Significance
Plants have evolved cell-wall integrity signaling pathways to maintain cell-wall homeostasis in response to stress conditions, but the components involved in the perception and transduction of cell-wall signals are largely unknown. Here we found that the Arabidopsis cell-wall–localized leucine-rich repeat extensins (LRX) 3/4/5 interact with RAPID ALKALINIZATION FACTOR (RALF) peptides RALF22/23. Mature RALF22/23 peptides interact with the plasma membrane-localized FERONIA (FER) and induce FER internalization. The lrx345 and fer mutants and RALF22/23 overexpressing transgenic plants display similar phenotypes such as retarded growth and increased sensitivity to salt stress. Our work thus reveals that the LRX3/4/5 proteins function with RALF22/23 and FER to define a signaling pathway that is critical for regulating plant growth and salt tolerance.
Keywords: stress tolerance, growth, cell-wall stress, receptor, RLK
Abstract
The perception and relay of cell-wall signals are critical for plants to regulate growth and stress responses, but the underlying mechanisms are poorly understood. We found that the cell-wall leucine-rich repeat extensins (LRX) 3/4/5 are critical for plant salt tolerance in Arabidopsis. The LRXs physically associate with the RAPID ALKALINIZATION FACTOR (RALF) peptides RALF22/23, which in turn interact with the plasma membrane-localized receptor-like protein kinase FERONIA (FER). The lrx345 triple mutant as well as fer mutant plants display retarded growth and salt hypersensitivity, which are mimicked by overexpression of RALF22/23. Salt stress promotes S1P protease-dependent release of mature RALF22 peptides. Treatment of roots with mature RALF22/23 peptides or salt stress causes the internalization of FER. Our results suggest that the LRXs, RALFs, and FER function as a module to transduce cell-wall signals to regulate plant growth and salt stress tolerance.
The plant cell wall not only serves as a structural support, determining growth and shape of cells and conferring mechanical properties to plant tissues but also plays important roles in the transduction of environmental cues into the cell interior (1–4). Biotic as well as abiotic stresses can perturb or damage the cell wall, which are thought to be perceived by plasma membrane-localized cell-wall sensors to induce downstream stress responses (3, 5, 6). Several receptor-like kinases, such as FERONIA (FER), THESEUS1, and WAK1, have been identified as potential cell-wall sensors (6–9), but the mechanisms by which they sense the cell-wall perturbations are not well understood.
FER, a member of the CrRLK1L family of receptor-like kinases in Arabidopsis, was initially identified as a regulator in male–female communication during pollen tube reception (10). Later, it was shown that FER has pleiotropic functions in a variety of cellular processes, including the preservation of cell integrity in tip-growing cells (7, 11), regulation of hormone-signaling pathways (12, 13), and response to biotic and abiotic stresses (6, 14, 15). FER was identified as a receptor of RALF (RAPID ALKALINIZATION FACTOR) peptides, and several studies have shown that RALF peptides inhibit the root elongation of wild type (WT) but not fer mutant plants (7, 15, 16). Treatment of plants with RALF23 peptides decreases ligand-induced association of the FLS2/EFR-BAK1 complex via the inhibition of FER protein (15). These versatile functions of FER are likely attributed to its role in the sensing and relay of cell-wall integrity signals. Recently, it was reported that FER is required for the recovery of root growth after plants are exposed to high salinity stress, and FER may sense salt-induced cell-wall changes through direct binding to pectin (6).
Leucine-rich repeat extensins (LRXs) are a group of cell-wall proteins that harbor an N-terminal leucine-rich repeat (LRR) domain and a C-terminal extensin domain (17). The LRR domain is predicted to recognize and bind a ligand, and the extensin domain, which is highly glycosylated, is probably involved in the cross-linking to cell-wall components, such as pectins (18, 19). In Arabidopsis, there are 11 LRX proteins, which can be generally divided into two clades based on their tissue-specific expression patterns. LRX1–LRX7 are expressed mainly in vegetative tissues, while LRX8–LRX11 are expressed mainly in pollens (20). LRX1 and LRX2 are required for cell-wall formation in root hairs (21, 22). LRX3, LRX4, and LRX5 are redundant proteins involved in the regulation of plant growth and cell-wall formation (23). Recently, several groups reported that LRX8–LRX11 proteins are important for cell-wall integrity regulation during pollen tube growth (24–27). Mutations in these four LRX genes result in severe defects in pollen germination and pollen tube growth. Interestingly, the LRX8–LRX11 proteins function together with RALF4/19 peptides to regulate pollen tube growth (27).
In this study, we found that the lrx345 triple mutant, the fer-4 mutant, and transgenic plants overexpressing RALF22 or RALF23, displayed similar phenotypes, including retarded growth and increased sensitivity to salt stress. The RALF peptides are physically associated with the LRX and FER proteins. Salt stress causes the S1P protease-dependent release of mature RALF22 peptides, which in turn induce the internalization of FER via an endosomal pathway. Taken together, our results suggest that the LRXs, RALFs, and FER form a signaling module that connects salt-stress–induced cell-wall changes to the regulation of growth and salt stress tolerance.
Results
LRX3, LRX4, and LRX5 Proteins Are Required for Salt Tolerance.
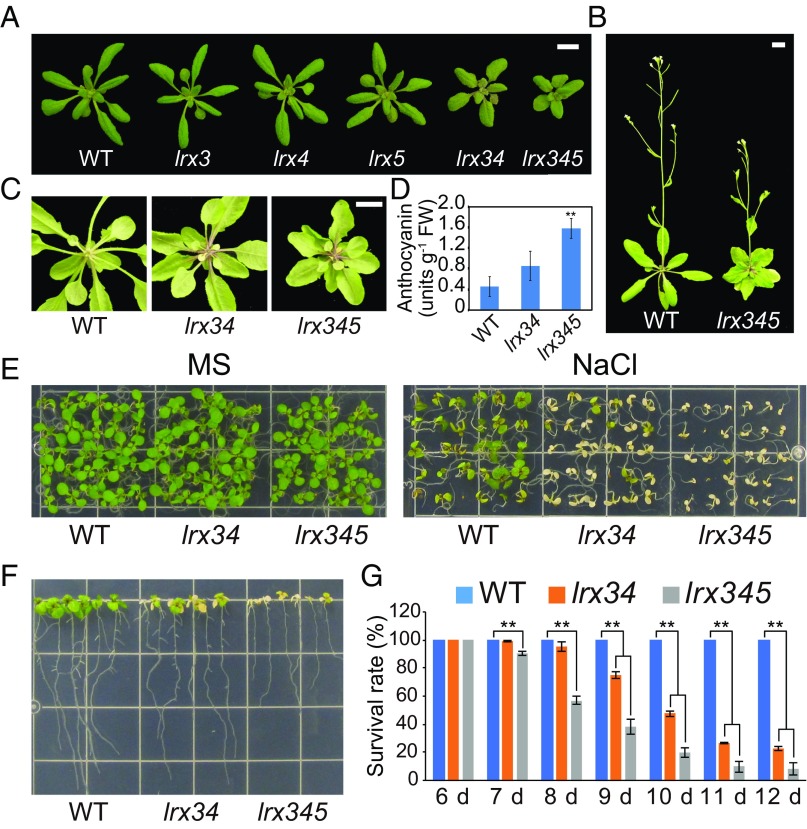
It has been shown that the Arabidopsis lrx3 lrx4 (lrx34) double and lrx3 lrx4 lrx5 (lrx345) triple mutants are defective in cell-wall composition and display growth retardation (23) (Fig. 1 A and B). We found that lrx34 and lrx345 mutant plants also displayed increased anthocyanin accumulation, especially in petioles (Fig. 1 C and D). To test whether the altered cell wall in lrx mutants may affect salt stress tolerance, we grew these mutants in the presence of NaCl and found that both lrx34 and lrx345, but not any of the lrx single mutants, exhibited severe salt hypersensitivity (Fig. 1 E–G and SI Appendix, Fig. S1A). This salt hypersensitivity phenotype was most dramatic in the lrx345 triple mutant, which showed significant lethality under 100 mM or more NaCl (Fig. 1 F and G). The results suggest that the three LRX proteins function redundantly to promote plant survival under salt stress. The retarded growth and salt-hypersensitive phenotypes of the lrx34 mutant were complemented by expressing a WT LRX3-coding sequence (CDS) (SI Appendix, Fig. S1 B and C), supporting that the lrx mutations are the cause of the observed mutant phenotypes.
Fig. 1.
LRX3, LRX4, and LRX5 affect plant growth and responses to salt stress. (A) Rosette morphology of WT and lrx single, double, and triple mutants grown in soil for 4 wk under long-day conditions. (B) Phenotype of WT and the lrx345 mutant at the flowering stage. (C) Accumulation of anthocyanin in the petioles of plants grown in soil for 4 wk. (Scale bars: A–C, 1 cm.) (D) Quantification of anthocyanin content in 12-d-old seedlings of WT and lrx34 and lrx345 mutants. Values are means ± SD (n = 3); **P < 0.01 (Student’s t test). (E) Phenotype of WT and lrx34 and lrx345 mutants germinated on MS and MS + NaCl (120 mM) media. (F) Effect of salt stress shock on plant survival. Four-day-old seedlings of WT, lrx34, and lrx345 mutants grown on MS medium were transferred to MS + NaCl (100 mM) medium. Photograph was taken 7 d after the transfer. (G) Effect of salt stress on plant survival. WT, lrx34, and lrx345 mutants were sown on MS + NaCl (120 mM) medium. Survival rates of seedlings at the indicated time point after germination were calculated. Values are means ± SD (n = 3); **P < 0.01 (Student’s t test).
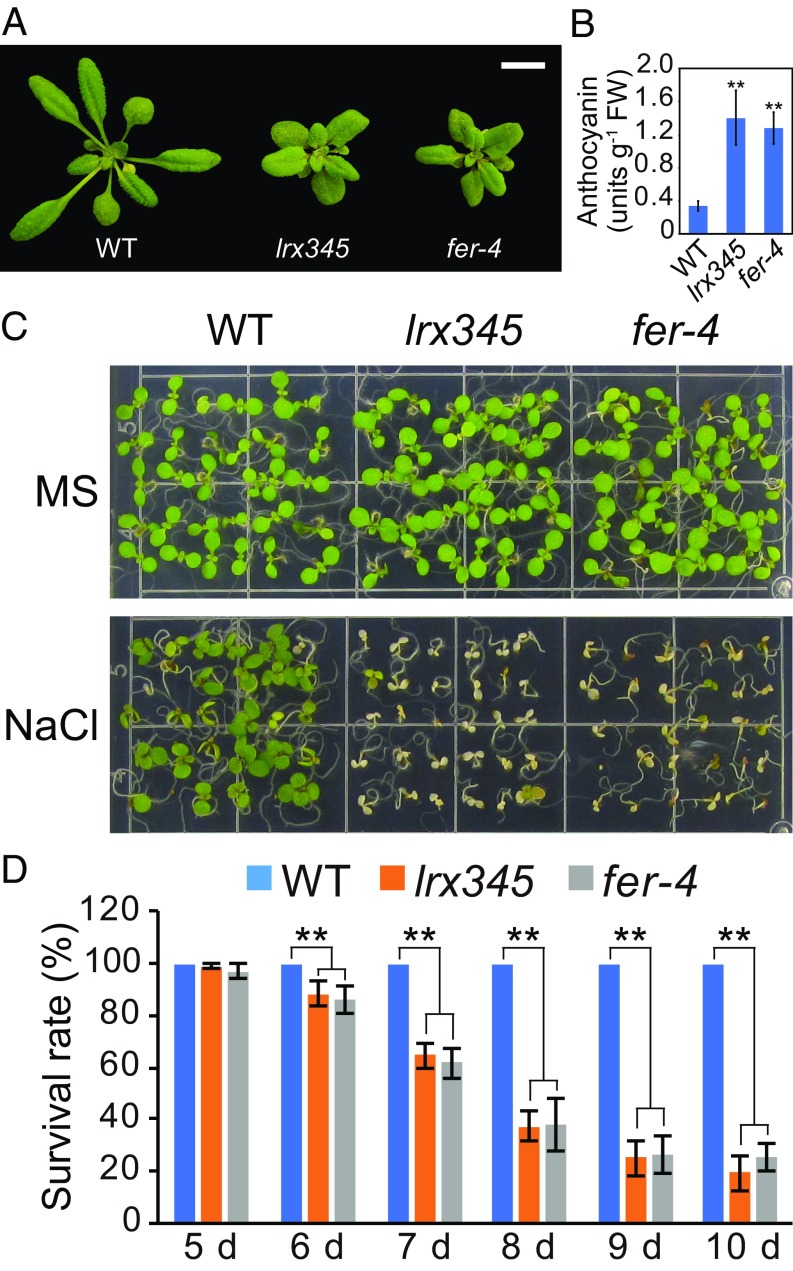
Because FER is required for cell-wall integrity and salt tolerance (6), we set out to compare the phenotypes of the fer-4 mutant to those of lrx345. We found that the lrx345 and fer-4 mutants exhibited very similar phenotypes, including retarded growth, increased anthocyanin accumulation and markedly increased sensitivity to NaCl (Fig. 2). The salt-hypersensitive phenotype of fer-4 could be complemented by expression of a (WT) FER (SI Appendix, Fig. S2A). These results suggest that LRX3/4/5 and FER may function in the same pathway for growth control and salt tolerance. Of note, fer-4 mutant plants display additional phenotypes, such as a larger seed size and deficiency in 1-naphthaleneacetic acid–induced root hair development (16, 28), which were not observed in the lrx345 mutant (SI Appendix, Fig. S2 B–D), indicating that some of the functions of FER are not shared by LRX3/4/5.
Fig. 2.
lrx345 and fer-4 mutants show similar phenotypes. (A) Rosette morphology of WT and lrx345 and fer-4 mutants grown in soil for 4 wk. (Scale bar, 1 cm.) (B) Quantification of anthocyanin accumulation in 12-d-old seedlings of WT, lrx345, and fer-4 mutants. Values are means ± SD (n = 3); **P < 0.01 (Student’s t test). (C) Phenotypes of WT, lrx345, and fer-4 seedlings grown on MS and MS + NaCl (120 mM) media. (D) Survival rates of WT, lrx345, and fer-4 seedlings on MS + NaCl (120 mM) medium. Values are means ± SD (n = 3); **P < 0.01 (Student’s t test).
The LRX3/4/5 Proteins Interact with RALF Peptides.
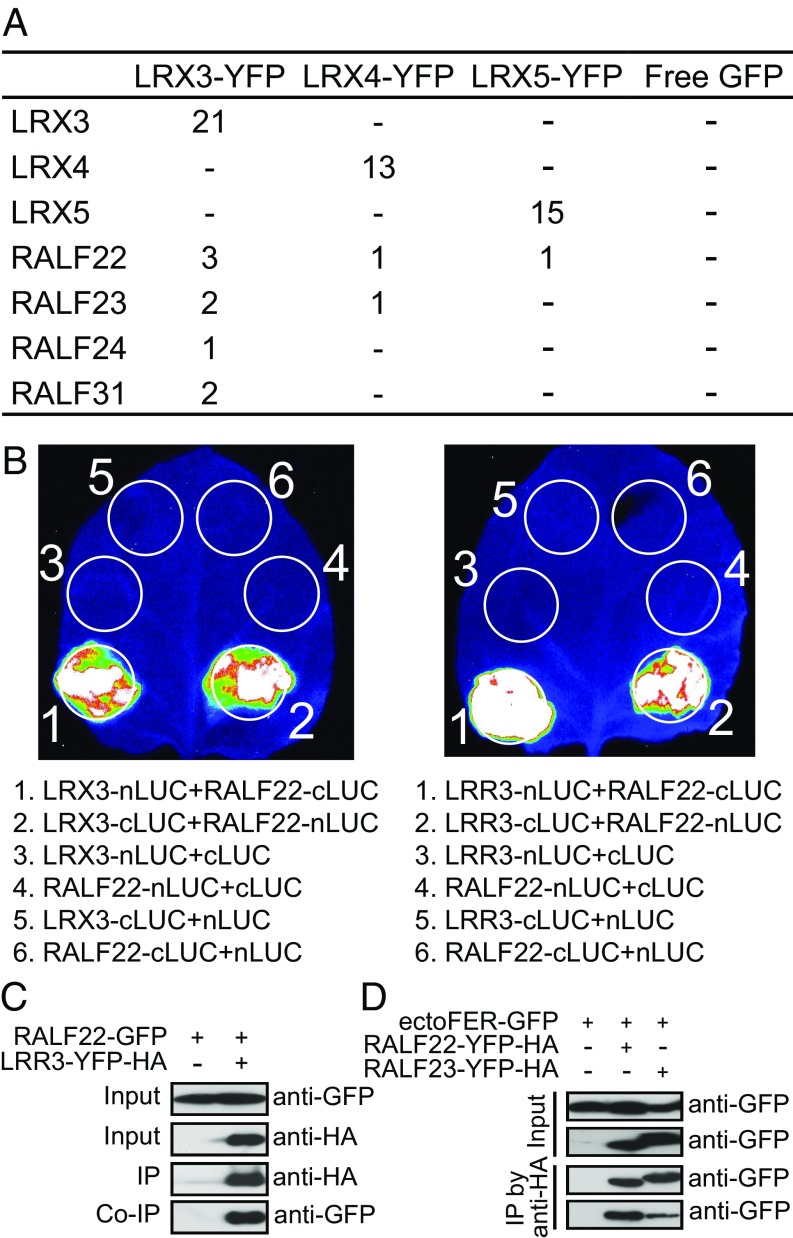
To investigate the mechanisms underlying the function of the LRX proteins in growth regulation and salt tolerance, we performed immunoprecipitation–mass spectrometry (IP–MS) analysis using 35S::LRX3-YFP-HA, 35S::LRX4-YFP-HA, and 35S::LRX5-YFP-HA transgenic plants to identify potential interacting partners of the LRXs. The transgenic plants expressing 35S::GFP were used as a control. Interestingly, peptides belonging to the four phylogenetically related RALF peptides RALF22, RALF23, RALF24, and RALF31 (27) were identified in the IP–MS samples from the LRX transgenic plants, but not in the control sample (Fig. 3A and Dataset S1). Split luciferase (split-LUC) complementation assays confirmed the interactions of LRX3 and LRX4 with all these four RALF peptides, and the LRR domain of LRX3 and LRX4 (named LRR3 and LRR4) was sufficient for the interactions (Fig. 3B and SI Appendix, Fig. S3A). We tested whether LRR3 may be able to form a homo-dimer by using the split-LUC assay, but no luciferase activity was detected (SI Appendix, Fig. S3A). Coimmunoprecipitation (Co-IP) assays further confirmed the interactions of LRR3 and LRR4 with RALF22 and RALF23 (Fig. 3C and SI Appendix, Fig. S3 B and C), and an in vitro pull-down assay supported that LRR3 directly interacts with RALF22 (SI Appendix, Fig. S3D). In agreement with published results, we found that FER interacts with RALF23 (15), as well as with RALF22 (Fig. 3D). To investigate whether the FER protein might be physically associated with the LRX proteins, we examined the IP–MS data generated from 35S::LRX3-YFP-HA, 35S::LRX4-YFP-HA, and 35S::LRX5-YFP-HA transgenic plants, but no FER peptides were detected (Dataset S1). We also performed Co-IP assays by expressing LRR3 and ectoFER in Nicotiana benthamiana leaves, but did not observe an interaction between these two proteins in the assay. These results suggest that LRX3/4/5 and FER do not exist in a complex.
Fig. 3.
RALF peptides are associated with LRX3/4/5 and FER. (A) Peptides of LRX and RALF proteins identified in IP–MS assays. Proteins were extracted from 35S::LRX3-YFP-HA, 35S::LRX4-YFP-HA, and 35S::LRX5-YFP-HA transgenic plants. The transgenic plants overexpressing free GFP were used as a control. The isolated proteins were incubated with anti-GFP antibodies overnight and then with protein G for an additional 2 h. The immunoprecipitated samples were analyzed by mass spectrometry. The number of peptides identified for each protein is shown, and a dash “-” indicates that the peptides were not identified. (B) Split luciferase complementation assays showing the interaction between RALF22 and LRX3 or the LRR domain of LRX3 (named LRR3). The constructs to express the indicated fusion proteins were transformed to N. benthamiana leaves through Agrobacterium infiltration. Luciferase activity was determined at 48 h after infiltration. (C) Coimmunoprecipitation assay showing the interaction between LRR3 and RALF22. LRR3-YFP-HA and RALF22-GFP were expressed in N. benthamiana. Immunoprecipitation was performed by using anti-HA antibodies. Immunoblottings were conducted by using anti-GFP and anti-HA antibodies. (D) ectoFER-GFP, RALF22-YFP-HA, and RALF23-YFP-HA were transiently expressed in N. benthamiana. Total proteins were extracted and immunoprecipitations were performed using anti-HA antibodies. Immunoblottings were conducted using anti-GFP antibodies.
RALF22 Overexpressing Plants Phenocopy the lrx345 and fer-4 Mutants.
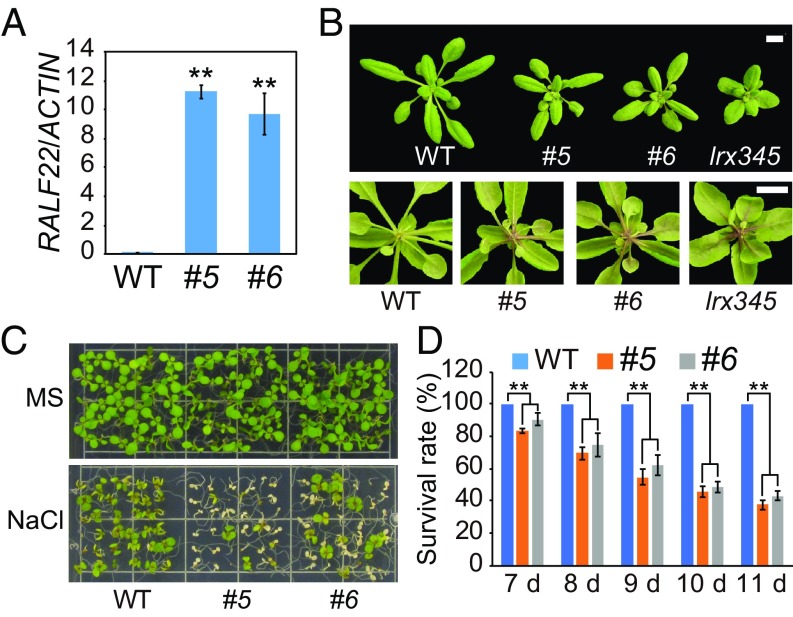
To understand the biological roles of the RALF peptides associated with LRX3/4/5 and FER, we generated transgenic plants overexpressing the RALF22 gene. Two independent transgenic lines with high expression levels of the RALF22 gene were examined (Fig. 4A). Similar to lrx345 and fer-4 mutants, the transgenic plants overexpressing RALF22 displayed retarded growth, increased accumulation of anthocyanin, and hypersensitivity to NaCl (Fig. 4 B–D). Similar phenotypes were also observed in transgenic plants overexpressing the RALF23 gene (SI Appendix, Fig. S4). We crossed the lrx345 triple mutant with a knockdown mutant of RALF22 (SI Appendix, Fig. S5 A and B) and obtained a quadruple mutant. Our results show that, although the ralf22 single mutant did not have an obvious phenotype on salt medium (SI Appendix, Fig. S5C), the ralf22 mutation partially suppressed the salt hypersensitivity of the lrx345 mutant (SI Appendix, Fig. S5 D and E), suggesting that the salt-hypersensitive phenotype of the lrx345 mutant is mediated by the RALF peptides.
Fig. 4.
RALF22 overexpressing plants phenocopy lrx345 and fer-4 mutants. (A) qRT-PCR analysis of the transcript levels of RALF22 in WT and two independent RALF22 overexpressing lines (#5 and #6). ACTIN8 was used as the internal control. Values are means ± SD (n = 3); **P < 0.01 (Student’s t test). (B) Rosette morphology (Top) and anthocyanin accumulation (Bottom) of WT and two independent RALF22 overexpressing lines (#5 and #6) grown in soil for 4 wk. (Scale bar, 1 cm.) (C) Phenotypes of WT and RALF22 overexpressing plants (#5 and #6) grown on MS and MS + NaCl (120 mM) media. (D) Survival rates of WT and RALF22 overexpressing plants on MS + NaCl medium (120 mM). Values are means ± SD (n = 3); **P < 0.01 (Student’s t test).
To test whether salt stress may affect the association between the LRXs and RALFs, we coinfiltrated Agrobacteria expressing LRX3-nLUC and RALF22-cLUC into two spots of one N. benthamiana leaf and sprayed water or 200 mM NaCl on the infiltrated spots. After the treatment for 1 h, the luciferase activities of the infiltrated spots were examined. The spot sprayed with NaCl showed a substantially lower luciferase activity than that sprayed with water (SI Appendix, Fig. S3E), which suggests that the LRXs and RALFs disassociate under salt stress.
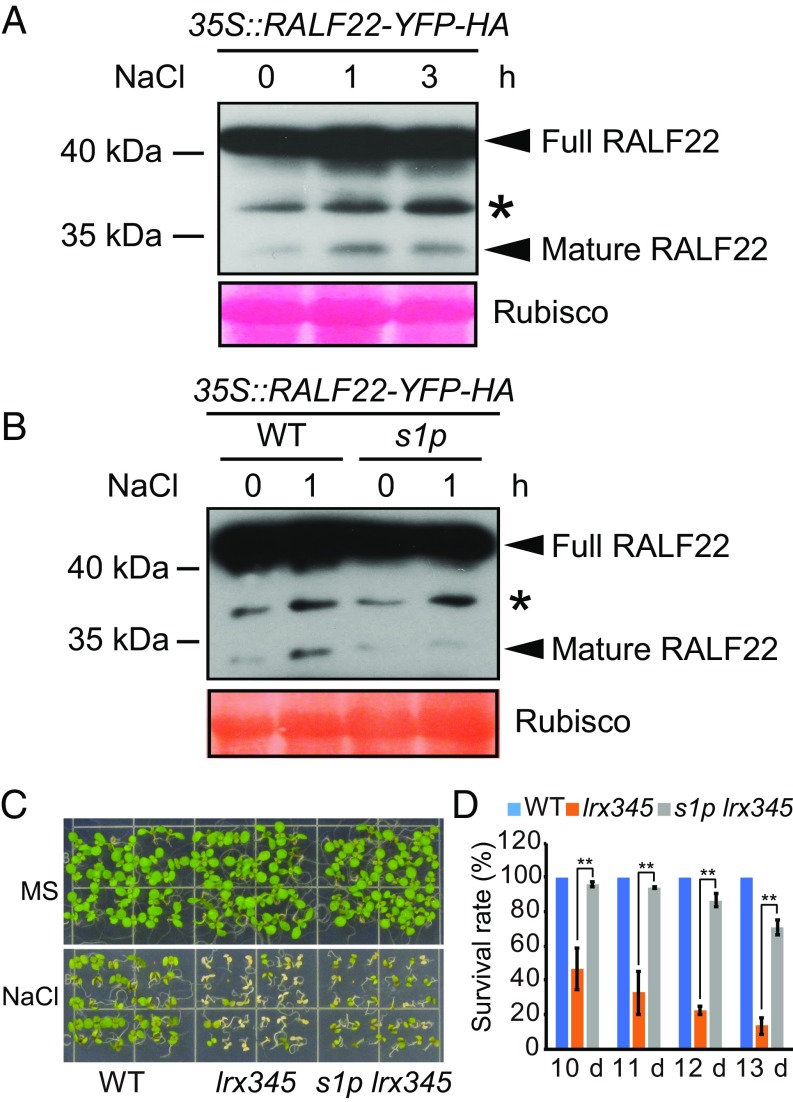
RALFs are small secreted peptides that need to be processed to mature active forms via cleavage at sites with the RRXL motif (29), and it has been shown that SITE-1 PROTEASE (S1P) is required for the cleavage (15, 30). Here, we found that salt stress caused an enhanced accumulation of mature RALF22 peptides (Fig. 5A) in a manner that depends on S1P (Fig. 5B). The salt-hypersensitive phenotype of lrx345 mutant seedlings was largely suppressed by s1p mutation (Fig. 5 C and D), suggesting that the enhanced salt sensitivity of lrx345 mutant plants is mediated by the mature active form of RALF peptides.
Fig. 5.
Salt stress induces S1P protease-dependent release of mature RALF22 peptide. (A) Ten-day-old 35S::RALF22-YFP-HA transgenic seedlings were harvested after treatment with NaCl (150 mM) at the indicated time points. Total proteins were extracted, and immunoblotting was performed using anti-GFP antibodies. (B) Ten-day-old 35S::RALF22-YFP-HA transgenic seedlings in WT or a s1p mutant background were treated without or with NaCl (150 mM) for 1 h. Immunoblotting was performed by using anti-GFP antibodies. The bands marked by an asterisk in A and B may represent an intermediate form of RALF22 cleavage. The large subunit of Rubisco is shown as a protein loading control. These experiments were repeated three times with similar results. (C) Phenotypes of seedlings grown on MS and MS + NaCl (120 mM) media. (D) Survival rates of seedlings on MS + NaCl (120 mM) medium. Values are means ± SD (n = 3); **P < 0.01 (Student’s t test).
RALF Peptides and Salt Stress Promote the Internalization of FER Protein.
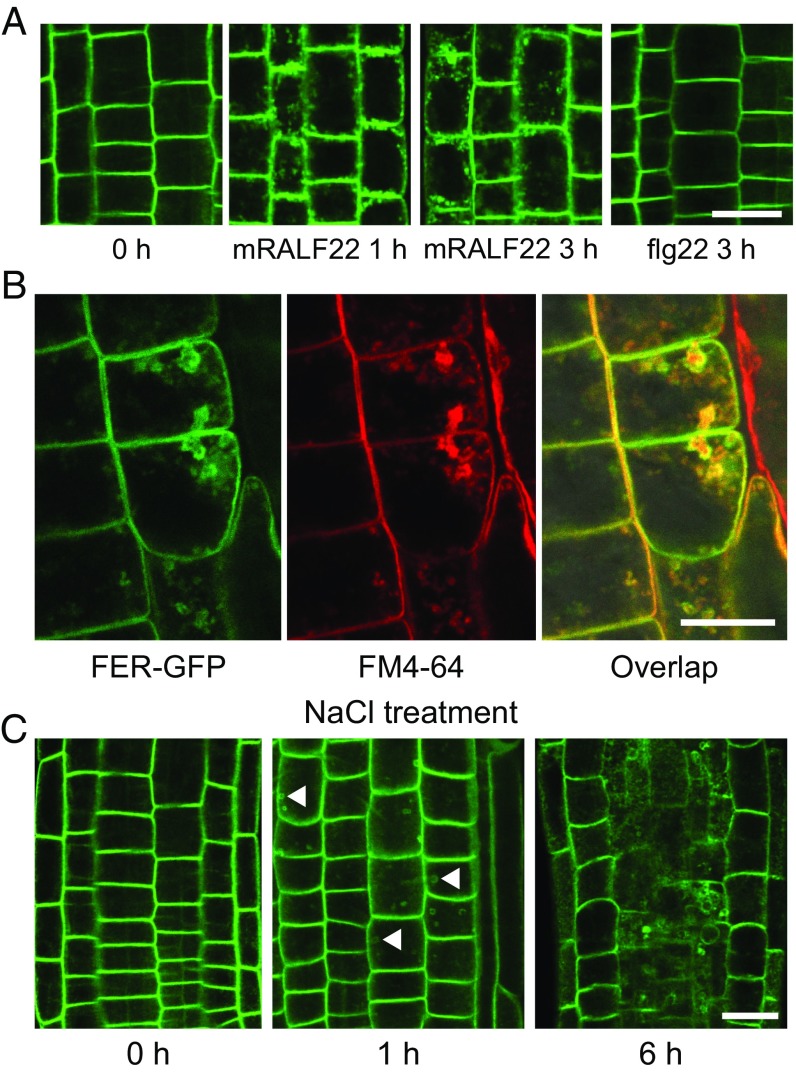
A previous study has shown that RALF23 negatively regulates FER in plant immunity (15). Our results above showed that RALF22 and RALF23 overexpressing plants phenocopy the loss of function of FER in terms of plant growth and salt sensitivity, suggesting that these RALF peptides may negatively regulate FER function in salt tolerance. Upon ligand binding, several RLKs have been shown to undergo internalization through endocytosis (31–34), which may remove signaling-competent receptor molecules from the membrane pool (35). To investigate whether the RALF22 and RALF23 peptides may induce the internalization of FER, we treated pFER::FER-GFP transgenic plants with synthesized mature RALF22 and RALF23 peptides and then examined the subcellular localization of FER-GFP in the treated roots. Upon treatment with mature RALF22 and RALF23, FER-GFP could be observed in intracellular compartments in a time-dependent manner, whereas under basal conditions FER-GFP was localized mainly at the plasma membrane (Fig. 6A and SI Appendix, Fig. S6). This observation indicates that mature RALF22/23 can induce the internalization of FER. The internalized FER-GFP was colocalized with the endocytic tracer FM4-64 (Fig. 6B), suggesting that FER proteins traffic along the endosomal pathway. As a control, the bacterial pathogen-associated molecular pattern flagellin (flg22), which can induce the internalization of its cognate RLK FLAGELLIN SENSING2 (FLS2) (31), did not induce the internalization of FER (Fig. 6A). These results suggest that the internalization of FER is induced specifically by the RALF peptides. The internalization of FER-GFP was also detected following treatment with NaCl (Fig. 6C), which is consistent with the NaCl-induced accumulation of mature RALFs.
Fig. 6.
Mature RALF22 peptide and salt stress promote the internalization of FER protein. (A) Effect of mature RALF22 (mRALF22) treatment on FER-GFP subcellular localization. The pFER::FER-GFP transgenic plants were treated with synthesized mRALF22 (1 μM) or flg22 (1 μM) peptide. Fluorescence in root cells was detected at the indicated time point after treatment using confocal microscopy. (B) pFER::FER-GFP transgenic plants were treated with synthesized mature RALF22 peptide (1 μM) for 1 h. Staining with the endocytic tracer FM4-64 is shown. (C) pFER::FER-GFP transgenic plants were treated with NaCl (150 mM). Fluorescence in root cells was detected at the indicated time point after treatment by using confocal microscopy. Identical parameters, including the same laser strength and the same pinhole, were applied for all samples. Arrowheads indicate the internalized compartments. (Scale bars, 10 μm.)
Discussion
Extensive studies in the past few years have demonstrated critical roles of FER in the regulation of multiple cellular processes (6, 7, 10, 11, 14, 15). Thus, how the FER protein is able to achieve so many distinct functions is of great interest. In this study, we found that lrx345 and fer-4 mutants share some similar phenotypes including retarded growth and increased sensitivity to salt stress, suggesting that LRX3/4/5 and FER likely function in the same pathways for growth control and salt tolerance. Considering that FER is a plasma-membrane–localized protein (11) and that the LRX proteins function in the cell wall (23), it is likely that FER functions downstream of the LRX proteins, and the phenotypes of lrx345 mutant plants are likely caused by the inhibition of FER function. LRX3/4/5 interacted with several RALF peptides including RALF22 and RALF23, and transgenic plants overexpressing RALF22 or RALF23 phenocopied lrx345 and fer-4 mutants in the aforementioned phenotypes. Application of synthesized RALF22 or RALF23 peptide promoted the internalization of FER, probably via endocytosis. Based on these results, we propose that LRX3/4/5, RALF22/23, and FER function as a module to sense and transmit cell-wall integrity signals and thereby to regulate plant growth and salt stress response.
Recently, Feng et al. (6) found that FER protein may sense salt-induced disruption of pectin cross-linking and induce cell-wall repair signaling by triggering a rapid cell-autonomous increase in cytosolic [Ca2+]. The extracellular domain of FER protein can interact with pectin, so FER may directly sense changes in the pectin network (6). LRX proteins consist of an N-terminal LRR domain that binds to RALF peptides and a C-terminal extensin domain (17, 36). Extensins are structural cell-wall proteins that interact with other cell-wall components including pectins through ionic interactions as well as covalent cross-linking (37). The combination of LRR and extensin domains places the LRX proteins in an ideal position to sense cell-wall signals and to relay this information to downstream components. The severe salt hypersensitivity of the lrx345 mutant suggests that the LRX3/4/5 are important sensors of cell-wall integrity signals. Under salt stress, cell-wall perturbations may somehow trigger the release of mature RALF peptides, which negatively regulate FER by causing FER internalization.
FER has been identified as the receptor of RALF peptides (7, 15), but how RALF peptides regulate the function of FER is not fully understood. Previous studies have shown that RALF1 activates FER by inducing its phosphorylation (7, 14), but our data indicate that RALF22 and RALF23 peptides may negatively regulate the function of FER in salt tolerance by inducing its internalization. The RALF peptide-induced internalization of FER may be a widespread phenomenon, which might underlie the observation that treatment with mature RALF23 can inhibit the scaffolding role of FER in FLS2/EFR-BAK1 complexes (15), as well as the inhibitory role of RALF peptides on root elongation and pollen tube growth (7, 27, 38). Ligand-induced activation and internalization have been reported for many other receptor-like kinases (31, 32, 39, 40). One of the well-studied examples in Arabidopsis is FLS2. FLS2 is a pattern recognition receptor, which specifically recognizes a 22-amino-acid epitope of bacterial flagellin (fls22) (41). fls22 induces the association of FLS2 with BAK1 to trigger downstream defense responses (42). Upon activation, FLS2 is internalized via clathrin-dependent endocytosis (31, 32) and finally degraded through an ubiquitination-mediated process (43). Whether the internalized FER is degraded and which E3 ligase may be involved in this process need to be investigated in the future.
LORELEI-LIKE GPI-anchored protein 1 (LLG1) is a chaperone of FER and is required for trafficking FER to the plasma membrane (16, 44). Loss of LLG1 results in cytoplasmic retention of FER. The llg1 mutants display indistinguishable growth, development, and salt-hypersensitive phenotypes from the fer-4 mutant (6, 16). Here we found that transgenic plants overexpressing the RALF22 or RALF23 also displayed similar phenotypes as the fer-4 mutant, which is likely caused by RALF peptide-induced internalization of FER. These results suggest that the dynamic regulation of the subcellular location of FER may contribute to a balanced coordination of cell-wall integrity, growth, and stress responses by controlling the pool of active FER.
In summary, our study suggests that specific cell-wall structural proteins (LRXs), small peptides (RALFs), and a plasma-membrane–localized receptor-like kinase (FER) function as a module to coordinate cell-wall integrity, plant growth, and stress responses. Under normal conditions, the LRX3/4/5 proteins interact with the RALF22/23 peptides, perhaps to prevent the association of the RALF22/23 peptides with the FER protein and thus to inhibit the internalization of the FER protein. Under salt stress, the LRX3/4/5 proteins may directly sense salt-induced changes in the cell wall, and RALF peptides are dissociated from the LRXs to transduce the cell-wall signals to the FER protein. In this module, FER acts as an executor to trigger intracellular signaling (SI Appendix, Fig. S7). This model raises several questions. It is unclear what specific salt-stress–induced cell-wall alterations are sensed by the LRX proteins. It is also unclear whether the LRXs may have a role in the S1P-dependent accumulation of mature RALFs under salt stress. The retarded growth phenotypes of lrx345 and fer-4 mutants suggest that the LRX-RALF-FER module represents a major cell-wall-integrity–sensing pathway for plant growth and that the mutants may be kept alive by unknown residual cell-wall-integrity–sensing pathways. It is possible that salt-stress–induced signaling may somehow inhibit the residual cell-wall-integrity–sensing and –repair pathways that remain in the lrx345 and fer mutants, causing complete growth inhibition and plant death.
Materials and Methods
Plant Materials.
The Col-0 ecotype of Arabidopsis thaliana was used as the WT. Plants were grown at 23 °C with a long-day light cycle (16 h light/8 h dark). The lrx3 (SALK_094400), lrx4 (GABI_017A08), lrx5 (SALK_013968), lrx34, lrx345, fer-4, and s1p mutants have been described previously (7, 23, 30). The ralf22 mutant (GK-293H09) was obtained from the Arabidopsis Biological Resource Center (Ohio State University). The s1p lrx345 and ralf22 lrx345 quadruple mutants were generated by crossing. Homozygous mutants were confirmed by PCR-based genotyping. Transgenic plants containing 35S::LRX3-YFP-HA, 35S::LRX4-YFP-HA, 35S::LRX5-YFP-HA, 35S::RALF22-YFP-HA, and 35S::RALF23-YFP-HA expression cassettes were generated by Agrobacterium tumefaciens-mediated transformation. The primers used for genotyping are listed in SI Appendix, Table S1.
Construction of Plasmids.
To generate constructs for transgenic plants and protein interaction assays, the whole corresponding CDSs of RALFs and the CDSs encoding the full length or the LRR domain of LRX genes were amplified from cDNA using gene-specific primers (SI Appendix, Table S1), and the amplified fragments were subcloned into pDONR207 ENTRY using BP clonase II kit (Life Technologies). The inserted fragments were recombined to destination vectors using LR clonase II kit (Life Technologies). The generated constructs were stably transformed to Arabidopsis or transiently transformed to N. benthamiana by A. tumefaciens-mediated transformation. For the pET-28a-mRALF22 construct, the CDS sequence encoding the mature peptide of RALF22 was amplified and cloned into the vector pET-28a by using BamHI and XhoI restriction sites. For the pGEX-4T-1-LRR3 construct, the LRR3 fragment was amplified and cloned into the vector pGEX-4T-1 by using SalI and NotI restriction sites.
Anthocyanin Measurement.
Anthocyanin content was assessed as previously described (45). In brief, 30 seedlings for each sample were collected in a 2-mL Eppendorf tube with 600 μL of 1% HCl in methanol (vol/vol). The seedlings were incubated overnight in the dark at 4 °C with gentle shaking. After extraction, 400 μL of water and 400 μL of chloroform were added, and the samples were vortexed. The tubes were centrifuged at 12,000 × g for 2 min, and the supernatant was transferred to a new tube. The absorbance of each supernatant was measured spectrophotometrically at 530 and 657 nm, respectively. The concentration of anthocyanin was calculated using the formula A530 − 0.25 A657.
Recombinant protein expression, in vitro pull-down, split luciferase complementation assay, synthetic peptides, RALF22 cleavage assay, coimmunoprecipitation assay, fluorescence assay, and LC-MS/MS analysis are described in SI Appendix, SI Materials and Methods. The sequences of mature RALF22 and RALF23 are listed in SI Appendix, Table S2.
Supplementary Material
Acknowledgments
We thank Rebecca Stevenson for technical assistance, Prof. Christoph Ringli for providing lrx34 and lrx345 seeds, Prof. Alice Y. Cheung for providing pFER::FER-GFP seeds, and Prof. Yiwei Jiang for assistance with anthocyanin measurement. This work was supported by the Strategic Priority Research Program (Grant XDB27040000) of the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816991115/-/DCSupplemental.
References
- 1.Le Gall H, et al. Cell wall metabolism in response to abiotic stress. Plants (Basel) 2015;4:112–166. doi: 10.3390/plants4010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 3.Wolf S, Hématy K, Höfte H. Growth control and cell wall signaling in plants. Annu Rev Plant Biol. 2012;63:381–407. doi: 10.1146/annurev-arplant-042811-105449. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J-K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenhaken R. Cell wall remodeling under abiotic stress. Front Plant Sci. 2015;5:771. doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng W, et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol. 2018;28:666–675.e5. doi: 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA. 2010;107:9452–9457. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung AY, Wu H-M. THESEUS 1, FERONIA and relatives: A family of cell wall-sensing receptor kinases? Curr Opin Plant Biol. 2011;14:632–641. doi: 10.1016/j.pbi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Escobar-Restrepo J-M, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 11.Duan Q, Kita D, Li C, Cheung AY, Wu H-M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu F, et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA. 2012;109:14693–14698. doi: 10.1073/pnas.1212547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, et al. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc Natl Acad Sci USA. 2016;113:E5519–E5527. doi: 10.1073/pnas.1608449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegmann M, et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science. 2017;355:287–289. doi: 10.1126/science.aal2541. [DOI] [PubMed] [Google Scholar]
- 16.Li C, et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:e06587. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borassi C, et al. An update on cell surface proteins containing extensin-motifs. J Exp Bot. 2016;67:477–487. doi: 10.1093/jxb/erv455. [DOI] [PubMed] [Google Scholar]
- 18.Cannon MC, et al. Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA. 2008;105:2226–2231. doi: 10.1073/pnas.0711980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi X, Behrens BX, West PR, Mort AJ. Solubilization and partial characterization of extensin fragments from cell walls of cotton suspension cultures. Evidence for a covalent cross-link between extensin and pectin. Plant Physiol. 1995;108:1691–1701. doi: 10.1104/pp.108.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumberger N, et al. Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol. 2003;131:1313–1326. doi: 10.1104/pp.102.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumberger N, Steiner M, Ryser U, Keller B, Ringli C. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003;35:71–81. doi: 10.1046/j.1365-313x.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 22.Baumberger N, Ringli C, Keller B. The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev. 2001;15:1128–1139. doi: 10.1101/gad.200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draeger C, et al. Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 2015;15:155. doi: 10.1186/s12870-015-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabrice TN, et al. LRX proteins play a crucial role in pollen grain and pollen tube cell wall development. Plant Physiol. 2018;176:1981–1992. doi: 10.1104/pp.17.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, et al. Pollen-expressed leucine-rich repeat extensins are essential for pollen germination and growth. Plant Physiol. 2018;176:1993–2006. doi: 10.1104/pp.17.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sede AR, et al. Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 2018;592:233–243. doi: 10.1002/1873-3468.12947. [DOI] [PubMed] [Google Scholar]
- 27.Mecchia MA, et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science. 2017;358:1600–1603. doi: 10.1126/science.aao5467. [DOI] [PubMed] [Google Scholar]
- 28.Yu F, et al. FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Mol Plant. 2014;7:920–922. doi: 10.1093/mp/ssu010. [DOI] [PubMed] [Google Scholar]
- 29.Murphy E, De Smet I. Understanding the RALF family: A tale of many species. Trends Plant Sci. 2014;19:664–671. doi: 10.1016/j.tplants.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava R, Liu J-X, Guo H, Yin Y, Howell SH. Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J. 2009;59:930–939. doi: 10.1111/j.1365-313X.2009.03926.x. [DOI] [PubMed] [Google Scholar]
- 31.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbengue M, et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc Natl Acad Sci USA. 2016;113:11034–11039. doi: 10.1073/pnas.1606004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postma J, et al. Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity. New Phytol. 2016;210:627–642. doi: 10.1111/nph.13802. [DOI] [PubMed] [Google Scholar]
- 34.Russinova E, et al. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irani NG, et al. Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat Chem Biol. 2012;8:583–589. doi: 10.1038/nchembio.958. [DOI] [PubMed] [Google Scholar]
- 36.Ringli C. The hydroxyproline-rich glycoprotein domain of the Arabidopsis LRX1 requires Tyr for function but not for insolubilization in the cell wall. Plant J. 2010;63:662–669. doi: 10.1111/j.1365-313X.2010.04270.x. [DOI] [PubMed] [Google Scholar]
- 37.Lamport DTA, Kieliszewski MJ, Chen Y, Cannon MC. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011;156:11–19. doi: 10.1104/pp.110.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge Z, et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science. 2017;358:1596–1600. doi: 10.1126/science.aao3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erwig J, et al. Chitin-induced and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) phosphorylation-dependent endocytosis of Arabidopsis thaliana LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE5 (LYK5) New Phytol. 2017;215:382–396. doi: 10.1111/nph.14592. [DOI] [PubMed] [Google Scholar]
- 41.Gómez-Gómez L, Boller T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 42.Chinchilla D, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 43.Lu D, et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Q, Bourdais G, Pan H, Robatzek S, Tang D. Arabidopsis glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc Natl Acad Sci USA. 2017;114:5749–5754. doi: 10.1073/pnas.1614468114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, et al. High ambient temperature represses anthocyanin biosynthesis through degradation of HY5. Front Plant Sci. 2017;8:1787. doi: 10.3389/fpls.2017.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.