Abstract
There is a growing need for more accurate biomarkers to facilitate the diagnosis and prognosis of patients with grade (G) 3 neuroendocrine carcinomas (NECs). In particular, the discrimination between well-differentiated neuroendocrine carcinomas (WD-NECs) and poorly differentiated neuroendocrine carcinomas (PD-NECs) is still an unmet need. We previously showed that 68Gallium-(68Ga-) PET/CT positivity is a prognostic factor in patients with gastroenteropancreatic (GEP) G3 NECs, correlating with a better outcome in terms of overall survival. Here, we hypothesize that 68Ga-PET/CT could help to discriminate between WD-NECs and PD-NECs, adding complementary information to that obtained from morphologic and biologic factors. A retrospective, single-institution study was performed on 11 patients with histologically confirmed, measurable G3 large- or small-cell GEP-NECs according to the 2017 WHO classification. The staging procedures included a 68Ga-PET/CT scan. Results of 68Ga-PET/CT were correlated in univariate analysis with loss of tissue immunohistochemical expression of DAXX/ATRX or RB1 frequently associated with WD-NECs or PD-NECs, respectively. None of the patients with positive 68Ga-PET/CT showed loss of RB1 expression, whereas among those (n = 6) with negative 68Ga-PET/CT, 4 showed loss of expression. A trend towards a correlation between loss of RB1 expression and negative 68Ga-PET/CT was observed. Our preliminary data support the hypothesis that PD-NECs carrying RB1 mutation and loss of its expression may be associated with negative 68Ga-PET/CT. If confirmed in a larger clinical trial, 68Ga-PET/CT would help in the stratification of G3 NECs.
1. Introduction
Poorly differentiated grade 3 (PD G3) gastroenteropancreatic (GEP) neuroendocrine carcinomas (NECs) are very rare malignancies that represent only 5%-10% of all neuroendocrine neoplasms (NENs) [1, 2]. These tumors are characterized by aggressive histological features such as high Ki67 index, extensive necrosis, and nuclear atypia [2]. At the time of diagnosis, patients are generally in poor conditions, with aggressive and diffuse disease [3, 4]. Due to the rarity of NECs, few dedicated prospective clinical or biological trials have been conducted. Furthermore, current NEC grading shows a number of controversies and discrepancies that highlight the need for more accurate biomarkers [5–9]. The revised 2010 World Health Organization (WHO) classification identified all GEP neuroendocrine tumors with Ki67 > 20% as grade 3 NECs [10]. Recent studies have shown that these tumors might actually include 2 heterogeneous subgroups with a different pathogenesis: well-differentiated neuroendocrine carcinomas (WD-NECs) characterized by mutations in MEN1, DAXX, and ATRX genes and poorly differentiated neuroendocrine carcinomas (PD-NECs) characterized by p53 and RB1 mutations probably derived from the neuroendocrine differentiation of adenocarcinomas [8, 11, 12]. There is evidence that these 2 subgroups also have a distinct prognosis and show different sensitivities to chemotherapy [3, 13]. A subdivision of tumors with Ki67 > 20% into G3 WD-NETs or G3 PD-NECs was proposed in the 2017 WHO classification for neuroendocrine neoplasms of pancreatic origin [14], leading to the identification of a new category comprising WD tumor morphology and Ki67 index > 20%, referred to as G3 pNETs. According to this classification, tumor grading is based on histopathologic morphology and on the assessment of the Ki67 index [15]. However, distinguishing G3 NETs from G3 NECs is often problematic due to the lack of well-defined histological criteria and differences in Ki67 assessment [16]. Moreover, the classification of NECs of different sites of origin has yet to be revised. According to international guidelines, the identification and evaluation of novel biomarkers is warranted. Tang et al. [8] reported that the 2 subgroups show a different positivity to 18F-fluorodeoxyglucose positron emission tomography/computerized tomography (18F-FDG-PET/CT) or octreoscan. In a recent study, we showed that 68Gallium- (68Ga-) PET/CT was a discriminating factor for patients with G3 GEP-NECs treated with first-line platinum-based chemotherapy. Patients with a positive 68Ga-PET/CT scan had a better outcome than those with a negative 68Ga-PET/CT (75% vs. 34.3% overall survival at 18 months, respectively) [17]. The identification of specific metabolic characteristics may be particularly useful when histological material is not available, and imaging studies could add complementary information to that obtained from morphologic and biologic factors. 68Gallium directly binds to somatostatin receptors (SSTRs) which are often overexpressed in the cell membrane of NENs, especially in WD tumors [18]. We hypothesized that 68Ga-PET/CT, reflecting the degree of neuroendocrine differentiation [19, 20], could help to distinguish between WD-NECs and PD-NECs. We conducted a preliminary study to assess whether 68Ga-PET/CT correlates with the specific mutations identified in the 2 subgroups, DAXX and ATRX for WD-NECs and RB1 for PD-NECs. Given that mutations in these genes are closely correlated with loss of immunolabeling [21, 22], we evaluated the tissue immunohistochemical expression of DAXX, ATRX, and RB1 in 11 patients with G3 GEP-NECs. We then compared the expression of these markers with results of 68Ga-PET/CT to look for potential correlations with metabolic parameters that could help to discriminate between WD-NECs and PD-NECs.
2. Materials and Methods
2.1. Study Design
We retrospectively evaluated 11 patients seen at our institute (IRST IRCCS, Meldola) between April 2010 and May 2018. The patients were required to have histologically confirmed, measurable G3 large- or small-cell GEP-origin NECs. All cases were revised by an expert pathologist and divided into poorly differentiated G3 NECs or well-differentiated G3 NETs according to the 2017 WHO classification for pancreatic NENs, as reported by Sorbye et al. [23]. Patients with mixed tumors were excluded. The study was reviewed and approved by IRST IRCCS Medical Scientific Committee and Ethics Committee. Staging procedures performed included physical examination, brain-chest-abdominal CT, and 68Ga- and FDG-PET/CT.
2.2. Immunohistochemical Analysis
Paraffin-embedded surgical or biopsy specimens of G3 neuroendocrine tumors were sliced with a rotating microtome (Leica Biosystems, Wetzlar, Germany) into 5 μM thick sections and mounted on SuperFrost Plus microslides (Thermo Fisher Scientific, Waltman, MA, USA). Immunolabeling reactions were carried out on a VENTANA BenchMark XT (Ventana Medical Systems Inc., Tucson, AZ, USA) automated slide strainer. The following antibodies were used according to the manufacturer's instructions: DAXX (HPA008736) (Sigma-Aldrich, St. Louis, MO, USA) 1 : 75, one hour at room temperature (RT); ATRX (HPA001906) (Sigma-Aldrich) 1 : 400, one hour at RT; and RB1 (Cell Signaling Technology, Beverly, Massachusetts, USA) 1 : 1000, one hour at RT. The stained sections were analyzed in blind by an expert pathologist in neuroendocrine neoplasms.
2.3. Imaging with 68Ga-Labeled Somatostatin Analogs
68Ga-labeled somatostatin analogs are generally short peptides linked to the positron emitter 68Ga by a bifunctional chelate, normally 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). 68Ga-DOTA peptides bind to SSTRs, in particular SSTR3 and SSTR5, both of which are usually overexpressed in neuroendocrine cells [24]. There are 3 main 68Ga-DOTA-peptides currently available for imaging procedures on the basis of their affinity for SSTR subtypes. We used 68Ga-DOTA-Phe1-Tyr3-octreotide (TOC), which has a high affinity for SSTR2 and SSTR5 [25].
2.4. Statistical Analysis
Continuous variables were expressed as mean and standard deviation (SD), while categorical variables were expressed as frequency. Fisher's exact test was used to evaluate the relationship between categorical variables. Median overall survival (OS) was estimated as an exploratory research objective using the Kaplan-Meier method (two-sided 95% confidence intervals (CIs)). Reported P values <0.05 were used as a threshold for significance. Statistical analyses were carried out with STATA/MP 10.1 for Windows (StataCorp LP, College Station, TX, USA).
3. Results
3.1. Clinical Features
The main clinical and histological characteristics of the 11 patients analyzed in this study are shown in Table 1. Six patients (54.5%) were males and 5 (45.5%) were females. Mean age at the time of diagnosis was 56.6 years (SD ± 13.1). The site of the primary tumor was pancreas in 5 patients (45.6%), stomach in 3 (27.2%), and colorectum in 3 (27.2%). Six patients (54.5%) had well-differentiated G3 NETs and 5 (45.5%) poorly differentiated NECs. Patients received first-line chemotherapy with platinum compounds and etoposide (8 with cisplatin and 2 with carboplatin). Of the 11 patients, 5 (45.6%) showed a partial response (PR), 3 (27.2%) stable disease (SD), and 3 (27.2%) progressive disease (PD). Median follow-up was 32 months (range 5-86). Median OS was 23 months (95% CI: 7-70). No difference in survival was observed in relation to gender or age (data not shown). Four patients had a positive 68Ga-PET/CT and 6 a negative 68Ga-PET/CT, while the 68Ga-PET/CT or octreoscan referral for the remaining patient was not available.
Table 1.
Clinical and histological characteristics of NEC patient samples.
| n (%) | |
|---|---|
| Age at diagnosis (years) | |
| Mean | 56.6 |
| Standard deviation | 13.1 |
| Gender | |
| Male | 6 (54.5) |
| Female | 5 (45.5) |
| Site of disease | |
| Stomach | 3 (27.2) |
| Colorectum | 3 (27.2) |
| Pancreas | 5 (45.6) |
| Histological classification | |
| G3 NET | 6 (54.5) |
| G3 NEC | 5 (45.5) |
| FDG-PET/CT | |
| Positive | 8 (72.7) |
| Not done | 3 (27.3) |
| 68Ga-PET/CT octreoscan | |
| Positive | 4 (36.4) |
| Negative | 6 (54.5) |
| Not done | 1 (9.1) |
| Chemotherapy | |
| CDDP | 8 (72.7) |
| Carboplatin | 2 (18.1) |
| Other | 1 (9.2) |
| Best response to first-line chemotherapy | |
| PD | 3 (27.2) |
| SD | 3 (27.2) |
| PR | 5 (45.6) |
| Median overall survival, months [range] | 23 [6-70] |
NEC: neuroendocrine carcinoma; NET: neuroendocrine tumor; G: grade; FDG-PET/CT: fluorodeoxyglucose-positron emission tomography/computerized tomography; 68Ga: Gallium-68; CDDP: cisplatin; PD: progressive disease; SD: stable disease; PR: partial response.
3.2. DAXX, ATRX, and RB1 Immunohistochemical Expression
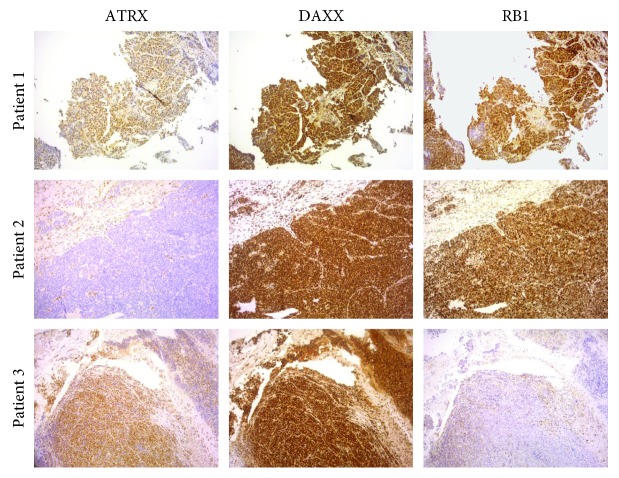
Expression of DAXX, ATRX, and RB1 in G3 neuroendocrine tumor tissue is shown in Table 2. All markers showed a strong nuclear localization, and stromal cells were used as an internal positive control for immunostaining (Figure 1). It was not possible to evaluate the expression of the three biomarkers in 2 patients due to insufficient bioptic material. DAXX was expressed in 100% of neuroendocrine tumor tissue, and no patient showed loss of IHC expression of this marker. ATRX was expressed in 66.7% of neuroendocrine tumor tissues, and 3 (33.3%) patients showed a loss of expression. Interestingly, all patients with loss of ATRX expression had NECs of gastrointestinal origin. DAXX and ATRX mutations are mutually exclusive. RB1 was expressed in 44.5% of neuroendocrine tumor tissue, and 5 (55.5%) patients showed a loss of expression. Of these, one had pancreatic NEC and 4 had gastrointestinal NECs.
Table 2.
Immunohistochemical expression of ATRX, DAXX, and RB1.
| Total (%) | Pancreatic (%) | GI (%) | |
|---|---|---|---|
| ATRX | |||
| Positive | 6 (66.7) | 3 (100.0) | 3 (50.0) |
| Negative | 3 (33.3) | 0 (0.0) | 3 (50.0) |
| Not evaluated | 2 | 2 | — |
| DAXX | |||
| Positive | 9 (100) | 3 (100.0) | 6 (100.0) |
| Negative | 0 (0) | 0 (0.0) | 0 (0.0) |
| Not evaluated | 2 | 2 | — |
| ATRX + DAXX | |||
| Positive | 6 (66.7) | 3 (100.0) | 3 (50.0) |
| Negative | 3 (33.3) | 0 (0.0) | 3 (50.0) |
| Not evaluated | 2 | 2 | — |
| RB1 | |||
| Positive | 4 (44.5) | 2 (66.7) | 2 (33.3) |
| Negative | 5 (55.5) | 1 (33.3) | 4 (66.7) |
| Not evaluated | 2 | 2 | — |
GI: gastrointestinal.
Figure 1.
NEC tissue immunostained for ATRX, DAXX, and RB1. Patient 1 showed positive immunostaining of all 3 markers. Patient 2 showed positive expression of DAXX and RB1 and loss of ATRX expression. Patient 3 showed positive expression of ATRX and DAXX and loss of RB1 expression. Magnification ×10.
3.3. Correlation between DAXX/ATRX and RB1 Expression and 68Ga-PET/CT
The correlation between DAXX/ATRX or RB1 expression and 68Ga-PET/CT scan is reported in Table 3. Bioptic material was not evaluable in 2 patients with a positive 68Ga-PET/CT. The other 2 68Ga-PET/CT-positive patients showed expression of ATRX/DAXX. Of the 6 patients with negative 68Ga-PET/CT, 4 showed ATRX/DAXX expression and 2 patients showed a loss of expression. With regard to RB1, patients with positive 68Ga-PET/CT showed expression of this marker. Among those with negative 68Ga-PET/CT, 2 showed RB1 expression and 4 patients a loss of expression.
Table 3.
Correlation between ATRX + DAXX and RB1 expression and 68Ga-PET/CT or octreoscan positivity.
| 68Ga-PET/CT or octreoscan results | |||||||
|---|---|---|---|---|---|---|---|
| Overall | Pancreatic | GI | |||||
| Negative (%) | Positive (%) | P value∗ | Negative (%) | Positive (%) | Negative (%) | Positive (%) | |
| Overall | 6 (66.7) | 2 (33.3) | 1 (33.3) | 2 (66.7) | 5 (100.0) | 0 (0.0) | |
| ATRX + DAXX | |||||||
| Negative | 2 (33.3) | 0 (0.0) | 0.536 | 0 (0.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) |
| Positive | 4 (66.7) | 2 (100.0) | 1 (100.0) | 2 (100.0) | 3 (60.0) | 0 (0.0) | |
| RB1 | |||||||
| Negative | 4 (66.7) | 0 (0.0) | 0.214 | 1 (100.0) | 0 (0.0) | 3 (60.0) | 0 (0.0) |
| Positive | 2 (33.3) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 2 (40.0) | 0 (0.0) | |
GI: gastrointestinal. ∗P-value was calculated on the overall number of patients.
3.4. Correlation between Histological Grading and 68Ga-PET/CT
The correlation between histological grading and 68Ga-PET/CT scan is reported in Table 4. Of the 4 patients with positive 68Ga-PET/CT, 3 had G3 NETs and one had G3 NEC. Of the 6 patients with negative 68Ga-PET/CT, 2 had G3 NETs and 4 had G3 NECs.
Table 4.
Correlation between histological classification and 68Ga-PET/CT or octreoscan positivity.
| 68Ga-PET/CT or octreoscan results | |||||||
|---|---|---|---|---|---|---|---|
| Overall | P value∗ | Pancreatic | GI | ||||
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | Negative (%) | Positive (%) | ||
| Overall | 6 (60.0) | 4 (40.0) | 1 (20.0) | 4 (80.0) | 5 (100.0) | 0 (0.0) | |
| Histological classification | |||||||
| NET G3 | 2 (33.3) | 3 (75.0) | 0.524 | 0 (0.0) | 3 (75.0) | 2 (40.0) | 0 (0.0) |
| NEC G3 | 4 (66.7) | 1 (25.0) | 1 (100.0) | 1 (25.0) | 3 (60.0) | 0 (0.0) | |
NET: neuroendocrine tumor; NEC: neuroendocrine carcinoma; GI: gastrointestinal.
3.5. Correlation between ATRX/DAXX and RB1 Expression, 68Ga-PET/CT, and Histological Grading and Best Response to First-Line Chemotherapy
The correlation between ATRX/DAXX or RB1 expression and best response to first-line chemotherapy is shown in Table 5. Of the 6 patients showing ATRX/DAXX expression, 3 had PR or SD and 3 PD. All 3 patients showing a loss of ATRX/DAXX expression had PR or SD, which is consistent with the less aggressive behavior of WD tumors. Of the 4 patients showing RB1 expression, 2 had PR or SD and 2 showed PD. Of the 5 patients with loss of RB1, 4 showed PR or SD and one PD. The correlation between 68Ga-PET/CT and best response to first-line chemotherapy is reported in Table 6. Of the 6 patients with negative 68Ga-PET/CT, 4 had PR or SD and 2 had PD. Of the 4 patients with positive 68Ga-PET/CT, 3 showed PR or SD and one had PD. The correlation between histological classification and best response to first-line chemotherapy is reported in Table 7. Of the 6 patients with G3 NETs, 4 had PR or SD and 2 showed PD. Of the 5 with G3 NECs, 4 had PR or SD and one had PD.
Table 5.
Correlation between ATRX + DAXX and RB1 expression and best response to first-line chemotherapy.
| Best response | |||||||
|---|---|---|---|---|---|---|---|
| Overall | P value∗ | Pancreatic | GI | ||||
| PD (%) | SD + PR (%) | PD (%) | SD + PR (%) | PD (%) | SD + PR (%) | ||
| Overall | 3 (33.3) | 6 (66.7) | 1 (33.3) | 2 (66.7) | 2 (33.3) | 4 (66.7) | |
| ATRX + DAXX | |||||||
| Negative | 0 (0.0) | 3 (50.0) | 0.464 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) |
| Positive | 3 (100.0) | 3 (50.0) | 1 (100.0) | 2 (100.0) | 2 (100.0) | 1 (25.0) | |
| RB1 | |||||||
| Negative | 1 (33.3) | 4 (66.7) | 0.524 | 0 (0.0) | 1 (50.0) | 1 (50.0) | 3 (75.0) |
| Positive | 2 (66.7) | 2 (33.3) | 1 (100.0) | 1 (50.0) | 1 (50.0) | 1 (25.0) | |
PD: progressive disease; SD: stable disease; PR: partial response; GI: gastrointestinal. ∗P-value was calculated on the overall number of patients.
Table 6.
Correlation between 68Ga-PET/CT or octreoscan and best response to first-line chemotherapy.
| Best response | |||||||
|---|---|---|---|---|---|---|---|
| Overall | P value∗ | Pancreatic | GI | ||||
| PD (%) | SD + PR (%) | PD (%) | SD + PR (%) | PD (%) | SD + PR (%) | ||
| Overall | 3 (30.0) | 7 (70.0) | 1 (20.0) | 4 (80.0) | 2 (40.0) | 3 (60.0) | |
| 68Ga-PET/CT or octreoscan | |||||||
| Negative | 2 (66.7) | 4 (57.1) | 0.667 | 0 (0.0) | 1 (25.0) | 2 (100.0) | 3 (100.0) |
| Positive | 1 (33.3) | 3 (42.9) | 1 (100.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | |
PD: progressive disease; SD: stable disease; PR: partial response; GI: gastrointestinal. ∗P value was calculated on the overall number of patients.
Table 7.
Correlation between histological classification and best response to first-line chemotherapy.
| Best response | |||||||
|---|---|---|---|---|---|---|---|
| Overall | P value∗ | Pancreatic | GI | ||||
| PD (%) | SD + PR (%) | PD (%) | SD + PR (%) | PD (%) | SD + PR (%) | ||
| Overall | 3 (27.3) | 8 (72.7) | 1 (20.0) | 4 (80.0) | 2 (33.3) | 4 (66.7) | |
| Histological classification | |||||||
| NET G3 | 2 (66.7) | 4 (50.0) | 1.000 | 1 (100.0) | 2 (50.0) | 1 (50.0) | 2 (50.0) |
| NEC G3 | 1 (33.3) | 4 (50.0) | 0 (0.0) | 2 (50.0) | 1 (50.0) | 2 (50.0) | |
PD: progressive disease: SD: stable disease; PR: partial response; GI: gastrointestinal; NET: neuroendocrine tumor; NEC: neuroendocrine carcinoma. ∗P-value was calculated on the overall number of patients.
3.6. PFS and OS according to DAXX/ATRX or RB1 Expression
The median PFS (mPFS) and OS (mOS) of the different subgroups on the basis of DAXX/ATRX and RB1 expression is shown in Table 8. mPFS was 6 months in the group with DAXX/ATRX-negative tumors and 3 months in those with DAXX/ATRX-positive disease. mPFS was 7 months in patients with RB1-negative tumors and 3 months in those with RB1-positive disease. mOS was 6 months in the group with DAXX/ATRX-negative tumors and 11 months in those with DAXX/ATRX-positive disease. mOS was 11 months in the group with RB1-negative tumors and 6 months for those with RB1 positivity.
Table 8.
PFS and OS according to ATRX + DAXX and RB1 expression.
| ATRX + DAXX | ||
| Negative (n = 3) | Positive (n = 6) | |
| Median PFS (95% CI) (months) | 6 (6-NE) | 3 (2-NE) |
| Median OS (95% CI) (months) | 6 (5-NE) | 11 (5-NE) |
|
| ||
| RB1 | ||
| Negative (n = 5) | Positive (n = 4) | |
| Median PFS (95% CI) (months) | 7 (2-NE) | 3 (3-NE) |
| Median OS (95% CI) (months) | 11 (6-NE) | 6 (5-NE) |
PFS: progression-free survival; OS: overall survival; CI: confidence interval; NE: not estimated.
4. Discussion
The updated 2017 WHO tumor classification divided pancreatic neuroendocrine carcinomas into G3 NETs characterized by Ki67 > 20% and a well-differentiated morphology, or G3 NECs characterized by Ki67%>20% and the absence of a low-grade component [14]. Given the lack of objective and well-defined histological criteria and consensus on Ki67 evaluation, more accurate biomarkers are needed. The authors of the NORDIC NEC study showed that G3 NEC patients with Ki67 > 55% were more sensitive to platinum-based chemotherapy but had poorer survival [3]. However, the study did not consider histopathological characteristics. Other trials have evaluated the diagnostic, prognostic, or predictive value of different biological markers such as serum plasma [26] and tissue [27] markers. Metabolic analysis plays an important role in the management of NEC patients in terms of diagnosis, staging, and therapeutic decision-making [28, 29]. In particular, 68Ga-PET/CT imaging provides information on SSTR expression [30], while 18F-FDG-PET/CT defines tumor metabolic status [31]. Although 18F-FDG-PET/CT has shown limited value in WD-NETs as they seldom show alterations in glucose turnover [32], the technique has emerged as a promising marker of aggressiveness and metastasis.
In the present study, we evaluated the potential correlation between 68Ga-PET/CT and loss of expression of tissue biomarkers specific for WD-NECs and PD-NECs in an attempt to define the value of 68Ga-PET/CT in discriminating between the 2 subgroups. In particular, we observed that none of the 68Ga-PET/CT-positive patients showed loss of RB1 expression, whereas among those with negative 68Ga-PET/CT, 4 (66.7%) showed loss of expression of RB1 and 2 (33.3%) normal expression. A trend towards a correlation between negative 68Ga-PET/CT and loss of RB1 expression emerged. Moreover, there was good agreement between 68Ga-PET/CT results and histological classification according to the 2017 WHO classification. Specifically, of the 4 patients with positive 68Ga-PET/CT, 3 (75.5%) had G3 NETs, while of the 6 patients with negative 68Ga-PET/CT, 4 (66.7%) had G3 NECs. These preliminary data support the hypothesis that negative 68Ga-PET/CT, reflecting a lower degree of neuroendocrine differentiation [19, 20], may be associated with the PD-NEC subgroup that frequently harbors an RB1 mutation and loss of its expression [8]. If confirmed in larger clinical trials, 68Ga-PET/CT could provide important complementary information to facilitate G3 NEC stratification. Given that NEC patients often present with metastatic disease and that histological material may thus not be available, metabolic imaging could substitute histological analysis in such cases. Further research is also needed to assess the impact of the proposed stratification on the definition of disease outcome in terms of PFS, OS, and response to therapy. The use of imaging analysis for the grading and prognosis assessment of NEN patients has been investigated by other groups. In a recent study by Zhao et al., pharmacokinetic parameters of dynamic contrast-enhanced magnetic resonance imaging were found to be predictive of NET grading, helping to distinguish between G1 and G2 tumors [33]. Another study reported that CT texture analysis and CT features were predictive of pancreatic NET aggressiveness and could be used to identify patients at risk of early disease progression after surgical resection [34]. We thus believe that in-depth research is warranted to investigate the role of radiologic and metabolic imaging as diagnostic, prognostic, or predictive tools in NEN patients.
In conclusion, the results from the present study show the potential value of investigating 68Ga-PET/CT as a marker to distinguish between WD-NETs and PD-NECs. Confirmation of our findings in larger case series, ideally in multicenter and prospective settings, would help to better define NEC patient prognosis and predict response to treatment.
Acknowledgments
The authors thank Gráinne Tierney and Cristiano Verna for editorial assistance.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Authors' Contributions
CL, AB, and TI designed the study; CL, AB, LM, FP, ADV, CS, GMi, FR, NR, SN, GMa, and SS acquired and analyzed all clinical and biological data; CL, AB, and TI performed all of the experiments and interpreted the results; FF carried out the statistical analyses; CL and AB drafted the manuscript; all authors approved the final version of manuscript for submission. Chiara Liverani and Alberto Bongiovanni contributed equally to this work.
References
- 1.Nilsson O., van Cutsem E., Delle Fave G., et al. Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic) Neuroendocrinology. 2007;84(3):212–215. doi: 10.1159/000098013. [DOI] [PubMed] [Google Scholar]
- 2.Inzani F., Petrone G., Fadda G., Rindi G. Cyto-histology in NET: what is necessary today and what is the future? Reviews in Endocrine and Metabolic Disorders. 2017;18(4):381–391. doi: 10.1007/s11154-017-9428-x. [DOI] [PubMed] [Google Scholar]
- 3.Sorbye H., Welin S., Langer S. W., et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Annals of Oncology. 2013;24(1):152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 4.Halfdanarson T. R., Rabe K. G., Rubin J., Petersen G. M. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annals of Oncology. 2008;19(10):1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vélayoudom-Céphise F.-L., Duvillard P., Foucan L., et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocrine-Related Cancer. 2013;20(5):649–657. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 6.Agaimy A., Erlenbach-Wünsch K., Konukiewitz B., et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Modern Pathology. 2013;26(7):995–1003. doi: 10.1038/modpathol.2013.40. [DOI] [PubMed] [Google Scholar]
- 7.Basturk O., Yang Z., Tang L. H., et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. The American Journal of Surgical Pathology. 2015;39(5):683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang L. H., Untch B. R., Reidy D. L., et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clinical Cancer Research. 2016;22(4):1011–1017. doi: 10.1158/1078-0432.CCR-15-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milione M., Maisonneuve P., Spada F., et al. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2016;104(1):85–93. doi: 10.1159/000445165. [DOI] [PubMed] [Google Scholar]
- 10.Rindi G., Klimstra D. S., Arnold R., Klöppel G., Bosman F. T., Komminoth P. WHO Classification of Tumours of the Digestive System. 4th. Lyon, France: WHO; 2010. Nomenclature and classification of neuroendocrine neoplasms of the digestive system WHO classification of tumours of the digestive system. [Google Scholar]
- 11.Jiao Y., Shi C., Edil B. H., et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yachida S., Vakiani E., White C. M., et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. The American Journal of Surgical Pathology. 2012;36(2):173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorbye H., Strosberg J., Baudin E., Klimstra D. S., Yao J. C. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120(18):2814–2823. doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd R. V., Osamura R. Y., Klöppel G., Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th. Lyon, France: IARC; 2017. [Google Scholar]
- 15.Kim J. Y., Hong S. M., Ro J. Y. Recent updates on grading and classification of neuroendocrine tumors. Annals of Diagnostic Pathology. 2017;29:11–16. doi: 10.1016/j.anndiagpath.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Tang L. H., Basturk O., Sue J. J., Klimstra D. S. A practical approach to the classification of WHO Grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. The American Journal of Surgical Pathology. 2016;40(9):1192–1202. doi: 10.1097/PAS.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bongiovanni A., Riva N., Ricci M., et al. First-line chemotherapy in patients with metastatic gastroenteropancreatic neuroendocrine carcinoma. OncoTargets and Therapy. 2015;2015(8):3613–3619. doi: 10.2147/OTT.S91971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cakir M., Dworakowska D., Grossman A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: part 1–molecular pathways. Journal of Cellular and Molecular Medicine. 2010;14(11):2570–2584. doi: 10.1111/j.1582-4934.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman M. S., Lau W. F. E., Hicks R. J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35(2):500–516. doi: 10.1148/rg.352140164. [DOI] [PubMed] [Google Scholar]
- 20.Mojtahedi A., Thamake S., Tworowska I., Ranganathan D., Delpassand E. S. The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. American Journal of Nuclear Medicine and Molecular Imaging. 2014;4(5):426–434. [PMC free article] [PubMed] [Google Scholar]
- 21.Tan H. L., Sood A., Rahimi H. A., et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clinical Cancer Research. 2014;20(4):890–903. doi: 10.1158/1078-0432.CCR-13-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wilde R. F., Heaphy C. M., Maitra A., et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Modern Pathology. 2012;25(7):1033–1039. doi: 10.1038/modpathol.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorbye H., Baudin E., Perren A. The problem of high-grade gastroenteropancreatic neuroendocrine neoplasms: well-differentiated neuroendocrine tumors, neuroendocrine carcinomas, and beyond. Endocrinology and Metabolism Clinics of North America. 2018;47(3):683–698. doi: 10.1016/j.ecl.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Cescato R., Schulz S., Waser B., et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. Journal of Nuclear Medicine. 2006;47(3):502–511. [PubMed] [Google Scholar]
- 25.Antunes P., Ginj M., Zhang H., et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? European Journal of Nuclear Medicine and Molecular Imaging. 2007;34(7):982–993. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 26.Freis P., Graillot E., Rousset P., et al. Prognostic factors in neuroendocrine carcinoma: biological markers are more useful than histomorphological markers. Scientific Reports. 2017;7(1, article 40609) doi: 10.1038/srep40609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalcanti E., Armentano R., Valentini A. M., Chieppa M., Caruso M. L. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death & Disease. 2017;8(8, article e3004) doi: 10.1038/cddis.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodei L., Sundin A., Kidd M., Prasad V., Modlin I. M. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology. 2015;101(1):1–17. doi: 10.1159/000367850. [DOI] [PubMed] [Google Scholar]
- 29.Adams S., Baum R., Rink T., Schumm-Dräger P. M., Usadel K. H., Hör G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. European Journal of Nuclear Medicine. 1998;25(1):79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 30.Cimitan M., Buonadonna A., Cannizzaro R., et al. Somatostatin receptor scintigraphy versus chromogranin A assay in the management of patients with neuroendocrine tumors of different types: clinical role. Annals of Oncology. 2003;14(7):1135–1141. doi: 10.1093/annonc/mdg279. [DOI] [PubMed] [Google Scholar]
- 31.Kayani I., Bomanji J. B., Groves A., et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112(11):2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 32.Sahani D. V., Bonaffini P. A., Fernández–del Castillo C., Blake M. A. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013;266(1):38–61. doi: 10.1148/radiol.12112512. [DOI] [PubMed] [Google Scholar]
- 33.Zhao W., Quan Z., Huang X., et al. Grading of pancreatic neuroendocrine neoplasms using pharmacokinetic parameters derived from dynamic contrast-enhanced MRI. Oncology Letters. 2018;15(6):8349–8356. doi: 10.3892/ol.2018.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canellas R., Burk K. S., Parakh A., Sahani D. V. Prediction of pancreatic neuroendocrine tumor grade based on CT features and texture analysis. American Journal of Roentgenology. 2018;210(2):341–346. doi: 10.2214/AJR.17.18417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.