Significance
Coffee consumption is linked with reduced risk of Parkinson’s disease (PD), and caffeine is generally believed to be the protective agent. However, several lines of evidence suggest the presence of additional compound(s) in coffee that can be protective as well. Here we show that eicosanoyl-5-hydroxytryptamide, which we purified from coffee as an agent that leads to enhanced enzymatic activity of the specific phosphatase PP2A that dephosphorylates the pathogenic protein α-synuclein, works in synergy with caffeine in protecting against mouse models of PD and Dementia with Lewy bodies. The mechanism of this synergy is also through enhancing PP2A, which is dysregulated in the brains of individuals with these α-synucleinopathies.
Keywords: α-synuclein, phosphorylation, Parkinson’s disease, Dementia with Lewy bodies, neuroprotection
Abstract
Hyperphosphorylated α-synuclein in Lewy bodies and Lewy neurites is a characteristic neuropathological feature of Parkinson’s disease (PD) and Dementia with Lewy bodies (DLB). The catalytic subunit of the specific phosphatase, protein phosphatase 2A (PP2A) that dephosphorylates α-synuclein, is hypomethylated in these brains, thereby impeding the assembly of the active trimeric holoenzyme and reducing phosphatase activity. This phosphatase deficiency contributes to the accumulation of hyperphosphorylated α-synuclein, which tends to fibrillize more than unmodified α-synuclein. Eicosanoyl-5-hydroxytryptamide (EHT), a fatty acid derivative of serotonin found in coffee, inhibits the PP2A methylesterase so as to maintain PP2A in a highly active methylated state and mitigates the phenotype of α-synuclein transgenic (SynTg) mice. Considering epidemiologic and experimental evidence suggesting protective effects of caffeine in PD, we sought, in the present study, to test whether there is synergy between EHT and caffeine in models of α-synucleinopathy. Coadministration of these two compounds orally for 6 mo at doses that were individually ineffective in SynTg mice and in a striatal α-synuclein preformed fibril inoculation model resulted in reduced accumulation of phosphorylated α-synuclein, preserved neuronal integrity and function, diminished neuroinflammation, and improved behavioral performance. These indices were associated with increased levels of methylated PP2A in brain tissue. A similar profile of greater PP2A methylation and cytoprotection was found in SH-SY5Y cells cotreated with EHT and caffeine, but not with each compound alone. These findings suggest that these two components of coffee have synergistic effects in protecting the brain against α-synuclein−mediated toxicity through maintenance of PP2A in an active state.
Parkinson’s disease (PD) and Dementia with Lewy bodies (DLB) are characterized by intraneuronal aggregates of α-synuclein in Lewy bodies and Lewy neurites (1). This normally soluble intrinsically disordered protein misfolds and forms amyloid fibrils in neuropathological hallmark inclusions. The initial oligomerization and eventual fibrillization are believed to be critical steps leading to neuronal dysfunction and death (2). Aggregates of α-synuclein can also propagate across neurons to synaptically connected brain regions (3), a phenomenon that explains the progressive nature of these disorders, their prodromal phase, and the emergence of additional clinical manifestations over time (4). Postmortem brain studies also show that α-synuclein in these aggregates is characteristically hyperphosphorylated at serine 129, and antibodies directed at phospho-Ser129-α-synuclein (p-α-Syn) are commonly used to detect these inclusions (5–7). Hyperphosphorylated α-synuclein is also a consistent feature of aggregates in transgenic rodent and Drosophila models expressing human α-synuclein (8–12) as well as aggregates deposited in remote brain regions following intracerebral inoculation of α-synuclein preformed fibrils (α-Syn PFF) (3). Additionally, α-synuclein phosphorylation at serine 129 accelerates its oligomerization and fibrillization in vitro (5). Accordingly, this posttranslational modification is of both pathogenetic and therapeutic interest in α-synucleinopathies.
While α-synuclein can be phosphorylated at serine 129 by multiple kinases (13–15), its dephosphorylation is carried out by a specific isoform of protein phosphatase 2A (PP2A) (8). This heterotrimeric holoenzyme is the primary serine/threonine phosphatase in the brain and consists of a structural A subunit, a catalytic C subunit, and one of multiple regulatory B subunits that determine substrate specificity (16–18). The isoform that dephosphorylates α-synuclein contains the B55α subunit, and its assembly and activity are highly regulated by reversible carboxyl methylation of Leu309 in the catalytic C subunit (8, 16–18). PP2A carboxyl methylation is regulated by the opposing activities of a PP2A-specific leucine carboxyl methyltransferase (LCMT-1) and a PP2A-specific methylesterase (PME-1) (19–21). Interestingly, the levels of these methylation-regulating enzymes are perturbed in PD and DLB brains, with down-regulation of LCMT-1 and up-regulation of PME-1. This profile is associated with reduced relative levels of methylated PP2A (methyl-PP2A), which is the enzymatically more active form of the phosphatase (22). Furthermore, treating α-synuclein transgenic mice (SynTg) with eicosanoyl-5-hydroxytryptamide (EHT), an inhibitor of the PME-1 methylesterase activity, increases both brain PP2A methylation and phosphatase activity, reduces the accumulation of phosphorylated α-synuclein aggregates, improves neuronal integrity, represses the neuroinflammatory response to the transgene, and preserves behavioral phenotype (8, 23).
It is of interest to note that we identified EHT initially in a series of extractions and purifications as a component of coffee with PME-1 methylesterase inhibitory activity in vitro (8). Subsequent studies showed that EHT also exhibits antioxidant and antiinflammatory activities (23). Structurally, EHT is unrelated to caffeine (CAF). It is a serotonin derivative wherein the C20 saturated fatty acid, eicosanoic acid, is linked to the serotonin amino group. Considering the epidemiological data linking coffee consumption with reduced risk of developing PD (24–27), the identification and characterization of the properties of EHT provide a mechanistic insight into the beneficial effects of this widely consumed beverage. Prior studies about the protective potential of coffee in PD have focused largely on CAF, because epidemiological data are consistent with CAF as a major source of neuroprotective activity (25, 27). In addition, CAF has been shown to protect mice against the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and this effect has been attributed to CAF acting as an adenosine A2A receptor antagonist (28). A recent study also reported protection against mutant α-synuclein fibril injections in the striatum (29). However, among patients with early PD, the amount of CAF consumption does not impact the rate of progression of the disease (30), and decaffeinated coffee has been found to be protective in Drosophila models of PD (31), raising some question about the protective effect of only CAF among the numerous other compounds in coffee.
The present study was carried out to test the potential synergy between EHT and CAF in protecting against α-synuclein−mediated pathology in two mouse models, transgenic mice expressing human α-synuclein panneuronally driven by the Thy1 promoter (SynTg) (32), and unilateral α-Syn PFF inoculation of the striatum (3). The potential synergy between these coffee components was also tested in cultured cells.
Results
Cotreatment with EHT and CAF Prevents the Behavioral Deficits of SynTg Mice.
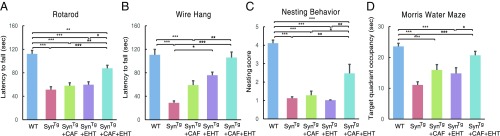
To test for a synergistic effect of EHT and CAF on α-synuclein−mediated pathology, we chose a relatively small dose of EHT in this study (12 mg/kg/d in chow). This is the smaller of the two doses we used previously in SynTg mice that resulted in partial amelioration of molecular, histochemical, and behavioral benefits after 9 mo of treatment (8). For CAF, a dose of 50 mg/kg/d in drinking water was selected based on extrapolations from the use of CAF in the MPTP model (28, 33) and our preliminary studies. This dose is about one-third of the dose that showed a protective effect in mice injected in the striatum with A53T mutant α-synuclein fibrils (29). In the present study, SynTg mice were treated with CAF and/or EHT starting upon weaning until 6 mo of age and were tested using four behavioral assessments that reflect the functions of different brain regions. Motor performance was evaluated on the rotarod and the Wire Hang test, which are controlled by the nigrostriatal pathway (Fig. 1 A and B) (34, 35); nesting behavior also reflects nigrostriatal function (Fig. 1C) (36); the Morris Water Maze test, which measures spatial learning and memory, reflects hippocampal function (Fig. 1D) (37).
Fig. 1.
EHT and CAF cotreatment prevents the behavioral deficits of SynTg mice. Behavioral performance of WT and SynTg mice (WT, n = 23; SynTg, n = 20; SynTg+CAF, n = 16; SynTg+EHT, n = 14; SynTg+CAF+EHT, n = 21) were tested at 6 mo of age on the (A) rotarod, (B) Wire Hang, (C) nesting behavior, and (D) Morris Water Maze. Data shown represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
As expected, the performance of SynTg mice was impaired on all four behavioral tests compared with wild-type (WT) mice (Fig. 1). CAF treatment alone in SynTg mice did not show any improvement on any of the tests, compared with untreated SynTg mice. EHT treatment alone in SynTg mice did not improve performance on the rotarod, nesting behavior, or Morris Water Maze, but did so only on the Wire Hang test, compared with untreated SynTg mice (Fig. 1B). On the other hand, cotreatment with both CAF and EHT improved behavioral performance of SynTg mice compared with untreated SynTg mice and compared with those treated with one compound on some of the tests (Fig. 1). These findings suggest that the combination of CAF and EHT, at doses that are individually ineffective, prevents the behavioral deficits of SynTg mice.
Cotreatment with EHT and CAF Reduces the Accumulation of Phosphorylated α-Synuclein Burden and Protects Against the Neuronal Damage and Neuroinflammation in SynTg Mice.
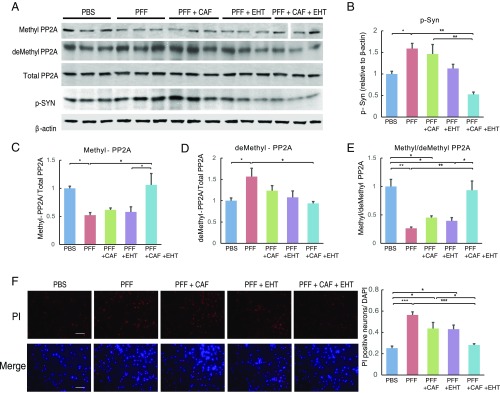
To investigate the effect of the treatments on α-synuclein phosphorylation, immunohistochemical stains for p-α-Syn were carried out. As expected, brains from SynTg mice had markedly increased p-α-Syn immunoreactivity in both the cerebral cortex and hippocampus compared with WT mice (Fig. 2 A and B). CAF treatment alone had no significant effect on the number of p-α-Syn immunoreactive cells compared with untreated SynTg mice (Fig. 2 A and B), while EHT treatment alone reduced p-α-Syn−positive cells in the hippocampus (Fig. 2B) but not the cortex (Fig. 2A). On the other hand, cotreatment with both EHT and CAF markedly reduced the number of p-α-Syn immunoreactive cells in both brain regions compared with untreated SynTg mice (Fig. 2 A and B). A similar profile of changes in p-α-Syn levels was found using Western blot analysis with cortical tissue lysates from five animals in each group (Fig. 2C). Consistent with immunohistochemistry, Western blots showed a sixfold increase in p-α-Syn levels in SynTg mice compared with WT mice (Fig. 2C). CAF or EHT treatments separately had no significant effect compared with untreated SynTg mice, whereas cotreatment with both compounds reduced p-α-Syn levels down to WT levels.
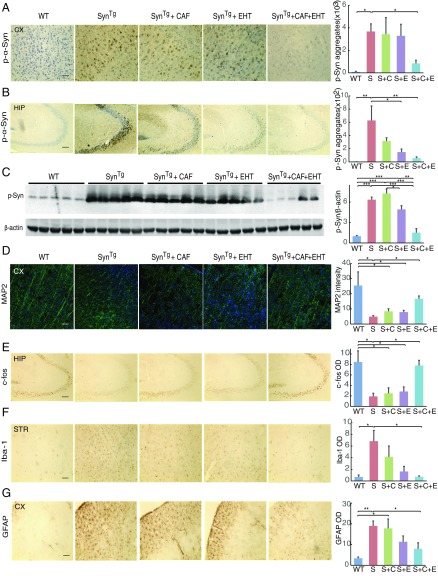
Fig. 2.
EHT and CAF cotreatment reduces p-α-Syn burden and protects against the neuronal damage and neuroinflammation in SynTg mice. (A and B) Representative images and quantification of immunohistochemical staining of p-α-Syn in the (A) cortex and (B) hippocampus. (C) Western blot analysis for p-α-Syn and β-actin with cortical brain tissue lysates from five groups and five animals per group. (D) Representative images and quantification of immunofluorescence staining of MAP2 in the cortex. (E) Representative images and quantification of immunohistochemical staining of c-fos in the hippocampus. (F) Representative images and quantification of immunohistochemical staining of Iba-1 in the striatum. (G) Representative images and quantification of immunohistochemical staining of GFAP in the cortex. (For all images, scale bar: 100 μm.) All bar graphs represent means ± SEM; n = 5 to 6 mice for immunohistochemical and immunofluorescence stains. *P < 0.05; **P < 0.01; ***P < 0.001.
The integrity of neuronal structure and activity was assessed next in the five groups of mice. As shown previously (8), SynTg mice have substantial depletion of the cytoskeletal microtubule associated protein 2, MAP2, in the cortex (Fig. 2D), suggestive of reduced dendritic complexity (38). Administration of CAF or EHT alone did not improve this profile, whereas cotreatment with both compounds restored neuritic integrity. A similar profile was observed for the immunoreactivity to the immediate early gene product c-fos in the hippocampus, a marker used as a surrogate of neuronal activity (39). Compared with WT mice, SynTg mice showed a marked loss of c-fos expression (Fig. 2E). Treatment of SynTg mice with either CAF or EHT failed to increase c-fos immunoreactivity significantly, but the combination preserved this marker to near WT levels (Fig. 2E). The latter finding is consistent with the improved performance of SynTg mice given both compounds on the Morris Water Maze test (Fig. 1D).
Neuroinflammation is one of the neuropathological features of PD (40) and models of α-synucleinopathy, including SynTg mice (8). Consistent with previous observations, untreated SynTg mice exhibited marked microglial activation (ionized calcium-binding adapter molecule 1) in the striatum and astrocytic proliferation (glial fibrillary acidic protein, GFAP) in the cortex that were partially but insignificantly attenuated by CAF or EHT treatment each given alone. However, the reduction of these markers was significant with the combined administration of both compounds (Fig. 2 F and G). These findings indicate that the cotreatment has a synergistic effect in protecting neuronal integrity and preventing the inflammatory response to the α-synuclein transgene in these mice.
Cotreatment with EHT and CAF Improves the Behavioral Performance of α-Syn PFF-Inoculated WT Mice.
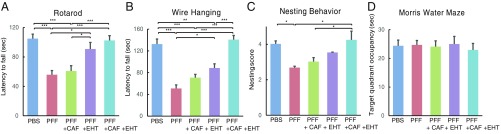
The effect of EHT and/or CAF was also tested in a second model of α-synucleinopathy, in which mouse α-Syn PFF injected in the dorsal striatum nucleate endogenous α-synuclein and propagate to anatomically linked brain regions, including nigral dopaminergic neurons (3). WT mice were placed, upon weaning, on a diet containing 12 mg/kg/d of EHT (or control diet), or given 50 mg/kg/d of CAF in drinking water (or normal water), or the two combined. These are the same doses used in SynTg mice described in Cotreatment with EHT and CAF Prevents the Behavioral Deficits of SynTg Mice. Animals were then injected at 2 mo of age with α-Syn PFF or PBS in the right striatum to induce PD-like pathology. Six months later (at 8 mo of age), behavioral tests were performed as described above. Untreated mice inoculated with α-Syn PFF showed significantly impaired performance in three behavioral tests (rotarod, Wire Hang, and nesting behavior) that are related to nigrostriatal function (Fig. 3 A–C). CAF treatment alone in α-Syn PFF-injected mice did not affect performance on any of the tests. EHT given alone improved performance on the rotarod and Wire Hang test but not nest building. On the other hand, the combination treatment improved performance on all three tests that were impacted by α-Syn PFF inoculation (Fig. 3 A–C). As noted previously with this model (3), learning and memory task on the Morris Water Maze, a test of hippocampal function, is not affected by α-Syn PFF inoculation or the treatments (Fig. 3D). These findings suggest that the coadministration of EHT and CAF protects against the behavioral deficits in the α-Syn PFF model better than each treatment alone.
Fig. 3.
EHT and CAF cotreatment improves the behavioral performance of α-Syn PFF-inoculated WT mice. Behavioral performance of PBS- and α-Syn PFF-inoculated mice (PBS, n = 14; PFF, n = 14; PFF+CAF, n = 19; PFF+EHT, n = 14; PFF+CAF+EHT, n = 15) was tested 6 mo postinoculation, at 8 mo of age on the (A) rotarod, (B) Wire Hang, (C) nesting behavior, and (D) Morris Water Maze. Bar graphs represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Cotreatment with EHT and CAF Prevents the Nucleation and Propagation of p-α-Syn−Positive Aggregates, Mitigates Neuroinflammation, and Protects Nigrostriatal Neurons in the α-Syn PFF Inoculation Model.
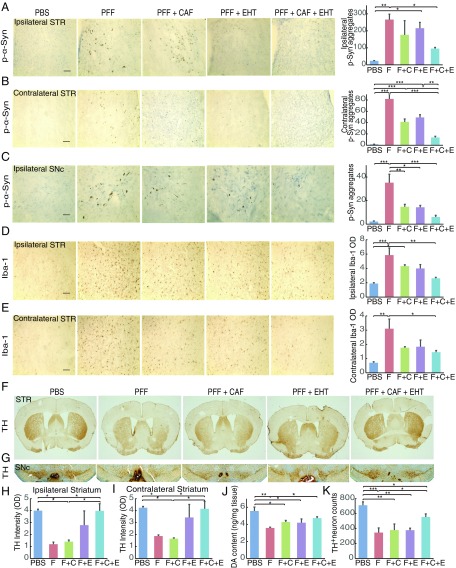
Following completion of behavioral assessments, mice were killed and their brains examined for p-α-Syn immunoreactivity, neuroinflammation, and the integrity of the nigrostriatal pathway. As described before (3), p-α-Syn immunoreactivity was found in the ipsilateral striatum and nigra as well as in the contralateral striatum in α-Syn PFF-inoculated mice but not in PBS-injected animals (Fig. 4 A–C). The p-α-Syn immunoreactivity in both ipsilateral and contralateral striata was partially but insignificantly reduced with each of CAF or EHT treatment given separately compared with untreated α-Syn PFF-inoculated mice, but the reduction with the combination treatment was significant (Fig. 4 A and B). Aggregates in the contralateral striatum were less abundant in cotreated animals compared with inoculated mice treated with each compound separately. In the ipsilateral nigra, p-α-Syn immunoreactivity was less abundant in all three treatment groups, but this effect was more pronounced in mice treated with both compounds (Fig. 4C). These findings suggest a synergistic effect of EHT and CAF in preventing the seeding and propagation of α-Syn PFF-induced pathologic aggregates.
Fig. 4.
EHT and CAF cotreatment prevents the formation of p-α-Syn−positive aggregates, mitigates neuroinflammation, and protects nigrostriatal neurons in the α-Syn PFF inoculation model. (A and B) Representative images and quantification of immunohistochemical staining of p-α-Syn in the (A) ipsilateral striatum and (B) contralateral striatum. (C) Representative images and quantification of immunohistochemical staining of p-α-Syn in the ipsilateral substantia nigra pars compacta (SNc). In A−C, n = 4 to 6 per group. (D and E) Representative images and quantification of immunohistochemical staining of Iba-1 in the (D) ipsilateral and (E) contralateral striatum. In D and E, n = 5 per group. (Scale bar: 100 μm for A–E.) (F) Representative images of immunohistochemical staining of TH in the striatum. (G) Representative images of immunohistochemical staining of TH in the SNc. (H and I) Quantification of (H) ipsilateral and (I) contralateral striatal TH staining; n = 5 per group. (J) DA content in the ipsilateral striatum analyzed by HPLC-MS; n = 5 to 6 per group. (K) Nigral TH-positive neuron count; n = 4 to 5 per group. All bar graphs represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. C, CAF; E, EHT; F, PFF.
α-Syn PFF inoculation induced microglial activation in the ipsilateral and contralateral striata (Fig. 4 D and E) as described previously (41, 42). The optical density of the Iba-1 signal in the ipsilateral striatum was about twofold higher than that in the contralateral side. Compared with the untreated α-Syn PFF-inoculated group, the prevention of microglial activation was significant in both striata only in mice cotreated with EHT and CAF, whereas each compound administered separately had only a partial effect that was statistically insignificant.
Assessment of the integrity of the nigrostriatal pathway revealed a similar profile (Fig. 4 F–K). Tyrosine hydroxylase (TH) staining of dopaminergic terminals in the striatum revealed bilateral depletion in untreated α-Syn PFF-inoculated mice compared with PBS-injected animals (Fig. 4 F, H, and I), consistent with previous observations (43). CAF alone had no effect on the decline of this marker, and EHT alone was associated with a nonsignificant increase compared with untreated α-Syn PFF-inoculated mice. The combination treatment, however, resulted in a significantly greater preservation of TH-positive terminals compared with untreated α-Syn PFF-inoculated mice.
Similarly, dopamine (DA) content in lysates of the ipsilateral striatum, measured by HPLC−mass spectrometry (HPLC-MS), was depleted by 36% in the α-Syn PFF-inoculated group compared with the PBS-injected group (Fig. 4J). Cotreatment with EHT and CAF preserved DA content by 32% compared with untreated α-Syn PFF-inoculated mice, while treatment with each compound alone did not have a significant benefit.
TH-positive dopaminergic neurons of the substantia nigra showed a similar profile whereby α-Syn PFF inoculation reduced the number of these neurons by 51% compared with PBS-injected mice (Fig. 4 G and K). EHT or CAF treatment individually did not prevent this reduction. However, cotreatment with EHT and CAF was associated with only 22% reduction compared with PBS-injected mice, representing a 59% protection compared with untreated α-Syn PFF-inoculated group.
EHT and CAF Exert Their Synergistic Neuroprotective Effects Through Regulating PP2A Activity.
EHT was identified and purified because of its ability to inhibit the activity of the PME-1, leading to enhanced methylation and activity of PP2A (8, 23). Considering the synergy between CAF and EHT in the behavioral and neurochemical profiles detailed above, we tested whether these two compounds also synergize in modulating PP2A methylation. Additionally, since PP2A is relatively demethylated in postmortem brains of α-synucleinopathy cases, including PD and DLB (22), we determined whether PP2A methylation changes also occur with α-synuclein overexpression and α-Syn PFF challenge in mice.
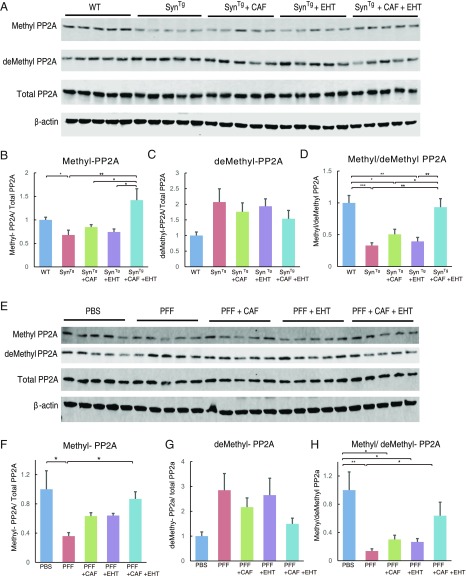
The state of PP2A methylation in the brains of SynTg mice without or with EHT and/or CAF treatment was assessed by Western blot analysis. Methyl-PP2A levels were lower in untreated SynTg mice compared with WT mice and did not change with either CAF or EHT administration given separately, but increased with the combination treatment (Fig. 5 A and B). Demethylated PP2A (demethyl-PP2A) tended to be higher in SynTg mice compared with WT mice, and was not affected by any of the treatments (Fig. 5 A and C). However, the ratio between methyl- and demethyl-PP2A was markedly lower in SynTg mice compared with WT mice and did not change by EHT or CAF treatments alone, but was significantly maintained at WT levels by coadministration of both compounds (Fig. 5D).
Fig. 5.
EHT and CAF exert their synergistic neuroprotective effects through regulating PP2A activity. (A) Western blots and (B−D) densitometric analysis for the indicated proteins with striatal tissue lysates from SynTg mouse brains. Bar graphs show (B) methyl-PP2A and (C) demethyl-PP2A levels that are normalized to total PP2A, and (D) the ratio of methylated PP2A over demethyl-PP2A. (E) Western blot and (F−H) densitometric analysis of ipsilateral striatal tissue lysates from α-Syn PFF-inoculated mice probed for the indicated proteins. Bar graphs show (F) methyl-PP2A and (G) demethyl-PP2A levels that are normalized to total PP2A, and (H) the ratio of methyl-PP2A over demethyl-PP2A. For all images, n = 5 per group. All bar graphs represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
In striatal tissue lysates following α-Syn PFF inoculation, methyl-PP2A levels as well as the methyl- to demethyl-PP2A ratio were reduced compared with PBS-injected striata (Fig. 5 E–H). CAF and EHT treatment given separately to these mice resulted in an insignificant increase in these measures compared with untreated α-Syn PFF-inoculated mice, but the effect of the combination treatment was significant. Thus, this profile of PP2A methylation changes is consistent with that seen in SynTg mice given these treatments (Fig. 5 A–D).
Combination of EHT and CAF Has Synergistic Effects in Up-Regulating PP2A Methylation and Attenuating Cytotoxicity Induced by α-Syn PFF in SH-SY5Y Cells.
Next, we confirmed the above in vivo findings in an analogous experiment with human neuroblastoma SH-SY5Y cells (Fig. 6 A–E). Challenging these cells with mouse α-Syn PFF reduced methyl-PP2A levels, with a reciprocal increase in demethyl-PP2A levels (Fig. 6 C and D). Adding either CAF or EHT to the medium partially reversed this trend, but the combination fully corrected these PP2A methylation changes to levels seen in PBS-treated cells. As a result, methyl- to demethyl-PP2A ratio, which was markedly reduced by α-Syn PFF challenge, was completely restored by EHT and CAF added together to the culture medium but not individually (Fig. 6E). These alterations in PP2A methylation profile were associated with parallel changes in p-α-Syn levels (Fig. 6B). Challenging cells with α-Syn PFF increased p-α-Syn levels, CAF alone had no effect, and EHT alone reduced it partially, but the combination of both compounds had a much bigger effect. Furthermore, the cytotoxicity of α-Syn PFF, assessed by propidium iodide (PI) exclusion, reflected a similar profile (Fig. 6F). The number of PI-positive cells increased 2.5-fold with α-Syn PFF challenge and was modestly protected by CAF and EHT added to the culture medium separately, but the combination of both compounds improved cell viability significantly compared with untreated α-Syn PFF-challenged cells (Fig. 6F). These findings support the hypothesis that EHT and CAF can act in synergy through enhancing steady-state levels of PP2A methylation, and hence phosphatase activity, associated with cytoprotection.
Fig. 6.
Combination of EHT and CAF has synergistic effects in up-regulating PP2A methylation and attenuating cytotoxicity induced by α-Syn PFF in SH-SY5Y cells. (A–E) SH-SY5Y cells were incubated with PBS or mouse α-Syn PFF and treated with CAF and/or EHT for 7 d. Cell lysates were subjected to Western blot analysis for the indicated proteins. The experiment was done in triplicates and repeated at least three times, yielding similar results. All lanes in the top Western blot (methyl-PP2A) were run on one gel. The vertical space between lanes 13 and 14 denotes where a size marker was loaded and was subsequently spliced out. (F) Representative images of PI and DAPI staining of SH-SY5Y cells incubated with PBS or mouse α-Syn PFF and treated with CAF and/or EHT for 7 d. Five random fields per well, and four independent wells per group, were counted for PI-positive cells. PI-positive cell numbers were normalized to DAPI count in the same field. Experiments were repeated twice, yielding similar results. (Scale bar: 100 μm.) All bar graphs represent means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The present findings demonstrate that subtherapeutic doses of CAF and EHT, two unrelated compounds found in coffee, can work in synergy to effect biochemical and molecular changes in the mouse brain leading to protection in two models of α-synucleinopathy. This is reflected in better behavioral performance of both SynTg mice and α-Syn PFF-inoculated mice treated chronically with a combination of these compounds for 6 mo, but not if each is given separately. In addition to preserved neuronal integrity and function, and dampened inflammatory response to α-synuclein, PP2A methylation is modulated by this cotreatment in a manner that favors enhanced phosphatase activity. This is associated with reduced accumulation of p-α-Syn. Similar biochemical changes occur in a cellular model challenged with α-Syn PFF and treated with the combination leading to increased PP2A methylation, reduced p-α-Syn levels, and cytoprotection.
These observations collectively suggest that CAF and EHT may function through a common mechanism. We purified EHT from coffee in an analytical assay set up to identify specifically compounds that inhibit the PP2A methylesterase PME-1 (8). EHT administered alone to SynTg mice for 9 mo also modulated PP2A methylation in brain tissue lysates in a dose-dependent manner in favor of the enzymatically active form and reduced the accumulation of p-α-Syn. This was more evident at a dose 10 times higher (120 mg/kg/d) than the dose used in the present study (8). CAF, on the other hand, is an adenosine A2a receptor antagonist, a property that is believed to mediate its protective function in the MPTP model of DA neuron degeneration (28). In addition, deleting the A2a receptor gene in mice has been shown to protect against dopaminergic neuron degeneration induced by human α-synuclein transgene containing two pathogenic mutations, A53T and A30P (44). Moreover, CAF was recently reported to protect against A53T mutant α-synuclein fibril injections in the striatum (29). By blocking A2a receptor signaling, CAF may enhance PP2A methylation through preventing cAMP-dependent protein kinase A/glycogen synthase kinase 3β-mediated activation of PME-1 (45–47). The combined effect of EHT and CAF is greater PP2A activity than either compound could achieve alone. The latter could underlie the synergy with EHT observed in the present study. Thus, these results suggest that chronic consumption of coffee and, therefore, chronic coingestion of both EHT and CAF have added benefits in α-synucleinopathy−prone brains. Additionally, the increase in methyl-PP2A with these treatments associated with neuroprotection, along with hypomethylation of PP2A in PD and DLB brains (22), suggests a pathogenetic significance of this phosphatase in these disorders.
The protection in the α-Syn PFF model with CAF and EHT coadministration provides some insight into the role of α-synuclein phosphorylation in nucleation and propagation of pathologic aggregates. This treatment was initiated upon weaning of WT mice, and α-Syn PFF inoculation occurred 6 wk later. Thus, the reduced phosphorylation level of α-synuclein in the brain appears to have decreased the ability of exogenous fibrils to nucleate endogenous α-synuclein at the site of striatal injection, leading to reduced propagation of aggregates to the substantia nigra pars compacta and the contralateral striatum. This may suggest that pharmacological interventions leading to reduced phosphorylation of α-synuclein may retard nucleation and propagation of pathology and slow down disease progression.
The present observations also have implications for the epidemiological data linking coffee consumption with reduced risk of PD (48–50) and attribution of this protection to CAF (24, 25). However, coffee is a complex chemical mixture containing more than a thousand different compounds (51). Thus, it is not unlikely that other components of coffee play a beneficial role as well. Factors that are not accounted for in epidemiological studies include the method of preparation of coffee. Different components of coffee are present in the final consumed product, depending on conditions of coffee growth and harvesting, methods of roasting and brewing, whether the coffee is filtered or not, and what kind of filter is used. EHT is a principal constituent of coffee wax that coats the coffee bean (52). The levels of EHT and closely related N-alkanoyl-5-hydoxytryptamides are appreciable in unfiltered coffee, whereas some preparation methods remove lipids such as EHT (53).
In addition to deficiencies in PP2A methylation in α-synucleinopathies (PD and DLB) (22), similar PP2A dysregulation is found in postmortem brains from patients with tauopathies such as Alzheimer’s disease (AD) and Progressive Supranuclear Palsy (PSP) (54, 55). Our present results, therefore, raise the possibility of similar synergy between EHT and CAF in both classes of chronic neurodegenerative diseases. Several epidemiologic studies have shown that greater consumption of coffee or CAF is associated with reduced risk of AD (56), and dietary supplementation with EHT is efficacious in two rodent models of AD: mice exposed to oligomeric beta-amyloid injections into the hippocampus (57) and rats injected in the lateral ventricle with viral vector expressing the endogenous PP2A inhibitor SET that exhibit tau hyperphosphorylation, elevated levels of cytoplasmic amyloid-β protein, and cognitive impairment (58). Additionally, in AβPPsw+PS1 transgenic mice, administration of caffeinated coffee increased plasma levels of cytokines, while neither CAF alone nor decaffeinated coffee had this effect, raising the likelihood that CAF synergizes with another component of coffee (59). Based on our present findings, we submit that at least one of the synergizing components is EHT.
Coffee is not the only botanical extract that contains agents that act to inhibit PME-1-mediated PP2A methylation. As we begin to unravel the polypharmacology of the micronutrients in commonly consumed botanical extracts such as coffee, it seems likely that it will be possible to optimize their composition to enhance efficacy so as to provide widely available, inexpensive, and effective therapeutics for the prevention and treatment of neurodegenerative diseases such as PD, DLB, PSP, and AD.
Materials and Methods
Detailed information on the experimental methods and materials used can be found in SI Appendix. All animal procedures were approved by the Rutgers Robert Wood Johnson Medical School Institutional Animal Care and Use Committee. SynTg on BDF1 background overexpressing human WT α-synuclein under the control of the Thy1 promoter were used as a model of diffuse α-synucleinopathy to study the effects of EHT and CAF treatments. The α-Syn PFF inoculation model was prepared using C57BL/6J mice (3). To prepare α-Syn PFF, plasmid pT7-7 encoding mouse α-synuclein cDNA was used to transform Escherichia coli BL21(DE3) strain (60, 61). EHT was synthesized at Signum Biosciences. Striatal DA content was measured using HPLC-MS. TH immunoreactive nigral neurons were counted using an automated, unbiased, context-intelligent neural network algorithm developed by Fimmic Oy.
Supplementary Material
Acknowledgments
We thank Eliezer Masliah (then at the University of California, San Diego) for providing SynTg mice, and acknowledge technical assistance from Gina M. Moriarty in Jean Baum’s laboratory (Rutgers University) with purifying recombinant mouse α-synuclein from plasmid pT7-7 originally obtained from Peter Lansbury. Technical assistance from Chelsea Bautista and Katherine Skibiel is also acknowledged. This study was supported by National Institutes of Health (NIH) Grant AT006868 (to M.M.M. and J.B.S.). Analyses conducted by the Rutgers Robert Wood Johnson Medical School Biological Mass Spectrometry Facility were supported by NIH Grant P30NS046593. E.J. is supported by NIH Grants NS070898 and NS095003 and the State of New Jersey. M.M.M. is the William Dow Lovett Professor of Neurology and is supported by the Michael J. Fox Foundation for Parkinson’s Research, the American Parkinson Disease Association, the New Jersey Health Foundation/Nicholson Foundation, and NIH Grants NS073994, NS096032, and NS101134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: J.B.S. has a financial interest in Signum Biosciences, which is developing PP2A phosphatase enhancing agents. M.V. is employed by Signum Biosciences. J.B.S. and M.M.M. are inventors of a patent application relevant to this work.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813365115/-/DCSupplemental.
References
- 1.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 3.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: Separating the wheat from the chaff. J Parkinsons Dis. 2017;7(Suppl 1):S71–S85. doi: 10.3233/JPD-179001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara H, et al. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 7.Oueslati A. Implication of alpha-synuclein phosphorylation at S129 in synucleinopathies: What have we learned in the last decade? J Parkinsons Dis. 2016;6:39–51. doi: 10.3233/JPD-160779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KW, et al. Enhanced phosphatase activity attenuates α-synucleinopathy in a mouse model. J Neurosci. 2011;31:6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinelli KJ, et al. Presynaptic alpha-synuclein aggregation in a mouse model of Parkinson’s disease. J Neurosci. 2014;34:2037–2050. doi: 10.1523/JNEUROSCI.2581-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi M, et al. Phosphorylation of alpha-synuclein characteristic of synucleinopathy lesions is recapitulated in alpha-synuclein transgenic Drosophila. Neurosci Lett. 2003;336:155–158. doi: 10.1016/s0304-3940(02)01258-2. [DOI] [PubMed] [Google Scholar]
- 11.Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: Resemblance to pathogenetic changes in Parkinson’s disease. J Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- 12.Neumann M, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii A, et al. Casein kinase 2 is the major enzyme in brain that phosphorylates Ser129 of human alpha-synuclein: Implication for alpha-synucleinopathies. FEBS Lett. 2007;581:4711–4717. doi: 10.1016/j.febslet.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 14.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 15.Inglis KJ, et al. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Bα subunit. Biochem J. 1999;339:241–246. [PMC free article] [PubMed] [Google Scholar]
- 17.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, et al. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Stock J. Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J Biol Chem. 1993;268:19192–19195. [PubMed] [Google Scholar]
- 20.Lee J, Chen Y, Tolstykh T, Stock J. A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc Natl Acad Sci USA. 1996;93:6043–6047. doi: 10.1073/pnas.93.12.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogris E, et al. A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem. 1999;274:14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ, et al. Dysregulation of protein phosphatase 2A in Parkinson disease and dementia with Lewy bodies. Ann Clin Transl Neurol. 2016;3:769–780. doi: 10.1002/acn3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KW, et al. Neuroprotective and anti-inflammatory properties of a coffee component in the MPTP model of Parkinson’s disease. Neurotherapeutics. 2013;10:143–153. doi: 10.1007/s13311-012-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross GW, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 25.Ascherio A, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 26.Ascherio A, et al. Coffee consumption, gender, and Parkinson’s disease mortality in the cancer prevention study II cohort: The modifying effects of estrogen. Am J Epidemiol. 2004;160:977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 27.Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson’s disease: A systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20(Suppl 1):S221–S238. doi: 10.3233/JAD-2010-091525. [DOI] [PubMed] [Google Scholar]
- 28.Chen JF, et al. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luan Y, et al. Chronic caffeine treatment protects against α-synucleinopathy by reestablishing autophagy activity in the mouse striatum. Front Neurosci. 2018;12:301. doi: 10.3389/fnins.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon DK, et al. NET-D Investigators Caffeine and progression of Parkinson disease. Clin Neuropharmacol. 2008;31:189–196. doi: 10.1097/WNF.0b013e31815a3f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinh K, et al. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J Neurosci. 2010;30:5525–5532. doi: 10.1523/JNEUROSCI.4777-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockenstein E, et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 33.Xu K, et al. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2006;26:535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozas G, Labandeira García JL. Drug-free evaluation of rat models of parkinsonism and nigral grafts using a new automated rotarod test. Brain Res. 1997;749:188–199. doi: 10.1016/S0006-8993(96)01162-6. [DOI] [PubMed] [Google Scholar]
- 35.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedelis M, et al. MPTP susceptibility in the mouse: Behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000;30:171–182. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- 37.Bennett MC, Rose GM. Chronic sodium azide treatment impairs learning of the Morris water maze task. Behav Neural Biol. 1992;58:72–75. doi: 10.1016/0163-1047(92)90967-9. [DOI] [PubMed] [Google Scholar]
- 38.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palop JJ, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blumenstock S, et al. Seeding and transgenic overexpression of alpha-synuclein triggers dendritic spine pathology in the neocortex. EMBO Mol Med. 2017;9:716–731. doi: 10.15252/emmm.201607305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boza-Serrano A, et al. The role of Galectin-3 in α-synuclein-induced microglial activation. Acta Neuropathol Commun. 2014;2:156. doi: 10.1186/s40478-014-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paumier KL, et al. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kachroo A, Schwarzschild MA. Adenosine A2A receptor gene disruption protects in an α-synuclein model of Parkinson’s disease. Ann Neurol. 2012;71:278–282. doi: 10.1002/ana.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku BM, et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3β pathways in U87MG human glioma cells. Mol Cells. 2011;31:275–279. doi: 10.1007/s10059-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim AR, et al. Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep. 2016;49:111–115. doi: 10.5483/BMBRep.2016.49.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao XQ, et al. Glycogen synthase kinase-3β regulates leucine-309 demethylation of protein phosphatase-2A via PPMT1 and PME-1. FEBS Lett. 2012;586:2522–2528. doi: 10.1016/j.febslet.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Hellenbrand W, et al. Diet and Parkinson’s disease. II: A possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47:644–650. doi: 10.1212/wnl.47.3.644. [DOI] [PubMed] [Google Scholar]
- 49.Fall PA, Fredrikson M, Axelson O, Granérus AK. Nutritional and occupational factors influencing the risk of Parkinson’s disease: A case-control study in southeastern Sweden. Mov Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 50.Benedetti MD, et al. Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: A case-control study. Neurology. 2000;55:1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 51.Higdon JV, Frei B. Coffee and health: A review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 52.Lang R, Hofmann T. A versatile method for the quantitative determination of bN-alkanoyl-5-hydroxytryptamides in roasted coffee. Eur Food Res Technol. 2005;220:638–643. [Google Scholar]
- 53.Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Curr Opin Clin Nutr Metab Care. 2007;10:745–751. doi: 10.1097/MCO.0b013e3282f05d81. [DOI] [PubMed] [Google Scholar]
- 54.Sontag E, et al. Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004;63:1080–1091. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- 55.Park HJ, et al. Protein phosphatase 2A and its methylation modulating enzymes LCMT-1 and PME-1 are dysregulated in tauopathies of progressive supranuclear palsy and Alzheimer disease. J Neuropathol Exp Neurol. 2018;77:139–148. doi: 10.1093/jnen/nlx110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: Systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187–S204. doi: 10.3233/JAD-2010-091387. [DOI] [PubMed] [Google Scholar]
- 57.Asam K, et al. Eicosanoyl-5-hydroxytryptamide (EHT) prevents Alzheimer’s disease-related cognitive and electrophysiological impairments in mice exposed to elevated concentrations of oligomeric beta-amyloid. PLoS One. 2017;12:e0189413. doi: 10.1371/journal.pone.0189413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basurto-Islas G, et al. Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2701–2712. doi: 10.1016/j.neurobiolaging.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao C, et al. Caffeine synergizes with another coffee component to increase plasma GCSF: Linkage to cognitive benefits in Alzheimer’s mice. J Alzheimers Dis. 2011;25:323–335. doi: 10.3233/JAD-2011-110110. [DOI] [PubMed] [Google Scholar]
- 60.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 61.Wu KP, Kim S, Fela DA, Baum J. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: Implication for aggregation. J Mol Biol. 2008;378:1104–1115. doi: 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.