Abstract
Salmonella enterica serotype Heidelberg is primarily a poultry adapted serotype of Salmonella that can also colonize other hosts and cause human disease. In this study, we compared the genomes of outbreak associated non-outbreak causing Salmonella ser. Heidelberg strains from diverse hosts and geographical regions. Human outbreak associated strains in this study were from a 2015 multistate outbreak of Salmonella ser. Heidelberg involving 15 states in the United States which originated from bull calves. Our clinicopathologic examination revealed that cases involving Salmonella ser. Heidelberg strains were predominantly young, less than weeks-old, dairy calves. Pre-existing or concurrent disease was found in the majority of the calves. Detection of Salmonella ser. Heidelberg correlated with markedly increased death losses clinically comparable to those seen in herds infected with S. Dublin, a known serious pathogen of cattle. Whole genome based single nucleotide polymorphism based analysis revealed that these calf isolates formed a distinct cluster along with outbreak associated human isolates. The defining feature of the outbreak associated strains, when compared to older isolates of S. Heidelberg, is that all isolates in this cluster contained Saf fimbrial genes which are generally absent in S. Heidelberg. The acquisition of several single nucleotide polymorphisms and the gain of Saf fimbrial genes may have contributed to the increased disease severity of these Salmonella ser. Heidelberg strains.
Electronic supplementary material
The online version of this article (10.1186/s13099-018-0279-0) contains supplementary material, which is available to authorized users.
Keywords: Salmonella Heidelberg, Outbreak, Genomic epidemiology, SNP, Fimbrial gene, Adhesion, Invasiveness, Multidrug resistance
Introduction
Nontyphoidal Salmonella spp. is the leading cause of death and hospitalization due to acquired food borne illnesses in the United States [1]. Episodes of salmonellosis and the resulting annual economic burden is very high in the United States [2]. Livestock and food commodities serve as reservoirs for the disease causing nontyphoidal Salmonella spp. Some of these serovars are linked to animal derived food commodities while others are linked to plant derived food commodities [3]. Salmonella enterica serovar Heidelberg is one of the common invasive nontyphoidal Salmonella associated with greater risk for severe disease [4, 5]. Salmonella ser. Heidelberg is among the top ten disease causing Salmonella serovars in the United States and the third most frequently isolated serovar in Canada [6–9]. Outbreaks of this serovar are attributed mainly to animal derived food commodities with less diversity in isolation sources [3]. This serovar is commonly isolated from eggs and poultry [3, 10–13].
In recent years, the Center for Disease Control and Prevention (CDC) reported six multistate outbreaks caused by Salmonella ser. Heidelberg in the United States. According to the CDC reports, five of these outbreaks were traced back to either chicken or turkey related products as the source of infection [13–21]. However, a very recent multistate outbreak caused by this serovar has been linked to contact with calves as the source of infection [22]. Epidemiological and laboratory investigation by the CDC identified Salmonella ser. Heidelberg as the outbreak-causing serovar emerging from dairy calves in Wisconsin which infected people in 15 states between 2015 and 2017. As per the CDC surveillance reports, Salmonella ser. Heidelberg strains involved in this outbreak are multidrug resistant [22]. During this period, the animal health labs in South Dakota and Wisconsin isolated Salmonella ser. Heidelberg strains from cases that were causing clinical infections in calves and were possibly related to human outbreaks. This raises the concern of whether Salmonella ser. Heidelberg has evolved as a bovine adapted lineage with increased colonization and virulence. Previous epidemiological studies on Salmonella ser. Heidelberg have mostly focused on outbreaks associated with poultry products [23–27]. Most of these analyses were carried out with a limited number of isolates from a small geographical region with a limited number of strains. To overcome the limitations of those studies and to understand the genomic changes that would have contributed to the outbreak-causing bovine origin S. Heidelberg, we performed whole genome sequence based comparison of the genomes of the isolates from our labs against more than six hundred Salmonella ser. Heidelberg strains with diverse host origins and geographical locations. Our analysis includes newly sequenced Salmonella ser. Heidelberg genomes from outbreak related bovine strains from Wisconsin and South Dakota, and isolates from wild birds. Our whole genome based phylogenomic analysis revealed the presence of a highly divergent multidrug resistant cluster of Salmonella ser. Heidelberg isolates which have acquired host cell adhesion related virulence functions, subsequently allowing increased bovine colonization.
Results
Clinical presentation of calves with S. Heidelberg
In the summer of 2016, several Midwestern premises raising bottle-fed calves experienced striking losses in dairy bulls purchased from sale barns. Death rates, usually dictated by neonatal diarrhea which is very common in sale barn calves, increased 5–10-fold. Illness was subsequently found in dairy heifers as well. Disease was apparent even when best practices were used: it affected quality calves given space and comfort, on a good plane of nutrition, with no failure of passive transfer. Sporadic cases were also found dating back to 2014 in our database (see Table 1).
Table 1.
Features of outbreak associated Salmonella ser. Heidelberg strains
| Assigned name | Biosample ID | Isolation location | Year | Host | Tissue | Lesions | safABCD |
|---|---|---|---|---|---|---|---|
| USA293 | SAMN04240660 | USA:IA | 2015 | Bovine | Lung, LN, intestine, kidney | Enteritis; lymphadenitis | + |
| USA420 | SAMN06330648 | USA:NE | 2016 | Bovine | Fecals | No tissues | + |
| USA520 | SAMN03734290 | USA:SD | 2014 | Bovine | Intestine, LN, liver | Ileitis | + |
| USA521 | SAMN03734314 | USA:SD | 2014 | Bovine | Lung, liver, intestine | Enteritis; lymphadenitis | + |
| USA522 | SAMN05781470 | USA:SD | 2016 | Bovine | Intestine, LN, liver, kidney | Villous atrophy | + |
| USA523 | SAMN05781531 | USA:SD | 2016 | Bovine | Intestine, abomasum | Enteritis | + |
| USA524 | SAMN06330649 | USA:SD | 2016 | Bovine | Intestine | No lesions | + |
| USA526 | SAMN06648142 | USA:SD | 2016 | Bovine | Intestine | No lesions, autolysis | + |
| USA585 | SAMN08150382 | USA:WI | 2016 | Bovine | Intestine, spleen, lung | Bronchopneumonia 3/5; lymphoid depletion | + |
| USA586 | SAMN08150383 | USA:WI | 2016 | Bovine | Intestine, LN, lung, bile, liver | Fibrinonecrotic enteritis | + |
| USA587 | SAMN08150384 | USA:WI | 2016 | Bovine | Intestine, Lung, LN | Mild enteritis; lymphoid depletion | + |
| USA588 | SAMN08150385 | USA:MN | 2017 | Bovine | Intestine, LN, lung, abomasum | Enteritis; lymphadenitis | + |
| USA084 | SAMN06231053 | USA | 2016 | bovine | N/A* | N/A* | + |
| USA089 | SAMN05916660 | USA | 2016 | bovine | N/A* | N/A* | + |
| USA099 | SAMN06012648 | USA | 2016 | bovine | N/A* | N/A* | + |
| USA397 | SAMN05752003 | USA:MN | 2016 | bovine | Fecals | N/A* | + |
| USA460 | SAMN05710975 | USA:NY | 2016 | bovine | Intestine | N/A* | + |
| USA538 | SAMN05757192 | USA:TX | 2011 | bovine | Comminuted beef | N/A* | + |
| USA582 | SAMN05852396 | USA:WV | 2009 | bovine | Raw ground beef | N/A* | + |
| USA114 | SAMN06290379 | USA | 2016 | Environmental | Swab | N/A* | + |
| USA115 | SAMN06290384 | USA | 2016 | Environmental | Swab | N/A* | + |
| USA116 | SAMN06290387 | USA | 2016 | Environmental | Swab | N/A* | + |
| USA117 | SAMN06290388 | USA | 2016 | Environmental | Swab | N/A* | + |
| USA074 | SAMN05773458 | USA | 2015 | Human | Stool | N/A* | + |
| USA080 | SAMN05714563 | USA | 2015 | Human | Stool | N/A* | + |
| USA094 | SAMN05773457 | USA | 2016 | Human | Urine | N/A* | + |
| USA104 | SAMN06235037 | USA | 2016 | Human | Blood | N/A* | + |
| USA107 | SAMN07177558 | USA | 2016 | Human | Blood | N/A* | + |
| USA113 | SAMN06290389 | USA | 2016 | Human | Blood | N/A* | + |
| USA120 | SAMN06688282 | USA | 2017 | Human | Stool | N/A* | + |
| USA088 | SAMN05916659 | USA | 2016 | Other | Intestine | N/A* | + |
| USA303 | SAMN07611477 | USA:IL | 2017 | Porcine | Raw ground pork | N/A* | + |
A comparison of virulence factors safA, safB, safC and safD as well as pathologic findings between newly sequenced bovine isolates and other members of cluster bovine
N/A* data not available
Pathologic findings
In the majority of calves, gross and histologic changes were typical of neonatal calf diarrhea caused by rotavirus, coronavirus or Cryptosporidium parvum. Lesions consisted of dehydration with sunken eyes and sticky tissues; mild enteritis due to crypt necrosis or villous atrophy, with the agent of cryptosporidiosis in some cases; mild lymphocytic abomasitis due to irritation of the mucosa secondary to severe metabolic abnormalities (dehydration, metabolic acidosis, electrolyte imbalances); and mild lymphoid depletion in spleen, lymph nodes and Peyer’s patches. In a small number of calves there was severe disease, with typical lesions of enteric salmonellosis: necrosis with fibrin deposition in the superficial small intestinal mucosa, fibrin thrombi in submucosal vessels, and transmural enteritis; also, fibrin, degenerate neutrophils and bacterial emboli in mesenteric lymph nodes; few, randomly scattered foci of necrosis in liver, with bacterial colonies; and severe lymphoid depletion of Peyer’s patches.
Conclusion of clinicopathologic observations
Predominantly young, < 3-weeks-old dairy calves were the source of S. Heidelberg. Pre-existing or concurrent disease was found in 9 of 12 younger calves and 3 of 4 older calves, but detection of Salmonella ser. Heidelberg correlated with markedly increased death losses clinically, comparable to those seen in herds infected with S. Dublin, a known serious pathogen of cattle. Salmonellosis was suspected at autopsy or by histopathologic examination in 5 of 12 young calves, and in 0 of 4 older calves, highlighting the importance of bacterial isolation. Since no lesions of salmonellosis were found in older calves, a carrier state cannot be ruled out.
Single nucleotide polymorphism (SNP) based phylogenetic analysis of S. Heidelberg
To determine the possible genomic changes that would have contributed to the increased virulence and its relationship to the strains causing human infections, a reference based SNP analysis of 634 Salmonella ser. Heidelberg strains was performed with Salmonella ser. Heidelberg str. SL476 (NCBI accession: NC_011083.1) as a reference genome. This whole genome sequence based SNP comparison revealed 1025 variant positions across the study isolates. Phylogenetic arrangement of genomes in the increasing order of branch length showed clusters of genetically related strains (Fig. 1). Based on our analysis, a chicken isolate from the USA (USA242) with lesser variants and branch length was positioned phylogenetically higher from all other isolates followed by isolate USA488, indicating chicken as the possible evolutionary origin of Salmonella ser. Heidelberg strains in this outbreak. Recently sequenced wild avian strains by our group (USA 481, USA482, and USA 483), collected in the early 90s, were positioned in the top few clusters closely related to chicken and human isolates.
Fig. 1.
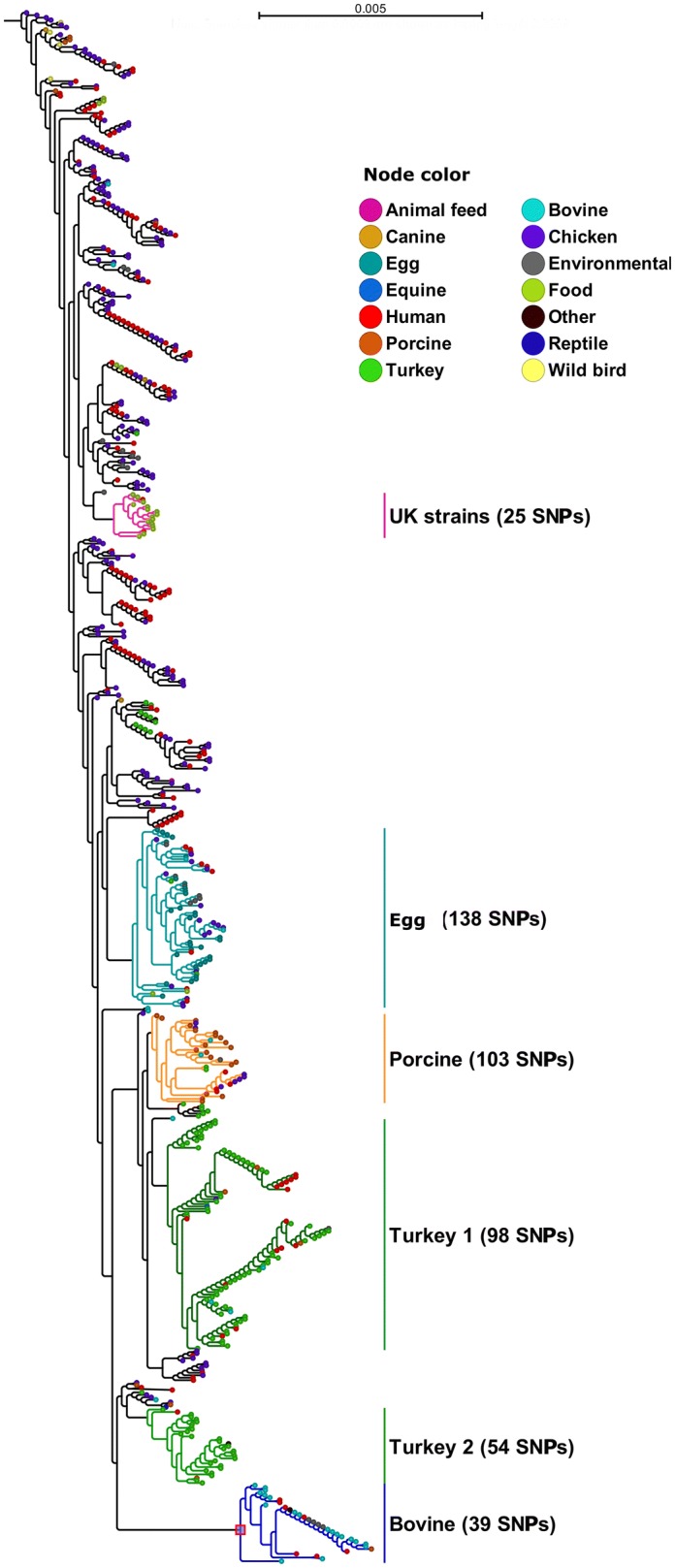
SNP based phylogram of 634 Salmonella ser. Heidelberg strains from various isolation sources and locations. Phylogenetic tree is arranged in the increasing order of branch length. The entire tree is distributed with a total of 1025 SNP positions. Branches of subtrees in which the major clusters were identified are shown in different colors corresponding to assigned cluster name. Values in bracket indicates the number of SNP positions residing in the corresponding cluster subtree. Cluster names were assigned based on either isolation location category or source country. Node color represents isolation source category of corresponding strain
As the branch length increased, we identified several major clusters in the SNP based phylogenetic analysis of our study isolates (Fig. 1). With increasing branch length, more clusters were differentiated mainly based on isolation sources. An exception was the cluster formed by around 80% of the isolates from the United Kingdom, which contained 25 SNPs that were unique to this cluster. Another interesting observation was that most of the egg isolates remained as a distinct clade having 138 SNPs unique to this cluster. Further down in the SNP tree, more than 50% of isolates from pigs formed a cluster which might be an indication of the expanding host colonization ability of Salmonella ser. Heidelberg strains. Two of the most divergent clusters, located at the bottom of Fig. 1 in our analysis, primarily originated from cattle, turkey and human cases. The bovine sub-cluster in this analysis was composed of 32 isolates and most were clonal. Defining features of these isolates is given in Table 1. Strain USA460, a bovine isolate, formed an outlier in this cluster indicating bovine origin of all isolates in this cluster. The bovine strains isolated from the animals believed to be originating from Wisconsin closely clustered with human and food isolates. For example, strains USA538 and USA582 originating from ground beef clustered closely with strains USA104 and USA107, which were isolated from human blood. Strains USA114, USA115, USA116 and USA117, environmental isolates from a livestock sale barn in Wisconsin, clustered in the middle of a clonal group of strains that were of human and bovine origin. The outgroup of this cluster was composed of strains that were isolated in the 1980s (USA 141, chicken isolate, 1987; USA128, porcine isolate, 1987) to recent years (Canada008, human isolate, 2012; Canada006, human isolate, 2012) and also from diverse hosts. Therefore, it appears that the strains related to the outbreak associated strains were present in a wider geographical region and a number of hosts other than cattle.
Virulence mapping of Salmonella ser. Heidelberg isolates
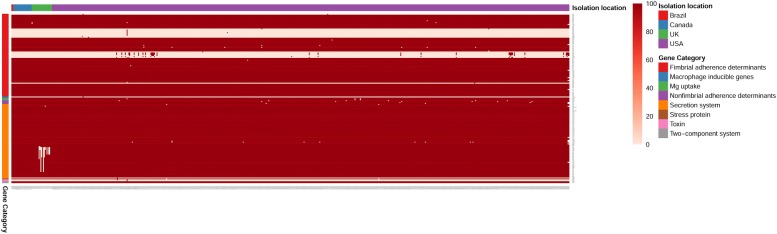
To understand the virulence repertoire of Salmonella ser. Heidelberg isolates, we carried out a virulence gene mapping of all 634 genomes. To predict the presence of virulence genes, we performed a local BLAST search of the genomes against virulence gene sequences obtained from the virulence factor database (VFDB) [28, 29]. We grouped 184 identified salmonella virulence genes into four groups (Fig. 2). As expected, the core set of Salmonella virulence genes were conserved among all isolates irrespective of isolation source and their geographical origin. However, isolates in the bovine cluster (Table 1) contained all the 4 fimbrial adherence determinants (safA, safB, safC and safD) of the Salmonella atypical fimbriae (Saf) gene cluster, while the full cluster was absent in all other isolates compared in this study. A detailed result of the virulence gene mapping across all genomes is given in Additional file 2: Tables 2A, B. Therefore, the virulence mapping of isolates revealed the main hallmark of outbreak associated strains was the presence of safABCD operon genes which were absent in all other isolates compared in this study.
Fig. 2.
Mapping of virulence determinants in S. Heidelberg. Heat map showing virulence factors identified in Salmonella ser. Heidelberg strains under this study. X axis represents sample name based on geographical origin of isolates. Y axis represents virulence gene names. Categories of virulence factors were grouped in to 5 groups. Group legend: 1. Fimbrial adherence determinants, 2. Macrophage inducible genes, Mg Uptake, and Non Fimbrial adherence determinants, 3. Secretion system, 4. Stress protein, Toxin, Two component system. A detailed description of predicted virulence data is available in Additional file 2: Table S2A, B. Color bar represents percentage sequence identity
Antimicrobial resistance gene profiling of Salmonella ser. Heidelberg isolates
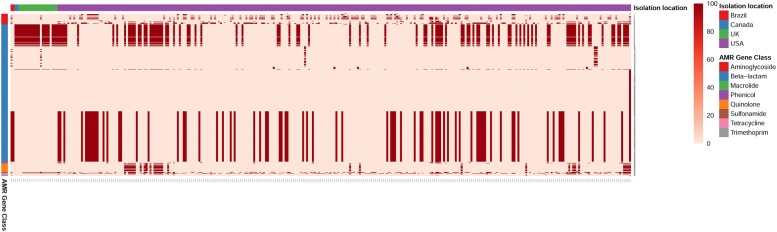
To determine the antibiotic resistance genotype among the Salmonella ser. Heidelberg isolates, we used a comparative genomic analysis approach. We performed a local BLAST search of all 634 of Salmonella ser. Heidelberg genomes against > 2000 antimicrobial resistance genes in the ResFinder database. A full list of resistance genes identified among these genomes with a minimum 95% sequence identity is given in Additional file 3: Tables 3A, B. Our results show that 50% of the study isolates (317/634) contained least one resistant gene (Fig. 3). Among isolates from chicken, 37% (75/202) were resistant while resistance was 83% (100/120) among turkey isolates. Highest resistance rate (93%) was identified among isolates of bovine origin. Among isolates of human origin, 30% percentage (47/154) contained at least one antibiotic resistance gene. One interesting observation was that, at 95% sequence identity, we could not predict any quinolone resistance genes in poultry isolates except in one turkey isolate (USA525) which was identified with genes qnrS2 and qnrS6. Comparison dataset in this analysis contained isolates dating back to 1980’s. When the resistance profiles of older isolates were compared to recent outbreak causing isolates, we find that the newer isolates contained higher number and type of resistance genes indicating acquisition of more antibiotic resistance among Salmonella ser. Heidelberg over these years.
Fig. 3.
Antimicrobial resistance (AMR) gene profiling of S. Heidelberg. Heat map showing antimicrobial resistance gene profile of 317 Salmonella ser. Heidelberg genomes that were predicted for at least one AMR gene. X axis represents sample name based on geographical origin of isolates. Y axis represents class of antimicrobial agents to which the resistance was predicted. A detailed description of predicted AMR is available in Additional file 3: Table S3A, B. Color bar represents percentage sequence identity
Discussion
Starting from January 2015, outbreaks involving Salmonella ser. Heidelberg were reported from 15 states in the United States. To date, 56 persons were diagnosed with Salmonella ser. Heidelberg infections in which 35% [17] were hospitalized and 15% [8] had invasive disease. People who became sick ranged in age from less than 1 year to 72, with a median age of 18, and 33% [18] were less than 5 years of age. Epidemiologic investigations demonstrated that 63% [34] of the infected people had contact with cattle, particularly recently purchased dairy and beef calves. Clinico-pathologic examination revealed pre-existing or concurrent disease in a majority of the calves. Detection of Salmonella ser. Heidelberg correlated with markedly increased death losses clinically, comparable to those seen in herds infected with Salmonella ser. Dublin, a known serious pathogen of cattle. Pathologic findings are intermediate between those caused by the host-adapted Salmonella ser. Dublin and disease due to Salmonella ser. Typhimurium, which has a broad host range [30]. Laboratory investigations, using polymorphism fragment gel electrophoresis (PFGE) and WGS, linked human isolates as being highly related to the bovine isolates [22]. The goal of this study was to determine the genomic changes that might have contributed to the virulence of Salmonella ser. Heidelberg strains infecting calves and people.
Whole genome based SNP clustering of human/bovine outbreak strains and other Salmonella ser. Heidelberg strains from different hosts and geographical regions revealed that human outbreak-associated strains formed a distinct cluster together with calf isolates (Fig. 1). This is not surprising since CDC outbreak investigations already have identified dairy and beef calves from livestock markets in Wisconsin as the likely source of most of the human infections [22]. However, some of the isolates in this cluster predates 2015, which is the beginning of the human outbreak. For example, strain USA520 was isolated from South Dakota in 2014. The WVDL and South Dakota Animal Disease Research and Diagnostic Laboratory have limited isolates prior 2014. The WVDL has an isolate dating back to 2009 that has the same MDR pattern, but the isolate was not available for sequencing. Therefore, it is possible that strains that contributed to this outbreak were present in the Midwest region before 2014.
The outgroup of the outbreak-specific cluster was composed of strains from multiple hosts including chicken, human, porcine, reptile and turkey (Fig. 1). This broad host range of isolations might be an indication that the SNP variations in this cluster may have allowed Salmonella ser. Heidelberg to better colonize hosts other than poultry. It is possible that these strains may have been the source for the human/bovine isolate causing the current outbreak.
When the virulence gene content was analyzed across all isolates, we find that most of the Salmonella virulence genes were present in a majority of the strains. However, the defining feature of the human/bovine outbreak-associated strains was that these isolates contained the presence of safABCD operon genes, which were absent in all other isolates (Table 1 and Fig. 2). Salmonella contain multiple fimbriae on their surface, many of which play a vital role in attachment with enterocytes to establish and maintain a successful infection. Salmonella atypical fimbriae (Saf) in Salmonella enterica is composed of four contiguous genes encoding the major subunit (safA), periplasmic chaperone (safB), outer membrane usher (safC) and minor subunit (safD) [31]. Saf operon is generally absent in Salmonella ser. Heidelberg [29] while it has been found important for pathogenesis in other serotypes such as Salmonella ser. Typhimurium [32]. Transcription of saf fimbrial genes has been found in blood samples from patients infected with Salmonella ser. Typhi [33] and Salmonella ser. Paratyphi A [34], which is indicative of a role for these genes in the human pathogenesis. Therefore, it is possible that the SNPs associated with the outbreak-causing cluster, together with the acquisition of Saf operon genes, could have contributed to the increased virulence of Salmonella ser. Heidelberg in both calves and people. However, further experimentation in animal models is required to verify this possibility.
Strains in our genomic comparison also included Salmonella ser. Heidelberg isolates dating back to the 1980s. The antibiotic resistance profile of the old isolates when compared to recent isolates revealed an overall increase in resistance in recent isolates (Fig. 3).
The resistance gene pattern and SNP based clustering did not show any specific correlation. This would indicate that while increased resistance could reduce the options for treatment, acquisition of SNPs in the core genome and virulence genes probably would have been the main driver in expansion of host range and increased infectivity of Salmonella ser. Heidelberg involved in the outbreak.
Materials and methods
Bacterial culture and genomic DNA isolation
Seventeen Salmonella ser. Heidelberg strains were isolated from cases submitted to the Animal Disease Research and Diagnostic Laboratory, South Dakota State University (ADRDL-SDSU) and Wisconsin Veterinary Diagnostic Laboratory (WVDL) per standard operating procedures. Pathological investigation was carried out following the guidelines set by the American Association of Veterinary Laboratory Diagnosticians. After Salmonella isolation and identification, strains were cultured overnight in Luria–Bertani broth. DNA was extracted from 1 mL of the overnight culture using Qiagen’s DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. Quality of DNA was assessed using Nanodrop One™ (Thermo scientific™, DE). Quantity of DNA was measured using Qubit® 3.0 (Thermo Fisher Scientific Inc., MA) fluorometer and stored at − 20 °C until further use.
Sequencing library preparation and genome sequencing
Sequencing library was prepared using Nextera XT kit (Illumina Inc. San Diego, CA). Concentration of the DNA samples were adjusted to 0.3 ng/µl prior to processing. After bead normalization, DNA libraries were pooled at equi-molar concentration. Pooled libraries were then sequenced on Illumina Miseq platform (Illumina Inc., CA) using V2 chemistry to generate 2× 250 paired end reads.
Genome data acquisition and comparative analysis
The raw data files for the seventeen isolates sequenced by our group were de-multiplexed and converted to FASTQ files using Casava v.1.8.2. (Illumina, Inc, San Diego, CA). For comparative analysis, we downloaded publicly available genome sequence data in Fastq format for 617 Salmonella ser. Heidelberg isolates from the National Center for Biotechnology Information Sequence Read Archive (NCBI-SRA) database using NCBI-SRA tool kit (version 2.8.1-2). To ensure a uniform analytic workflow, we considered only genomes sequenced using the Illumina platform. This constituted 634 genomes in total. Metadata for all genomes are given in Additional file 1: Table S1. Genome data was then imported into CLC Genomics work bench (version 9.5.3, Qiagen bioinformatics) and was further processed. Failed reads as well as reads having length below 200 and above 500 bases were removed. A quality based trimming, with a quality score limit of 0.05 as described by CLC Genomics workbench, was performed to ensure good quality sequence data without any ambiguous nucleotides. Trimmed reads were used for SNP based phylogenetic analysis while assembled genomes were used for virulence gene mapping and antimicrobial resistance gene profiling. For SNP analysis, we used quality trimmed reads mapped to reference genome Salmonella ser. Heidelberg str. SL476 (NCBI accession: NC_011083.1). This genome was selected as reference as it is a complete genome and has been used as reference for comparative genomics studies of Salmonella ser. Heidelberg previously [35–38]. For variant calls, fixed ploidy variant detection for bacterial genomes with a minimum 90% variant probability was used. Variant calls and read mappings results were then used to determine the SNP positions and to estimate the consensus sequence. Neighborhood joining method was used to create the phylogenetic tree composed of all 634 genomes. For virulence and antimicrobial gene mapping, we used local databases of Salmonella enterica virulence gene sequences available from Virulence Factor Database (VFDB) [28, 29] and ResFinder [39], respectively. Genome assemblies were then used for BLAST searching against these local gene databases with criteria of ≥ 95% minimum sequence identity and ≥ 50% minimum sequence length.
Additional files
Additional file 1: Table S1. Metadata for 634 Salmonella ser. Heidelberg strains used in this study. Genome sequence data accession and bio sample accession numbers were collected from the NCBI Sequence Read Archive (SRA) for all 634 strains. Assigned name indicates the name given to each strain based on the country from which they were isolated (source country). Isolation location indicates actual location in the source country from which the corresponding strain was isolated.
Additional file 2: Table S2A. List of virulence factors identified in Salmonella ser. Heidelberg isolates. Virulence factors identified in 634 Salmonella ser. Heidelberg strains when all genomes were BLAST searched the against Salmonella virulence gene sequences in Virulence Factor Database (VFDB). A) This table represents the tabular view of Fig. 2. Table shows a categorized view of identified virulence factors and the percentage sequence identity corresponding to each isolate. Coloring scheme is based on virulence categories. Values corresponding to each strain represent percentage sequence identity in BLAST search. Table S2B. A detailed BLAST search result of virulence genes identified in 634 Salmonella ser. Heidelberg genomes.
Additional file 3: Table S3A. List of antimicrobial resistance genes identified in Salmonella ser. Heidelberg isolates. Antimicrobial resistance genes identified in 634 Salmonella ser. Heidelberg isolates when all genomes were BLAST searched the against resistance gene sequences in ResFinder database. A) Table shows a categorized view of all the AMR genes predicted across 634 Salmonella ser. Heidelberg genomes. Sample names are given in first row. First and second column represents antimicrobial class and gene name respectively. Coloring is based on Class of antimicrobial agents. Values corresponding to each strain represent BLAST search percentage sequence identity. Table S3B. A detailed BLAST search result of AMR genes predicted in 634 Salmonella ser. Heidelberg genomes.
Authors’ contributions
JS and LA conceived and designed the study. LA performed the experiments and analyzed the data. MB, DS, NA, DM, MWA, JH and EN contributed materials and resources. JS, MB and LA wrote the manuscript with input from all authors. All authors read and approved the final manuscript.
Acknowledgements
We thank the Section of Bacteriology, Animal Disease Research and Diagnostic Laboratory, SD and the Bacteriology Section at the Wisconsin Veterinary Diagnostic Laboratory at the University of Wisconsin- Madison for the helping to collect bovine Salmonella ser. Heidelberg isolates. We also thank Dr. Milton Thomas for the help with sequencing the strains isolated by ADRDL, South Dakota.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Funding
This work was supported in part by the grants from the South Dakota Beef Industry Council (SDBIC) and South Dakota Governor’s Office of Economic Development awarded to JS, JH and EN, the USDA National Institute of Food and Agriculture Hatch projects SD00H532-14, SD00R540-15, and the United States Food and Drug Administration GenomeTrakr project subcontract awarded to JS.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.091101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandt CH, Fedorka-Cray PJ, Tewari D, Ostroff S, Joyce K, M’Ikanatha MN. A comparison of non-typhoidal Salmonella from humans and food animals using pulsed-field gel electrophoresis and antimicrobial susceptibility patterns. PLoS One. 2013;8(10):e77836. doi: 10.1371/journal.pone.0077836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. Outbreak-associated Salmonella enterica serotypes and food Commodities, United States, 1998–2008. Emerg Infect Dis. 2013;19(8):1239–1244. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump JA, Medalla FM, Joyce KW, Krueger AL, Hoekstra RM, Whichard JM, Barzilay EJ, Emerging Infections Program NARMS Working Group Emerging infections program: antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother. 2011;55(3):1148–1154. doi: 10.1128/aac.01333-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clothier KA, Byrne BA. Phenotypic and genotypic characterization of animal-source Salmonella Heidelberg isolates. J Vet Med. 2016;2016:6380890. doi: 10.1155/2016/6380890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.C. f. D. C. a. Prevention. National Antimicrobial Resistance Monitoring System (NARMS) 2014 Human Isolates Surveillance Report. 2014.
- 7.Andrysiak AK, Olson AB, Tracz DM, Dore K, Irwin R, Ng LK, Gilmour MW, C. Canadian Integrated Program for Antimicrobial Resistance Surveillance Genetic characterization of clinical and agri-food isolates of multi drug resistant Salmonella enterica serovar Heidelberg from Canada. BMC Microbiol. 2008;8:89. doi: 10.1186/1471-2180-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health Agency of Canada. National Enteric Surveillance Program (NESP) annual report. 2014. http://publications.gc.ca/collections/collection_2016/aspc-phac/HP37-15-2014-eng.pdf. Accessed June 2016.
- 9.Edirmanasinghe R, Finley R, Parmley EJ, Avery BP, Carson C, Bekal S, Golding G, Mulvey MR. A whole-genome sequencing approach to study cefoxitin-resistant Salmonella enterica serovar Heidelberg isolates from various sources. Antimicrob Agents Chemother. 2017;61(4):AAC-01919. doi: 10.1128/AAC.01919-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley SL, Lynne AM, Nayak R. Salmonella challenges: prevalence in swine and poultry and potential pathogenicity of such isolates. J Anim Sci. 2008;86(14 Suppl):E149–E162. doi: 10.2527/jas.2007-0464. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy TW, Cheng LH, Kassenborg H, Ahuja SD, Mohle-Boetani J, Marcus R, Shiferaw B, Angulo FJ, G. Emerging Infections Program FoodNet Working Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S237–S243. doi: 10.1086/381593. [DOI] [PubMed] [Google Scholar]
- 12.Chittick P, Sulka A, Tauxe RV, Fry AM. A summary of national reports of foodborne outbreaks of Salmonella Heidelberg infections in the United States: clues for disease prevention. J Food Prot. 2006;69(5):1150–1153. doi: 10.4315/0362-028X-69.5.1150. [DOI] [PubMed] [Google Scholar]
- 13.Folster JP, Pecic G, Rickert R, Taylor J, Zhao S, Fedorka-Cray PJ, Whichard J, McDermott P. Characterization of multidrug-resistant Salmonella enterica serovar Heidelberg from a ground Turkey-associated outbreak in the United States in 2011. Antimicrob Agents Chemother. 2012;56(6):3465–3466. doi: 10.1128/Aac.00201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinnell M, Provo G, Marsden-Haug N, Stigi KA, DeBess E, Kissler B, Crarey E, Tate H, Pringle J, Grass J, Folster J, Williams I, Gieraltowski L, Laufer AS. Outbreak of Salmonella Heidelberg infections linked to a single poultry producer-13 states, 2012–2013. Mmwr Morb Mortal Wkly Rep. 2013;62(27):553–556. [PMC free article] [PubMed] [Google Scholar]
- 15.Gieraltowski L, Higa J, Peralta V, Green A, Schwensohn C, Rosen H, Libby T, Kissler B, Marsden-Haug N, Booth H, Kimura A, Grass J, Bicknese A, Tolar B, Defibaugh-Chavez S, Williams I, Wise M, S. H. I. Team National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. Plos One. 2016;11(9):e0162369. doi: 10.1371/journal.pone.0162369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M, Zhao SH, Luo Y, Li C, Folster JP, Whichard J, Allard MW, Brown EW, McDermott PF. Genome sequences of five Salmonella enterica serovar Heidelberg isolates associated with a 2011 multistate outbreak in the United States. J Bacteriol. 2012;194(12):3274–3275. doi: 10.1128/Jb.00419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention: Multistate outbreak of human Salmonella Heidelberg infections linked to ground turkey. 2011. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/2011/ground-turkey-11-10-2011.html. Accessed 10 Nov 2011.
- 18.Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella Heidelberg infections linked to “Kosher Broiled Chicken Livers” from Schreiber Processing Corporation (Final Update). 2012. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/2011/chicken-liver-1-11-2012.html. Accessed 11 Jan 2012.
- 19.Centers for Disease Control and Prevention. Multistate outbreak of multidrug-resistant Salmonella Heidelberg infections linked to foster farms brand chicken (Final Update). 2014. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/Heidelberg-10-13/index.html. Accessed 31 July 2014.
- 20.Centers for Disease Control and Prevention. Multistate outbreak of Salmonella Heidelberg infections linked to chicken (Final Update). 2013. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/Heidelberg-02-13/index.html. Accessed 10 July 2013.
- 21.Centers for Disease Control and Prevention. Outbreak of Salmonella Heidelberg infections linked to tyson brand mechanically separated chicken at a correctional facility (Final Update). 2014. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/Heidelberg-01-14/index.html. Accessed 24 Feb 2014.
- 22.Centers for Disease Control and Prevention. Multistate outbreak of multidrug-resistant Salmonella Heidelberg infections linked to contact with dairy calves. 2017. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella/Heidelberg-11-16/index.html. Accessed 16 Feb 2018.
- 23.Routh JA, Pringle J, Mohr M, Bidol S, Arends K, Adams-Cameron M, Hancock WT, Kissler B, Rickert R, Folster J, Tolar B, Bosch S, Barton Behravesh C, Williams IT, Gieraltowski L. Nationwide outbreak of multidrug-resistant Salmonella Heidelberg infections associated with ground turkey: United States, 2011. Epidemiol Infect. 2015;143(15):3227–3234. doi: 10.1017/s0950268815000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease and Prevention Outbreak of Salmonella Heidelberg infections linked to a single poultry producer—13 states, 2012-2013. MMWR Morb Mortal Wkly Rep. 2013;62(27):553–556. [PMC free article] [PubMed] [Google Scholar]
- 25.Rebolledo J, Garvey P, Ryan A, O’Donnell J, Cormican M, Jackson S, Cloak F, Cullen L, Swaan CM, Schimmer B, Appels RW, Nygard K, Finley R, Sreenivasan N, Lenglet A, Gossner C, McKeown P. International outbreak investigation of Salmonella Heidelberg associated with in-flight catering. Epidemiol Infect. 2014;142(4):833–842. doi: 10.1017/S0950268813001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gieraltowski L, Higa J, Peralta V, Green A, Schwensohn C, Rosen H, Libby T, Kissler B, Marsden-Haug N, Booth H, Kimura A, Grass J, Bicknese A, Tolar B, Defibaugh-Chavez S, Williams I, Wise M, T. Salmonella Heidelberg Investigation National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS ONE. 2016;11(9):e0162369. doi: 10.1371/journal.pone.0162369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor AL, Murphree R, Ingram LA, Garman K, Solomon D, Coffey E, Walker D, Rogers M, Marder E, Bottomley M, Woron A, Thomas L, Roberts S, Hardin H, Arjmandi P, Green A, Simmons L, Cornell A, Dunn J. Multidrug-resistant Salmonella Heidelberg associated with mechanically separated chicken at a correctional facility. Foodborne Pathog Dis. 2015;12(12):950–952. doi: 10.1089/fpd.2015.2008. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Yang J, Yu J, Yao Z, Sun L, Shen Y, Jin Q. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33(Database issue):D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Zheng D, Liu B, Yang J, Jin Q. VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016;44(D1):D694–D697. doi: 10.1093/nar/gkv1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maxie G. Jubb Kennedy & Palmer’s pathology of domestic animals. Philadelphia: Saunders Ltd; 2016. [Google Scholar]
- 31.Zeng L, Zhang L, Wang P, Meng G. Structural basis of host recognition and biofilm formation by Salmonella Saf pili. Elife. 2017;6:e28619. doi: 10.7554/elife.28619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laniewski P, Baek CH, Roland KL, Curtiss R. Analysis of spleen-induced fimbria production in recombinant attenuated Salmonella enterica serovar Typhimurium vaccine strains. MBio. 2017 doi: 10.1128/mbio.01189-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhuiyan S, Sayeed A, Khanam F, Leung DT, Rahman Bhuiyan T, Sheikh A, Salma U, LaRocque RC, Harris JB, Pacek M, Calderwood SB, LaBaer J, Ryan ET, Qadri F, Charles RC. Cellular and cytokine responses to Salmonella enterica serotype Typhi proteins in patients with typhoid fever in Bangladesh. Am J Trop Med Hyg. 2014;90(6):1024–1030. doi: 10.4269/ajtmh.13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheikh A, Charles RC, Rollins SM, Harris JB, Bhuiyan MS, Khanam F, Bukka A, Kalsy A, Porwollik S, Brooks WA, LaRocque RC, Hohmann EL, Cravioto A, Logvinenko T, Calderwood SB, McClelland M, Graham JE, Qadri F, Ryan ET. Analysis of Salmonella enterica serotype paratyphi A gene expression in the blood of bacteremic patients in Bangladesh. PLoS Negl Trop Dis. 2010;4(12):e908. doi: 10.1371/journal.pntd.0000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fricke WF, Mammel MK, McDermott PF, Tartera C, White DG, Leclerc JE, Ravel J, Cebula TA. Comparative genomics of 28 Salmonella enterica isolates: evidence for CRISPR-mediated adaptive sublineage evolution. J Bacteriol. 2011;193(14):3556–3568. doi: 10.1128/JB.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann M, Zhao S, Pettengill J, Luo Y, Monday SR, Abbott J, Ayers SL, Cinar HN, Muruvanda T, Li C, Allard MW, Whichard J, Meng J, Brown EW, McDermott PF. Comparative genomic analysis and virulence differences in closely related Salmonella enterica serotype Heidelberg isolates from humans, retail meats, and animals. Genome Biol Evol. 2014;6(5):1046–1068. doi: 10.1093/gbe/evu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labbe G, Edirmanasinghe R, Ziebell K, Nash JH, Bekal S, Parmley EJ, Mulvey MR, Johnson RP. Complete genome and plasmid sequences of three Canadian isolates of Salmonella enterica subsp. enterica Serovar Heidelberg from human and food sources. Genome Announc. 2016 doi: 10.1128/genomea.01526-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timme RE, Pettengill JB, Allard MW, Strain E, Barrangou R, Wehnes C, Van Kessel JS, Karns JS, Musser SM, Brown EW. Phylogenetic diversity of the enteric pathogen Salmonella enterica subsp. enterica Inferred from genome-wide reference-free SNP characters. Genome Biol Evol. 2013;5(11):2109–2123. doi: 10.1093/gbe/evt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Metadata for 634 Salmonella ser. Heidelberg strains used in this study. Genome sequence data accession and bio sample accession numbers were collected from the NCBI Sequence Read Archive (SRA) for all 634 strains. Assigned name indicates the name given to each strain based on the country from which they were isolated (source country). Isolation location indicates actual location in the source country from which the corresponding strain was isolated.
Additional file 2: Table S2A. List of virulence factors identified in Salmonella ser. Heidelberg isolates. Virulence factors identified in 634 Salmonella ser. Heidelberg strains when all genomes were BLAST searched the against Salmonella virulence gene sequences in Virulence Factor Database (VFDB). A) This table represents the tabular view of Fig. 2. Table shows a categorized view of identified virulence factors and the percentage sequence identity corresponding to each isolate. Coloring scheme is based on virulence categories. Values corresponding to each strain represent percentage sequence identity in BLAST search. Table S2B. A detailed BLAST search result of virulence genes identified in 634 Salmonella ser. Heidelberg genomes.
Additional file 3: Table S3A. List of antimicrobial resistance genes identified in Salmonella ser. Heidelberg isolates. Antimicrobial resistance genes identified in 634 Salmonella ser. Heidelberg isolates when all genomes were BLAST searched the against resistance gene sequences in ResFinder database. A) Table shows a categorized view of all the AMR genes predicted across 634 Salmonella ser. Heidelberg genomes. Sample names are given in first row. First and second column represents antimicrobial class and gene name respectively. Coloring is based on Class of antimicrobial agents. Values corresponding to each strain represent BLAST search percentage sequence identity. Table S3B. A detailed BLAST search result of AMR genes predicted in 634 Salmonella ser. Heidelberg genomes.