Abstract
Vaccines impact antibiotic-resistant infections in two ways: through a direct reduction in the organisms and strains carrying resistant genes that are specifically targeted by the vaccine and also via a secondary effect through a reduction in febrile illnesses that often lead to the use of antibiotics. We review here the impact of pneumococcal conjugate vaccines (PCVs) on the prevalence of antibiotic-resistant disease and antibiotic usage as an example of the direct effect of vaccines on antibiotic resistance and the impact of influenza vaccination on antibiotic usage as an example of a secondary effect. A prelicensure study of a PCV in Africa demonstrated 67% fewer penicillin-resistant invasive disease episodes in the PCV group compared with controls. Similar studies in the United States and Europe demonstrated reductions in antibiotic use consistent with the vaccines’ impact on the risk of otitis media infections in children. Postlicensure reductions in the circulation of antibiotic-resistant strains targeted by the vaccines have been dramatic, with virtual elimination of these strains in children following vaccine introduction. In terms of a secondary effect, following influenza vaccination reductions of 13–50% have been observed in the use of antibiotics by individuals receiving influenza vaccine compared with controls. With the demonstrated effectiveness of vaccination programs in impacting the risk of antibiotic-resistant infections and the increasing threat to public health that these infections represent, more attention needs to be given to development and utilization of vaccines to address antibiotic resistance.
Keywords: antibiotic resistance, vaccines, pneumococcal vaccine, influenza vaccine
Soon after the beginning of the antibiotic era in the 1950s, resistance to newly developed antibiotics such as sulfonamides, tetracycline, and penicillin emerged. This phenomenon has continued with resistance to each antibiotic occurring shortly after its introduction (1). More recently, bacteria resistant to multiple antibiotics have emerged and become increasingly problematic, with some strains now resistant to almost all or even all antibiotics (2). It is now estimated that antibiotic resistance (ABR) currently causes 700,000 deaths annually (3). Without a dramatic intervention, this number is projected to grow in coming years with detrimental effects in both human and economic terms.
The last 25 years have seen an explosion in vaccine development. Vaccines have been developed to target major causes of morbidity and mortality globally, including vaccines directed against the pneumococci, Haemophilus influenzae type b (Hib), rotavirus, and the meningococci as well as improved vaccines for influenza (4). Vaccines against these targets have been developed because of the high burden of disease caused by each of these pathogens rather than any potential impact on ABR. However, many of these vaccines have had dramatic impacts on ABR. Vaccines in development such as respiratory syncytial virus (RSV) vaccines and group B streptococcal (GBS) vaccines may similarly further reduce antibiotic use and resistance. Here we review selected available data documenting the impact of existing vaccines on ABR and discuss the implications these data have for future vaccines specifically targeting ABR organisms.
Types of Vaccine Effects
Vaccines can impact ABR primarily in two ways. Vaccines exert their primary effect by reducing or eliminating the risk of infection due to antibiotic-resistant strains. Such effects can be achieved through antibody-mediated killing of an organism in the lung or blood stream, for example, or through the impact on the colonization and hence circulation of an organism, or both (5). Examples of such currently licensed vaccine interventions include conjugate vaccines for Hib and the pneumococcus. Future vaccines in development in this category include vaccines for GBS infection and staphylococci.
Vaccines can also have a secondary effect on ABR by obviating antibiotic use by reducing the rates of febrile illness and the likelihood of secondary infections following the prevented episode. Since a clear link has been demonstrated between increased antibiotic usage and increasing antibiotic resistance, such effects are important (6). Secondary effects can be seen with current vaccines targeting viral illnesses such as influenza and measles, which prevent not only febrile illness but also secondary bacterial infections, as well as potential future vaccines such as an RSV vaccine.
Here we explore the impact of two examples: pneumococcal conjugate vaccine (PCV) and influenza vaccine.
Pneumococcal Conjugate Vaccine
PCVs were first introduced with the seven-valent vaccine (PCV7) in 2000 (7). The PCV7 vaccine demonstrated more than 90% efficacy against invasive pneumococcal disease (IPD) in the United States in the primary target population of young children. Subsequently both 10- and 13-valent vaccines have been introduced and are now in widespread use globally (8). For all these vaccines, significant reductions in the carriage of the vaccine serotypes has been observed in children, leading to an indirect reduction in the rates of disease due to vaccine strains in individuals who did not receive the vaccine (“herd protection”) (9). In the United States, these reductions have been dramatic, especially in adults >65 y of age. In the 1990s, before the introduction of PCV in the United States, there were an estimated 60,000 cases of IPD/y. At that time, the proportion of cases due to antibiotic-resistant strains was increasing, and an increasing number of cases of disease were due to pneumococci resistant to three or more antibiotics. By 2007, 7 y following PCV7 introduction, an estimated 211,000 of these cases of disease (both antibiotic-susceptible and -resistant) caused by the seven serotypes in the vaccine had been prevented in the US population (10). Although some of this reduction might be due to reduced blood culture sampling, the virtual elimination of the circulation of vaccine strains in the population and the observed reductions of disease in unvaccinated adults make it likely that the observed reductions in disease in children were attributable to vaccine use (11).
It is important to note that while the primary selection criteria for serotypes selected to be included in PCVs was the estimated of burden of disease for each serotype, most of the selected strains also harbored antibiotic resistance. The higher-valency PCVs now target 10–13 pneumococcal strains, including 90% of the antibiotic-resistant strains causing disease in children at the time of PCV13 introduction in the United States (12). PCVs have also been shown to reduce antibiotic-resistant disease in unvaccinated adults because of the reduced circulation of these stains in the population after the introduction of childhood vaccination programs (13). While antibiotic use continues to select for ABR strains in the remaining nonvaccine serotypes, the burden of pneumococcal disease overall has been greatly reduced. Data from studies of these vaccines provide further information on their impact on ABR globally (Table 1).
Table 1.
Impact of PCV and influenza vaccine on ABR
| Country | Vaccine | Observed impact |
| Prelicensure pneumococcal efficacy studies showing impact on incidence of resistance | ||
| Finland | PCV10 | 8% reduction in prescriptions for antibiotics for first episode of otitis media |
| Higher reduction in children with more frequent otitis media (17) | ||
| South Africa | PCV9 | 67% fewer penicillin-resistant IPD episodes |
| 56% fewer co-trimoxazole–resistant episodes | ||
| 56% fewer infections resistant to any antibiotic compared with controls (15). | ||
| United States | PCV7 | 5.4% (95% CI 4.0–6.7%) fewer antibiotic prescriptions written compared with controls. |
| The use of second-line antibiotics was reduced by 12.6% (95% CI 9.6–15.6%). | ||
| PCV7 prevented 35 antibiotic prescriptions per 100 children vaccinated in 3.5 y of follow-up (14). | ||
| Postlicensure observational studies of PCV impact | ||
| Australia | PCV13 | Following PCV introduction, ABR remains low in children and adults with 12% penicillin nonsusceptible in 2011 and 10% in 2012 (34) |
| Iceland | PCV10 | Isolates from the vaccine-eligible cohort had lower penicillin minimum inhibitory concentrations, less resistance to erythromycin, and less multidrug resistance than isolates from the control group (35). |
| Japan | PCV7 | Proportion of penicillin-resistant isolates declined from 54.7% before widespread PCV use to 5.1% in 2012. |
| Increases in 15B and 35B ABR serotypes (36) | ||
| Korea | PCV7 | Of multiply-resistant strains, the proportion due to PCV7 serotypes decreased from 65.2% to 21.7% following availability of PCV7 as an optional vaccine (37). |
| South Africa | PCV7 followed by PCV13 | Rates of IPD due to penicillin-nonsusceptible strains declined 82% (95% CI = −85, −78) |
| Ceftriaxone-nonsusceptible IPD strains declined 85% (95% CI = −91, −77) (38) | ||
| Sweden | PCV10 and PCV13 | Between 2005 and 2016 IPD in vaccinated children decreased by 68.5% and by 13.5% in the whole population |
| No reduction in the elderly where NVT strains became prominent | ||
| For PCV10 and PCV13, respectively, penicillin nonsusceptibility increased from 2.5% and 4.0% in 2007 to 3.7% and 6.6% in 2013–2016, primarily driven by expansion of nonsusceptible NVTs (24). | ||
| Switzerland | PCV7 followed by PCV13 | Between 2004 and 2014, the lowest nonsusceptibility rates were found in the recent years 2013–2014, 3 and 8 y, respectively, after the PCV13 and PCV7 recommendations for children aged <2 y (39). |
| United States | PCV7 | Reduction of 81% in the proportion of penicillin-nonsusceptible IPD strains in children aged <2 y |
| Reduction of 49% in the proportion of penicillin-nonsusceptible IPD strains in adults aged >65 y | ||
| For multidrug-resistant strains, a 57% reduction in IPD overall (12) | ||
| United States | PCV13 | Percent nonsusceptible by meningitis breakpoint decreased from 53.9% to 35.3% for penicillin and from 9.3% to 1.7% for cefotaxime (39) |
| Efficacy studies of influenza vaccine impact on antibiotic use | ||
| Turkey | Influenza | Episodes of acute otitis media and otitis media with effusions occurred in 2.3% and 22.8% of influenza vaccine recipients, respectively, vs. 5.2% and 31.1% of controls during influenza season; P < 0.001 (26) |
| Italy | Influenza | In a control study of influenza vaccination, vaccinated children had 13.2% fewer antibiotic prescriptions during the 6-mo observation period in their study; study children had 54.8% fewer otitis media episodes, which likely contributed to the reduction in antibiotic use (27) |
| Europe | Influenza | In this randomized, controlled study in 6- to 36-mo-old children, influenza vaccinees had 50% fewer antibiotic prescriptions and 47% fewer confirmed influenza infections during five consecutive seasons (28) |
| Observational studies of influenza vaccine impact on antibiotic use | ||
| Canada | Influenza | Influenza vaccine recipients had 64% fewer antibiotic prescriptions than controls (29) |
| United Kingdom | Influenza (live attenuated virus vaccine) | In vaccinated children, 14.5% fewer amoxicillin prescriptions were given during the period of influenza virus circulation compared with other time periods (30) |
In the California PCV7 licensure trial, it was noted that the children receiving PCV7 had 5.4% (95% CI 4.0–6.7%) fewer antibiotic prescriptions written compared with controls. The use of second-line antibiotics was reduced by 12.6% (95% CI 9.6–15.6%). From the receipt of dose one to age 3.5 y in children vaccinated per protocol, PCV7 prevented 35 antibiotic prescriptions per 100 children vaccinated (14). For the current US birth cohort of ∼4 million children, this would be equivalent to an estimated 1.4 million avoided antibiotic treatment courses per birth cohort over a 3.5-y period.
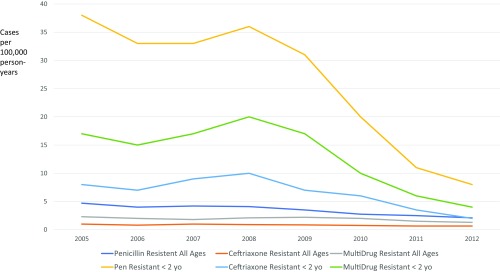
In the South African clinical trial, PCV9 was evaluated for its impact on antibiotic-resistant invasive disease. In this study, there were 67% fewer penicillin-resistant IPD episodes, 56% fewer co-trimoxazole–resistant episodes, and 56% percent fewer infections resistant to any antibiotic in the vaccine group compared with controls (15). Trends for disease in children <2 y of age due to both penicillin-susceptible and -nonsusceptible strains in South Africa following the introduction of PCV7 and then PCV13 are shown in Fig. 1 (16). As can be seen, this decline was dramatic for penicillin-nonsusceptible disease in both HIV-infected and -noninfected children. In addition to the reduction in penicillin resistance, these data show the rarity of ceftriaxone-resistant pneumococci post-PCV13 in South Africa. These data are clinically relevant as they argue the safety of empiric ceftriaxone therapy alone for presumed pneumococcal meningitis post-PCV13 in South Africa, thus reserving the use of vancomycin only for cases failing to respond clinically within 24 h rather than the empirical use of the combination in all cases.
Fig. 1.
Trends in invasive pneumococcal disease in South Africa pre- and post-PCV introduction. Adapted from figure S4 in ref. 16.
In a cluster-randomized clinical trial of PCV10 in Finland, the number of outpatient antibiotic prescriptions filled was monitored in the vaccine group and compared with controls. Consistent with the California PCV7 study, vaccinees had an 8% reduction in prescriptions for antibiotics after a first episode of otitis media with double that reduction in children with more frequent disease (17). In an observational Finnish study of invasive and noninvasive pneumococcal disease in children and adults in Helsinki between 2009 and 2014, the proportion of penicillin-nonsusceptible isolates in children <5 y old declined from 25% to 13% following PCV10 introduction with multidrug-resistant isolates declining from 22% to 6% (18). Of note, this trend had not yet been observed in Finnish adults. The most recent Finnish study addressing this issue focused on young children, in whom otitis media was the most common driver of antibiotic use. In evaluating the rate of antibiotic prescribing before and after the introduction of the routine use of PCV10 in children, it was noted that the diagnosis of otitis media accounted for 84% of prescribing in children. In the cohort of children that had been vaccinated with PCV10, there was a 17.5% reduction in prescriptions written compared with the reference cohort. Reductions began in young childhood and persisted up through 45 mo of age (19).
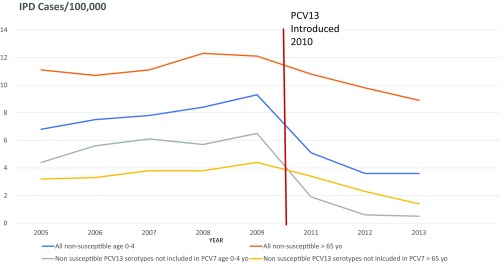
In the United States, the Centers for Disease Control and Prevention monitors pneumococcal disease as part of its ABC surveillance program. Trends for penicillin-nonsusceptible disease in two age groups pre- and post-PCV13 introduction are shown in Fig. 2. As can be seen, rates of ABR were rising before the introduction of PCV13 but have fallen after PCV13 introduction in both age groups, illustrating the dramatic effect of this vaccine on ABR (20).
Fig. 2.
US trends in invasive non–penicillin-susceptible pneumococcal disease 2005–2013.
The emergence of new serotypes and fluctuation in the rates of disease caused by them has been observed since pneumococcal surveillance and serotyping began early in the last century. Following the introduction of PCV7, an increase in disease due to pneumococcal serotype 19A was observed globally. While clones of this serotype were more likely to be antibiotic-susceptible before 2000, over the last decade both the disease incidence of 19A and the proportion of strains resistant to one or more antibiotics have increased (21). This serotype was not included in the PCV7 or PCV10 formulations, which hoped to rely on cross-protection with serotype 19F, which is included in both vaccines. Following identification of this problem, serotype 19A was included in the PCV13 formulation. Importantly, the emergence of 19A does not represent the emergence of vaccine resistance per se (since the serotype was not included in the original vaccine) but rather indicates that pneumococcal vaccine formulations need to be reviewed and revised to address changing epidemiology and newly emerging serotypes, particularly those that are resistant to current antibiotics.
As ABR remains a significant selective advantage for surviving pneumococcal lineages post-PCV10 and PCV13, it is instructive to consider the acquisition of ABR in the remaining nonvaccine types (NVTs). Subsequent to the introduction of PCV13, ABR has now been observed in cases of disease due to previously less common serotypes such as 15A (22) and 35B (23). For example, in Sweden ABR among invasive pneumococci has traditionally been among the lowest observed in Western countries. Following the introduction of PCV vaccination, while the number of cases of IPD due to vaccine types has been reduced, there has been sufficient acquisition of resistance in NVTs so that the level of nonsusceptibility rose from 1% in 2007 to 4.1% in 2016 (24).
Secondary Effect of Influenza Vaccine on ABR
Antiviral vaccines have no direct effect on the organisms causing antibiotic-resistant disease. However, because antiviral vaccines largely target viral diseases that cause acute febrile illnesses, a reduction in the rates of these illnesses should result in a reduction of antibiotics prescribed (often inappropriately) for these episodes. In addition, influenza infection is known to increase the risk of secondary bacterial infections such as pneumonia and otitis media, which then require antibiotic treatment (25). In a prospective blinded study in children, influenza vaccination significantly reduced the risk of otitis media during the influenza season (26). In a study comparing children who received influenza vaccine with a control group that did not, Marchisio et al. observed that vaccinated children had 13.2% fewer antibiotic prescriptions during the 6-mo observation period in their study. Study children had 54.8% fewer otitis media episodes, which likely contributed to the reduction in antibiotic use (27).
In a recent multicenter European trial, children 6–36 mo of age were randomized to receive either quadrivalent influenza vaccine or placebo during five consecutive influenza seasons. The incidence of RT-PCR–confirmed influenza was 47% lower in the vaccine group. This was consistent with the 50% reduction in antibiotic use observed in children receiving influenza vaccine in this study (28). A similar observational study in Canada following the introduction of universal influenza vaccination in children in Ontario comparing antibiotic-usage patterns in Ontario with the patterns in other provinces without universal vaccination revealed 64% fewer antibiotic prescriptions in the vaccinated children in Ontario (29). In the United Kingdom in a self-control study using The Health Improvement Network (THIN) database, there was a 14.5% reduction in amoxicillin prescribing during the period of influenza virus circulation in vaccinated children. This occurred although not all children in the cohort were vaccinated (30).
Given the annual recurrence of influenza epidemics and the high frequency of influenza disease, the potential impact of influenza vaccine on ABR is high. However, this may be just the tip of the iceberg, in that it has been estimated that half of all antibiotic prescriptions are inappropriately written for viral respiratory illnesses in the United States (31). Hence, future vaccines targeting other respiratory pathogens such as RSV or rhinovirus could result in an even more dramatic effect in reducing antibiotic use and consequent drug pressure in inducing ABR.
Discussion
Over the last decade we have observed a dramatic increase in serious disease due to organisms resistant to multiple antibiotics. Some Gram-negative bacterial strains have been susceptible only to colistin, and transmissible colistin resistance now threatens even that last-resort antibiotic (32). Obviously, the emergence of these strains poses a serious public health threat. This is especially challenging because the pipeline for new antibiotics has largely run dry, with no new classes of antibiotics licensed in the last decade (33). Because of the need to address this problem, the Wellcome Trust and the Gates Foundation, among others, have called for the development of vaccines targeting these antibiotic-resistant strains. With the availability of whole-genome sequencing for bacteria to define conserved genes and the demonstrated effectiveness of newer vaccine-development techniques such as structural biology and reverse vaccinology, this should be feasible. Here we have presented two examples of vaccines that have had significant impact against ABR. This demonstrated effectiveness of existing vaccines, including PCVs and influenza vaccines, against ABR as presented here provide scientific justification for such an effort.
To move forward expeditiously to address ABR, in the future the designated endpoints of pre- and postlicensure vaccine trials should include ABR as a prespecified outcome with recognition by regulators, recommending bodies, and manufacturers that such trials are not only desirable and feasible but are critical to moving forward to address the threat of ABR. The vaccine-development effort should be undertaken concomitantly with ongoing surveillance of strains causing disease because such monitoring of ABR may need to guide the development of evolving vaccines that address the shifting target that bacteria are likely to provide. In addition, the development of vaccines for common respiratory viral pathogens should be considered, as these are a major driver of antibiotic use. Only by moving in these directions can we optimally address the growing threat of ABR to public health.
Footnotes
Conflict of interest statement: S.B. is a consultant for GSK, Merck, Sutrovax, and WHO.
This article is a PNAS Direct Submission. R.C. is a guest editor invited by the Editorial Board.
References
- 1.Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos A-P, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Jasovský D, Littmann J, Zorzet A, Cars O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups J Med Sci. 2016;121:159–164. doi: 10.1080/03009734.2016.1195900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappuoli R, Pizza MG, Del Giudice G, Gregorio E. Vaccines for a new society. Proc Natl Acad Sci USA. 2014;111:12288–12293. doi: 10.1073/pnas.1402981111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: Mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32:199–206. doi: 10.1016/j.ijantimicag.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Bronzwaer SL, et al. European Antimicrobial Resistance Surveillance System A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;8:278–282. doi: 10.3201/eid0803.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Progress in introduction of pneumococcal conjugate vaccine–Worldwide, 2000-2008. MMWR Morb Mortal Wkly Rep. 2008;57:1148–1151. [PubMed] [Google Scholar]
- 8.Black S, et al. Northern California Kaiser Permanente Vaccine Study Center Group Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54:893–897. [PubMed] [Google Scholar]
- 10.Hampton LM, et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis. 2012;205:401–411. doi: 10.1093/infdis/jir755. [DOI] [PubMed] [Google Scholar]
- 11.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 12.Kyaw MH, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 13.Moore MR, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fireman B, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10–16. doi: 10.1097/00006454-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Klugman KP, et al. Vaccine Trialists Group A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 16.von Gottberg A, de Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 17.Palmu AA, et al. Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: A double-blind, cluster randomised phase 3-4 trial. Lancet Infect Dis. 2014;14:205–212. doi: 10.1016/S1473-3099(13)70338-4. [DOI] [PubMed] [Google Scholar]
- 18.Sihvonen R, et al. Streptococcus pneumoniae antimicrobial resistance decreased in the Helsinki Metropolitan Area after routine 10-valent pneumococcal conjugate vaccination of infants in Finland. Eur J Clin Microbiol Infect Dis. 2017;36:2109–2116. doi: 10.1007/s10096-017-3033-5. [DOI] [PubMed] [Google Scholar]
- 19.Palmu AA, Rinta-Kokko H, Nohynek H, Nuorti JP, Jokinen J. Impact of national ten-valent pneumococcal conjugate vaccine program on reducing antimicrobial use and tympanostomy tube placements in Finland. Pediatr Infect Dis J. 2018;37:97–102. doi: 10.1097/INF.0000000000001810. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention 2017 Surveillance and Reporting. Available at https://www.cdc.gov/pneumococcal/surveillance.html. Accessed November 1, 2017.
- 21.Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis. 2009;199:776–785. doi: 10.1086/597044. [DOI] [PubMed] [Google Scholar]
- 22.Sheppard C, et al. Rise of multidrug-resistant non-vaccine serotype 15A Streptococcus pneumoniae in the United Kingdom, 2001 to 2014. Euro Surveill. 2016;21:30423. doi: 10.2807/1560-7917.ES.2016.21.50.30423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olarte L, et al. Emergence of multidrug-resistant pneumococcal serotype 35B among children in the United States. J Clin Microbiol. 2017;55:724–734. doi: 10.1128/JCM.01778-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naucler P, et al. Comparison of the impact of PCV10 or PCV13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65:1780–1789. doi: 10.1093/cid/cix685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kash JC, Taubenberger JK. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol. 2015;185:1528–1536. doi: 10.1016/j.ajpath.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozgur SK, et al. Effectiveness of inactivated influenza vaccine for prevention of otitis media in children. Pediatr Infect Dis J. 2006;25:401–404. doi: 10.1097/01.inf.0000217370.83948.51. [DOI] [PubMed] [Google Scholar]
- 27.Marchisio P, et al. Efficacy of injectable trivalent virosomal-adjuvanted inactivated influenza vaccine in preventing acute otitis media in children with recurrent complicated or noncomplicated acute otitis media. Pediatr Infect Dis J. 2009;28:855–859. doi: 10.1097/INF.0b013e3181a487b4. [DOI] [PubMed] [Google Scholar]
- 28.Dbaibo G, et al. 2017 Inactivated quadrivalent influenza vaccine reduces influenza associated healthcare, antibiotic use and parent-child absenteeism during a randomized controlled trial in healthy children aged 6-35 months. The 35th meeting of the European Society for Paediatric Infectious Diseases. Available at https://espid2017.kenes.com/Documents/ESPID17%20abstracts.pdf. Accessed May 7, 2018.
- 29.Kwong JC, Maaten S, Upshur RE, Patrick DM, Marra F. The effect of universal influenza immunization on antibiotic prescriptions: An ecological study. Clin Infect Dis. 2009;49:750–756. doi: 10.1086/605087. [DOI] [PubMed] [Google Scholar]
- 30.Hardelid P, et al. Effectiveness of live attenuated influenza vaccine in preventing amoxicillin prescribing in preschool children: A self-controlled case series study. J Antimicrob Chemother. 2018;73:779–786. doi: 10.1093/jac/dkx463. [DOI] [PubMed] [Google Scholar]
- 31.Fleming-Dutra KE, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864–1873. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 32.Liu YY, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 33.Gupta SK, Nayak RP. Dry antibiotic pipeline: Regulatory bottlenecks and regulatory reforms. J Pharmacol Pharmacother. 2014;5:4–7. doi: 10.4103/0976-500X.124405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toms C, de Kluyver R. Enhanced Invasive Pneumococcal Disease Surveillance Working Group for the Communicable Diseases Network Australia Invasive pneumococcal disease in Australia, 2011 and 2012. Commun Dis Intell Q Rep. 2016;40:E267–E284. [PubMed] [Google Scholar]
- 35.Sigurdsson S, et al. Pneumococcal vaccination: Direct and herd effect on carriage of vaccine types and antibiotic resistance in Icelandic children. Vaccine. 2017;35:5242–5248. doi: 10.1016/j.vaccine.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Chiba N, et al. Invasive Pneumococcal Diseases Surveillance Study Group Changes in capsule and drug resistance of Pneumococci after introduction of PCV7, Japan, 2010-2013. Emerg Infect Dis. 2014;20:1132–1139. doi: 10.3201/eid2007.131485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho EY, et al. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolated from invasive infections after optional use of the 7-valent conjugate vaccine in Korea, 2006-2010. Diagn Microbiol Infect Dis. 2014;78:481–486. doi: 10.1016/j.diagmicrobio.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 38.von Gottberg A, et al. GERMS-SA Investigators Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 39.Hauser C, Kronenberg A, Allemann A, Mühlemann K, Hilty M. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus pneumoniae, Switzerland, 2004 to 2014. Euro Surveill. 2016;21:30239. doi: 10.2807/1560-7917.ES.2016.21.21.30239. [DOI] [PubMed] [Google Scholar]
- 40.Gaviria-Agudelo CL, Jordan-Villegas A, Garcia C, McCracken GH., Jr The effect of 13-valent pneumococcal conjugate vaccine on the serotype distribution and antibiotic resistance profiles in children with invasive pneumococcal disease. J Pediatric Infect Dis Soc. 2017;6:253–259. doi: 10.1093/jpids/piw005. [DOI] [PMC free article] [PubMed] [Google Scholar]