Abstract
Objective
The pathophysiologic mechanism of how thyroid function is related to the development and prognosis of acute myocardial infarction (AMI) remains under explored, and there has been a lack of clinical investigations. In this study, we investigate the relationship between triiodothyronine (T3) level and cardiac ejection fraction (EF) as well as probrain natriuretic peptide (NT-proBNP) on admission and subsequent prognosis in AMI patients.
Methods
We measured admission thyroid function, NT-proBNP, and EF by echocardiography in 345 patients diagnosed with AMI. Simple and multiregression analyses were performed to investigate the correlation between T3 level and EF as well as NT-proBNP. Major adverse cardiovascular events (MACE), including new-onset myocardial infarction, acute heart failure, and cardiac death, were documented during the follow-up. 248 participants were separated into three groups based on T3 and free triiodothyronine (FT3) levels for survival analysis during a 2-year follow-up.
Results
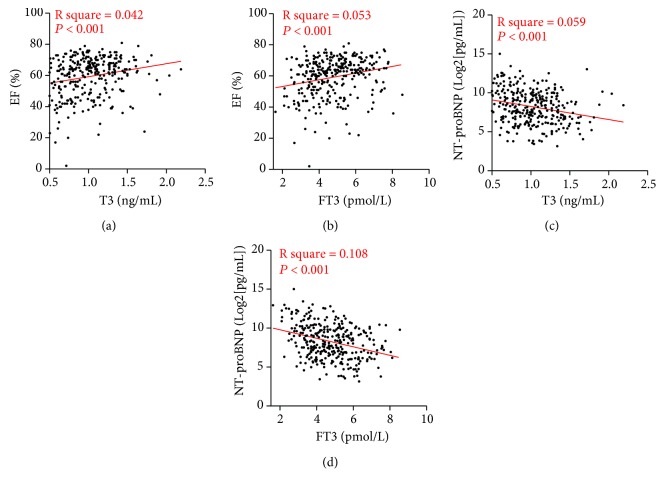
345 patients diagnosed with AMI were included in the initial observational analysis. 248 AMI patients were included in the follow-up survival analysis. The T3 levels were found to be significantly positively correlated with EF (R square = 0.042, P < 0.001) and negatively correlated with admission NT-proBNP levels (R square = 0.059, P < 0.001), which is the same with the correlation between FT3 and EF (R square = 0.053, P < 0.001) and admission NT-proBNP levels (R square = 0.108, P < 0.001). Kaplan-Meier survival analysis revealed no significant difference with regard to different T3 or FT3 levels at the end of follow-up.
Conclusions
T3 and FT3 levels are moderately positively correlated with cardiac function on admission in AMI patients but did not predict a long-time survival rate. Further studies are needed to explain whether longer-term follow-up would further identify the prognosis effect of T3 on MACE and all-cause mortality.
1. Introduction
Acute myocardial infarction (AMI) is one of the leading health-threatening diseases worldwide [1, 2]. Although it has been well known that atherosclerosis contributes to the pathogenesis of AMI [3, 4], metabolism disorders including deregulated thyroid function have also been reported to play direct and indirect effects in the heart and vasculature lesions [5–7]. However, the pathophysiologic mechanism of how thyroid function is related to the development and prognosis of myocardial infarction remains under explored, and there has been a lack of clinical investigations.
Biologically active thyroid hormones include thyroxine (T4) and triiodothyronine (T3) [8, 9]. It has been reported that thyroid hormone activity in the cardiomyocyte regulates myocardial contractility, diastolic, and systolic function. Moreover, thyroid hormones also exert profound effects on the heart and on cardiovascular hemodynamics, maintaining cardiac mass and wall stress while preserving ellipsoid left ventricular geometry [10, 11].
While T4 is solely a product of the thyroid gland, T3 is a product of the thyroid and of many other tissues. Low T3 level has been proven to be correlated with myocardial damage in animal models [12] and is associated with a poor cardiovascular prognosis in human [11, 13, 14]. Previous studies have indicated that subclinical hypothyroidism could be a predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure [14–16], and low T3 has been correlated with worse hospital outcomes in patients with acute HF [11, 14]. However, most of the studies focused on the function of T3 in patients with heart failure, while there has been a lack of evidence with regard to patients with acute myocardial infarction.
In this prospective cohort study, we investigated the relationship between triiodothyronine (T3) level and cardiac ejection fraction (EF) as well as N-terminal fragment of probrain natriuretic peptide (NT-proBNP) on admission. We then carried out survival analysis to investigate the prognostic value of T3 and FT3 level on long-term mortality and morbidity in AMI patients.
2. Research Design and Methods
2.1. Study Design and Participants
This was a single-centre prospective cohort study. Consecutive patients admitted to the cardiology department of the First Affiliated Hospital of Xi'an Jiaotong University for AMI between January 2013 and December 2016 were selected. The inclusion criteria and exclusion criteria were as previously described [17]. AMI were defined based on the universal definition criteria by the American Cardiology College [18].
2.2. Assessment of Thyroid Function, Cardiac EF, and NT-proBNP
Blood thyroid function of all patients was measured by Immulite 2000 (Bio DPC, Los Angeles, USA) within 24 h of admission after overnight fasting. The reference intervals of our laboratory were as follows: T3 0.78–2.20 ng/mL, T4 4.2–13.5 μg/dL, FT3 2.91–9.08 pmol/L, FT4 9.05–25.5 pmol/L, TSH 0.25–5 mIU/mL, TGAB < 30%, and TMAB < 20%. The patients were further divided into 3 groups, respectively, according to tertiles of T3 and FT3. NT-proBNP levels of all patients were measured using immunoassay (Elecsys® ProBNP, Roche Diagnostics, Indianapolis, IN, USA) upon initial admission without fasting, and the reference interval was 0–125 pg/mL. Because NT-proBNP values were not normally distributed, NT-proBNP levels were log-transformed to the base 2, and analyses were reported per doubling of NT-proBNP. Echocardiographs were performed on admission by experienced echo cardiologists, and systolic function was expressed as the ejection fraction (EF), which was calculated using Simpson's method.
2.3. Statistical Analysis
All statistical analyses were performed by using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL). Data were presented as frequencies and percentages for categorical variables and mean ± SD for continuous variables unless otherwise indicated. One-way ANOVA was used to compare continuous variables. Simple linear analysis was used for calculating correlation between T3 level and EF, T3 level and NT-proBNP, FT3 level and EF, and FT3 level and NT-proBNP, respectively. To ascertain the independent contribution to EF and NT-proBNP, multivariate regression analysis was conducted. Kaplan-Meier survival curve analysis was used to represent the proportional risk of all-cause mortality and MACE for the admission T3 and FT3 values in AMI patients. Patients were divided into three groups based on tertiles of T3 and FT3 levels. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
From January 2013 till December 2016, a total of 2054 patients were enrolled in the study; 345 patients, including 139 STEMI and 206 non-STEMI patients, provided consent for full screen and were included in the initial observational analysis, while 248 patients were included in the follow-up survival analysis (Figure 1). All of the patients have received coronary angiography, and 95.36% patients had coronary intervention. Patients were divided into three groups based on tertiles of T3 and FT3 levels for the survival analysis. Baseline patients' characteristics are shown in Table 1 based on the T3 levels (0.500–0.860 ng/mL, 0.862–1.140 ng/mL, and 1.150–2.190 ng/mL) and in Table 2 based on the FT3 levels (1.62–3.98 pmol/L, 3.99–5.33 pmol/L, and 5.34–8.55 pmol/L). The mean age was 58.766 ± 10.313, 59.477 ± 10.039, and 59.725 ± 10.237 years in respective T3 groups and was 59.625 ± 10.468, 58.351 ± 10.107, and 59.694 ± 10.490 in FT3 groups. No significant difference in risk factors at baseline was seen in different T3 and FT3 groups except for aspartate transaminase (AST), EF, NT-proBNP, and thyroid hormones.
Figure 1.
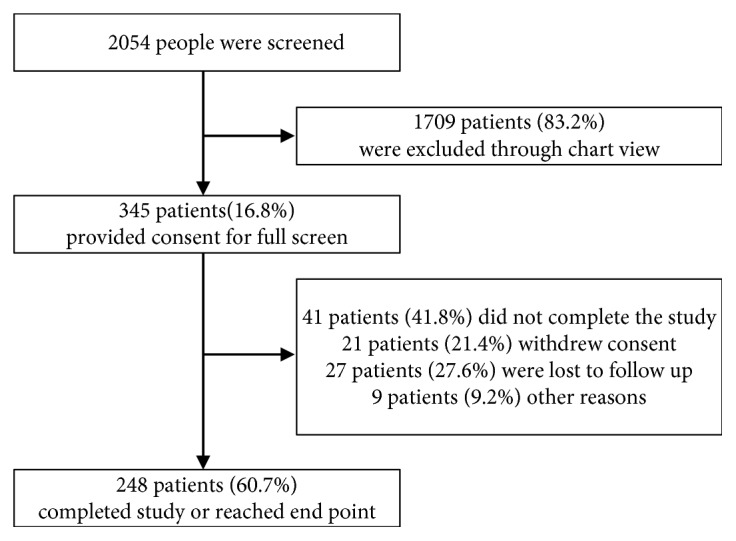
Enrolment and outcomes.
Table 1.
Basic characteristics based on T3 levels in AMI patients.
| Triiodothyronine levels (ng/mL) | P value | |||
|---|---|---|---|---|
| 0.500–0.860 | 0.862–1.140 | 1.150–2.190 | ||
| Female (%) | 22.02% | 24.77% | 24.77% | |
| GRACE score | 112.252 ± 42.084 | 127.159 ± 40.671 | 129.294 ± 38.054 | <0.01 |
| Age (y) | 58.766 ± 10.313 | 59.477 ± 10.039 | 59.725 ± 10.237 | 0.860 |
| HR (bpm) | 70.355 ± 21.222 | 71.336 ± 16.876 | 70.706 ± 14.019 | 0.933 |
| Onset of AMI (h) | 20.077 ± 13.130 | 13.538 ± 16.033 | 18.385 ± 30.321 | 0.602 |
| CKMB (U/L) | 33.371 ± 58.571 | 48.333 ± 70.930 | 41.862 ± 60.303 | 0.210 |
| HbA1C (%) | 5.748 ± 0.340 | 5.744 ± 0.361 | 5.718 ± 0.345 | 0.453 |
| sBP (mmHg) | 124.542 ± 21.078 | 126.453 ± 16.723 | 125.303 ± 14.810 | <0.05 |
| dBP (mmHg) | 79.075 ± 13.482 | 78.189 ± 10.272 | 78.083 ± 10.610 | 0.061 |
| EF (%) | 55.059 ± 14.298 | 61.697 ± 10.699 | 61.879 ± 11.132 | <0.001 |
| AST (U/L) | 77.610 ± 79.248 | 44.818 ± 49.386 | 34.039 ± 32.576 | <0.001 |
| Creatine (μmol/L) | 69.688 ± 16.694 | 67.767 ± 15.181 | 69.090 ± 14.364 | 0.708 |
| NT-proBNP (pg/mL) | 1561.706 ± 3620.966 | 552.291 ± 758.825 | 570.607 ± 1117.933 | <0.001 |
| T4 (μg/dL) | 6.380 ± 1.666 | 7.151 ± 1.545 | 8.053 ± 1.808 | <0.001 |
| T3 (ng/mL) | 0.700 ± 0.102 | 1.009 ± 0.078 | 1.377 ± 0.195 | <0.001 |
| FT4 (pmol/L) | 13.738 ± 2.894 | 15.028 ± 2.991 | 16.350 ± 3.136 | <0.001 |
| FT3 (pmol/L) | 4.079 ± 1.124 | 4.792 ± 1.149 | 5.628 ± 1.145 | <0.001 |
| TSH (μIU/mL) | 2.782 ± 7.471 | 1.960 ± 1.408 | 2.748 ± 3.519 | 0.315 |
| TGAB (%) | 9.932 ± 13.510 | 6.797 ± 9.021 | 7.567 ± 10.325 | 0.117 |
| TMAB (%) | 7.295 ± 8.741 | 5.323 ± 6.282 | 5.595 ± 6.718 | 0.119 |
| Previous history of hypertension (%) | 47.71% | 54.13% | 52.29% | |
| CHF (%) | 10.09% | 7.34% | 5.50% | |
| Myocardial infarction (%) | 17.43% | 19.27% | 16.51% | |
| PCI or CABG (%) | 22.02% | 22.02% | 21.10% | |
| In-hospital treatment | ||||
| Aspirin (%) | 95.41% | 97.25% | 97.25% | |
| β-Blocker (%) | 81.65% | 88.07% | 79.82% | |
| Statin (%) | 97.25% | 99.08% | 93.58% | |
| CCB (%) | 19.27% | 23.85% | 17.43% | |
Abbreviations: GRACE: the Global Registry of Acute Coronary Events; HR: heart rate; CKMB: MB isoenzyme of creatine kinase; HbA1c: hemoglobin A1c; BP: blood pressure; EF: ejection fraction; AST: aspartate transaminase; NT-proBNP: probrain natriuretic peptide; T4: thyroxine; T3: triiodothyronine; FT4: free thyroxine; FT3: free triiodothyronine; TSH: thyroid-stimulating hormone; TGAB: thyroglobulin; TMAB: thyroid microsomal antibody; CHF: chronic heart failure; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; CCB: calcium channel blocker. Data are mean ± SD and number (%).
Table 2.
Basic characteristics based on FT3 levels in AMI patients.
| Free triiodothyronine levels (pmol/L) | P value | |||
|---|---|---|---|---|
| 1.62–3.98 | 3.99–5.33 | 5.34–8.55 | ||
| Female (%) | 18.35% | 18.35% | 22.94% | |
| GRACE score | 116.098 ± 43.037 | 121.991 ± 40.497 | 128.117 ± 38.421 | 0.091 |
| Age (y) | 59.625 ± 10.468 | 58.351 ± 10.107 | 59.694 ± 10.490 | 0.550 |
| HR (bpm) | 69.732 ± 14.248 | 72.728 ± 22.362 | 69.856 ± 13.955 | 0.344 |
| Onset of AMI (h) | 17.538 ± 32.545 | 17.769 ± 30.339 | 16.692 ± 18.481 | 0.911 |
| CKMB (U/L) | 40.333 ± 66.052 | 40.266 ± 61.909 | 41.398 ± 60.269 | 0.989 |
| HbA1C (%) | 124.027 ± 19.070 | 124.513 ± 18.211 | 125.973 ± 16.001 | 0.272 |
| sBP (mmHg) | 76.929 ± 11.943 | 78.796 ± 11.498 | 78.369 ± 11.353 | 0.700 |
| dBP (mmHg) | 5.693 ± 0.356 | 5.744 ± 0.357 | 5.767 ± 0.328 | 0.454 |
| EF (%) | 67.939 ± 14.945 | 69.152 ± 16.936 | 69.203 ± 14.298 | <0.001 |
| AST (U/L) | 55.486 ± 13.468 | 60.057 ± 11.187 | 62.135 ± 11.974 | <0.05 |
| Creatine (μmol/L) | 68.437 ± 75.677 | 53.158 ± 57.580 | 40.438 ± 45.711 | 0.192 |
| NT-proBNP (pg/mL) | 1780.395 ± 3700.982 | 739.469 ± 1208.398 | 411.908 ± 537.265 | <0.001 |
| T4 (μg/dL) | 6.464 ± 1.641 | 7.005 ± 1.687 | 7.911 ± 1.875 | <0.001 |
| T3 (ng/mL) | 0.845 ± 0.238 | 1.015 ± 0.277 | 1.218 ± 0.286 | <0.001 |
| FT4 (pmol/L) | 13.432 ± 2.918 | 14.832 ± 2.836 | 16.540 ± 3.075 | <0.001 |
| FT3 (pmol/L) | 3.336 ± 0.512 | 4.690 ± 0.394 | 6.295 ± 0.750 | <0.001 |
| TSH (μIU/mL) | 2.812 ± 7.305 | 2.238 ± 3.348 | 2.241 ± 1.618 | 0.585 |
| TGAB (%) | 8.873 ± 12.438 | 6.765 ± 9.865 | 8.271 ± 10.397 | 0.335 |
| TMAB (%) | 6.567 ± 8.205 | 5.184 ± 6.313 | 6.202 ± 7.025 | 335.000 |
| Previous history of hypertension (%) | 44.95% | 50.46% | 54.13% | |
| CHF (%) | 6.42% | 1.83% | 1.83% | |
| Myocardial infarction (%) PCI or CABG (%) | 13.76% | 13.76% | 14.68% | |
| In-hospital treatment | 18.35% | 16.51% | 20.18% | |
| Aspirin (%) | 95.41% | 97.25% | 97.25% | |
| β-Blocker (%) | 78.90% | 88.99% | 80.73% | |
| Statin (%) | 97.25% | 99.08% | 92.66% | |
| CCB (%) | 15.60% | 19.27% | 15.60% | |
Abbreviations: GRACE: the Global Registry of Acute Coronary Events; HR: heart rate; CKMB: MB isoenzyme of creatine kinase; HbA1c: hemoglobin A1c; BP: blood pressure; EF: ejection fraction; AST: aspartate transaminase; NT-proBNP: probrain natriuretic peptide; T4: thyroxine; T3: triiodothyronine; FT4: free thyroxine; FT3: free triiodothyronine; TSH: thyroid-stimulating hormone; TGAB: thyroglobulin; TMAB: thyroid microsomal antibody; CHF: chronic heart failure; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; CCB: calcium channel blocker. Data are mean ± SD and number (%).
3.2. Association between T3 and Cardiac Function
As baseline characters exhibited a significant alteration of EF and NT-proBNP based on different T3 and FT3 levels, which were crucially important to evaluate cardiac function during AMI, we then carried on to investigate the relationship between T3 and cardiac function by regression analysis. The T3 levels were found to be significantly positively correlated with EF (R square = 0.042, P < 0.001) and negatively correlated with admission NT-proBNP levels (R square = 0.059, P < 0.001), which is the same with the correlation between FT3 and EF (R square = 0.053, P < 0.001) and admission NT-proBNP levels (R square = 0.108, P < 0.001) (Figure 2). Multiregression analysis was then utilized to further explain the association of T3 and cardiac function. Interestingly, although both T3 and FT3 levels were found to be significantly positively correlated with EF in AMI patients (P < 0.05), only FT3 was found to be significantly negatively correlated with NT-proBNP (P < 0.05). In addition, TSH, thyroid-stimulating hormone, was also found to be positively correlated with EF (P < 0.05) (Table 3). It is noteworthy that the TSH, T4, and FT4 did not exhibit a significant correlation with cardiac function in the present cohort.
Figure 2.
Linear analysis between heart function and triiodothyronine level. (a) Simple linear regression model with heart ejection fraction in relation to triiodothyronine level. (b) Simple linear regression model with heart ejection fraction in relation to free triiodothyronine level. (c) Simple linear regression model with NT-proBNP in relation to triiodothyronine level. (d) Simple linear regression model with NT-proBNP in relation to free triiodothyronine level.
Table 3.
Multiregression analysis of NT-proBNP and EF.
| Factors | Coefficient | SEM | P value |
|---|---|---|---|
| Multiregression analysis of NT-proBNP | |||
| Age (y) | −0.001 | 14.246 | 0.994 |
| HR (bpm) | 0.161 | 7.271 | <0.05 |
| GRACE score | 0.106 | 3.986 | 0.141 |
| T3 (ng/mL) | −0.094 | 486.295 | 0.155 |
| FT3 (pmol/L) | −0.195 | 115.569 | <0.05 |
| TSH (μIU/mL) | −0.024 | 25.870 | 0.666 |
| CKMB (U/L) | −0.045 | 2.294 | 0.480 |
| Creatine (μmol/L) | 0.014 | 8.706 | 0.807 |
| HbA1C (%) | −0.039 | 377.367 | 0.495 |
| Multiregression analysis of EF | |||
| Age (y) | −0.051 | 0.081 | 0.432 |
| HR (bpm) | 0.087 | 0.041 | 0.134 |
| GRACE score | 0.017 | 0.023 | 0.816 |
| T3 (ng/mL) | 0.132 | 2.687 | <0.05 |
| FT3 (pmol/L) | 0.152 | 0.633 | <0.05 |
| TSH (μIU/mL) | 0.121 | 0.145 | <0.05 |
| CKMB (U/L) | 0.021 | 0.013 | 0.744 |
| Creatine (μmol/L) | −0.117 | 0.052 | 0.054 |
| HbA1C (%) | 0.034 | 2.126 | 0.564 |
Abbreviations: HR: heart rate; GRACE: the Global Registry of Acute Coronary Events; T3: triiodothyronine; FT3: free triiodothyronine; TSH: thyroid-stimulating hormone; CKMB: MB isoenzyme of creatine kinase; HbA1c: hemoglobin A1c.
3.3. All-Cause Mortality and MACE
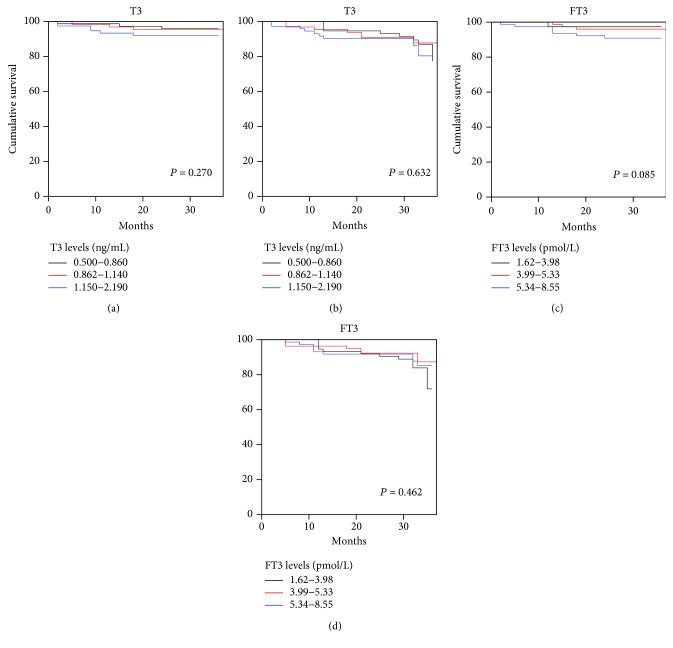
During the follow-up period, 12 (5.2%) patients died for all cause, 9 (4.0%) for cardiac cause, 28 (11.6%) for acute heart failure, and 12 (4.4%) had new-onset myocardial infarction. We also examined the prognostic significance of T3 and FT3 in AMI. As demonstrated in the Kaplan-Meier plots, the analysis revealed no significant difference with regard to different T3 or FT3 levels, as shown in Figure 3.
Figure 3.
Kaplan-Meier survival curves for freedom from all-cause mortality and MACE judging by triiodothyronine level. (a) Kaplan-Meier survival curve for freedom from all-cause mortality judging by triiodothyronine level. P = 0.270. (b) Kaplan-Meier survival curves for freedom from MACE judging by triiodothyronine level. P = 0.632. (c) Kaplan-Meier survival curves for freedom from all-cause mortality judging by free triiodothyronine level. P = 0.085. (d) Kaplan-Meier survival curves for freedom from MACE judging by free triiodothyronine level. P = 0.462. There is no significantly higher event-free survival rate in high triiodothyronine and free triiodothyronine levels.
4. Discussion
In this study, serum FT3 level was found to be moderately associated with the cardiac function, as indicated by EF and NT-proBNP levels, on admission in patients with acute myocardial infarction. However, neither T3 nor FT3 exhibited effects on all-cause mortality rate and MACE in AMI patients during 2-year follow-up.
The important implication of the present study is that T3 levels are found to be correlated with the cardiac function as indicated by EF and NT-proBNP in AMI patients. In vitro studies have shown that T3 activates and regulates cardiac genes encoding proteins such as the myosin heavy chain isoforms and the sarcoplasmic reticulum calcium-activated ATPase pump [19–21], indicating a possible relationship between T3 level and cardiac function. Previous study has also indicated that T3 predicts outcomes in patients with acute heart failure [11]. In addition, low T3 is found out to be associated with elevation of N-terminal probrain natriuretic peptide (NT-proBNP) and mortality in dialysis patients [13, 22, 23]. However, few studies have addressed the question whether thyroid function is related to cardiac function during acute myocardial infarction. The major outcome of this study shows a positive correlation between T3 and FT3 levels and cardiac ejection fraction, indicating that T3 level is a potential therapeutic target for cardiac function improvement during acute myocardial infarction.
In the present analysis, it is noteworthy that the TSH, T4, and FT4 did not exhibit a significant correlation. The results correlate with previous publications that low T3 level has been proven to be correlated with myocardial damage in animal models. It has been known that T4 is solely a product of the thyroid gland, while T3 is a product of the thyroid and of many other tissues. Thus, the difference in the correlation results among thyroid hormones might reflect differential thyroid metabolism and function in AMI patients and requires further mechanism investigation.
Our study also shows that T3 and FT3 levels do not predict adverse cardiovascular outcomes in AMI patients. There is conflicting evidence regarding thyroid hormone levels and cardiovascular outcomes [24–26]. It has been reported that thyroid function tests predict disability in advanced old age patients, with higher serum TSH levels predicting better outcomes [27]. Moreover, in patients with acute heart failure, subclinical hypothyroidism on admission is an independent predictor of adverse cardiovascular outcomes [24]. In this study, thyroid function is not associated with all-cause mortality and MACE in AMI patients over a 2-year follow-up, indicating that the interaction between T3 and cardiac function might be more significant during acute phase of myocardial infarction. Meanwhile, it is also noteworthy that the less clear association between T3 and cardiovascular outcomes could be due to a limited number of patients with a relatively short follow-up in the present study, and its association with long-term prognosis may be stronger. As a result, more well-designed and long-term studies as well as systemic analysis are needed to investigate whether thyroid function will play an important role in the prognosis of AMI.
The present study also has several limitations. On the one hand, this is a single-centre-based observational cohort study. As the sample size in this study is relatively small, thus, the comparisons of subgroups may lack power to detect significant differences for selected variables, especially in Kaplan-Meier survival analysis. Notably, the association between T3 levels and cardiac function as interpreted by EF and NT-proBNP exhibits only low correlation coefficients, indicating at best a moderate association. On the other hand, the onset of AMI and the coronary intervention vary among patients. As a result, the correlation between AMI progression, iodine intake, and thyroid function could lead to a confounding bias and limit the interpretation of pathophysiology of thyroid function during the onset and progression of AMI. Therefore, multicentre-based clinical observations and subgroup analysis are necessary to further evaluate the predictive value of T3 for MACE and all-cause mortality, so as to explore the therapeutic value of modulating thyroid function during AMI.
In conclusion, in patients with AMI, T3 and FT3 levels are moderately positively correlated with cardiac function on admission in AMI patients but did not predict a long-time survival rate. The results of this study further provide therapeutic view that thyroid function control could be one of the treatment targets for AMI patients. Further studies are needed to determine whether longer-term follow-up would further identify the prognosis effect of T3 on MACE and all-cause mortality.
Acknowledgments
The authors acknowledge the support of China Scholarship Council (CSC) and Deutscher Akademischer Austauschdienst (DAAD). This study was also supported by the Central University Basic Science Foundation of China (1191329724), the National Natural Science Foundation of China (81570406, 81800390), and the Natural Science Foundation of Shaanxi Province (2018KW067).
Abbreviations
- AMI:
Acute myocardial infarction
- T3:
Triiodothyronine
- FT3:
Free triiodothyronine
- T4:
Thyroxine
- FT4:
Free thyroxine
- TSH:
Thyroid-stimulating hormone
- TGAB:
Thyroglobulin
- TMAB:
Thyroid microsomal antibody
- EF:
Cardiac ejection fraction
- NT-proBNP:
N-terminal probrain natriuretic peptide
- MACE:
Major adverse cardiovascular events
- GRACE:
The Global Registry of Acute Coronary Events
- HR:
Heart rate
- CKMB:
MB isoenzyme of creatine kinase
- HbA1c:
Hemoglobin A1c
- BP:
Blood pressure
- AST:
Aspartate transaminase
- CHF:
Chronic heart failure
- PCI:
Percutaneous coronary intervention
- CABG:
Coronary artery bypass graft
- CCB:
Calcium channel blocker.
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was approved by the ethics committee of the First Affiliated Hospital of Xi'an Jiaotong University.
Consent
Written informed consent was obtained from all study participants.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All listed authors have contributed significantly to the research and manuscript. JS, JF, YW, and ZY participated in the design of the study. YD and LS collected the written informed consent and patients' data. LS and YX performed the statistical analysis. JS and JF drafted the manuscript. All authors approved the final manuscript. Jianqing She, Jiahao Feng, and Yangyang Deng contributed equally to this work.
References
- 1.She J., Hu Z., Deng Y., Liu F., Yuan Z. Chest pain after aortic valve replacement: rupture of right sinus of Valsalva presenting as myocardial infarction. Cardiology. 2016;134(1):22–25. doi: 10.1159/000443266. [DOI] [PubMed] [Google Scholar]
- 2.Hollander J. E., Than M., Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134(7):547–564. doi: 10.1161/CIRCULATIONAHA.116.021886. [DOI] [PubMed] [Google Scholar]
- 3.Vernon S. T., Coffey S., Bhindi R., et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. European Journal of Preventive Cardiology. 2017;24(17):1824–1830. doi: 10.1177/2047487317720287. [DOI] [PubMed] [Google Scholar]
- 4.Kidd S. K., Bonaca M. P., Braunwald E., et al. Universal classification system type of incident myocardial infarction in patients with stable atherosclerosis: observations from thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events (TRA 2°P)-TIMI 50. Journal of the American Heart Association. 2016;5(7) doi: 10.1161/JAHA.116.003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin S. S., Daya N., Lutsey P. L., et al. Thyroid function, cardiovascular risk factors, and incident atherosclerotic cardiovascular disease: the Atherosclerosis Risk in Communities (ARIC) Study. The Journal of Clinical Endocrinology and Metabolism. 2017;102(9):3306–3315. doi: 10.1210/jc.2017-00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling Y., Jiang J., Gui M., et al. Thyroid function, prevalent coronary heart disease, and severity of coronary atherosclerosis in patients undergoing coronary angiography. International Journal of Endocrinology. 2015;2015:9. doi: 10.1155/2015/708272.708272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Guan H., Gerdes A. M., Iervasi G., Yang Y., Tang Y. D. Thyroid status, cardiac function, and mortality in patients with idiopathic dilated cardiomyopathy. The Journal of Clinical Endocrinology and Metabolism. 2015;100(8):3210–3218. doi: 10.1210/jc.2014-4159. [DOI] [PubMed] [Google Scholar]
- 8.Hennemann G., Docter R., Friesema E. C. H., de Jong M., Krenning E. P., Visser T. J. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocrine Reviews. 2001;22(4):451–476. doi: 10.1210/edrv.22.4.0435. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y. C., Chen S. A., Chen Y. J., Chang M. S., Chan P., Lin C. I. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. Journal of the American College of Cardiology. 2002;39(2):366–372. doi: 10.1016/S0735-1097(01)01731-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang W., Guan H., Fang W., et al. Free triiodothyronine level correlates with myocardial injury and prognosis in idiopathic dilated cardiomyopathy: evidence from cardiac MRI and SPECT/PET imaging. Scientific Reports. 2016;6(1, article 39811) doi: 10.1038/srep39811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothberger G. D., Gadhvi S., Michelakis N., Kumar A., Calixte R., Shapiro L. E. Usefulness of serum triiodothyronine (T3) to predict outcomes in patients hospitalized with acute heart failure. The American Journal of Cardiology. 2017;119(4):599–603. doi: 10.1016/j.amjcard.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Lehnen T. E., Santos M. V., Lima A., Maia A. L., Wajner S. M. N-acetylcysteine prevents low T3 syndrome and attenuates cardiac dysfunction in a male rat model of myocardial infarction. Endocrinology. 2017;158(5):1502–1510. doi: 10.1210/en.2016-1586. [DOI] [PubMed] [Google Scholar]
- 13.Prado-Uribe M. d. C., Ventura M.-d.-J., Ávila-Díaz M., et al. Low triiodothyronine is associated with elevation of N-terminal pro-brain natriuretic peptide (NT-proBNP) and mortality in dialysis patients. Nefrología. 2017;37(6):598–607. doi: 10.1016/j.nefro.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T., Hasegawa T., Kanzaki H., et al. Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC Heart Failure. 2016;3(3):168–176. doi: 10.1002/ehf2.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okayama D., Minami Y., Kataoka S., Shiga T., Hagiwara N. Thyroid function on admission and outcome in patients hospitalized for acute decompensated heart failure. Journal of Cardiology. 2015;66(3):205–211. doi: 10.1016/j.jjcc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Ziman M. E., Bui H. T., Smith C. S., et al. The pharmacists’ role in improving guideline compliance for thyroid function testing in patients with heart failure. Journal of Pharmacy Practice. 2012;25(2):195–200. doi: 10.1177/0897190011416008. [DOI] [PubMed] [Google Scholar]
- 17.She J., Deng Y., Wu Y., et al. Hemoglobin A1c is associated with severity of coronary artery stenosis but not with long term clinical outcomes in diabetic and nondiabetic patients with acute myocardial infarction undergoing primary angioplasty. Cardiovascular Diabetology. 2017;16(1):p. 97. doi: 10.1186/s12933-017-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine G. N., Bates E. R., Bittl J. A., et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123–e155. doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 19.Mai W., Janier M. F., Allioli N., et al. Thyroid hormone receptor alpha is a molecular switch of cardiac function between fetal and postnatal life. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(28):10332–10337. doi: 10.1073/pnas.0401843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson E. A., Gloss B., Belke D. D., Kaneshige M., Cheng S. Y., Dillmann W. H. Cardiac expression and function of thyroid hormone receptor β and its PV mutant. Endocrinology. 2003;144(11):4820–4825. doi: 10.1210/en.2003-0522. [DOI] [PubMed] [Google Scholar]
- 21.Ojamaa K., Kenessey A., Klein I. Thyroid hormone regulation of phospholamban phosphorylation in the rat heart. Endocrinology. 2000;141(6):2139–2144. doi: 10.1210/endo.141.6.7514. [DOI] [PubMed] [Google Scholar]
- 22.Pinelli M., Bindi M., Cassetti G., et al. Relationship between low T3 syndrome and NT-proBNP levels in non-cardiac patients. Acta Cardiologica. 2007;62(1):19–24. doi: 10.2143/AC.62.1.2019366. [DOI] [PubMed] [Google Scholar]
- 23.Chuang C. P., Jong Y. S., Wu C. Y., Lo H. M. Impact of triiodothyronine and N-terminal pro-B-type natriuretic peptide on the long-term survival of critically ill patients with acute heart failure. The American Journal of Cardiology. 2014;113(5):845–850. doi: 10.1016/j.amjcard.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Altamirano Ufion A., Zulfiqar B., Hassan A., Habibi R., Boddu P. Subclinical hypothyroidism and its association with increased cardiovascular mortality. Cardiology Research and Practice. 2017;2017:5. doi: 10.1155/2017/7539735.7539735 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Bano A., Chaker L., Mattace-Raso F. U. S., et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the Rotterdam Study. Circulation Research. 2017;121(12):1392–1400. doi: 10.1161/CIRCRESAHA.117.311603. [DOI] [PubMed] [Google Scholar]
- 26.Stott D. J., Gussekloo J., Kearney P. M., et al. Study protocol; thyroid hormone replacement for untreated older adults with subclinical hypothyroidism - a randomised placebo controlled trial (TRUST) BMC Endocrine Disorders. 2017;17(1):p. 6. doi: 10.1186/s12902-017-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce S. H. S., Razvi S., Yadegarfar M. E., et al. Serum thyroid function, mortality and disability in advanced old age: the Newcastle 85+ study. The Journal of Clinical Endocrinology and Metabolism. 2016;101(11):4385–4394. doi: 10.1210/jc.2016-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.