Significance
Humans are constantly confronted with multiple stressors, to which the bodily response and adaptation are essential. The adrenal gland plays a major role in the response to physiological challenges. Maintenance of the adrenal is partly accomplished by proliferation and differentiation of adult progenitors and stem cells in the cortex and medulla. In this study, we have isolated and characterized a subpopulation of adrenocortical progenitors, which are interconnected with adrenomedullary stress-dependent progenitors. Under stress, the adrenocortical progenitors are also activated and they mobilize, giving rise to steroidogenic cells. Our findings demonstrate the coordinated action of stress-inducible stem cells to ensure tissue remodeling and cellular and functional adaptation to stress.
Keywords: adrenal, cortex, progenitors, stem cells, stress
Abstract
The adrenal gland is a master regulator of the human body during response to stress. This organ shows constant replacement of senescent cells by newly differentiated cells. A high degree of plasticity is critical to sustain homeostasis under different physiological demands. This is achieved in part through proliferation and differentiation of adult adrenal progenitors. Here, we report the isolation and characterization of a Nestin+ population of adrenocortical progenitors located under the adrenal capsule and scattered throughout the cortex. These cells are interconnected with progenitors in the medulla. In vivo lineage tracing revealed that, under basal conditions, this population is noncommitted and slowly migrates centripetally. Under stress, this migration is greatly enhanced, and the cells differentiate into steroidogenic cells. Nestin+ cells cultured in vitro also show multipotency, as they differentiate into mineralocorticoid and glucocorticoid-producing cells, which can be further influenced by the exposure to Angiotensin II, adrenocorticotropic hormone, and the agonist of luteinizing hormone-releasing hormone, triptorelin. Taken together, Nestin+ cells in the adult adrenal cortex exhibit the features of adrenocortical progenitor cells. Our study provides evidence for a role of Nestin+ cells in organ homeostasis and emphasizes their role under stress. This cell population might be a potential source of cell replacement for the treatment of adrenal insufficiency.
Stress is defined as a threat to homeostasis. The pituitary and the adrenal glands are the major organs of the human body involved in the response to stress. During an acute stress response, catecholamines such as epinephrine and norepinephrine are produced in the sympathetic nervous system and the adrenal medulla within seconds. Instantly, the hypothalamo-pituitary-adrenocortical (HPA) axis responds by producing glucocorticoids, which support the action of catecholamines. Proper control of the stress response and the restoration of homeostasis is of critical importance, as inappropriate or prolonged HPA axis activation is linked with numerous physiological and psychological disease states (1). Tissue and organ maintenance in the body is partly accomplished by proliferation and differentiation of adult stem cells, by division of differentiated cells, or by conversion of cell lineage. Adult stem cells reside in different tissues and organs of the body, where they contribute to the renewal of organ-specific cells. A proper balance between the proliferation and differentiation of such stem/progenitor cells is crucial, as a dysregulation of this mechanism might result in organ failure (reviewed in ref. 2). Stem cells are dynamically regulated by signals originating from their niches, helping to regulate appropriate proliferation and differentiation. We have previously demonstrated a direct effect of adrenocortical growth factors and androgens on proliferation and differentiation of adult adrenomedullary chromaffin cells (3) and chromaffin progenitor cells (4). We have also shown that Nestin+ stem-like cells in the adrenal medulla play an important role under stress, predominantly differentiating into chromaffin cells (5). The adrenal cortex of the mouse is formed by an outer layer, the zona glomerulosa (zG), and an inner layer, the zona fasciculata (zF). Several studies have demonstrated that distinct populations of adrenocortical progenitors are located in the subcapsular region of the adrenal cortex, possibly in the zG (6–8), where they contribute to regeneration of the adrenal gland (9). These cells are displaced centripetally until they reach the cortical–medullary boundary, where they become apoptotic (6, 10). The Sonic hedgehog (SHH) signaling pathway plays an important role in the development of the adrenal cortex, and SHH+ subcapsular cells represent a population of undifferentiated cells that activate GLI1 (Glioma-Associated Oncogene Homolog 1)-expressing progenitor cells in the adrenal capsule (9, 11, 12). The GLI1+/SF1− progenitors descend from the fetal FAdE-utilizing SF1+ cells of the adrenal primordium (ref. 13; reviewed in refs. 14 and 15). This is supported by lineage tracing and specific deletion of SF1 in the zG, which results in a disorganized zG, but a normal zF (16). This suggests that progenitors might reside outside the zG. Accordingly, Pignatti et al. (17) have recently suggested that other cell populations may function to maintain adult homeostasis, as there is a discrepancy between the continuous need for new cortical cells and the infrequent capsular contribution of well-characterized GLI1+/SF1− adrenocortical stem cells.
Previously, we have demonstrated that Nestin+ cells are present in the adult human (18, 19) and murine (5) adrenal medulla. In the present paper, we have characterized a distinct population of Nestin+ progenitors in the murine adrenal cortex and analyzed its role in stress. Under basal conditions, these progenitors very slowly migrate centripetally through the different zones of the adrenal cortex to the cortical–medullary boundary. However, under stress, the progenitors migrate faster and differentiate into steroidogenic cells. In addition, we have isolated the Nestin+ cells from the adrenal cortex and characterized them in vitro. Thereby, we have shown that they display progenitor characteristics and are able to generate functional cells producing steroid hormones.
Results
Cells in the Adrenal Cortex Express Nestin-GFP.
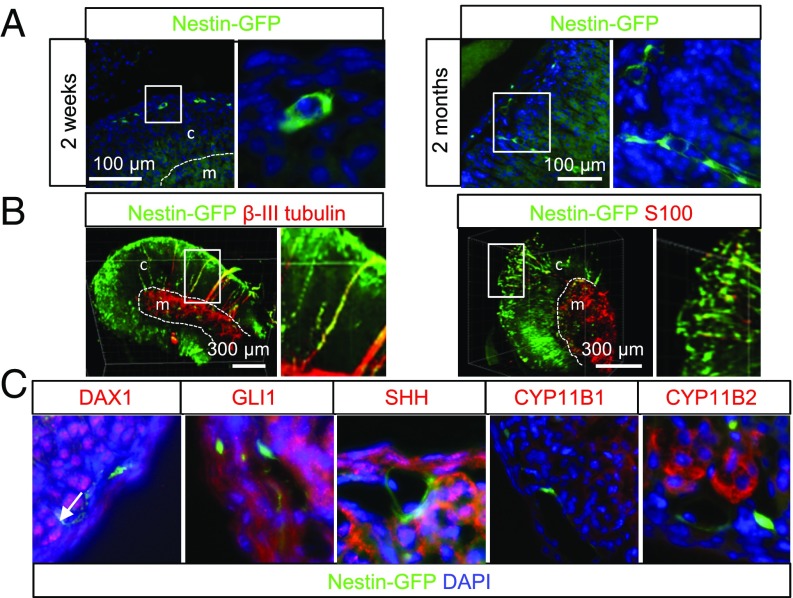
To investigate Nestin+ cells in the adrenal cortex, we used the Nestin-GFP transgenic mouse model (20), which has previously been validated to denote progenitors in a range of tissues (5, 21, 22). Immunohistochemistry showed that, in addition to the adrenal medulla, Nestin+ cells were also present in the cortex. In young mice [postnatal day (P) 14], almost all Nestin+ cells were located just underneath the capsule (Fig. 1A and SI Appendix, Fig. S1C). In adult mice, the majority of Nestin+ cells were found under the capsule, but some cells were also scattered through the zG and the zF (Fig. 1A). To perform 3D imaging of 2–5-mm adrenal sections and further characterize Nestin+ cells, we have developed a lipid clearing protocol for murine adrenals based on the method developed by Tomer et al. (23). Imaging of these adrenal sections showed that Nestin+ cells in the adult adrenal cortex and medulla were extensively interconnected (Fig. 1B and Movies S1 and S2). Nestin+ cells with long cellular protrusions/extensions positive for β-III tubulin or closely connected to β-III tubulin-positive neurons crossed the whole adrenal, thereby connecting the medulla with the capsule and making direct signaling possible. Furthermore, Nestin+ cells just underneath the capsule appeared to be interconnected all around the capsule (Fig. 1B and Movies S1 and S2). In adult mice, nearly all Nestin+ cells under the capsule were positive for the glial marker S100 (Fig. 1B and ref. 24), which has previously been reported to also mark progenitors of the adrenal medulla (5), neural progenitors of the intermediate zone of the developing cerebral cortex (25), and progenitors of hormone-producing cells in the anterior pituitary (26). In young (P14) mice, the number of double-positive cells was lower (SI Appendix, Fig. S1D).
Fig. 1.
Nestin-GFP–positive cells in the adrenal cortex. (A) Localization of Nestin+ cells in young (P14) and adult (2 mo old) mice. (B) Three-dimensional imaging of cleared adrenal sections costained with antibodies against β-III tubulin or S100. (C) Costaining with a panel of known progenitor/stem cell markers and steroidogenic enzymes. Double-positive cells are marked with arrows. Dashed lines mark the border between the cortex (c) and medulla (m).
Expression of Stem Cell Markers in Adrenocortical Nestin-Positive Cells.
To elucidate if Nestin marks a known population of progenitors to steroidogenic cells, we performed immunohistochemistry with a range of progenitor/stem cell and steroid markers. DAX1 is critical for the maintenance of adrenocortical progenitor cells (15), and we have previously shown that DAX1 is expressed in progenitors of the bovine adrenal cortex (27). In adult mice, double-positive cells were rarely observed (Fig. 1C and SI Appendix, Fig. S1A), and, in P14 mice, all Nestin+ cells were DAX1-negative (SI Appendix, Fig. S1D). In young and adult mice, we observed only a partial double staining with GLI1 and SHH (Fig. 1C and SI Appendix, Fig. S1A). Flow cytometry showed that, in the adrenal cortex of adult mice, 1–2% of the cells were Nestin+ (SI Appendix, Fig. S1E). The overlap with the cell surface markers CD44, CD73, CD90, and CD105 identifying mesenchymal stem cells (MSCs) (28) was also limited as observed by flow cytometry of cells isolated from whole adrenals (SI Appendix, Fig. S1F). Under normal conditions, the Nestin+ cells were negative for steroidogenic markers such as CYP11B1 and CYP11B2 (Fig. 1C and SI Appendix, Fig. S1B), SF1, and CYP11A1 (24). These results show that Nestin+ cells in the adrenal cortex are undifferentiated and differ from the known stem/progenitor populations in the adrenal cortex. Furthermore, they are highly connected around the capsule and throughout the cortex.
Nestin-Positive Cells from the Adrenal Cortex Show Progenitor Characteristics in Vitro.
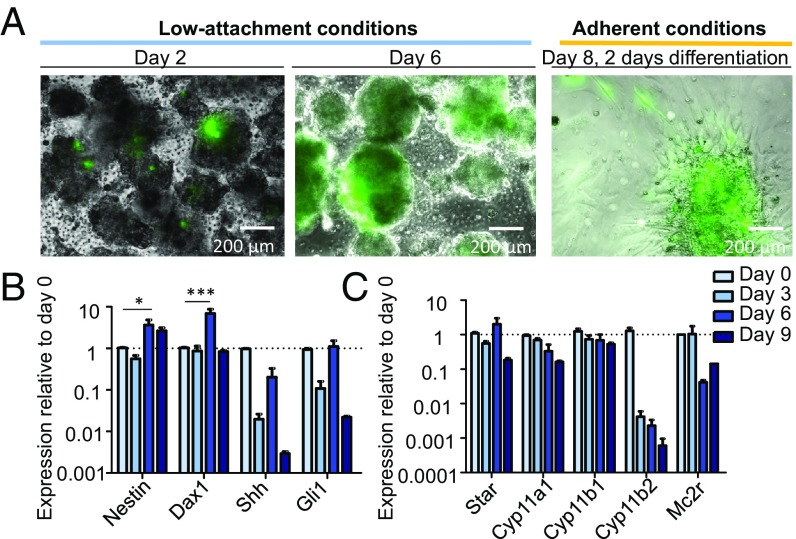
To characterize the properties of adrenocortical Nestin+ cells in vitro, the entire adrenal cortex from Nestin-GFP mice was carefully separated from the medulla to obtain a purely cortical culture (see ref. 24). Isolated cells, cultured under low-attachment conditions, started to form aggregates on the first day after isolation (Fig. 2A). The expression of GFP in these aggregates was initially heterogeneous, but, after 6 d in culture, the GFP expression was increased. A statistically significant increase in the expression of Nes (Nestin) was also seen at the transcriptomic level (Fig. 2B). The highest expression was found on day 6. An increase in the expression of Nr0b1 (Dax1) was also observed, whereas the expression of Shh and Gli1 decreased. The expression of all steroidogenic markers and the adrenocorticotropic hormone (ACTH) receptor Mc2r was decreased after 9 d of culture under low-attachment conditions (Fig. 2C), albeit not significantly. This suggests an enrichment of Nestin+ cells within the aggregates, as the expression of Nestin was maintained at a high level from day 6 of culture. We have previously shown that the Nestin+ cells are able to proliferate and self-renew (24). However, to elucidate if the Nestin+ cells are able to form clonal spheres alone or if other cells are necessary for the maintenance of the progenitors, adrenocortical cells from Nestin-GFP mice were flow-sorted into GFP+ and GFP− populations and cultured in stem cell-promoting cell culture medium. After 4 d, the GFP+ cells readily formed spheres (SI Appendix, Fig. S2A), indicating that the Nestin+ cells have proliferative potential that does not depend on the GFP− cell fraction. In contrast, only ∼10% of the GFP− cells formed spheres (SI Appendix, Fig. S2A). The GFP+ cells continued to express GFP during culture (SI Appendix, Fig. S2A). Surprisingly, the GFP− cells began to express GFP after 3–4 d in culture, suggesting that other stem cell populations might express Nestin at later time points or that the cell culture conditions can induce dedifferentiation in these cells. On the contrary, when this experiment was repeated with adrenomedullary cells from Nestin-GFP mice, the GFP− cells stayed negative during the culture period (SI Appendix, Fig. S2A). This suggests a higher plasticity of the cortical cells.
Fig. 2.
In vitro culture of adrenocortical progenitors. (A) Adrenocortical cells of Nestin-GFP mice were isolated and cultured under low-attachment conditions. On day 6, the culture conditions were changed and differentiation was induced. The expression of GFP was tracked throughout the experiment, and representative images are shown. (B) qRT-PCR showing the relative expression of various stem cell markers and (C) steroidogenic markers plus the ACTH receptor at 3-d intervals following isolation and culture. Data in B and C are presented as mean ± SEM (n ≥ 3). *P < 0.05; ***P < 0.001.
Cortical Nestin-Positive Progenitors Differentiate into Steroidogenic Cells in Vitro.
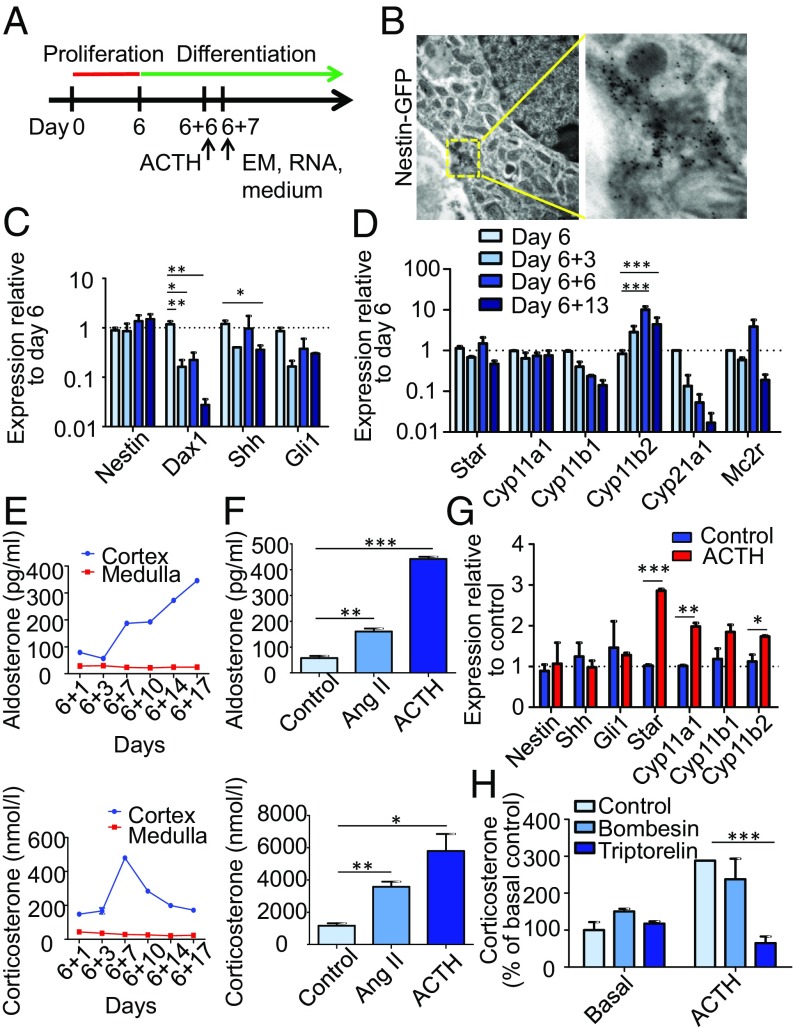
To further investigate the progenitor characteristics of the Nestin+ cells, we decided to test their differentiation capability. As we noted the highest expression of stem cell markers at day 6 of proliferation, we started the differentiation at that day. Thus, we changed the culture conditions by transferring the spheres from nonadherent plates to plates coated with poly-d-lysine and fibronectin. Furthermore, basic FGF (bFGF) was removed from the culture medium to promote differentiation (Fig. 3A). After 1–2 d in culture, the adherent cells started to migrate from the spheres and to differentiate (Fig. 2A). By using bright-field microscopy, we previously observed that a portion of the differentiated cells contained lipid droplets (24). Here, we show by EM that differentiated cells also contain numerous mitochondria, which is typical for steroid-producing cells of the zG. Immunogold labeling of GFP demonstrated that these mitochondria-rich cells were indeed the Nestin+ cells (Fig. 3B). qRT-PCR showed that the expression of Nes was unchanged during differentiation, whereas the expression of Nr0b1 and Shh decreased significantly. The expression of Gli1 also decreased, albeit not significantly (Fig. 3C). The expression of Star and Cyp11a1 was stable, whereas the expression of Cyp11b2 was significantly increased during differentiation. The expression of Cyp11b1 and Cyp21a1 decreased, albeit not significantly (Fig. 3D). By ELISA, we observed an increase in corticosterone secreted into the growth medium until day 7 of differentiation, with a subsequent decrease. The level of aldosterone was augmented during the whole differentiation process (Fig. 3E). Further steroid profiling was performed by liquid chromatography/tandem MS. Here, we measured various steroids secreted to the culture medium (SI Appendix, Fig. S3A). Cortisol, 11-desoxycortisol, cortisone, and 17-OH-progesterone were not detectable, which showed that, as in adult mice in vivo (29), CYP17A1 was not expressed in vitro. Pregnenolone, 11-desoxycorticosterone, and corticosterone showed the highest levels at day 7 of differentiation, as also shown by ELISA for corticosterone. The progesterone level also had a peak at day 7, but, after a decrease until day 17, the level started to increase. The aldosterone level increased until day 14. These results demonstrate that adrenocortical progenitors can be isolated and differentiated into zG and zF steroidogenic cells in vitro. During extended differentiation conditions, adrenocortical cells isolated from WT C57BL/6N or Nestin-GFP mice partly lost their Nestin expression at the protein level while becoming positive for StAR and CYP11B2 (SI Appendix, Fig. S4A and ref. 24). Therefore, we decided to isolate adrenocortical cells from the tamoxifen-inducible Nes-CreERT/R26R-eYFP mouse line and induce recombination in vitro, as the Nestin-derived cells will remain YFP+ (described in ref. 5). After 6 d of proliferation, cells in spheres were all YFP+ (SI Appendix, Fig. S4B). One week of differentiation revealed that YFP+ cells became positive for steroidogenic markers like StAR, SF1, CYP11A1, CYP11B1, and CYP11B2, but negative for chromogranin A, a marker for chromaffin cells (SI Appendix, Fig. S4C). To determine if the Nestin+ cells are able to differentiate into steroidogenic cells without signals from other populations, Nestin-GFP+ cells were flow-sorted, cultured under conditions promoting their proliferation for 9 d, and subsequently cultured under conditions promoting their differentiation for 11 d. We observed that the GFP signal was diminished followed by acquisition of steroidogenic markers like StAR and CYP11B2, indicating their differentiation capacity (SI Appendix, Fig. S2 B and C).
Fig. 3.
In vitro differentiation of adrenocortical progenitors. (A) Adrenocortical cells isolated from Nestin-GFP mice were allowed to proliferate for 6 d before differentiation was induced. (B) EM image shows Immunogold labeling of GFP on day 7 of differentiation. (C) qRT-PCR depicts the relative expression of stem cell markers and (D) steroidogenic markers plus the ACTH receptor at different time points following culture in differentiation conditions. (E) Aldosterone and corticosterone levels in the media as measured by ELISA. (F) Aldosterone and corticosterone levels after 6 d of differentiation and exposure to Ang II or ACTH for 24 h. (G) qRT-PCR shows the relative expression of stem cell and steroidogenic markers in ACTH-treated cells compared with control cells. (H) Corticosterone levels in control and ACTH-stimulated cells treated with triptorelin and bombesin. Data in C, D, and F–H are presented as mean ± SEM (n ≥ 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Response to Angiotensin II and ACTH.
Angiotensin II (Ang II) is the key enzyme in the renin–angiotensin–aldosterone system and known to regulate the biosynthesis of aldosterone in the zG through the induction of HSD3B and CYP11B2 (30). Stimulation with Ang II for 24 h on day 6 of differentiation increased the levels of aldosterone (180%) and corticosterone (200%; Fig. 3F).
ACTH produced in the pituitary during stress affects differentiation and steroidogenesis partly through SF1-induced transcription of steroidogenic enzymes leading to an increase in glucocorticoids: cortisol in humans and corticosterone in mice (31). The expression of the ACTH receptor Mc2r during the differentiation of adrenocortical progenitors was investigated. We observed that the highest expression was seen on day 6 of differentiation, and the expression subsequently decreased (Fig. 3D). Therefore, we decided to look at the responses to ACTH on day 6 of differentiation. The expression of the stem cell markers Nes, Shh, and Gli1 was unchanged, but that of the steroidogenic enzymes Star, Cyp11a1, and Cyp11b2 was significantly increased (Fig. 3G). The levels of aldosterone and corticosterone secreted into the culture medium were greatly augmented; the aldosterone concentration increased by 670% and the corticosterone concentration by 400% (Fig. 3F).
Previously, we tested various pharmacological agents for their ability to influence the properties of a bioartificial bovine adrenal cortex. In this former study, two peptides, triptorelin and bombesin, were shown to increase cell functionality and proliferative potential when applied to bovine adrenocortical cells encapsulated in alginate (27). When we applied bombesin to our differentiating cells, the production of corticosterone was unchanged. With triptorelin alone, no changes were observed, but, in combination with ACTH, the stimulatory effect of ACTH on corticosterone production was significantly inhibited (Fig. 3H).
Nestin-Expressing Cortical Progenitors Migrate Centripetally Toward the Medulla.
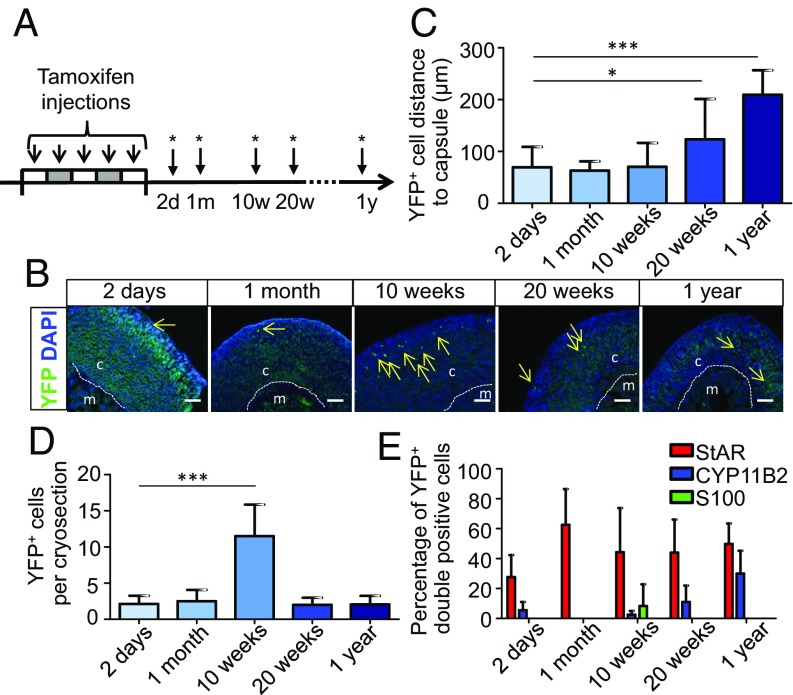
To determine the fate of Nestin+ cells in the adrenal cortex in vivo, we lineage-traced these cells by using the Nes-CreERT/R26R-eYFP mouse model. In these animals, the administration of tamoxifen transiently activated Cre in a proportion of Nestin+ cells, resulting in a permanent expression of YFP in those cells and their descendants. Mice treated with tamoxifen were analyzed at 2 d, 1 mo, 10 wk, 20 wk, and 1 y after the injections (Fig. 4A). For 10 wk, the YFP+ cells were mainly located in the zG underneath the capsule (Fig. 4 B and C). However, after 10 wk, the numbers of YFP+ cells had increased significantly, indicating that the cells were proliferating (Fig. 4D). After 20 wk, the distance of YFP+ cells to the capsule had augmented significantly (Fig. 4C), even though the number of YFP+ cells decreased (Fig. 4D), indicating that a proportion of the cells had undergone apoptosis at the cortical–medullary boundary. After 1 y, nearly all remaining YFP+ cells were located in the zF close to the adrenal medulla (Fig. 4 B and C). Two days after the injections of tamoxifen, all cells lost their expression of S100 and a part became StAR-positive (∼25%) or CYP11B2-positive (∼5%; Fig. 4E and SI Appendix, Fig. S5A). After 1 y, ∼50% of the descendants of Nestin+ cells were StAR-positive and ∼30% were CYP11B2-positive (Fig. 4E and SI Appendix, Fig. S5A). These results clearly demonstrate a pattern of slow centripetal migration toward the adrenal medulla, whereby a proportion of the Nestin+ progenitors proliferate, differentiate, and acquire steroidogenic markers.
Fig. 4.
Tracing of Nestin-positive cells in vivo. (A) Nes-CreERT/R26R-eYFP mice were injected with tamoxifen for five consecutive days to induce recombination. Mice were killed at different time points as indicated with asterisks. (B) YFP+ cells in the adrenals are indicated with arrows. (Scale bars, 100 µm.) (C) The distance of YFP+ cells to the adrenal capsule. *P < 0.05; ***P < 0.001. (D) Number of YFP+ cells per cryosection. ***P < 0.001. (E) Quantification of double-positive cells (n ≥ 3 mice per time point, n ≥ 3 cryosections per adrenal). Data in C–E are presented as mean ± SEM. Dashed lines mark the border between the cortex (c) and medulla (m).
Stress Promotes the Differentiation and Migration of Nestin-Expressing Cells in the Adrenal Cortex.
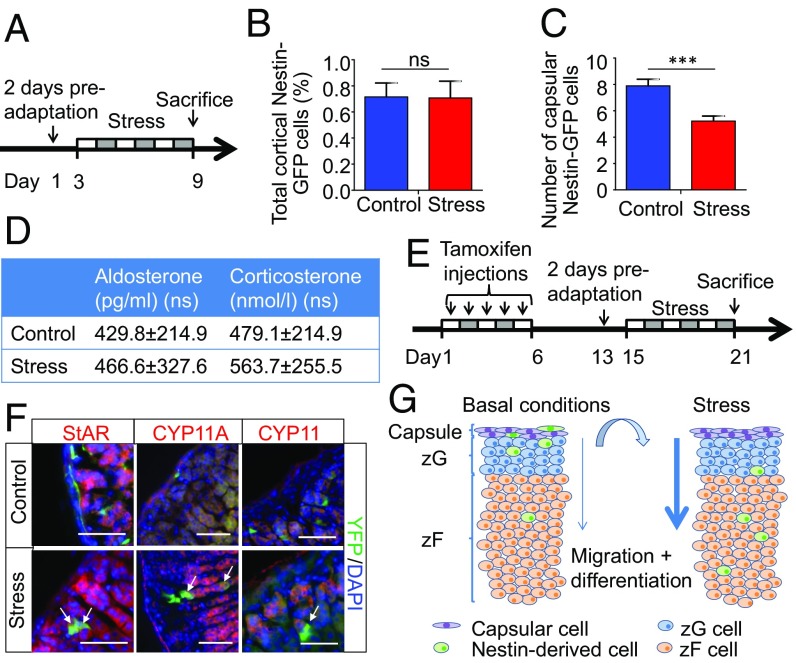
Previously, we have addressed the role of adrenomedullary progenitors in stress (5). Nestin-GFP mice were exposed to repeated stress of immobilization (2 h of restraint stress per day for six consecutive days; Fig. 5A). In addition, mice from the stress group were isolated in individual cages 2 d before starting the stress experiments, whereas the mice in the control group were kept together in one cage. Mice exposed to stress lost body weight, whereas their adrenal weights increased significantly compared with control mice (5). By using the same stress protocol, we characterized the adrenocortical progenitors. We observed that the total count of Nestin+ cells in the cortex was unchanged (Fig. 5B), whereas the number of capsular Nestin+ cells decreased (Fig. 5C), indicating fast centripetal migration under stress. The concentrations of corticosterone and aldosterone in the serum were the same in control mice and mice exposed to the stress of immobilization (Fig. 5D). To lineage trace Nestin+ cells under stress, we used Nes-CreERT/R26R-eYFP mice, in which the expression of YFP was induced upon tamoxifen administration for 5 d (Fig. 5E). After a resting period of 7 d, mice were subjected to 6 d of immobilization stress as described here earlier. As in the case of Nestin-GFP cells, the YFP+ cells migrated centripetally from the capsule in the direction of the medulla. During this migration, they became positive for the steroidogenic markers StAR, CYP11A1, and CYP11B2 (Fig. 5F and SI Appendix, Fig. S5B). These results indicate that, under stress, the migration of Nestin+ cells from the adrenal capsule in the direction of the adrenal medulla and their subsequent differentiation into steroidogenic cells are greatly increased (Fig. 5G). This reveals an important supporting role of Nestin+ progenitors in stress situations.
Fig. 5.
Stress induces the generation of steroidogenic cells from the Nestin-population. (A) Nestin-GFP mice were subjected to restraint stress for 2 h per day for 6 d. (B) On the last day, mice were killed, and adrenals were processed for immunostaining. The total number of Nestin+ cells was counted. (C) Number of capsular Nestin+ cells. (D) Plasma corticosterone and aldosterone levels as measured by ELISA (n = 6). (E) To trace Nestin+ cells in vivo after stress, Nes-CreERT/R26R-eYFP mice were injected with tamoxifen for five consecutive days. After a resting period, the mice were subjected to restraint stress. (F) Immunostaining of adrenals from control mice and mice injected with tamoxifen and subjected to immobilization stress (n = 5). Double-positive cells are marked with arrows. Representative images are shown. (G) Schematic representation of the function of Nestin+ progenitors in the adrenal cortex. Under normal conditions, Nestin+ progenitors are mainly located in the capsule or subcapsular region. Over time, they very slowly migrate centripetally in the direction of the adrenal medulla. Under stress, migration and differentiation are greatly increased. Data in B and C are presented as mean ± SEM. ns, not significant; ***P < 0.001.
Discussion
Taken together, these in vitro and in vivo experiments suggest that, under normal conditions, Nestin+ progenitors with long processes are mainly located in the capsule or subcapsular region of the adrenal. These cells are connected under the adrenal capsule, and also physically interact with Nestin+ cells in the medulla. Our results indicate that Nestin+ cells in the cortex and medulla form a network of interconnected cells but belong to two different populations, as their differentiation potentials are distinct. In the dentate gyrus, Nestin+ adult radial glia with neural stem cell properties also have long processes showing an intimate relationship with synapses, blood vessels, and astrocytes, providing a link between their local niche and hippocampal neurogenesis (22). Nestin+ MSCs in the bone marrow also display this morphology (32), as do anterior pituitary stem cells (33). Furthermore, catecholaminergic nerve fibers and hematopoietic stem cells were shown to be closely associated with Nestin+ MSCs, indicating a niche, which is regulated by paracrine cell–cell signaling and endocrine signaling from hormones and the autonomic nervous system (32). In addition, vascular Nestin+ cells in the testis, whichtransform into Leydig cells, exhibit protrusions connecting them with the vessels from which they were derived (34). These observations fit with our observations in the adrenal, where we see that the Nestin+ cells seem to be closely associated with the nerve fibers crossing the adrenal cortex, thereby enabling paracrine cell–cell signaling in the adrenal but also endocrine signaling from the brain.
The lack of costaining with well-known adrenocortical stem cell markers like DAX1, SHH, and GLI1 indicates that the Nestin+ cells do not belong to the SHH+ or GLI1+ progenitors known to be involved in daily cell renewal and regeneration in the adrenal cortex (9). However, as the expression of Shh and Gli1 decreased during culture of the total adrenocortical progenitors and a proportion of Nestin− cells acquired expression of Nestin, it is possible that Nestin+ progenitors are descendants of a subpopulation of SHH+ or GLI1+ progenitors. As the Nestin+ progenitors have the potential to migrate and differentiate into steroid-producing cells, an ability greatly enhanced during stress, and also are highly reactive to ACTH, we suggest that they have a unique role during stress adaptation. We have previously described a similar situation in the adrenal medulla, where Nestin+ progenitors under normal conditions are able to differentiate into glia and neuronal and chromaffin cells (5). However, under stress, in which the adrenal shows an extremely adaptive response, they preferentially differentiate into chromaffin cells. As the adrenal medulla and cortex are two cellular systems united under the capsule, it is obvious that, if one undergoes changes, the other has to adapt in response. This is supported by the facts that, during stress, when an acute activation of the adrenal medulla by the splanchnic nerves triggers the release of epinephrine and various neuropeptides, the release of adrenal glucocorticoids and mineralocorticoids is also mediated in a paracrine way (35). In reality, there is an active cellular and functional interaction of cortical and chromaffin cells within the gland. Whereas adrenocortical glucocorticoids are required for the biosynthesis of adrenomedullary epinephrine, catecholamines regulate the release of steroids and the cellular function of the adrenal cortex (36). Furthermore, patients with disorders of the adrenal cortex such as Addison’s disease or congenital adrenal hyperplasia display a dysfunction of the adrenal medulla, resulting in an impaired stress response (37–39). Therefore, we suggest that there could be a joint subset of progenitors in the adrenal medulla and cortex that, in a synergistic way, share cellular pathways for coordinating regeneration and adaptation to stress (Fig. 6). The proposed model integrates the population of Nestin+ stress-dependent subgroup of progenitors in the overall concept of stem cell regulation. The proliferation- and differentiation-inducing potential of stress on stem-like cells is of particular interest because it cannot be excluded that hyperactivation of those cells can lead to the development of cancer. Recently, we also introduced the concept of stress-induced stem cells. Here, we propose that the effects of stress on stem/progenitor cells from a range of tissues, during the early stages of postnatal development, may predispose to adult disease (40). Interestingly, we did not observe any increase in the serum corticosterone and aldosterone levels after stress. A possible explanation is that the levels measured in control mice were already very high compared with earlier reported values (41). This might be a result of the CO2/O2 anesthesia, which has previously been shown to increase serum corticosterone levels (42).
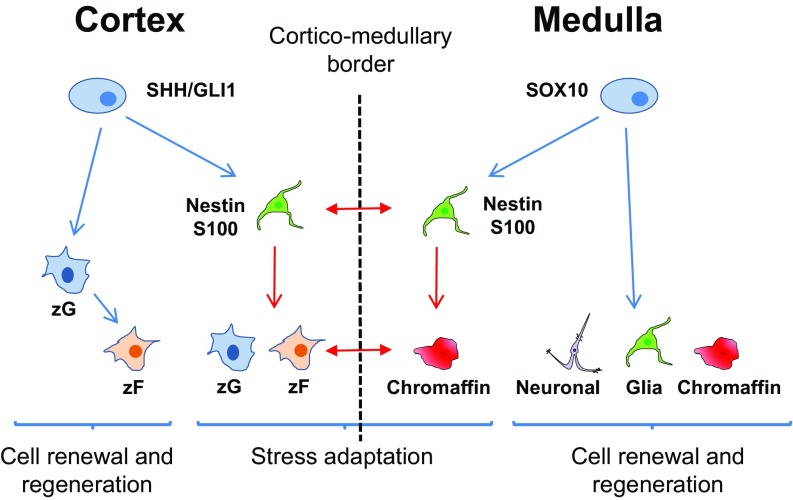
Fig. 6.
Progenitor cells in the adrenal. The adrenal cortex and medulla are two cellular systems under the adrenal capsule. In the cortex, SHH+ and GLI1+ progenitors are involved in daily cell renewal and regeneration and first differentiate into zG cells and then transdifferentiate into zF cells. In the medulla, SOX10+ progenitors are fundamental for regeneration by differentiating into neuronal, glial, and chromaffin cells. Under stress, Nestin+ progenitors, which are potentially subpopulations of SHH/GLI1 cells in the cortex and SOX10 cells in the medulla, are important for synergetic remodeling and adaptation. Although the Nestin+ progenitors in the cortex and medulla are distinct subpopulations, they are both induced by stress in a coordinated manner. Blue arrows mark differentiation under normal conditions, red arrows mark differentiation under stress, and red double arrows mark cell–cell interactions under stress.
We have been able to isolate the adrenocortical Nestin+ progenitors and culture them in vitro. The differential expression of various progenitor markers in adrenocortical cells, isolated from mice, is similar to what we observed in cultures of bovine adrenocortical cells, in which we also saw the highest expression on days 6–7 of culture (27). On day 7 of differentiation, the cells were highly reactive to Ang II and ACTH, indicating that, at this point, there is a mixture of zG and zF cells. However, in the culture conditions used in this study, the differentiation into corticosterone-producing cells was stopped after 7 d. The cell phenotype with few lipid droplets, numerous mitochondria, and the elevated expression of CYP11B2 also indicates that we mostly saw zG-like cells. However, as murine adrenal cells do not produce cortisol, and aldosterone is synthesized from corticosterone (SI Appendix, Fig. S3B), it is not possible to draw a conclusion. By optimizing the culture conditions and growth factors, this process can probably be refined and further controlled. When we added the synthetic peptide triptorelin, an LHRH agonist, which, in addition to stimulating the secretion of luteinizing hormone and follicle-stimulating hormone from the pituitary, can increase the release of cortisol from adrenocortical cells (43, 44), we did not observe any change in corticosterone production under basal conditions. However, triptorelin inhibited the stimulatory effect of ACTH on corticosterone production. Another compound, the neuropeptide and growth factor bombesin that activates GLI1 and regulates proliferation in many progenitor cell types (45), did not show any effect, again suggesting that the Nestin+ cells are distinct from the GLI1+ cells and that bombesin receptors are not expressed on the Nestin+ progenitors.
Lineage-specific stem cells from adult tissues are important sources for cell-replacement therapies. Recently, we have shown that the expression of progenitor markers is associated with the functionality of a bioartificial adrenal cortex (27). Our demonstration that Nestin+ progenitors can be cultured in vitro and differentiated into mineralocorticoid- and glucocorticoid-producing cells therefore provides a source of cells to address, for example, adrenal insufficiency, which makes this study very relevant clinically.
Materials and Methods
Detailed materials and methods are described in SI Appendix, SI Materials and Methods. All animal experiments were approved according to the German Animal Welfare Act by the Landesdirektion Sachsen, Germany.
Supplementary Material
Acknowledgments
We thank Uta Lehnert and Linda Friedrich for technical assistance, Doreen Streichert for performing the EM experiments, and Celso Gomez-Sanchez for the CYB11B2 antibodies. This study was supported by the TransCampus initiative between Technische Universität Dresden and King’s College London and by the DFG Grants CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” (to C.S., C.L.A., and S.R.B.), IRTG2251 (to C.S., C.L.A., and S.R.B.), SFB655 (to S.R.B.), and CRC252 (to S.R.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814072115/-/DCSupplemental.
References
- 1.Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30:1433–1440. doi: 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge Y, Fuchs E. Stretching the limits: From homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet. 2018;19:311–325. doi: 10.1038/nrg.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicard F, et al. Age-dependent regulation of chromaffin cell proliferation by growth factors, dehydroepiandrosterone (DHEA), and DHEA sulfate. Proc Natl Acad Sci USA. 2007;104:2007–2012. doi: 10.1073/pnas.0610898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KF, Qin N, Androutsellis-Theotokis A, Bornstein SR, Ehrhart-Bornstein M. Effects of dehydroepiandrosterone on proliferation and differentiation of chromaffin progenitor cells. Mol Cell Endocrinol. 2011;336:141–148. doi: 10.1016/j.mce.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Rubin de Celis MF, et al. Multipotent glia-like stem cells mediate stress adaptation. Stem Cells. 2015;33:2037–2051. doi: 10.1002/stem.2002. [DOI] [PubMed] [Google Scholar]
- 6.Chang SP, et al. Cell proliferation, movement and differentiation during maintenance of the adult mouse adrenal cortex. PLoS One. 2013;8:e81865. doi: 10.1371/journal.pone.0081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto H, Mitani F, Mukai K, Suematsu M, Ishimura Y. Studies on cytogenesis in adult rat adrenal cortex: Circadian and zonal variations and their modulation by adrenocorticotropic hormone. J Biochem. 1999;126:1175–1183. doi: 10.1093/oxfordjournals.jbchem.a022564. [DOI] [PubMed] [Google Scholar]
- 9.Finco I, Lerario AM, Hammer GD. Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology. 2018;159:579–596. doi: 10.1210/en.2017-03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertholet JY. Proliferative activity and cell migration in the adrenal cortex of fetal and neonatal rats: An autoradiographic study. J Endocrinol. 1980;87:1–9. doi: 10.1677/joe.0.0870001. [DOI] [PubMed] [Google Scholar]
- 11.Finco I, LaPensee CR, Krill KT, Hammer GD. Hedgehog signaling and steroidogenesis. Annu Rev Physiol. 2015;77:105–129. doi: 10.1146/annurev-physiol-061214-111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol Cell Endocrinol. 2011;336:117–122. doi: 10.1016/j.mce.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood MA, et al. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140:4522–4532. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerario AM, Finco I, LaPensee C, Hammer GD. Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Front Endocrinol (Lausanne) 2017;8:52. doi: 10.3389/fendo.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walczak EM, Hammer GD. Regulation of the adrenocortical stem cell niche: Implications for disease. Nat Rev Endocrinol. 2015;11:14–28. doi: 10.1038/nrendo.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman BD, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pignatti E, Leng S, Carlone DL, Breault DT. Regulation of zonation and homeostasis in the adrenal cortex. Mol Cell Endocrinol. 2017;441:146–155. doi: 10.1016/j.mce.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung KF, et al. Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem Cells. 2009;27:2602–2613. doi: 10.1002/stem.180. [DOI] [PubMed] [Google Scholar]
- 19.Langton K, et al. Hypertensive crisis in pregnancy due to a metamorphosing pheochromocytoma with postdelivery Cushing’s syndrome. Gynecol Endocrinol. 2018;34:20–24. doi: 10.1080/09513590.2017.1379497. [DOI] [PubMed] [Google Scholar]
- 20.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 21.Jiang MH, et al. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24:1466–1485. doi: 10.1038/cr.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss J, et al. Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci USA. 2016;113:E2536–E2545. doi: 10.1073/pnas.1514652113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomer R, Ye L, Hsueh B, Deisseroth K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc. 2014;9:1682–1697. doi: 10.1038/nprot.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steenblock C, et al. Adrenal cortical and chromaffin stem cells: Is there a common progeny related to stress adaptation? Mol Cell Endocrinol. 2017;441:156–163. doi: 10.1016/j.mce.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Vinci L, et al. Immunohistochemical markers of neural progenitor cells in the early embryonic human cerebral cortex. Eur J Histochem. 2016;60:2563. doi: 10.4081/ejh.2016.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higuchi M, et al. GFP-expressing S100β-positive cells of the rat anterior pituitary differentiate into hormone-producing cells. Cell Tissue Res. 2014;357:767–779. doi: 10.1007/s00441-014-1890-0. [DOI] [PubMed] [Google Scholar]
- 27.Balyura M, et al. Expression of progenitor markers is associated with the functionality of a bioartificial adrenal cortex. PLoS One. 2018;13:e0194643. doi: 10.1371/journal.pone.0194643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niehage C, et al. The cell surface proteome of human mesenchymal stromal cells. PLoS One. 2011;6:e20399. doi: 10.1371/journal.pone.0020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keeney DS, Jenkins CM, Waterman MR. Developmentally regulated expression of adrenal 17 alpha-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology. 1995;136:4872–4879. doi: 10.1210/endo.136.11.7588219. [DOI] [PubMed] [Google Scholar]
- 30.Nogueira EF, Bollag WB, Rainey WE. Angiotensin II regulation of adrenocortical gene transcription. Mol Cell Endocrinol. 2009;302:230–236. doi: 10.1016/j.mce.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sewer MB, et al. Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54(nrb)/NonO, protein-associated splicing factor, and SF-1, a complex that also participates in repression of transcription. Endocrinology. 2002;143:1280–1290. doi: 10.1210/endo.143.4.8748. [DOI] [PubMed] [Google Scholar]
- 32.Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andoniadou CL, et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. doi: 10.1016/j.stem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Davidoff MS, et al. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA, Pfeiffer EF, Holst JJ. Effects of splanchnic nerve stimulation on the adrenal cortex may be mediated by chromaffin cells in a paracrine manner. Endocrinology. 1990;127:900–906. doi: 10.1210/endo-127-2-900. [DOI] [PubMed] [Google Scholar]
- 36.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 37.Bornstein SR, et al. Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 39.Merke DP, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 40.Bornstein SR, et al. Stress-inducible-stem cells: A new view on endocrine, metabolic and mental disease? Mol Psychiatry. September 21, 2018 doi: 10.1038/s41380-018-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Foong D, Cooper MS, Seibel MJ, Zhou H. Comparison of blood sampling methods for plasma corticosterone measurements in mice associated with minimal stress-related artefacts. Steroids. 2018;135:69–72. doi: 10.1016/j.steroids.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Altholtz LY, Fowler KA, Badura LL, Kovacs MS. Comparison of the stress response in rats to repeated isoflurane or CO2:O2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci. 2006;45:17–22. [PubMed] [Google Scholar]
- 43.Nekola MV, Pedroza E, Schally AV. Paradoxical effects of D-Trp6-LHRH in immature female rats correlated with changes in ACTH, prolactin, and corticosterone levels. Biol Reprod. 1981;24:505–511. doi: 10.1095/biolreprod24.3.505. [DOI] [PubMed] [Google Scholar]
- 44.Balyura M, et al. Transplantation of bovine adrenocortical cells encapsulated in alginate. Proc Natl Acad Sci USA. 2015;112:2527–2532. doi: 10.1073/pnas.1500242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castellone MD, et al. Cross talk between the bombesin neuropeptide receptor and Sonic hedgehog pathways in small cell lung carcinoma. Oncogene. 2015;34:1679–1687. doi: 10.1038/onc.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.