Significance
New international regulatory guidelines aim to better protect bees from harmful exposures to agrochemicals used in crop protection. Our results provide evidence that the laboratory bioassays in these guidelines can discriminate harmful bioaccumulative insecticides that had formerly been approved for agricultural use. As a test case, we investigated insecticides implicated in the mass mortalities of honey bees in France in the 1990s. We demonstrate that a hitherto overlooked insecticide with a strong capacity to bioaccumulate was very likely responsible.
Keywords: bee health, ecotoxicology, neonicotinoids, fipronil, pesticides
Abstract
Mass mortalities of honey bees occurred in France in the 1990s coincident with the introduction of two agricultural insecticides, imidacloprid and fipronil. Imidacloprid, a neonicotinoid, was widely blamed, but the differential potency of imidacloprid and fipronil has been unclear because of uncertainty over their capacity to bioaccumulate during sustained exposure to trace dietary residues and, thereby, cause time-reinforced toxicity (TRT). We experimentally quantified the toxicity of fipronil and imidacloprid to honey bees and incorporated the observed mortality rates into a demographic simulation of a honey bee colony in an environmentally realistic scenario. Additionally, we evaluated two bioassays from new international guidance for agrochemical regulation, which aim to detect TRT. Finally, we used analytical chemistry (GC-MS) to test for bioaccumulation of fipronil. We found in demographic simulations that only fipronil produced mass mortality in honey bees. In the bioassays, only fipronil caused TRT. GC-MS analysis revealed that virtually all of the fipronil ingested by a honey bee in a single meal was present 6 d later, which suggests that bioaccumulation is the basis of TRT in sustained dietary exposures. We therefore postulate that fipronil, not imidacloprid, caused the mass mortalities of honey bees in France during the 1990s because it is lethal to honey bees in even trace doses due to its capacity to bioaccumulate and generate TRT. Our results provide evidence that recently proposed laboratory bioassays can discriminate harmful bioaccumulative substances and, thereby, address evident shortcomings in a regulatory system that had formerly approved fipronil for agricultural use.
Conspicuous mass mortalities of honey bees were observed in France between 1994 and 1998 (1). Their onset coincided with the introduction of two new-to-market systemic insecticides, imidacloprid (released in 1994) and fipronil (released in 1993), which were used widely on French sunflower (Helianthus annuus) crops (2). Imidacloprid is a neonicotinoid pesticide that disrupts the insect nervous system by acting on nicotinic acetylcholine receptors (nAChRs) (3), and fipronil is a phenylpyrazole insecticide that acts on γ-aminobutyric acid (GABA) receptors (4). Applied as seed dressings, these systemic insecticides are taken up by the growing plant and distributed throughout its tissues, including the flowers (5). Consequently, honey bees are exposed to low-level dietary residues when feeding on nectar and pollen from systemically treated bee-attractive crops (6). Despite being used across similar acreages (7), it was generally believed that the mass mortalities were caused by imidacloprid (1), but the case against imidacloprid is weak for two reasons. First, dietary imidacloprid at environmentally realistic levels does not appear to be able to cause mass mortality in honey bees. Neonicotinoid residues in the nectar and pollen of bee-attractive crops are typically less than 6 ppb (parts per billion) (8), but the consensus dose–response relationship from four previous laboratory studies (9–11) (SI Appendix, section S1) indicates that lethality is infrequent in this range (ca. <5% mortality) even after a 10-d dietary exposure and only dietary concentrations of in excess of 100 times the environmentally realistic level cause substantial mortality (dietary concentration for 50% mortality, LC50 = 1,750 μg·L−1, or ca. 1,350 ppb). Continuous experimental exposures of honey bee colonies to 5 pbb imidacloprid-laced syrup over 6-wk periods under field conditions (12) found no mass mortality and report only minor sublethal impacts on cardinal indicators of colony performance, such as hive mass and the population size of adult bees.
Second, dietary imidacloprid does not appear to bioaccumulate in individual bees, which decreases the potential harmfulness of the low-level residues that typify its presence in the nectar and pollen of treated crops. Potentially, an insecticide that is present as trace dietary residues eventually may accumulate to a lethal level during a sustained exposure, which is possible in bees because their adult lifespan and the blooming period of mass-flowering crops like sunflower and canola both extend over several weeks. However, honey bees clear ingested imidacloprid rapidly from their bodies (92% within 48 h) (13) and the elimination half-life of imidacloprid and its toxic metabolites is ∼24 h (14). In theory, the bodily concentration of an ingested toxicant reaches steady state in ∼4 elimination half-lives (15), so imidacloprid is unlikely to be bioaccumulative during exposures at an ecologically relevant timescale, which is measured in weeks. Further, it appears that imidacloprid does not accumulate locally at its target sites by binding irreversibly to receptors in the insect nervous system. Instead, rapid postexposure recovery is observed in honey bees (13) and other insects including cockroaches (16), termites (17), and bumble bees (18, 19), which clearly indicates reversible binding. Taken together, this evidence suggests that it is unlikely that even a sustained exposure to dietary imidacloprid at environmentally realistic levels can be the cause of mass fatalities.
By contrast, fipronil appears more likely than imidacloprid to have caused the mass mortalities among honey bees observed in France because it is more potent in sustained exposures. After ad libitum feeding on dosed diets, the 10-d LD50 for fipronil is 3 ng·bee−1 compared with 189 ng·bee−1 for imidacloprid (9). Furthermore, the fact that the LD50 for exposed honey bees is lower in sustained than acute exposures (10-d LD50 = 3 ng·bee−1 vs. 48-h LD50 = 123 ng·bee−1; ref. 9) strongly implicates bioaccumulation because each ingested unit of a bioaccumulative toxicant has greater opportunity to injure the subject in a prolonged exposure than each unit of a nonbioaccumulative toxicant, which spends a much shorter time in the subject’s body. However, it is unclear whether fipronil and imidacloprid are differentially toxic in an environmentally realistic in-hive scenario involving mixed-age groups of honey bees taken directly from outdoor hives rather than in the newly emerged, well nourished, disease-free honey bees conventionally used in the laboratory tests to establish the various LD50 values for each toxicant. Further, it has been unclear whether the effects measured on individual bees in the laboratory are sufficient to cause mass mortality in a colony. We therefore set out to experimentally quantify the differential toxicity of fipronil and imidacloprid to bees taken from outdoor hives, and we incorporated the emergent dose-appropriate mortality rates into a demographic simulation model (20) to evaluate their potential to cause colony-level impacts under an environmentally realistic scenario.
Our experiments also provided an opportunity to test whether the high toxicity of fipronil is due to bioaccumulation. The mark of a bioaccumulative toxicant is that it is increasingly injurious as the exposure is prolonged because its bodily levels increase, which is termed “time-reinforced toxicity” (TRT) (21). One possible explanation for the historical mass mortalities of honey bees in France is that they were caused by the accumulation of trace residues of a TRT-capable pesticide. We therefore investigated each pesticide’s capacity to generate TRT as suggested in proposed international guidelines for regulatory testing (22) by evaluating Haber’s “constant product” rule. Specifically, we used the results of our experiments to evaluate the exponent, b, in the following constant–product relationship:
| [1] |
where C denotes the concentration of the toxicant at its target site and t denotes the duration of the exposure in the dose-duration combinations that produce a specified injury, such as fatality. In theory, the exponent takes the value b = 1 if the toxicant reaches steady state and b = 2 if the toxicant bioaccumulates (SI Appendix, section S2). The evaluation of the exponent in Haber’s constant product rule is a widely recognized approach in toxicology (23) and risk assessment (24) that has been recently recommended for understanding bee–pesticide interactions (21).
We additionally employed a second test for TRT based on recent European Food Safety Authority (EFSA) draft guidance (25). When experimental exposures are conducted at a range of doses and mortality is recorded, toxicokinetic inferences can be made by comparing the ingested mass that precedes fatality across the different doses. A nonbioaccumulative toxicant with a short in-body residence will cause the same injury per unit ingested irrespective of dose (because each unit has approximately the same in-body residence), which means that each bee must ingest the same total mass of toxicant to cause its fatality irrespective of the duration of the exposure. In contrast, a bioaccumulative toxicant retained in the bee’s body has longer to cause injury in the longer-lived bees feeding on lower doses, so these bees need to consume less of the toxicant in total to be killed. Hence, a second signature of TRT is evident when the fatal mass of ingested toxicant declines as the duration of exposure increases, which can be evaluated by testing the ingestion-vs.-longevity relationship for a negative slope.
In addition to investigating the toxicity of the focal historical compounds imidacloprid and fipronil, we also examined two other pesticide compounds in widespread current use, thiamethoxam (a neonicotinoid) and cypermethrin (a pyrethroid). Whereas thiamethoxam has been used worldwide, ongoing concerns over bee health have led the European Union recently to ban the use of neonicotinoids and fipronil on bee-attractive crops (26), but until recently, derogations in the United Kingdom have allowed some farmers to use neonicotinoids (including thiamethoxam) on oilseed rape (Brassica napus) (27). Meanwhile, other farmers are instead using nonsystemic pyrethroid foliar sprays with active ingredients such as cypermethrin, which acts on the sodium channels in the postsynaptic membrane of insect nerve cells (28). Using data from experimental exposures, we estimated demographic mortality rates of all four candidate pesticides and evaluated their potential impact on a computer-simulated colony (SI Appendix, section S3). We investigated whether fipronil’s high potency in sustained exposures (9) emerged from bioaccumulation by testing for the two signatures of time-reinforced toxicity and by using gas chromatography-mass spectrometry (GC-MS) to establish its bioaccumulative potential. Additionally, we used our approach to confirm that neither imidacloprid, thiamethoxam nor cypermethrin generate TRT.
Results
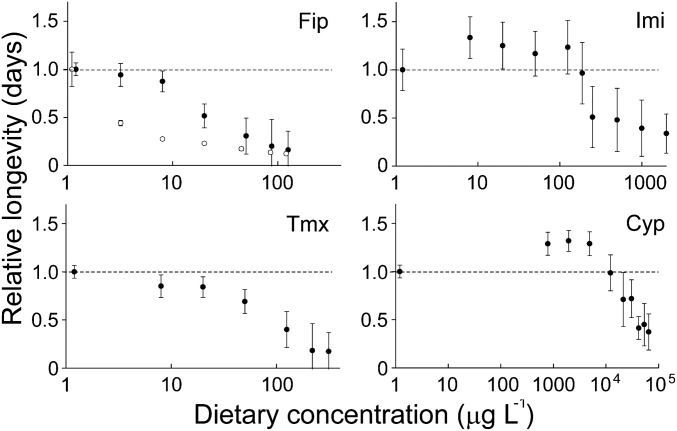
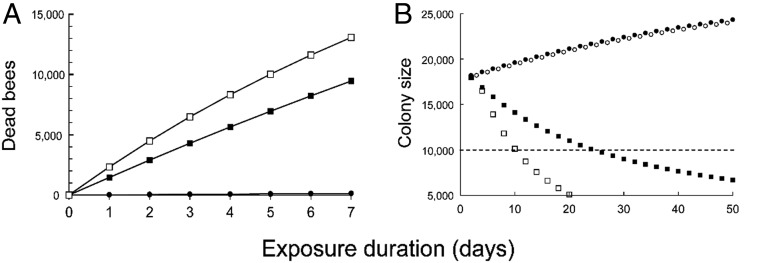
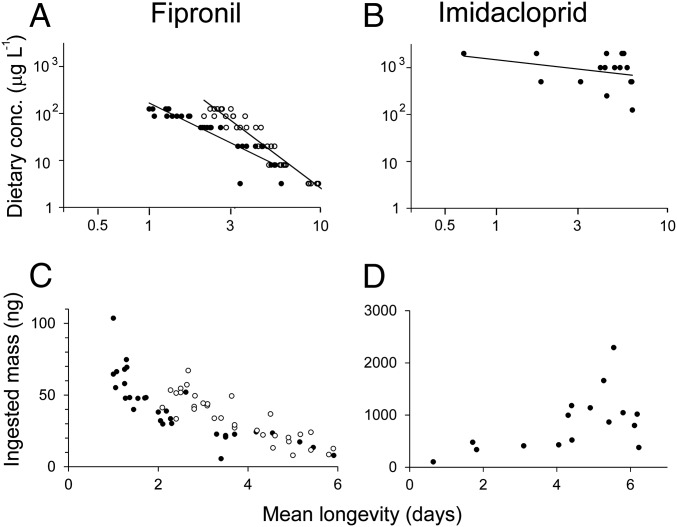
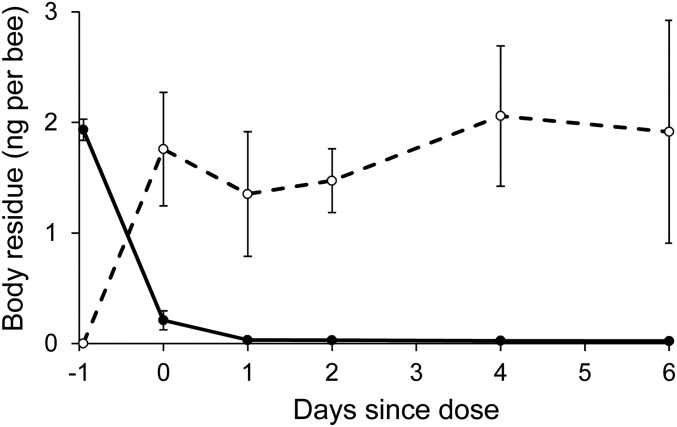
Experimental exposure to dietary fipronil caused dose-dependent reductions in the longevity (days of exposure survived) of adult honey bees (Fig. 1 and SI Appendix, Fig. S4.1), and residues in the environmentally realistic range produced a demographic effect that was consistently strong across both experiments (daily per capita mortality rate due to pesticide, 2013 exposure: Mpesticide = 0.045, 2015 exposure: Mpesticide = 0.099; SI Appendix, Table S3.1). When this effect was applied to the simulated colony, fipronil caused a mass mortality of adult bees. Specifically, the demographic simulation predicted the death of between 4,000 and 9,000 additional bees (between ∼20% and 50% of the original population) over the first week of exposure to fipronil (Fig. 2A), which can account for “un tapis d’abeilles mortes” (a carpet of dead bees) in front of each colony, a symptom that characterized the affected French apiaries during the 1990s (29). In a prolonged exposure, fipronil caused the simulated colony to fail within 2 or 3 wk (Fig. 2B). Laboratory exposures to fipronil exhibited both of the signatures of TRT (C-vs.-t relationship, regression analysis, 2013 exposure: b = 1.8 ± 0.14, 2015 exposure: b = 2.8 ± 0.12, Fig. 3A; ingestion-vs.-longevity relationship, Spearman’s correlation analysis, 2013 exposure: rho = −0.92, P < 0.001; 2015 exposure: rho = −0.90, P < 0.01; Fig. 3C). Using GC-MS analysis of honey bee whole-body residues, we established that the highly toxic sulfone metabolite produced from a single fipronil-laced meal persisted undiminished in honey bees for at least 6 d (Fig. 4), which confirms and extends a recent 48-h study (30). Consequently, fipronil sulfone appears very likely to bioaccumulate if the dietary intake were to be sustained. Taken together, these findings suggest that bioaccumulation of fipronil metabolites is the cause of the time-reinforced toxicity observed in our experiments.
Fig. 1.
Dose-dependent variation in the longevity of adult honey bees during dietary exposures to four pesticides (Cyp, cypermethrin; Fip, fipronil; Imi, imidacloprid; Tmx, thiamethoxam). Each datum indicates the relative longevity (y axis: mean days of exposure survived relativized against undosed controls) observed in an experimental cage at a given dietary dose (x axis: dietary concentration of toxicant in µg·L−1). Error bars indicate 95% confidence intervals (1.96 SEM, n = 7 cages per level of dose). Fipronil exposures were made twice (2013 = ●, 2015 = ○); the effects of low fipronil doses are strongest in relative terms in 2015 because of the greater longevity of the bees, which apparently afforded TRT a greater opportunity to develop; mean longevity of controls, 2013 = 7.3 d; 2015 = 21.2 d.
Fig. 2.
Impacts of dietary exposure to fipronil and imidacloprid on a simulated honey bee colony. (A) Model predictions of the number of dead bees (y axis: number of dead adult worker bees) to be found outside a hive over a 7-d period (x axis: time in days) under control conditions (symbol: filled circles) and during environmentally realistic dietary exposures to fipronil (squares, filled = 2013 experiment, open = 2015). (B) Model predictions of colony size (y axis: number of adult worker bees) over a 7-wk period (x axis: time in days) under control conditions (symbol: filled circles) and during environmentally realistic dietary exposures to imidacloprid (open circles) or fipronil (squares, filled = 2013 experiment, open = 2015). The dashed line indicates the assumed minimum for colony survival. Data points of control and imidacloprid-exposed colonies have been slightly shifted in the x plane for ease of inspection.
Fig. 3.
TRT indicators for fipronil and imidacloprid. (A and B) Fipronil and imidacloprid evaluated for time-reinforced toxicity by their C-vs.-t relationships. Each datum indicates the mean longevity (days of exposure survived) in a cage of dosed bees. Separate experiments involving fipronil are indicated by closed (2013) and open (2015) symbols. Fitted curves show log(C)-vs.-log(t) relationships between dietary concentration (y axis: C, µg·L−1) and time-to-effect (x axis: t, mean time until death of honey bees in an experimental cage). (C and D) Fipronil and imidacloprid evaluated by their ingestion-vs.-longevity relationships. Each datum represents a single cage of honey bees based on: the total mass of toxicant consumed by the bees before their deaths (y axis: mass ingested, ng) and the mean longevity of the exposed bees (x axis: mean days of exposure survived). Separate experiments involving fipronil are indicated by closed (2013) and open (2015) symbols. Only fipronil produced significant negative trends (Spearman correlation analysis, P < 0.001). These data include only cages of dosed bees where the reduced longevity could be attributed to toxicity (SI Appendix, Fig. S2.8).
Fig. 4.
Time-course of whole-body residues of fipronil (solid line) and its sulfone metabolite (dashed line) in honey bees after a single fipronil-laced meal. Body residues (y axis: mean ng·bee−1) were measured in bees sampled separately at intervals over a 6-d period (x axis: days since dose) after a single acute dietary exposure to fipronil (20 μL·bee−1 of syrup with 145 µg of fipronil·L−1, or ca. 3 ng·bee−1). Day = 0 indicates samples collected immediately after dosing and Day = −1 indicates the estimated initial fipronil ingestion. Error bars denote ±1 SEM. Mean residues are connected for ease of inspection only. Concentrations in undosed bees were less than 0.02 ng·bee−1 fipronil and 0.11 ng·bee−1 fipronil sulfone.
Experimental exposure to dietary imidacloprid caused a hormetic response in the mean longevity of adult honey bees (i.e., low-dose stimulation coupled with high-dose inhibition; Fig. 1 and SI Appendix, Fig. S4.1). As expected, dietary imidacloprid produced only a slight increase in the level of mortality at an environmentally realistic dose with a correspondingly small demographic effect (Mpesticide = 0.004; SI Appendix, Table S3.1) that was ∼10 times smaller than the effect of fipronil. When this effect was applied to the simulated colony, imidacloprid caused only a small increment in the colony-wide mortality of adult bees (∼400 additional deaths over the first week of exposure; SI Appendix, Fig. S5.1A), which is not sufficiently large to be considered as a mass mortality. Additionally, exposure to imidacloprid had virtually no effect on colony growth (Fig. 2B). The mortality rate due to exposure to dietary imidacloprid measured in our present study corresponds very closely to the rate that can be estimated from a metaanalysis of previous laboratory studies in honey bees (SI Appendix, section S1), which supports the general inference that trace levels of dietary imidacloprid do not cause fatality in adult workers. Also, as expected, exposures to imidacloprid exhibited neither signature of TRT (C-vs.-t relationship, regression analysis, b = 0.4 ± 0.34, Fig. 3B; ingestion-vs.-longevity relationship: Spearman’s correlation analysis, rho = 0.42, P > 0.05; Fig. 3D), which indicates that it is unlikely to be bioaccumulative in honey bees.
Dietary thiamethoxam reduced the mean longevity of adult honey bees in exposures to residues in the environmentally realistic range (Fig. 1 and SI Appendix, Fig. S4.1) with a moderate demographic effect (Mpesticide = 0.0086; SI Appendix, Table S3.1) that was ∼5 times smaller than the effect of fipronil. When this effect was applied to the simulated colony, thiamethoxam caused a moderate increase in the mortality of adult bees (ca. 700 additional deaths over the first week of exposure, SI Appendix, Fig. S5.1B), which suppressed colony growth (SI Appendix, Fig. S5.1C). Thiamethoxam exhibited neither signature of TRT (C-vs.-t relationship, regression analysis, b = 0.7 ± 0.13; SI Appendix, Fig. S6.1A; ingestion-vs.-longevity relationship: correlation analysis, Spearman’s rho = 0.04, P > 0.05; SI Appendix, Fig. S6.1C).
Dietary cypermethrin caused a hormetic response in mean longevity (Fig. 1 and SI Appendix, Fig. S4.1) and an environmentally realistic exposure caused a minute increase to the level of mortality (Mpesticide = 0.00001; SI Appendix, Table S3.1) that had negligible impact on the simulated colony. Cypermethrin exhibited neither signature of TRT (C-vs.-t relationship, regression analysis, b = 0.4 ± 0.13; SI Appendix, Fig. S6.1B; ingestion-vs.-longevity relationship: Spearman’s correlation analysis, rho = 0.62, P ≤ 0.001; SI Appendix, Fig. S6.1D). We speculate that the positive slope of the ingestion-vs.-duration relationship for cypermethrin indicates that the bees were detoxifying this substance more effectively at lower doses.
Discussion
Based on our findings, we postulate that fipronil is a credible cause of the mass mortalities of honey bees that were associated with agricultural sunflower in France during the 1990s. Fipronil can be lethal to honey bees in dietary exposures to the trace residues that typify those in nectar and pollen from treated crops, due in part to its capacity to generate TRT. We estimate that the resulting increase in the demographic mortality rate is capable of causing mass mortality among adult bees. Our present study has examined in detail only a lethal endpoint, but fipronil may also have various sublethal impacts, such as detrimental effects on foraging intensity and homing success (31), which could further accelerate colony failure. Our hypothesis that fipronil is capable of causing major impacts on honey bees is supported by the occurrence of occasional mass mortalities of honey bees, such as the 2014 event that involved 172 hives across 23 apiaries in the Canton of Bern, Switzerland (32). Despite the ongoing ban on the use of fipronil in European agriculture, the accident in Bern arose from fipronil residues present as an accidental contaminant in a batch of fungicide that had been used to treat nearby fruit trees, which were foraged by honey bees. It is not yet possible to identify that fipronil definitively was a culprit in the French incidents because there is a lack of data to prove the historical levels and prevalence of its residues in nectar and pollen, but the findings of our laboratory experiments provide strong evidence that justifies a future program of field experimentation to further test the hypothesis.
Imidacloprid and Mass Mortalities of Honey Bees.
Our results suggest that dietary exposure to imidacloprid in nectar and pollen is an unlikely cause of mass mortality in adult honey bees because trace dietary residues at environmentally realistic levels cause only low levels of mortality, even in sustained exposures. This finding is consistent with the levels of mortality that have been reported by previous researchers (SI Appendix, section S1). Of course, some recent mass mortalities of honey bees were instead caused by the release of insecticidal neonicotinoid dust created when treated maize seeds were planted by pneumatic drilling machinery, such as the 2008 incident in Baden-Würtemberg in Germany (33), but dust emission cannot account for the mass mortalities that coincided with the midsummer bloom of French sunflower crops, however, because agricultural sowing (including maize) occurs earlier in the year.
Imidacloprid’s low toxicity in sustained dietary exposures to trace residues (12) appears to be due in part to its rapid elimination by bees, which makes it unable to bioaccumulate and thereby generate TRT. Bees can eliminate ingested imidacloprid in part because it binds reversibly to its target receptor, which is indicated by two lines of evidence. First, in vitro experiments using radio-labeled ligands show that imidacloprid can be displaced from the neuroreceptors of stable flies, Stomoxys calcitrans (34) (SI Appendix, section S7), and that this process can be rapid (dissociation half-life of ∼10 min) in house flies (Musca domestica) (35) and aphids (Myzus persicae) (36). In principle, similar reversible ligand-receptor binding therefore appears likely in bees. Second, the timescale of postexposure recovery (24–48 h) in honey bees (13) and bumble bees (18, 19) coincides with the timescale of metabolic elimination (elimination half-life ∼ 24 h) (13, 14, 19), which logically suggests that imidacloprid increasingly dissociates from its receptors as detoxification reduces the concentration of its unbound form. Taken together, this collection of evidence indicates that imidacloprid binds reversibly to its receptors in bees and, if so, toxicodynamic-kinetic theory relating to toxicants with a short elimination half-life (15) predicts the absence of TRT in experimental exposures to imidacloprid, just as we observed in the present study. Our conclusion that imidacloprid fails to cause TRT is further supported by the results of a previous laboratory exposure (9), which also produced results that indicate the absence of TRT (C-vs.-t relationship, Haber exponent b = 1.1; SI Appendix, section S8).
While our results demonstrate that dietary imidacloprid is not lethal to honey bees at environmentally relevant levels, we emphasize that this should not be taken to mean that it is harmless to bees. We found that dietary imidacloprid produced a hormesis in longevity (days of exposure survived) in honey bees, which is not unexpected; various chemical stressors produce hormesis in insects (37) and imidacloprid itself causes hormetic responses in stink bugs (38), aphids (39) and, notably, in the longevity of spider mites (40). However, the increased longevity that we observed at low doses should be viewed as an intoxication symptom, which is likely to displace affected honey bees from their normal physiological equilibria (37, 39). Finally, we note that although our present study did not consider the potentially detrimental impact of imidacloprid on other demographic variables that are relevant to colony health, such as queen fecundity and larval performance, the lack of mass mortalities in exposed colonies under field conditions (12) suggests that we have not overlooked a crucial factor.
We add two further notes about the imidacloprid hormesis. First, a hormetic increase in mean longevity in the low-dose range is not incompatible with a small increase in daily mortality rate in the same range (such as we observed), because the increased lifespan of the surviving majority more than offsets the days lost by infrequent deaths. Second, the increased longevity observed in our lowest doses confirms the presence of the active substance and, in conjunction with dose-dependent mortality that conforms closely to previous studies (SI Appendix, section S9), validates our dose preparations.
Inferences About Cypermethrin and Thiamethoxam.
Based on our findings, cypermethrin has the lowest potential for impact on colony performance by directly causing adult mortality. In individual honey bees, detoxicative enzyme systems can reduce harm from ingested pyrethroids (41) and preclude bioaccumulative toxicity, or TRT, which may explain the low mortality rate due to cypermethrin in our present study. In contrast, thiamethoxam appears capable of causing a more substantive impact on colony performance by elevating the mortality rate among adult workers. Nevertheless, thiamethoxam produced neither signature of TRT. It appears probable that thiamethoxam does not bioaccumulate in honey bees because it is subject to detoxicative metabolism, like the closely similar neonicotinoid clothianidin (42). Performing the analytical chemistry to clarify the toxicokinetics of thiamethoxam in honey bees should be a target for future research. The relatively low levels of mortality in adult honey bee workers caused by dietary exposures to thiamethoxam and cypermethrin do not preclude harmful sublethal effects on colony performance (43).
Future Research and Regulatory Implications.
We used laboratory toxicology and demographic simulation to predict the potential of four dietary pesticides to produce mass mortality of adult honey bees. The postulated absence of mass mortality in environmentally realistic exposures to 5 ppb imidacloprid is already supported by the outcome of in-hive exposures under field conditions (12), but further research is necessary to provide a similar experimental evaluation of the other predictions, especially the proposition that fipronil has the capacity to cause mass mortality in honey bees. Various insects besides honey bees forage on the flowers of pesticide-treated crops, including other kinds of bees that may be affected more strongly (44). Consequently, other species should be tested to assess more broadly the impacts of pesticides on farmland insect faunas.
When Haber’s Rule successfully describes the manifestation of toxic injuries, it suggests underlying proportionalities between exposure concentration, bodily concentration, and the accrual rate of injury. We speculate that these proportionalities arise when physical processes (e.g., diffusion, concentration-dependent association-dissociation of ligand–receptor complexes) fundamentally determine the levels of toxic effects. In itself, however, Haber’s Rule cannot elucidate the details of pharmacological mechanisms and a comprehensive theory of bee-pesticide toxicology, e.g., pharmacokinetic/pharmacodynamic models, should be a goal for future research.
The potentially severe impact of dietary fipronil highlights the need to identify agrochemicals that cause TRT before they are used widely in agriculture because TRT enables trace contaminants to become disproportionately harmful by sustained exposure. Formerly, international regulatory procedures for the risk assessment of plant protection products have relied on short-term laboratory exposures of honey bees (so-called “first tier” tests), which do not take account of the possible harm that results from TRT during realistically sustained exposures. Our findings illustrate the potential value of a bioassay aimed at revealing TRT. Specifically, we show that TRT can be detected both by evaluating the exponent of Haber’s Rule [i.e., evaluation of b in the log(t)-vs.-log(C) relationship] and by testing for exposure dependence of the lethal dose (i.e., evaluation of the ingestion-vs.-longevity relationship), which endorses the value of these analyses as specified in the newly formulated draft guidelines issued by both the EFSA for risk assessment in bees (25) and the Organisation for Economic Co-operation (OECD) (22). By explicitly including an evaluation of TRT due to dietary exposure, future risk assessments will enable regulators to better protect farmland bees and the valuable ecosystem services that they provide in pollinating crops and wild flowers.
Materials and Methods
Honey Bee Demographic Model.
To evaluate the impact of dietary pesticides on honey bee colonies, we simulated the population dynamics of a control (unexposed) colony using a published demographic model (20) and then perturbed the mortality rate according to effects that we quantified experimentally. The previous application of the model to a toxicological perturbation (45) investigated only the loss of intoxicated foragers through homing failure, but we instead explored the case where all adult bees experience an elevated rate of mortality by feeding on either nectar or stored honey that contains a dietary pesticide. We therefore modified the original model to apply mortality due to pesticide to all adult workers in the colony (SI Appendix, Fig. S3.1). The population dynamics of the control colony were described using previously determined parameter values [L = 2000, alpha = 0.25, theta = 0.75 (20); MB = 0.154 (46); w = 22,000 (46, 47)] so that its population of bees increased by ∼25% over 30 d from an initial size of 18,000 (13,500 hive bees, 4,500 foragers), which simulates the rates of development typical in France coincident with the blooming of sunflower and canola (46).
The model of Khoury et al. (20) was modified (SI Appendix, Fig. S3.1) so that foragers die at a rate, MB+P = Mtotal, that compounds the baseline rate, MB = Mbase, and the rate due to pesticide exposure, Mpesticide (SI Appendix, Eq. S3.1). Hive bees die only when exposed to pesticides, at a rate of MP = Mpesticide. Values of Mpesticide for each pesticide were determined from experimental toxicity data (SI Appendix, section S3).
We simulated a colony’s exposure to each of four dietary pesticides by perturbing the mortality rate according to our experimental observations. To estimate the per capita daily mortality rate of bees feeding on each diet, we used the mean proportion dying daily, which was calculated across the time span for which the total number of experimental bees alive was three or more individuals. To determine the pesticide mortality rate applied in the demographic model, we use the environmentally realistic residue concentrations of 5 ppb for imidacloprid, thiamethoxam, and fipronil and 100 ppb for cypermethrin (SI Appendix, section S3.3).
For predicting the number of dead bees found outside a hive (Fig. 2A and SI Appendix, Fig. S5.1 A and B), we assume that 2.5% of natural mortalities in control colonies occur at the hive (47), whereas under pesticide exposure, all mortalities occur at the hive.
Testing for Time-Reinforced Toxicity.
Imidacloprid was obtained as a solution in acetonitrile (analytical standard, PESTANAL, product code: 46341; Sigma Aldrich). A vacuum concentrator (ScanSpeed MaxiVac Beta; LaboGene ApS) was used to completely remove the acetonitrile solvent, and the imidacloprid was dissolved in deionized water to form a stock solution of 10 mg·L−1. Thiamethoxam, fipronil, and cypermethrin (analytical standards, PESTANAL, product codes: 37924, 46451, 36128, respectively; Sigma Aldrich) were dissolved in water (thiamethoxam) and acetone (fipronil and cypermethrin) to form stock solutions (10 mg·L−1, 10 mg·L−1 and 400 mg·L−1, respectively) before being combined with 50% wt/vol aqueous sugar solution (Attraker: 1.27 kg·L−1 fructose/glucose/saccharose solution; Koppert B.V.). Doses of cypermethrin contained a maximum of 0.95% acetone vol/vol, reducing with cypermethrin concentration (control doses contained 0.95% acetone vol/vol). Doses of fipronil contained a maximum of 1.25% acetone vol/vol, reducing with fipronil concentration (control doses contained 1.25% acetone vol/vol).
Adult worker honey bees (Apis mellifera) of various ages were obtained from two well-managed apiaries in Devon, United Kingdom, which were separated by over 25 km. We conducted exposures on fipronil, thiamethoxam, and cypermethrin using bees from apiary A, which were collected in June and July 2013. We made a single collection from apiary B in August 2015, which we randomly split to make a comparative series of exposures to imidacloprid and fipronil. Each experiment involving a single pesticide was conducted on bees taken from a single hive in an apiary, which were collected by opening the hive and scooping them from the top boards and shaking them from the frames. Newly eclosed bees were not used because we wanted to determine the effects of pesticide on a demographically representative sample of adults, which is an environmentally realistic scenario. Honey bees were caged in groups of 10 (cage dimensions: ∼0.10 m diameter × 0.04 m height) in plastic containers, with seven replicate cages per dose. Sample sizes were not chosen a priori based on a statistical power, but they equal or exceed those used in comparable published studies. Cages of bees were randomly assigned to doses and randomly positioned in the laboratory with respect to dose. Bees were kept in a semicontrolled environment (daily mean temperature ± SE = 24.4 °C ± 0.18; mean relative humidity = 35.9% ± 0.76; 12:12 h of low-light:darkness), but bees were capable of maintaining warmer body temperatures (>30 °C) due to nonflight thermogenesis (SI Appendix, section S10). Bees were fed ad libitum on syrup containing either imidacloprid (dosages: 0.00, 8.00, 20.00, 50.00, 125.00, 187.50, 250.00, 500.00, 1,000.00 or 2,000.00 µg·L−1), thiamethoxam (0.00, 8.00, 20.00, 50.00, 125.00, 218.75 or 312.50 µg·L−1), fipronil (0.00, 3.20, 8.00, 20.00, 50.00, 87.50 or 125.00 µg·L−1) or cypermethrin (0.00, 0.78, 1.95, 4.88, 12.21, 21.36, 30.52, 41.99, 53.46 or 64.94 mg·L−1). Each cage received one of the doses, whose collective range spanned and exceeded the environmentally realistic concentrations. Bees were monitored daily for mortality (corpses were removed daily), and syrup consumption was measured daily by weighing syrup feeders for the first 10 d of treatment, and every 2–3 d thereafter (SI Appendix, section S11). Syrup feeders were replaced completely at least every 2 or 3 d to ensure a continuous ad libitum supply.
The power law relationship between dietary concentration of pesticide and mean longevity was fitted on log-transformed axes, and the slope of the relationship (parameter b) was determined by linear regression. We used mean longevity because a sample mean is inherently less prone to statistical error as a measure of central tendency than the median (SI Appendix, Fig. S2.6). Haber’s Rule (Eq. 1) predicts an infinite lifespan for exposed subjects as dose (C) approaches zero. However, this model of toxicity cannot fit lifespan data from experiments with real animals because lifespan is constrained by the organism’s senescence at the lowest doses and so the dose–longevity relationship is necessarily hockey stick-shaped (SI Appendix, Fig. S2.7). To objectively exclude the nonlinearity, we used the lower confidence interval on the longevity (days of exposure survived) of control bees to define the range used to fit the straight-line log(C)-vs.-log(t) relationship of Haber’s Rule (SI Appendix, section S2.7). Note that we were evaluating whether the longevity of an individual dosed cage belonged to the control population, hence the confidence interval was calculated using the SD and not the SE.
Honey Bee Whole-Body Residue Assay.
Of the four focal pesticides, only fipronil was tested for bioaccumulation within honey bee bodies as it alone exhibited time-reinforced toxicity. Our methods were based on the OECD guideline (No. 213) for the honey bee acute oral toxicity test (48). Adult worker honey bees of varied age were starved for 2 h, each cage of 10 bees was then fed 200 µL of either control syrup or syrup containing fipronil at a concentration of 145 µg·L−1 (i.e., 2.9 ng·bee−1). Cages were sampled over a 6-d period (full methods and results, SI Appendix, section S12). Residues of fipronil and its main toxic metabolite (fipronil sulfone) were measured in samples each comprising the bees collected from a single cage using GC-MS (SI Appendix, section S13). Bees fed control syrup were analyzed only for residues of fipronil sulfone.
Supplementary Material
Acknowledgments
P.J.H. was supported by a doctoral studentship (Grant 1200662) from the Natural Environment Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The datasets generated and analyzed during the current study are available in the University of Exeter ORE repository, https://ore.exeter.ac.uk/repository/ (doi: 10.24378/exe.943).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804934115/-/DCSupplemental.
References
- 1.Maxim L, van der Sluijs JP. Expert explanations of honeybee losses in areas of extensive agriculture in France: Gaucho (R) compared with other supposed causal factors. Environ Res Lett. 2010;5:014006. [Google Scholar]
- 2.Aubert M, Faucon J-P, Chauzat M-P, Martel A-C. Recherches sur les mortalités d’abeilles et prévention des risques liés aux insecticides [Research on bee mortalities and insecticide risk prevention] Bull Epidemiol. 2006;20:1–4. [Google Scholar]
- 3.Matsuda K, et al. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2001;22:573–580. doi: 10.1016/s0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- 4.Law RJ, Lightstone FC. Gaba receptor insecticide non-competitive antagonists may bind at allosteric modulator sites. Int J Neurosci. 2008;118:705–734. doi: 10.1080/00207450701750216. [DOI] [PubMed] [Google Scholar]
- 5.Nauen R, Jeschke P. Basic and applied aspects of neonicotinoid insecticides. In: Lopez O, Fernandez-Bolanos JG, editors. Green Trends in Insect Control. R Soc Chem; Cambridge, UK: 2011. pp. 132–162. [Google Scholar]
- 6.Chauzat MP, et al. An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France. Environ Toxicol Chem. 2011;30:103–111. doi: 10.1002/etc.361. [DOI] [PubMed] [Google Scholar]
- 7.Food and Environment Research Agency 2014 Pesticide usage statistics. Available at https://secure.fera.defra.gov.uk/pusstats/index.cfm. Accessed May 1, 2017.
- 8.Godfray HC, et al. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc Biol Sci. 2014;281:20140558. doi: 10.1098/rspb.2014.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department for Environment, Food and Rural Affairs 2007. Assessment of the risk posed to honeybees by systemic pesticides (Dep Environ Food Rural Aff, London), Project PS2322.
- 10.Decourtye A, Lacassie E, Pham-Delègue MH. Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag Sci. 2003;59:269–278. doi: 10.1002/ps.631. [DOI] [PubMed] [Google Scholar]
- 11.Dechaume Moncharmont FX, Decourtye A, Hennequet-Hantier C, Pons O, Pham-Delègue MH. Statistical analysis of honeybee survival after chronic exposure to insecticides. Environ Toxicol Chem. 2003;22:3088–3094. doi: 10.1897/02-578. [DOI] [PubMed] [Google Scholar]
- 12.Meikle WG, et al. Sublethal effects of imidacloprid on honey bee colony growth and activity at three sites in the U.S. PLoS One. 2016;11:e0168603. doi: 10.1371/journal.pone.0168603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schott M, et al. Temporal dynamics of whole body residues of the neonicotinoid insecticide imidacloprid in live or dead honeybees. Sci Rep. 2017;7:6288. doi: 10.1038/s41598-017-06259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suchail S, De Sousa G, Rahmani R, Belzunces LP. In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag Sci. 2004;60:1056–1062. doi: 10.1002/ps.895. [DOI] [PubMed] [Google Scholar]
- 15.Rozman KK, Doull J, Hayes WJ. Dose, time, and other factors influencing, toxicity. In: Krieger RI, Krieger WC, editors. Handbook of Pesticide Toxicology. 2nd Ed. Academic; London: 2001. pp. 1–95. [Google Scholar]
- 16.Kaakeh W, Reid BL, Kaakeh N, Bennett GW. Rate determination, indirect toxicity, contact activity, and residual persistence of lufenuron for the control of the German cockroach (Dictyoptera: Blattellidae) J Econ Entomol. 1997;90:510–522. [Google Scholar]
- 17.Thorne BL, Breisch NL. Effects of sublethal exposure to imidacloprid on subsequent behavior of subterranean termite Reticulitermes virginicus (Isoptera: Rhinotermitidae) J Econ Entomol. 2001;94:492–498. doi: 10.1603/0022-0493-94.2.492. [DOI] [PubMed] [Google Scholar]
- 18.Laycock I, Cresswell JE. Repression and recuperation of brood production in Bombus terrestris bumble bees exposed to a pulse of the neonicotinoid pesticide imidacloprid. PLoS One. 2013;8:e79872. doi: 10.1371/journal.pone.0079872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cresswell JE, Robert FX, Florance H, Smirnoff N. Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris) Pest Manag Sci. 2014;70:332–337. doi: 10.1002/ps.3569. [DOI] [PubMed] [Google Scholar]
- 20.Khoury DS, Myerscough MR, Barron AB. A quantitative model of honey bee colony population dynamics. PLoS One. 2011;6:e18491. doi: 10.1371/journal.pone.0018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tennekes H, Sanchez-Bayo F. Time-dependent toxicity of neonicotinoids and other toxicants: Implications for a new approach to risk assessment. J Environ Anal Toxicol. 2011;4:001. [Google Scholar]
- 22.Organisation for Economic Cooperation and Development . Proposal for a New Guideline for the Testing of Chemicals: Honey Bee (Apis mellifera L.), Chronic Oral Toxicity Test 10-Day Feeding Test in the Laboratory. Organ Econ Coop Dev; Paris: 2016. [Google Scholar]
- 23.Miller FJ, Schlosser PM, Janszen DB. Haber’s rule: A special case in a family of curves relating concentration and duration of exposure to a fixed level of response for a given endpoint. Toxicology. 2000;149:21–34. doi: 10.1016/s0300-483x(00)00229-8. [DOI] [PubMed] [Google Scholar]
- 24.Doull J, Rozman KK. Using Haber’s law to define the margin of exposure. Toxicology. 2000;149:1–2. doi: 10.1016/s0300-483x(00)00226-2. [DOI] [PubMed] [Google Scholar]
- 25.European Food Safety Authority Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees) EFSA J. 2013;11:3295. doi: 10.2903/j.efsa.2023.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Commission July 16, 2013 EUROPA press release - Bee health: EU takes additional measures on pesticides to better protect Europe’s bees. Available at europa.eu/rapid/press-release_IP-13-708_en.htm. Accessed April 12, 2018.
- 27.Eisenstein M. Pesticides: Seeking answers amid a toxic debate. Nature. 2015;521:S52–S55. doi: 10.1038/521S52a. [DOI] [PubMed] [Google Scholar]
- 28.Davies TGE, Field LM, Usherwood PNR, Williamson MS. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life. 2007;59:151–162. doi: 10.1080/15216540701352042. [DOI] [PubMed] [Google Scholar]
- 29.de Villiers P. 2004. Quand les abeilles meurent, les jours de l’homme sont comptes [When bees die, man’s days are numbered] (Editions Albin Michel S.A., Paris), p 173.
- 30.Department for Environment, Food and Rural Affairs 2016. Interpretation of pesticide residues in honeybees (Dep Environ Food Rural Aff, London), Project PS2370.
- 31.Decourtye A, et al. Honeybee tracking with microchips: A new methodology to measure the effects of pesticides. Ecotoxicology. 2011;20:429–437. doi: 10.1007/s10646-011-0594-4. [DOI] [PubMed] [Google Scholar]
- 32.Der Grosse Rat des Kantons Bern 2014 Bienensterben und Bienenvergiftungen: Massnahmen sind fällig! [Bee mortality and bee poisoning: Measures are due!]. Available at https://www.rr.be.ch/rr/de/index/rrbonline/rrbonline/suche_rrb/beschluesse-detailseite.gid-d16ca3178adf420d99e563cdaf686860.html. Accessed November 3, 2017.
- 33.Nuyttens D, Devarrewaere W, Verboven P, Foqué D. Pesticide-laden dust emission and drift from treated seeds during seed drilling: A review. Pest Manag Sci. 2013;69:564–575. doi: 10.1002/ps.3485. [DOI] [PubMed] [Google Scholar]
- 34.Abbink J. The biochemistry of imidacloprid. Pflanzenschutz Nachr Bayer. 1991;44:183–195. [Google Scholar]
- 35.Liu MY, Casida JE. High affinity binding of [3H]imidacloprid in the insect acetylcholine receptor. Pestic Biochem Physiol. 1993;46:40–46. [Google Scholar]
- 36.Lind RJ, Clough MS, Reynolds SE, Earley FGP. [H-3]imidacloprid labels high- and low-affinity nicotinic acetylcholine receptor-like binding sites in the aphid Myzus persicae (Hemiptera: Aphididae) Pestic Biochem Physiol. 1998;62:3–14. [Google Scholar]
- 37.Jager T, Barsi A, Ducrot V. Hormesis on life-history traits: Is there such thing as a free lunch? Ecotoxicology. 2013;22:263–270. doi: 10.1007/s10646-012-1022-0. [DOI] [PubMed] [Google Scholar]
- 38.Haddi K, et al. Sexual success after stress? Imidacloprid-induced hormesis in males of the neotropical stink bug Euschistus heros. PLoS One. 2016;11:e0156616. doi: 10.1371/journal.pone.0156616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayyanath M-M, Cutler GC, Scott-Dupree CD, Sibley PK. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS One. 2013;8:e74532. doi: 10.1371/journal.pone.0074532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James DG, Price TS. Fecundity in twospotted spider mite (Acari: Tetranychidae) is increased by direct and systemic exposure to imidacloprid. J Econ Entomol. 2002;95:729–732. doi: 10.1603/0022-0493-95.4.729. [DOI] [PubMed] [Google Scholar]
- 41.Pilling ED, Bromleychallenor KAC, Walker CH, Jepson PC. Mechanism of synergism between the pyrethroid insecticide λ-cyhalothrin and the imidazole fungicide prochloraz, in the honeybee (Apis mellifera L.) Pestic Biochem Physiol. 1995;51:1–11. [Google Scholar]
- 42.Sgolastra F, et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag Sci. 2017;73:1236–1243. doi: 10.1002/ps.4449. [DOI] [PubMed] [Google Scholar]
- 43.Sandrock C, et al. Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLoS One. 2014;9:e103592. doi: 10.1371/journal.pone.0103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cresswell JE, et al. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid) Zoology (Jena) 2012;115:365–371. doi: 10.1016/j.zool.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Henry M, et al. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- 46.Cresswell JE, Thompson HM. Comment on “A common pesticide decreases foraging success and survival in honey bees”. Science. 2012;337:1453; author reply 1453. doi: 10.1126/science.1224618. [DOI] [PubMed] [Google Scholar]
- 47.Porrini C, et al. Use of honey bees as bioindicators of environmental pollution in Italy. In: Devillers J, Pham-Delegue M-H, editors. Honey Bees: The Environmental Impact of Chemicals. Taylor & Francis; London: 2002. pp. 186–247. [Google Scholar]
- 48.Organisation for Economic Cooperation and Development . Guideline for the Testing of Chemicals, No. 213: Honey Bees: Acute Oral Toxicity Test. Organ Econ Coop Dev; Paris: 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.