Significance
Circadian rhythms are not simply a passive consequence of cyclic fluctuations in the environment, but instead originate within the organism. Accumulating evidence indicates that disruption of circadian rhythms contributes to the development of many diseases. Here, we demonstrate that removal of the clock gene Bmal1 from the retina has multiple effects on rod and cone pathways. In the cone pathway, the primary effect is on cone viability, an effect accentuated in aging mice. In the rod pathway, we observed that retinal Bmal1 removal caused stunting of rod bipolar cell dendrites and thinning of the outer plexiform layer in both young and old mice. Hence, our data suggest that circadian clock dysfunction contributes to aberrant visual function during development and aging.
Keywords: circadian rhythm, retina, development, aging, cone photoreceptor
Abstract
The mammalian retina contains an autonomous circadian clock system that controls many physiological functions within this tissue. Previous studies on young mice have reported that removal of the key circadian clock gene Bmal1 from the retina affects the circadian regulation of visual function, but does not affect photoreceptor viability. Because dysfunction in the circadian system is known to affect cell viability during aging in other systems, we compared the effect of Bmal1 removal from the retina on visual function, inner retinal structure, and photoreceptor viability in young (1 to 3 months) and aged (24 to 26 months) mice. We found that removal of Bmal1 from the retina significantly affects visual information processing in both rod and cone pathways, reduces the thickness of inner retinal nuclear and plexiform layers, accelerates the decline of visual functions during aging, and reduces the viability of cone photoreceptors. Our results thus suggest that circadian clock dysfunction, caused by genetic or other means, may contribute to the decline of visual function during development and aging.
All mammalian organs, including the retina, contain circadian clocks that are essential for normal physiological function (1). In the mammalian retina, multiple circadian rhythms are controlled by autonomous circadian clocks in various cell types, in which the circadian genes are heterogeneously distributed (2–6). Among the many different clock genes (1), however, the only gene whose removal can abolish circadian rhythmicity is Bmal1 (also known as Arntl) (7). Although Bmal1 is expressed in many retinal cell types (2, 8), including cone photoreceptors and rod bipolar cells, it is not expressed in rods (8). In Bmal1 knockout (KO) mice, a large fraction of the more than 1,000 genes that normally show daily rhythms have reduced amplitude or are no longer rhythmically expressed. In addition, in both Bmal1 KO mice and mice lacking only retinal Bmal1 (Chx10Cre;Bmal1fl/fl) the day/night changes in amplitude of the photopic b wave are no longer present (9). Retinal removal of Bmal1 also affects the amplitude of the b wave of both scotopic and photopic electroretinograms (ERGs), suggesting that the transmission of light information from the photoreceptors to the ON bipolar cells for both rods and cones is affected by Bmal1 disruption (9). Collectively, these results show that retinal Bmal1 is required for the circadian rhythm in visual processing. Additional studies have shown that the loss of Bmal1 affects the distribution of short- and medium-wavelength opsins in the mouse retina (10). Although aging is known to affect retinal function, studies of the effect of circadian disruption on aged Bmal1 KO mice have not been carried out. To investigate the basis of the changes in retinal function and how they might be affected by aging, we examined the effects of retinal Bmal1 removal in both young and older mice. In contrast to global Bmal1 KO mice (11), which have a reduced life span (about 9 mo), Chx10Cre;Bmal1fl/fl mice have a normal life span and are thus ideal for studying the long-term effects of circadian dysfunction within the retina. In this study, we investigated visual function, retinal circuitry, and photoreceptor viability in both young and older mice to determine the interaction of retinal Bmal1 removal with retinal development and the aging process.
Results
Retina-Specific Disruption of Bmal1.
The retina-specific disruption of Bmal1 was generated using a conditional Bmal1 allele (in which the exon encoding the basic helix-loop-helix domain was floxed) and a Chx10-Cre recombinase driver, as reported by Storch et al. (9). Disruption of Bmal1 was confirmed by Southern blot, and loss of BMAL1 protein by Western blot (9). To further confirm the loss of BMAL1, we used PCR to examine the floxed and KO alleles of the Chx10Cre;Bmal1fl/fl mice and Bmal1fl/fl controls (SI Appendix, Fig. S1A). In the retina from floxed control mice, only the floxed allele was observed. From the retina of the Chx10Cre;Bmal1fl/fl mice, the major band corresponded to the KO allele; there was a very faint band corresponding to the floxed allele, indicating that floxed alleles were excised in the majority of retinal cells. In addition, we examined immunofluorescence staining of retinas from Chx10Cre;Bmal1fl/fl mice and Bmal1fl/fl controls (SI Appendix, Fig. S1B). We found the staining for BMAL1 to be greatly reduced, but not completely eliminated, in the retinas of Chx10Cre;Bmal1fl/fl mice compared with Bmal1fl/fl control mice.
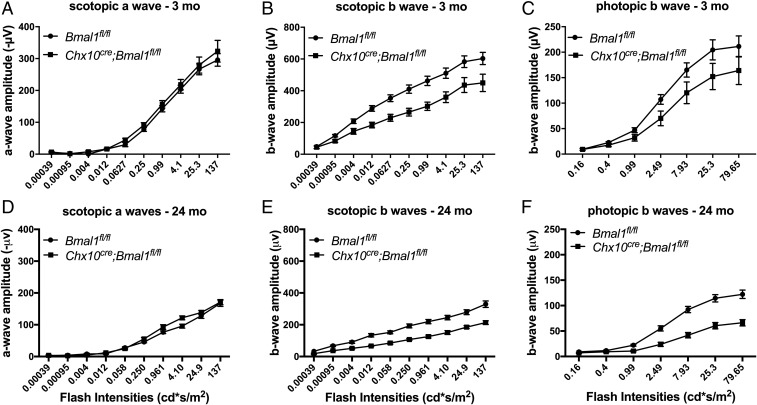
Disruption of Bmal1 in the Retina Reduces the b-Wave Amplitudes of the ERGs of Young and Aged Mice.
Examination of ERGs in Chx10Cre;Bmal1fl/fl mice compared with littermate Bmal1fl/fl controls showed that both scotopic (rod-based) and photopic (cone-based) ERGs in Chx10Cre;Bmal1fl/fl mice exhibited significantly lower b-wave amplitudes in comparison with Bmal1fl/fl mice at 3 mo of age (Fig. 1). The scotopic a-wave amplitude was not different between the two genotypes (Fig. 1A), whereas the b-wave amplitude of the scotopic ERGs was significantly lower in Chx10Cre;Bmal1fl/fl mice than in Bmal1fl/fl littermate controls (P < 0.01; Fig. 1B). The decrease in b-wave amplitudes in Chx10Cre;Bmal1fl/fl mice was not due to off-target effects of the Chx10Cre driver, as the scotopic and photopic b-wave amplitudes of the Chx10Cre;Bmal1+/+, Bmal1fl/fl, and C57BL/6 mice were all similar and all significantly greater than the amplitudes of the Chx10Cre;Bmal1fl/fl mice (SI Appendix, Fig. S2). Thus, both rod and cone retinal pathways are affected by retinal Bmal1 disruption.
Fig. 1.
Scotopic and photopic ERGs in Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice. Scotopic and photopic ERGs were obtained from young (A–C) and old (D–F) mice. The scotopic and photopic b waves, but not a waves, were affected in Chx10Cre;Bmal1fl/fl mice compared with littermate Bmal1fl/fl controls (P < 0.05; n = 5 to 8). The amplitudes of both scotopic and photopic ERGs decline with age (P < 0.01), and there was a trend for this decline to be more pronounced in Chx10Cre;Bmal1fl/fl mice compared with Bmal1fl/fl mice.
When 24-mo-old Bmal1fl/fl mice were examined, both scotopic a- and b-wave and photopic b-wave amplitudes were significantly decreased compared with those of young animals (P < 0.001), indicating an effect of aging on photoresponses both at the photoreceptor level and the bipolar cell level. When the responses of aged Chx10Cre;Bmal1fl/fl animals were examined and compared with those of aged Bmal1fl/fl animals, no difference was observed in the scotopic a-wave amplitude between the Chx10Cre;Bmal1fl/fl and Bmal1fl/fl animals (P > 0.5; Fig. 1 A and D), suggesting that removal of Bmal1 did not change the age-related decrease in the rod photoreceptor response. In addition, the age-related decrease in the scotopic b wave was similar in both aged Chx10Cre;Bmal1fl/fl mice (45% decrease at the highest flash intensity) and Bmal1fl/fl mice (53% decrease at the highest flash intensity) (Fig. 1 B and E). When the photopic response was examined, however, the age-related decline in the photopic b wave showed a trend for a larger decrease in Chx10Cre;Bmal1fl/fl KO mice (60% at the highest flash intensity) compared with Bmal1fl/fl mice (42% at the highest flash intensity). Overall, these results suggest that aging diminishes the ERG response of both rod- and cone-based retinal pathways and that disruption of the retinal circadian rhythm may increase the decline during aging of cone-based bipolar responses, but not of those in the rod pathway.
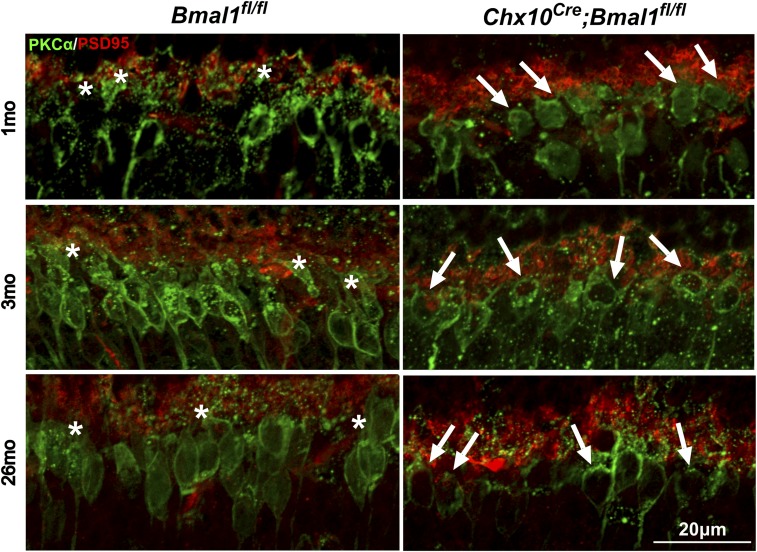
Removal of Bmal1 Affects Retinal Circuitry.
The ERG data show that at both young and old ages, the bipolar cell response to scotopic stimulation is reduced by removal of retinal Bmal1. This is somewhat surprising as the amplitude of the scotopic b wave is not regulated as a circadian rhythm (12, 13). To investigate the cellular basis of this effect, we performed a detailed analysis of the outer plexiform layer (OPL) of Chx10Cre;Bmal1fl/fl mice and littermate Bmal1fl/fl controls at 1, 3, and 26 mo of age using immunohistochemistry (Fig. 2 and SI Appendix, Fig. S3). Our results show that although the overall cellular organization of retinas of Chx10Cre;Bmal1fl/fl mice was apparently normal at 3 and 26 mo of age (SI Appendix, Fig. S3), the dendritic processes of rod bipolar cells were stunted at both ages (Fig. 2), whereas the dendrites of cone bipolar cells appeared normal. The dendritic arbors of rod bipolar cells of Chx10Cre;Bmal1+/+control mice appeared normal (SI Appendix, Fig. S4) and indistinguishable from those of the Bmal1fl/fl controls (Fig. 2). The dendritic abnormality of the Chx10Cre;Bmal1fl/fl mice was also observed at 1 mo of age, suggesting a developmental mechanism. Our analysis also revealed that in old, but not young, Chx10Cre;Bmal1fl/fl mice, the morphology of the cone outer segments and pedicles was altered (see cone arrestin staining, SI Appendix, Fig. S3). These results suggest that the effects of BMAL1 removal on rod and cone retinal visual pathways are mediated by different mechanisms.
Fig. 2.
The dendritic processes of rod bipolar cells are affected in Chx10Cre;Bmal1fl/fl mice. Synaptic connections between photoreceptor cells (anti-PSD95, red) and bipolar cells (anti-PKCα, green) in Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice at the ages of 1, 3, and 26 mo. White asterisks show the presence of dendritic arborization in Bmal1fl/fl mice; white arrows show the stunted dendritic arborizations in Chx10Cre;Bmal1fl/fl mice at all of the ages tested.
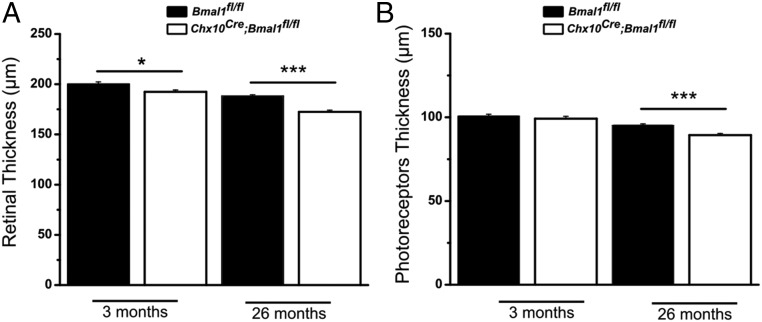
Effects of Bmal1 Disruption on Inner and Outer Retinal Structure.
We used spectral-domain optical coherence tomography (SD-OCT) and immunohistochemistry to further investigate the effect of Bmal1 removal on the retina. This analysis showed that the overall thickness of the retina was slightly reduced in Chx10Cre;Bmal1fl/fl mice compared with Bmal1fl/fl mice in young and old animals (P < 0.05 at 3 mo and P < 0.001 at 24 mo; Fig. 3A and SI Appendix, Fig. S5). Although the thickness of the photoreceptor layer (PRL) in young mice of both genotypes was the same, we observed a modest but significant reduction of the PRL thickness in older Chx10Cre;Bmal1fl/fl mice (P < 0.001 at 24 mo; Fig. 3B). To further investigate the effects of Chx10Cre;Bmal1fl/fl disruption on retinal thickness, detailed segmentation analysis of retinal layers was performed (SI Appendix, Table S1). As early as 3 mo of age, the OPL, the inner nuclear layer, and the inner plexiform layer were significantly thinner in the conditional KO mice compared with age-matched Bmal1fl/fl littermate controls. These differences became larger with increasing age. At 3 mo of age, there was no significant effect of genotype on the ganglion cell/nerve fiber layer (GC/NFL) or outer nuclear layer (ONL) thickness, although there was a trend for reduced thickness of the ONL in Chx10Cre;Bmal1fl/fl mice. However, by 12 mo of age, the ONL and GC/NFL thicknesses were also significantly reduced in Chx10Cre;Bmal1fl/fl mice compared with age-matched Bmal1fl/fl mice, and this effect increased with advancing age. The thinner retina of Chx10Cre;Bmal1fl/fl mice was also observed compared with Chx10Cre mice lacking the floxed Bmal1 alleles (Chx10Cre;Bmal1+/+; SI Appendix, Fig. S6).
Fig. 3.
Quantification of SD-OCT. (A) A small but significant difference in the total thickness of the retina was detected between Bmal1fl/fl and Chx10Cre;Bmal1fl/fl genotypes at both ages investigated (*P < 0.05 at 3 mo and ***P < 0.001 at 24 mo). (B) No difference in the thickness of PRL was observed between young Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice, whereas a significant reduction in the thickness of the PRL was detected in older Chx10Cre;Bmal1fl/fl mice with respect to the Bmal1fl/fl mice (***P < 0.001 at 24 mo).
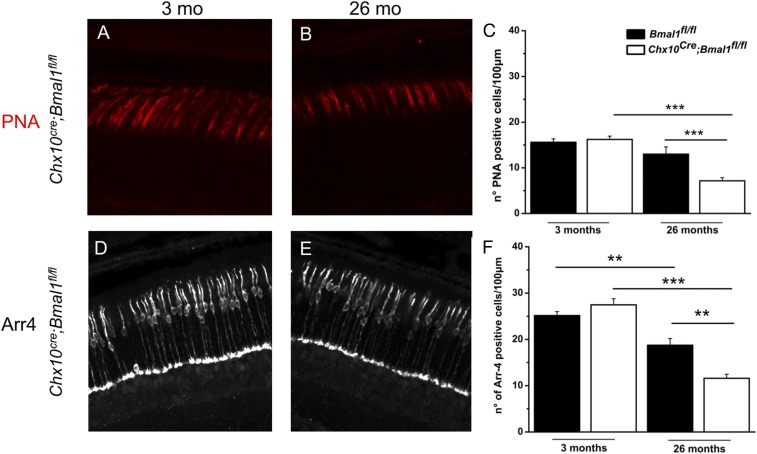
Cone Photoreceptor Viability During Aging Is Reduced in Chx10Cre;Bmal1fl/fl Mice.
Because Bmal1 protein is expressed in mouse cone photoreceptors, but not in rods (8), we investigated the effect of Bmal1 removal on cone viability with aging (Fig. 4 A–F). We found no effect of genotype on cone photoreceptor number at 3 mo of age. However, consistent with SD-OCT results, Chx10Cre;Bmal1fl/fl mice at 26 mo showed a reduction of about 60% in the number of cone outer segments (P < 0.001; Fig. 4C) and cone nuclei (P < 0.01; Fig. 4F) compared with younger mice. A significantly smaller reduction in the number of cones (25 to 30%, P < 0.01) was seen in old Bmal1fl/fl mice (Fig. 4 C and F). In addition, the remaining cones in the 26-mo-old Chx10Cre;Bmal1fl/fl mice had significantly shorter outer-segment (OS) plus inner-segment (IS) lengths compared with Bmal1fl/fl mice (Fig. 4B and SI Appendix, Fig. S7). These results, combined with the photopic ERG phenotype, show that conditional deletion of Chx10Cre;Bmal1fl/fl results in accelerated age-related disruption of cone cell structure, function, and viability.
Fig. 4.
Morphological evaluation of cone photoreceptors in young and old Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice. Peanut agglutinin (PNA) labeling in the central retina of young (A) and old (B) Chx10Cre;Bmal1fl/fl mice. (C) Quantification of PNA-labeled cones of Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice retinas at 3 and 26 mo of age. A significant change in the number of PNA-positive cells is observed between Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice at 26 mo of age (***P < 0.001). Cone arrestin (Arr4) staining in young (D) and old (E) Chx10Cre;Bmal1fl/fl mice. (F) Quantification of cone arrestin staining in Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice retinas at 3 and 26 mo of age. A significant change in the number of arrestin-positive cells is observed between Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice at 26 mo of age (**P < 0.01). A significant reduction in the number of cones is also observed in both genotypes during aging (Bmal1fl/fl, **P < 0.01; Chx10Cre;Bmal1fl/fl, ***P < 0.001). Each bar represents the mean ± SEM (n = 3 to 4).
Chx10Cre;Bmal1fl/fl Removal Results in Lower Contrast Sensitivity in Both Young and Aged Mice.
To determine whether visual function is affected by Bmal1 gene disruption in the retina, an optomotor response test was used to measure contrast sensitivity and visual acuity (14), the latter defined by spatial frequency threshold (15) in young and old Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice. A small but significant difference was observed in visual acuity between Bmal1fl/fl and Chx10Cre;Bmal1fl/fl mice at both ages tested (P < 0.05 and P < 0.01, respectively; SI Appendix, Fig. S8A). Bmal1fl/fl mice exhibited significantly higher contrast sensitivity than Chx10Cre;Bmal1fl/fl mice (P < 0.001; SI Appendix, Fig. S8B). As expected, contrast sensitivity decreased with age in both genotypes (P < 0.001; SI Appendix, Fig. S8B), but even in 24-mo-old mice, contrast sensitivity was higher in Bmal1fl/fl mice than in Chx10Cre;Bmal1fl/fl mice (P < 0.001). Advanced age did not accentuate the difference in contrast sensitivity between genotypes.
Discussion
The retinal clock and its circadian outputs play an important role in the regulation of retinal functions, as demonstrated by previous studies (9, 13, 14, 16–19). We have extended these findings by examining the effects of Bmal1 disruption on retinal function as a function of age. Our results show that Bmal1 removal affects both rod- and cone-mediated pathways in the retina, consistent with previous results (9) and, further, that Bmal1 removal also produces changes during aging in both pathways. Interestingly, the multiple effects of Bmal1 removal on visual signaling appear to be mediated by multiple, separate mechanisms.
The principal evidence for the effect of Bmal1 on retinal aging comes from cone photoreceptor analyses in young and older Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice, supported by electroretinography studies. The ERG, consisting mainly of an a wave and a b wave, is a commonly used method to assess retinal functioning. In the dark-adapted (scotopic) ERGs, the a wave represents the response of the rod photoreceptors to a flash of light, and the b wave represents the response of the rod bipolar cells. Similarly, the light-adapted (photopic) ERGs can be used to detect alterations in the cone pathway. Thus, a-wave and b-wave amplitudes can be used to determine the effects of aging, genetic mutations, or pharmacological treatments on specific retinal cell types (20–22).
Because we observed a significant reduction of the scotopic b-wave amplitude in Chx10Cre;Bmal1fl/fl mice with no effect on the a-wave amplitude, we concluded that the effects of Bmal1 removal should be observable at the level of the OPL rather than on the photoreceptors directly. Indeed, our SD-OCT and immunohistochemistry analyses revealed that the OPL was significantly thinner and that the rod bipolar cells have stunted dendritic processes in Chx10Cre;Bmal1fl/fl mice (Fig. 2 and SI Appendix, Table S1). Interestingly, this phenotype was already present in Chx10Cre;Bmal1fl/fl mice as early as 28 to 30 d of age (Fig. 2), suggesting that the lack of dendritic processes in these cells is likely due to the action of BMAL1 in the bipolar cells during retinal development rather than to a degenerative process occurring in postnatal life.
Consistent with this interpretation, deletion of Bmal1 or Clock delays the establishment of inhibitory neuronal networks in the visual cortex during a critical period of development (23). Because we did not observe similar dendritic abnormalities in cone ON bipolar cells of Chx10Cre;Bmal1fl/fl mice, our electroretinography, SD-OCT, and cone viability results suggest that BMAL1 removal may directly affect the cones.
The absence of Bmal1 also affected visual acuity and contrast sensitivity (SI Appendix, Fig. S5). Interestingly, mice lacking dopamine D4 receptors (D4Rs) also have disrupted circadian rhythms of contrast sensitivity and light-adapted ERG responses, with decreases during the subjective daytime, suggesting that dopamine signaling promotes daytime enhancement of visual function (13). The observation that D4R KO mice and Chx10Cre;Bmal1fl/fl mice show similar phenotypes suggests that removal of Bmal1 from dopaminergic cells within the retina may also be involved in the modulation of these functions (9).
Several studies have reported a decrease in photoreceptor function during aging (20–22), and our results indicate that in mice lacking Bmal1, photoreceptor to bipolar cell signaling is significantly impaired with respect to age-matched Bmal1fl/fl mice. We found no difference, however, in the response of rod photoreceptors with aging, as the a-wave amplitude of the scotopic ERGs was not different between Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice at the two ages tested (Fig. 1). This observation is consistent with the apparent absence of circadian clock proteins in rod photoreceptors (8). Although the amplitudes of the scotopic a and b waves steadily decreased in both genotypes with age, the magnitude of the decreases was not different between Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice (a wave, 47% and 44%; b wave, 53% and 45%, respectively). On the other hand, the amplitude of the b wave of the photopic ERG appeared to decrease more with age in Chx10Cre;Bmal1fl/fl mice (60%) compared with Bmal1fl/fl mice (42%, Fig. 1). Aging also significantly affected visual acuity and contrast sensitivity, and old Bmal1fl/fl mice performed significantly better than Chx10Cre;Bmal1fl/fl mice with respect to both functions (SI Appendix, Fig. S5). Therefore, the results obtained both with the ERGs and the optomotor response test demonstrate that removal of Bmal1 from the retina accelerates the aging of visual perception in the retina.
In previous work, Storch et al. (9) observed no difference in the morphology and/or number of photoreceptor cells between young Chx10Cre;Bmal1fl/fl and Bmal1fl/fl mice. In contrast, using SD-OCT, we detected a small but significant reduction of PRL and ONL thicknesses in Chx10Cre;Bmal1fl/fl mice, but only in older mice (Fig. 3 and SI Appendix, Table S1). The observation that the a-wave amplitude of the scotopic ERG was not reduced in Chx10Cre;Bmal1fl/fl mice compared with age-matched Bmal1fl/fl mice, together with the observation that BMAL1 protein is detectable in the cones, but not in the rods (8), suggested that cone viability was selectively affected by removal of Bmal1. Indeed, we detected a significant age-related decrease in the number of cones (outer segments and nuclei) in mice lacking Bmal1 (Fig. 4), suggesting that these cells are directly affected by Bmal1 removal. We also observed that the remaining cones in old Chx10Cre;Bmal1fl/fl mice had shorter OS+IS lengths (SI Appendix, Fig. S7). Sawant et al. (10) found that the ratio of OS length to OS+IS length was already reduced at 3 mo of age, suggesting that there may be an early-onset shortening of outer segments before reduction in IS length. These researchers also showed that retinal Bmal1 disruption affects the spectral identity of cones during development. Thus, BMAL1 appears to play important roles in both cone development and cone viability during aging. Whether these two roles are related is unknown. Cones are known to be among the cells with highest metabolism within the body (24) and, therefore, alteration of metabolic processes within these cells is likely to affect their health status and viability. It is now well established that the circadian clock controls cellular metabolism (1).
Finally, it is worth noting that disruption of the retinal melatonin system (a key circadian output in the eye) also produces a significant reduction in the number of cone photoreceptors (about 30%) during aging (25). Hence, it appears that a significant feature of the dysfunction of the retinal circadian system produced by genetic mutation of the clock genes or its circadian output is the effect on cone viability.
In conclusion, our results demonstrate that removal of Bmal1 from the retina has multiple effects on both rod and cone pathways and that several of these effects are importantly related to aging. In the cone pathway, the primary effect is on cone viability, an effect that is accentuated in aging mice. In the rod pathway, we observed that retinal Bmal1 removal caused stunting of rod bipolar cell dendrites and thinning of the OPL in both young and old mice, a result that has been previously unreported. Because Bmal1 is not expressed in rod photoreceptors, this may be an indirect effect mediated through synaptic signaling pathways or may indicate that Bmal1 expression in rod bipolar cells is necessary for their normal development.
Methods
Animals.
Retinal Bmal1 KO (Chx10Cre;Bmal1fl/fl) and control (Bmal1fl/fl or Chx10Cre;Bmal1+/+) mice on C57BL/6 background (9) were bred and housed in the animal facility at Emory University School of Medicine. Mice were genotyped by PCR analysis of genomic DNA. The mice were kept under standard laboratory conditions in a 12-h light/12-h dark cycle (illumination with fluorescent strip lights, 200 lx at cage level during the day); water and food were provided ad libitum. Animal experimentation was carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (26) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Institutional Animal Care and Use committees of Morehouse School of Medicine and Emory University.
Immunohistochemistry.
Eyes for immunohistochemistry were isolated and fixed in paraformaldehyde 4% for 1 h and cryoprotected in sucrose 30% overnight at 4 °C. Cryosections (14 μm thick) were washed three times for 10 min in PBS and then incubated for 45 min in 1% BSA and 0.4% Triton-X100 in PBS to permeabilize membranes and block unspecific binding. Sections were incubated overnight at 4 °C with primary antibodies diluted in 1% BSA and 0.03% Triton-X100 (SI Appendix, Table S2), washed in PBS, and then incubated for 2 h at room temperature in secondary antibodies (anti-mouse or anti-rabbit conjugated with Alexa Fluor 488, 1:1,000, or with Alexa Fluor 568, 1:1,000; Molecular Probes) diluted in 1% BSA in PBS. Nuclear staining was obtained with propidium iodide or DAPI. After washing in PBS, slides were coverslipped with VECTASHIELD (Vector Laboratories). Retinal sections were visualized with a confocal microscope (Zeiss LSM 700); files were processed with image software (GIMP 2.8). All of the images shown are Z projections of four slices (22).
Analysis of the OS+IS length was performed on the same sections that we used to count the peanut agglutinin-positive cones. The measurements were performed in the central retina within approximately 1 mm from the optic nerve. The relative OS+IS length represents the value of the combined OS and IS lengths at 26 mo of age relative to the values obtained in retinas of 3-mo-old Bmal1fl/fl mice. The combined cone OS+IS lengths were measured using ImageJ on 100-μm-wide retinal segments.
Electroretinography.
Dark- and light-adapted ERG recordings were performed with an LKC Technologies UTAS E-3000 Visual Electrodiagnostic System. Before testing, mice were housed on a 12-h light/12-h dark cycle. All mice were dark-adapted the night before electroretinography; measurements were made in subjective midday between circadian times 4 and 8. Mice were anesthetized with an i.p. injection of ketamine (10 mg/mL) and xylazine (1.5 mg/mL), which was injected at 10 µL/g of mouse weight. Topical 0.5% proparacaine HCL eye drops (Alcon Laboratories, Inc.) were applied and pupils were dilated with 1% tropicamide ophthalmic solution (Bausch & Lomb). Their eyes were kept moist with Refresh Tears (0.5% carboxymethylcellulose sodium) eye drops. Core body temperature was maintained at ∼37.0 °C using a heating pad (TC-1000 Temperature Controller; CWE). Needle electrodes placed in the cheek and the tail served as reference and ground leads, respectively. Dawson–Trick–Litzkow (DTL) fiber electrodes were used for recording ERG responses. The recording epoch was 250 ms, with a 20-ms prestimulation baseline. Stimulus flashes were presented in a UTAS BigShot ganzfeld (LKC Technologies). A total of 10 stimulus intensities, ranging from 0.00039 to 137 cd⋅s⋅m−2, were used under dark-adapted conditions. Each flash duration was 20 μs, and stimuli were presented in order of increasing intensity. As flash intensity increased, retinal dark adaptation was maintained by increasing the interstimulus interval from 10 to 63 s.
For light-adapted ERG recordings, a steady background-adapting field (29.76 cd⋅s⋅m−2) was presented inside the ganzfeld to saturate the rod photoreceptors for 10 min. After 10 min of light adaptation, light flashes were presented on top of the background light. A total of seven stimulus intensities, ranging from 0.16 to 79.65 cd⋅s⋅m−2, were presented. Time between flashes was 0.476 s. All other test parameters were similar to the dark-adapted ERG. The a-wave and b-wave amplitudes were analyzed off-line. The amplitude of the a wave was measured from the prestimulus baseline to trough, and that of the b wave from the trough of the a wave to the peak of the b wave.
Visual Psychophysical Testing.
Mice were tested for visual function (spatial frequency threshold and contrast sensitivity) in the OptoMotry device (CerebralMechanics, Inc.) (14); measurements were made in the middle of the day, 4 to 8 h after light onset of the light/dark cycle, as described previously (12, 13). Contrast sensitivity threshold was measured at a spatial frequency of 0.064 cycles per degree, the peak of the contrast sensitivity function in the middle of the day (12, 13).
SD-OCT.
Chx10Cre;Bmal1fl/fl, Bmal1fl/fl, and Chx10Cre;Bmal1+/+ mice were anesthetized with a mixture of ketamine (10 mg/mL) and xylazine (1.5 mg/mL), which was given at 10 µL/g of mouse weight. Topical 0.5% proparacaine HCl eye drops (Alcon Laboratories, Inc.) were applied and pupils dilated with 1% tropicamide ophthalmic solution (Bausch & Lomb). Corneas were kept moist with regular application of the sterile lubricant GenTeal (Novartis). Fundus photographs and SD-OCT images were obtained with the Μm-IV imaging system (Phoenix Research Laboratories, Inc.). To control for regional differences in retinal thickness, SD-OCT measurements were made of a linearized circle with a diameter of 0.57 mm centered on the optic nerve (see SI Appendix, Fig. S5). The average thickness of retinal layers was measured using Adobe Photoshop.
Statistical Analysis.
Data were analyzed with one- or two-way ANOVA. Post hoc multiple comparisons of interactions were performed with the Student–Newman–Keuls test (SigmaPlot) or Tukey’s test (GraphPad Prism). Significance level was set at P = 0.05 with a power >0.8. Data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Dr. Zach Hall for helpful comments and suggestion on the previous version of the manuscript. This work was supported by the National Institutes of Health Grants GM116760 (to K.B.), R01EY026291 (to G.T.), 5U54NS083932 (to Morehouse School of Medicine), R01EY004864, R01EY027711, and P30EY006360 (to P.M.I.); the Abraham J. and Phyllis Katz Foundation; and by an unrestricted Emory Department of Ophthalmology grant from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808137115/-/DCSupplemental.
References
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 3.Ruan G-X, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan G-X, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba K, Sengupta A, Tosini M. Contreras-Alcantara S, Tosini G. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010;16:2605–2611. [PMC free article] [PubMed] [Google Scholar]
- 7.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storch KF, et al. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawant OB, et al. The circadian clock gene Bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep. 2017;21:692–706. doi: 10.1016/j.celrep.2017.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson CR, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012;32:9359–9368. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron MA, et al. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23:489–501. doi: 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- 14.Hwang CK, et al. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J Neurosci. 2013;33:14989–14997. doi: 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 16.LaVail MM. Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 17.Tosini G, Menaker M. The clock in the mouse retina: Melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 18.Pozdeyev N, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ait-Hmyed Hakkari O, et al. Rev-Erbα modulates retinal visual processing and behavioral responses to light. FASEB J. 2016;30:3690–3701. doi: 10.1096/fj.201600414R. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Cheng M, Yang H, Peachey NS, Naash MI. Age-related changes in the mouse outer retina. Optom Vis Sci. 2001;78:425–430. doi: 10.1097/00006324-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Gresh J, Goletz PW, Crouch RK, Rohrer B. Structure-function analysis of rods and cones in juvenile, adult, and aged C57BL/6 and Balb/c mice. Vis Neurosci. 2003;20:211–220. doi: 10.1017/s0952523803202108. [DOI] [PubMed] [Google Scholar]
- 22.Baba K, et al. Age-related changes in the daily rhythm of photoreceptor functioning and circuitry in a melatonin-proficient mouse strain. PLoS One. 2012;7:e37799. doi: 10.1371/journal.pone.0037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Ye Z, Hensch TK. Clock genes control cortical critical period timing. Neuron. 2015;86:264–275. doi: 10.1016/j.neuron.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianesini C, Hiragaki S, Laurent V, Hicks D, Tosini G. Cone viability is affected by disruption of melatonin receptors signaling. Invest Ophthalmol Vis Sci. 2016;57:94–104. doi: 10.1167/iovs.15-18235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.