chosen value of threshold, the relationship of both parameters in a dimensionless space has been analyzed theoretically. This analysis has provided two useful indices that describe the applicability of SSD-sorting methods for a given sample of pupae, i.e. the quality of the biological material and the rearing conditions. As SDI increases, the purity-performance function becomes more optimal (better performance with higher purity). The SDI index has two components, the SSD and the sample variance. Consequently, an increase in SSD and a reduction in variance increase the efficiency of any SSD-sorting method, as will be discussed later. Index SHI modifies the slope of the curve. The higher the SHI value, the more flattened purity-performance curve is obtained. This parameter describes the difference in variance between males and females, which is difficult to control during the rearing process. SDI and SHI can be used for long-term monitoring of the quality control of the sorting process.
The purity and performance are inversely correlated. From the applied point of view, any sorting system must choose a size threshold considering the trade-off between performance and purity. From Equations 8 and 9, it is possible to estimate, for a given sample, the threshold of size needed to obtain a desired value of purity or performance. Unfortunately, it is not possible to calculate a single constant size threshold for sex sorting that keeps constant the purity and performance levels across different batches. It is known that there is heterogeneity in size in the production units (rearing containers) that affects the outcome of the sorting methods [8, 20, 21, 52]. For instance, the three Ae. aegypti GSS SSD batches varied in purity (99.2-100%) and performance (66-94%) when separated by the same threshold value (see Table 4).
Keeping the purity as a constant parameter, and assuming heterogeneity in size, the outcome of the sorting will have a variable percent recovery of males. Since this heterogeneity is important in the output of the sex sorting, we analyzed and quantified the sources of variation in size in the experimental sample. Then, we used the parameters directly estimated from the samples to predict the expected values of purity and performance for each batch of pupae. Changing the value of these parameters in the fitted models allowed us to simulate the outcome of SSD-sorting methods under different scenarios. The partitioning of variance showed two different patterns of relative importance in respect to the source of heterogeneity in size. For the Aedes species, the main source of variation was the sexual difference, followed by the residual, the differences between batches and finally the interaction sex-batch. For Anopheles, the effect of sex was of less importance, and the residual accounted for most of the variation.
The SSD, as the absolute difference between mean size of each sex, is the main factor that explains the interspecific differences in the applicability of SSD-sorting methods. A higher SSD will produce higher performance independently of the scale, and for all the size thresholds considered, yielding a higher SDI. The two species with higher SSD (Ae. aegypti and Ae. polynesiensis) are known to yield better results than Ae. albopictus when separated with plate separators. On the other hand, An. arabiensis, as expected [11, 12], showed a poor suitability for the SSD-sorting methods. The experimental samples of An. arabiensis showed an average SSD of 0.52 √pixels, while the Aedes species ranged from 3.3 to 3.9 √pixels. Likely, even achieving a reduction in the heterogeneity would not be enough to make SSD-sorting methods suitable for An. arabiensis or related species. For example, a SSD-sorting method was used to sort An. albimanus in a trial in El Salvador [54], and resulted in 14% of female contamination in the released mosquitoes which would be currently unacceptable.
The residual variance is the second important source of variation in the Aedes group. It accounts for the unexplained variation due to other factors, mainly the natural heterogeneity in size that can be found in any pupal batch. The heterogeneity in size in a given batch (rearing container) has a strong effect on the performance. As an example, our simulations (Table 4) with the GSS strain predict that a 20 % increase in the standard deviation of the experimental value reduces the performance by about 17 %. Conversely, a reduction of the same magnitude produces an increase in male recovery of 7.6 %. The heterogeneity in size could be due to genetic and/or environmental factors [55, 56]. It is not expected that the genetic heterogeneity of laboratory populations has a major effect given that it has been drastically reduced by the colonization process [55]. On the other hand, it is known that intraspecific asymmetric density-dependent factors can increase the variability in size in other insects which have an aquatic larval developmental stage [57, 58]. Given that the mosquito larvae are usually kept at high densities in the artificial rearing containers, the heterogeneity in size could potentially be reduced by adjusting the larval density.
The variance between rearing containers is also an important factor to consider in SSD-sorting. In a real mass production context, there is variation in size between batches that is present in our experiments as well. This variation affected mainly the average size of the pupae, but also at some extent the absolute SSD magnitude (Table 5, Interaction term Batch:Sex). It has been reported that the food availability or the water pollution by conspecifics does not affect the absolute SSD magnitude in Aedes [38, 43] while larval competition could produce some degree of sexual allometry in size [40, 42]. The intraspecific variation in SSD is a complex issue [35–37] out of the scope of this article, but worth to be investigated in mosquitoes in the context of SSD-sorting methods. The variability between rearing containers is of major applied significance because the threshold of size needed to obtain a desired degree of purity is specific for each batch. The use of a common fixed threshold for all the production batches would produce a variable output in respect to purity and performance, and it is therefore not recommended (Table 4). In a mass production context, it can be sometimes useful to mix the pupae production of different rearing containers before the sex sorting, but this would likely increase the size heterogeneity. This is clearly shown in the simulations presented in Tables 3 and 4. For example, mixing the Ae. aegypti GSS pupae production from three rearing trays reduced the performance in about 17 % (with purity level of 99.5 %).
Two main strategies are presently used for SSD sorting methods. First, sieves [8], rows of slots [21] or openings between plates [22], which are based on fixed size thresholds, were developed through a trial and error process. This means that the purity and performance are not controlled and they entirely depend on the rearing conditions. On the other hand, plate separators [23], which rely on a visual adaptive size threshold election system, exhibit better performance [52] at the expense of productivity [21]. Both strategies can be optimized using the appropriate analytical tool. For the fixed threshold methods, a more optimal threshold based on the actual range of variation in size between batches of pupae may be required. The plate separator could be optimized by the determination of less subjective threshold election criteria. Finally, the analytical framework proposed here can be integrated in large scale mechanized sex-sorters of high precision.
Conclusions
The distribution of size in mosquito pupae can be modeled by a mixture of two Gaussian distribution functions. This approach, combined with the parameters obtained from laboratory samples, can be useful to understand and optimize the mechanisms of the SSD-sorting methods. Purity and performance, which are the most relevant features of sex sorting devices, can be directly calculated from the presented model. Two additional dimensionless parameters, SDI and SHI, which are good descriptors of the suitability of a species/strain under given rearing conditions for its sorting with SSD-based methods are proposed. This approach can be applied to all the SSD-sorting methods. The output of the SSD-sorting methods can be improved by reducing the heterogeneity in size within the rearing containers. The heterogeneity between batches can affect the quality of sex sorting when different batches are mixed before the sorting or when a common separation threshold is determined for a series of batches. For new designs of sex-sorting devices based on SSD, we recommend the following: (i) use of an adaptive and precise threshold selection method based on automatic measurement systems and the proposed formulas; and (ii) a specific threshold size for each batch to maintain the purity at a constant level. In this way, the heterogeneity in size will be resulting to a variable male recovery (performance). From the practical point of view, this study shows that enhanced SSD-based sex sorting methods can be applied to Aedes mosquito mass-rearing facilities that depend on lateral area to distinguish sexes to efficiently produce batches of male-only pupae with a male recovery ranging between 70% and 99% and female contamination under 0.5%, with the lower values of male recovery being obtained when different batches are mixed or when larval rearing conditions are not standardized.
Acknowledgements
We would like to thank the staff members of the Insect Pest Control Laboratory of the Joint FAO/IAEA Division, Antonios Augustinos, Nanwintoum S. Bimbile-Somda, Danilo Carvalho, Anna Konczal, Hamidou Maïga, Wadaka Mamai, Gulizar Pillwax and Asha Wijegunawardana, for their great support during the preparation of the samples used in this study. We are particularly grateful to Ángela Aguado and José Félix Descalzo for their essential assistance. We thank to Enrique Torrente and Jose Antonio Herrador their indispensable support to carry out this research. This study was supported by the International Atomic Energy research contact no. 17939 as part of the Coordinated Research Project “Exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes”. MZ acknowledges support from Universidad Católica de Valencia "San Vicente Mártir" (grant 2018-253-002)
Funding
This research was financially supported by GRUPO TRAGSA. Publication costs for this study was provided by the International Atomic Energy Agency as part of the Coordinated Research Project “Exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes”.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
About this supplement
This article has been published as part of Parasites & Vectors Volume 11 Supplement 2, 2018: Exploring genetic molecular, mechanical and behavioural methods of sex separation in mosquitoes. The full contents of the supplement are available online at https://parasitesandvectors.biomedcentral.com/articles/supplements/volume-11-supplement-2.
Abbreviations
- GSS
Genetic sexing strain
- IIT
Incompatible insect technique
- RIDL
Release of insects carrying a dominant lethal
- SDI
Sexual dimorphism index
- SHI
Sexual homoscedasticity index
- SIT
Sterile insect technique
- SSD
Sexual size dimorphism
Authors’ contributions
MZ, GS, DA, CT, RA, IP designed the study. HB and KB provided the strains. GS designed and built the acquisition data system based on artificial vision. DA and CT performed the data collection. DA and MZ analyzed the data and wrote the manuscript. MZ proposed the theoretical model for data analysis. GS, CT, RA, IP and KB contributed to the manuscript drafting. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mario Zacarés, Email: mario.zacares@ucv.es.
Gustavo Salvador-Herranz, Email: gsalva@uchceu.es.
David Almenar, Email: dalmenar@tragsa.es.
Carles Tur, Email: ctur@tragsa.es.
Rafael Argilés, Email: R.Argiles-Herrero@iaea.org.
Kostas Bourtzis, Email: K.Bourtzis@iaea.org.
Hervé Bossin, Email: hbossin@ilm.pf.
Ignacio Pla, Email: ipla@tragsa.es.
References
- 1.Lees RS, Gilles JR, Hendrichs J, Vreysen MJ, Bourtzis K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci. 2015;10:156–162. doi: 10.1016/j.cois.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/S1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 3.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, et al. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourtzis K, Lees RS, Hendrichs J, Vreysen MJB. More than one rabbit out of the hat: Radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 2016;157:115–130. doi: 10.1016/j.actatropica.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Dyck VA, Hendrichs J, Robinson AS. Sterile insect technique: principles and practice in area-wide integrated pest management. Dordrecht: Springer; 2006.
- 6.Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol. 2012;30:828–830. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- 7.Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- 8.Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol. 2013;50:317–325. doi: 10.1603/ME12048. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. 2015;9:e0003864. doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mains JW, Brelsfoard CL, Rose RI, Dobson SL. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci Rep. 2016;6:33846. doi: 10.1038/srep33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilles JRL, Schetelig MF, Scolari F, Marec F, Capurro ML, Franz G, et al. Towards mosquito sterile insect technique programmes: exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes. Acta Trop. 2014;132:S178–S187. doi: 10.1016/j.actatropica.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Papathanos PA, Bossin HC, Benedict MQ, Catteruccia F, Malcolm CA, Alphey L, et al. Sex separation strategies: past experience and new approaches. Malar J. 2009;8 Suppl 2:S5. doi: 10.1186/1475-2875-8-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caceres C. Mass rearing of temperature sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata). Genetica 2002;116:107–116. [DOI] [PubMed]
- 14.Robinson A, Franz G, Fisher K. Genetic sexing strains in the medfly, Ceratitis capitata: development, mass rearing and field application. Trends Entomol. 1999;2:81–104. [Google Scholar]
- 15.McCombs SD, Saul SH. Translocation-based genetic sexing system for the oriental fruit fly (Diptera: Tephritidae) based on pupal color dimorphism. Ann Entomol Soc Am. 1995;88:695–698. doi: 10.1093/aesa/88.5.695. [DOI] [Google Scholar]
- 16.Zepeda-Cisneros CS, Meza Hernández JS, García-Martínez V, Ibañez-Palacios J, Zacharopoulou A, Franz G. Development, genetic and cytogenetic analyses of genetic sexing strains of the Mexican fruit fly, Anastrepha ludens Loew (Diptera: Tephritidae) BMC Genet. 2014;15:S1. doi: 10.1186/1471-2156-15-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustinos AA, Targovska A, Cancio-Martinez E, Schorn E, Franz G, Cáceres C, et al. Ceratitis capitata genetic sexing strains: laboratory evaluation of strains from mass-rearing facilities worldwide. Entomol Exp Appl. 2017;164:305–317. doi: 10.1111/eea.12612. [DOI] [Google Scholar]
- 18.Yamada H, Benedict MQ, Malcolm CA, Oliva CF, Soliban SM, Gilles JR. Genetic sex separation of the malaria vector, Anopheles arabiensis, by exposing eggs to dieldrin. Malar J. 2012;11:208. doi: 10.1186/1475-2875-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada H, Jandric Z, Chhem-Kieth S, Vreysen MJB, Rathor MN, Gilles JRL, et al. Anopheles arabiensis egg treatment with dieldrin for sex separation leaves residues in male adult mosquitoes that can bioaccumulate in goldfish (Carassius auratus auratus) Environ Toxicol Chem. 2013;32:2786–2791. doi: 10.1002/etc.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho DO, Nimmo D, Naish N, McKemey AR, Gray P, Wilke ABB, et al. Mass production of genetically modified Aedes aegypti for field releases in Brazil. J Vis Exp JoVE. 2014. 10.3791/3579. [DOI] [PMC free article] [PubMed]
- 21.Mccray EMJ. A mechanical device for the rapid sexing of Aedes aegypti. J Econ Entomol. 1961;54:4–819.
- 22.Gerberg E, Hopkins TM, Gentry JW. others. Mass rearing of Culex pipiens L. Mosq News. 1969;29:382–385. [Google Scholar]
- 23.Focks DA. An improved separator for the developmental stages, sexes and species of mosquitoes (Diptera, Culicidae) J Med Entomol. 1980;17:567–568. doi: 10.1093/jmedent/17.6.567. [DOI] [PubMed] [Google Scholar]
- 24.Benedict MQ, Knols BG, Bossin HC, Howell PI, Mialhe E, Caceres C, et al. Colonisation and mass rearing: learning from others. Malar J. 2009;8:S4. doi: 10.1186/1475-2875-8-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinwascher K. Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environ Entomol. 1982;11:150–153. doi: 10.1093/ee/11.1.150. [DOI] [Google Scholar]
- 26.Armbruster P, Hutchinson RA. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) J Med Entomol. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- 27.Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus. J Vector Ecol. 2000;25:212–217. [PubMed] [Google Scholar]
- 28.Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008;79:688–695. doi: 10.4269/ajtmh.2008.79.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyimo EO, Koella JC. Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology. 1992;104:233–237. doi: 10.1017/S0031182000061667. [DOI] [PubMed] [Google Scholar]
- 30.Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos. Peru. J Med Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- 31.Virginio F, Oliveira Vidal P, Suesdek L. Wing sexual dimorphism of pathogen-vector culicids. Parasit Vectors. 2015;8:159. doi: 10.1186/s13071-015-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lardeux F, Tetuanui A. Larval growth of Aedes polynesiensis and Aedes aegypti (Diptera: Culicidae) Mosq Syst. 1995;27:118–124. [Google Scholar]
- 33.Gouws EJ, Gaston KJ, Chown SL. Intraspecific body size frequency distributions of insects. PLOS ONE. 2011;6:e16606. doi: 10.1371/journal.pone.0016606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamps J. Sexual size dimorphism in species with asymptotic growth after maturity. Biol J Linn Soc. 1993;50:123–145. doi: 10.1111/j.1095-8312.1993.tb00921.x. [DOI] [Google Scholar]
- 35.Fairbairn DJ, Blanckenhorn WU, Székely T. Sex, size and gender roles: evolutionary studies of sexual size dimorphism. New York: Oxford University Press; 2007.
- 36.Teder T, Tammaru T. Sexual size dimorphism within species increases with body size in insects. Oikos. 2005;108:321–334. doi: 10.1111/j.0030-1299.2005.13609.x. [DOI] [Google Scholar]
- 37.Blanckenhorn WU, Meier R, Teder T. Rensch’s rule in insects: patterns among and within species. In: Fairbairn, D Blanckenhorn W, Szekely T, editors. Sex, size and gender roles. Oxford: Oxford University Press; 2007. p. 60–70.
- 38.Wormington JD, Juliano SA. Sexually dimorphic body size and development time plasticity in Aedes mosquitoes (Diptera: Culicidae) Evol Ecol Res. 2014;16:223–234. [PMC free article] [PubMed] [Google Scholar]
- 39.Hapairai LK, Marie J, Sinkins SP, Bossin HC. Effect of temperature and larval density on Aedes polynesiensis (Diptera: Culicidae) laboratory rearing productivity and male characteristics. Acta Trop. 2014;132:S108–S115. doi: 10.1016/j.actatropica.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. J Evol Biol. 2003;16:721–730. doi: 10.1046/j.1420-9101.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 41.Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol Exp Appl. 1992;63:265–271. doi: 10.1111/j.1570-7458.1992.tb01583.x. [DOI] [Google Scholar]
- 42.Agnew P, Haussy C, Michalakis Y. Effects of density and larval competition on selected life history traits of Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2000;37:732–735. doi: 10.1603/0022-2585-37.5.732. [DOI] [PubMed] [Google Scholar]
- 43.Bédhomme S, Agnew P, Sidobre C, Michalakis Y. Pollution by conspecifics as a component of intraspecific competition among Aedes aegypti larvae. Ecol Entomol. 2005;30:1–7. doi: 10.1111/j.0307-6946.2005.00665.x. [DOI] [Google Scholar]
- 44.Yamada H, Parker A, Oliva C, Balestrino F, Gilles J. X-ray-induced sterility in Aedes albopictus (Diptera: Culicidae) and male longevity following irradiation. J Med Entomol. 2014;51:811–816. doi: 10.1603/ME13223. [DOI] [PubMed] [Google Scholar]
- 45.Zheng M-L, Zhang D-J, Damiens DD, Lees RS, Gilles JRL. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae) - II - Egg storage and hatching. Parasit Vectors. 2015;8:348. doi: 10.1186/s13071-015-0951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damiens D, Benedict MQ, Wille M, Gilles JRL. An inexpensive and effective larval diet for Anopheles arabiensis (Diptera: Culicidae): eat like a horse, a bird, or a fish? J Med Entomol. 2012;49:1001–1011. doi: 10.1603/ME11289. [DOI] [PubMed] [Google Scholar]
- 47.Soma DD, Maïga H, Mamai W, Bimbile-Somda NS, Venter N, Ali AB, et al. Does mosquito mass-rearing produce an inferior mosquito? Malar J. 2017;16:357. doi: 10.1186/s12936-017-2012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balestrino F, Puggioli A, Gilles JRL, Bellini R. Validation of a new larval rearing unit for Aedes albopictus (Diptera: Culicidae) mass rearing. PLOS ONE. 2014;9:e91914. doi: 10.1371/journal.pone.0091914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puggioli A, Balestrino F, Damiens D, Lees RS, Soliban SM, Madakacherry O, et al. Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2013;50:819–825. doi: 10.1603/ME13011. [DOI] [PubMed] [Google Scholar]
- 50.Szeliski R. Computer vision: algorithms and applications. London: Springer Science & Business Media; 2010.
- 51.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 52.Zhang D, Zhang M, Wu Y, Gilles JRL, Yamada H, Wu Z, et al. Establishment of a medium-scale mosquito facility: optimization of the larval mass-rearing unit for Aedes albopictus (Diptera: Culicidae) Parasit Vectors. 2017;10:569. doi: 10.1186/s13071-017-2511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brust RA. Weight and development time of different stadia of mosquitoes reared at various constant temperatures. Can Entomol. 1967;99:986–993. doi: 10.4039/Ent99986-9. [DOI] [Google Scholar]
- 54.Lofgren CS, Dame DA, Breeland SG, Weidhaas DE, Jeffery G, Kaiser R, et al. Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. Am J Trop Med Hyg. 1974;23:288–297. doi: 10.4269/ajtmh.1974.23.288. [DOI] [PubMed] [Google Scholar]
- 55.Schneider JR, Chadee DD, Mori A, Romero-Severson J, Severson DW. Heritability and adaptive phenotypic plasticity of adult body size in the mosquito Aedes aegypti with implications for dengue vector competence. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2011;11:11–16. doi: 10.1016/j.meegid.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehmann T, Dalton R, Kim EH, Dahl E, Diabate A, Dabire R, et al. Genetic contribution to variation in larval development time, adult size, and longevity of starved adults of Anopheles gambiae. Infect Genet Evol. 2006;6:410–416. doi: 10.1016/j.meegid.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Peckarsky BL, Cowan CA. Consequences of larval intraspecific competition to stonefly growth and fecundity. Oecologia. 1991;88:277–288. doi: 10.1007/BF00320823. [DOI] [PubMed] [Google Scholar]
- 58.Gribbin SD, Thompson DJ. Asymmetric intraspecific competition among larvae of the damselfly Ischnura elegans (Zygoptera: Coenagrionidae) Ecol Entomol. 1990;15:37–42. doi: 10.1111/j.1365-2311.1990.tb00781.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.