Abstract
Background:
Human Papillomavirus (HPV) infection persistence is the necessary but not sufficient cause of invasive cervical cancer (ICC). The effects of Human Immunodeficiency Virus (HIV) co-infection have been well documented. The purpose of this study was to describe our experience on the clinico-pathological characteristics of patients with cervical cancer and HIV status at a tertiary Hospital in Nigeria.
Materials and Methods:
This was a descriptive study among ICC patients presenting for clinical staging and biopsy for histological diagnosis at the Obstetrics and Gynaecology outpatient theatre of our hospital between January 2009 and February 2011.
Results:
Sixteen (6.8%) of the 248 patients with histologically confirmed ICC in this study were HIV positive. The mean age of all the participants was 55.4 (SD±10.2) years with the HIV positive patients’ younger than the HIV-negative and those that declined HIV testing. Coitarche was at lower age (18 [SD±4.4] vs 22[SD±3.4] years vs 24.5[SD±4.4], respectively). The modal lifetime sexual partners were four, one and two, respectively. Clinically, more HIV positive patients, presented at advanced stage of ≥ 2B. Also, the adenocarcinoma histological variant was slightly more among the HIV positive patients.
Conclusion:
HIV seemed relatively common among ICC patients and they presented at lower ages, at more advanced stages, earlier coitarche and more lifetime sexual partners. The proportion of adenocarcinoma histological types was slightly higher among the HIV positive patients compared with seronegative patients and those with unknown HIV status. Larger studies to substantiate these findings and ICC-HIV causal relationship are required.
Keywords: HPV, HIV, Co-Infection, Invasive cervical cancer
Introduction
Cancer of the cervix is a major public health problem all over the world. It is the second most common reproductive tract cancer and a major cause of cancer morbidity and mortality in women (Rostad et al., 2003; Ferlay et al., 2012). The global burden of this preventable disease is disproportionately high among patients in developing countries where 85 per cent of the estimated 493, 000 new cases and 273, 000 deaths occur worldwide (Kay et al., 2003; Anorlu et al., 2004;). However, this common genital cancer is one of the few cancers where effective vaccination against the precursor agent, human papillomavirus (HPV) is available and screening can identify precancerous lesions with an association existing between effective screening and a decline in mortality (Bosch et al., 2002; WHO. 2005). Also, the pre-cancerous and early invasive stages are easily treated with an almost 100% survival rate at five years (ACS, 2002). During the last century, knowledge about the carcinogenesis and aetiology of cervical carcinoma has increased immensely. Studies have shown some changes in the distribution of cervical cancer by age, stage, stage and histopathology distribution (ACS, 2002; Nygard et al., 2005; Quinn et al., 2006).
Some of these changes may be an effect of the screening activities (WHO. 2005) and the increasing uptake of the HPV vaccination globally. For developing countries, the contributions of the pandemic of human immunodeficiency virus (HIV) infection might be a major contributor.
Infection with HIV is known to be associated with several neoplasms including invasive cervical cancer (ICC). Invasive cervical cancer is an important factor in the definition of the AIDS stage (Robbins et al., 2014). Since the advent of ART, the incidence of these AIDS-defining cancers has dropped especially for Kaposi’s sarcoma (KS) and non-Hodgkin’s lymphoma (NHL) (Engels et al., 2008; Shiels 2009). Despite the over six million patients on treatment, access to ART and subsequently considerable increase in survival of those treated in the last ten to fifteen years in sub-Saharan Africa (Newton et al., 1996; Toure et al., 2008; UNAIDS 2011), the association of cancer, especially ICC, with HIV infection remains poorly understood. Our aim was to describe the relationship between co-infection with HIV with socio-demographic characteristics, clinical stage and histopathological distributions of cervical cancer among patients with suspected cases presenting for routine diagnosis in a single tertiary health care level in Nigeria.
Materials and Methods
Design and Study Population
This prospective study was conducted among patients referred with clinical suspicion of cervical cancer and presenting to the Outpatient Gynaecological theatre of Department of Obstetrics and Gynaecology of University of Ibadan/University College Hospital, Ibadan, Nigeria for examination under anaesthesia (EUA), clinical staging and cervical biopsy between January 2009 and February 2011. After obtaining written informed consent from eligible patients, participants were administered a structured questionnaire by the attending physician scheduled to perform the EUA for the day according to the institution protocol. The questionnaire assessed socio-demographic and sexual (socio-sexual) characteristics of the patients which included the age, level of education, age at first sexual exposure, lifetime number of sexual partners, smoking and/or tobacco use history; and clinical characteristics like gravidity and parity, presenting symptoms and duration. To determine the HIV status of the patients, we asked the patients if they know their HIV status and documented the results if known. Participating patients with unknown HIV status, and who consented to have HIV test were linked with the HIV counselling and testing unit within the outpatient clinic complex. They were screened for HIV according to Nigerian Government protocol for routine HIV testing services (HTS) (FMOH, 2016). Also, were those with known negative but tested more than six months before presentation? For diagnostic purposes of cervical cancer, the routine examinations under anaesthesia, clinical staging and biopsies according to the institution protocol were performed. The specimens obtained were sent to the institutions histopathology department for histological diagnosis.
Data Management and Statistical Analysis
Completed questionnaires were edited daily by the Investigators. The socio-demographic, sexual and medical data as well as the histological results of only confirmed cases of ICC were entered and analysed by descriptive statistics using statistical software SPSS version 22 (SAS Institute Inc, Cary, NC, USA). Summary statistics, such as frequencies, means and percentages, were used to summarize variables. For this study, patients’ HIV status is classified as Negative, positive or declined.
Ethical consideration
Ethical approval for the study was obtained from the Joint Ethics Committee of the University of Ibadan and University College Hospital, Ibadan (Awolude et al., 2016) and informed consent obtained from all patients.
Results
Socio-demographic Characteristics
Two hundred and forty-eight eligible, consenting and histologically confirmed patients with invasive cervical cancer were recruited for the study. The mean age of respondents was 55.4 (SD±10.2) years. Majority, 73% (n=181) were married, 25.0% (n=62) were widows while 2.0% (n=5) were single. Sixty-two (37.1%) never had western education, 29.4%, 20.6% and 12.9% had completed primary, secondary and tertiary education respectively. The modal gravidity was 6 (Range: 0-14). Majority of the patients (76.6%) contributed little or nothing to their household income hence they were not as financially independent and 38 % with at least one adult directly depending on them and 43. 7 % had 1 to 5 children as direct dependants. Of these participants with suspected invasive cervical cancer, prior HIV status were known in 35 (14.1%) of these patients (4 positive; 31 negative). The remaining 213 were, therefore, counselled for screening for HIV of which 14 (6.0%) declined. Of the remaining 199 patients with unknown HIV status that were subsequently tested 12 (6.5%) were diagnosed HIV positive. In all, the overall HIV positivity rate among the patients with invasive cancer and known HIV status in this study was 6.8% (Figure 1).
Figure 1.
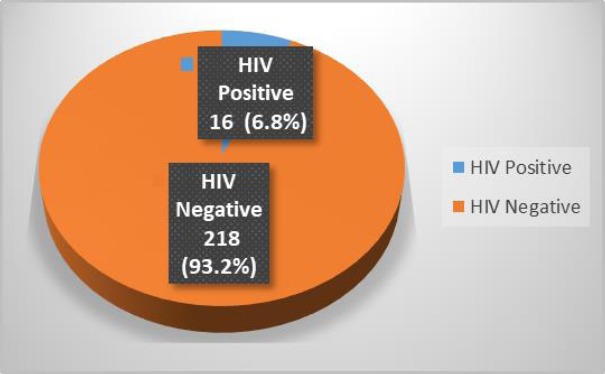
HIV Positivity Rate among the Patients
The 4 patients with known positive HIV status prior to their enrolment in this study were already on ART. However, all the participants were included in this analysis for comparison purpose. The characteristics of the participating patients according to HIV status are presented in table 1. The 16 patients identified as HIV positive were younger than the remaining 218 HIV-negative patients and 14 patients that declined HIV testing with mean age of 44.6 years [SD±11.7], 56.2 [SD±9.8] and 54.6 [SD±8.6] years, respectively, coitarche was at 18 years [SD±4,4], 22 years [SD±3.4] and 24.5 years [SD±4.4], respectively for HIV positive, negative and for those that declined HIV testing while the modal number of lifetime sexual partners were four, one and two. In table 2, the clinical stage at diagnosis, according to the International Federation of Gynaecology and Obstetrics (FIGO) classification, among the cases showed that 72 (29.1%) were in early stages of 1b-2a while the remaining 176 (70.6%) were in advanced stages of 2b-4b (9.3% were in stage 1b, 19.8% in stage 2a, 12.5% in stage 2b, 5.5% in stage 3a, 20.2% in stage 3b, 2.0% in stage 4a and 0.8% in stage 4b. In terms of HIV status, 93.8%, 68.3% and 85.7% presented in advanced stage of ≥ 2b among the HIV positive, negative and those that declined HIV test, respectively. Histologically confirmed cases as invasive cervical carcinoma included 220 (88.7%) squamous cell carcinomas, 18 (7.3%) adenocarcinomas, 6 (2.4%) adenosquamous carcinoma and 4 (1.6%) other variants which included endometriod adenocarcinoma with cervical extension and small cell carcinoma. The adenocarcinoma variant was slightly higher among the HIV positive patients compared with the HIV seronegative and the unknown HIV status patients (Table 3).
Table 1.
Sociodemographic Characteristics of Respondents
| Characteristics | Aggregate | Positive N=16 | Negative N=218 | Declined N=14 | |
|---|---|---|---|---|---|
| Age (Years) | 55.4 [SD±10.2] | 44.6 [SD±11.7] | 56.2 [SD±9.8] | 54.6 [SD±8.6] | |
| Coitarche (Years) | 21 [SD±10.2] | 18 [SD±4.4] | 22 [SD±3.4]. | 24.5 [SD±4.4] | |
| Number of sexual partners (Modal) | 4 | 1 | 2 | ||
| Prior Cervical Cancer Screening | Yes | 24 (9.7%) | 3 (18.8%) | 19 (8.7%) | 2 (14.3%) |
| No | 224 (90.3%) | 13(81.3%) | 199(91.3%) | 12 (85.7%) | |
| Marital status | Married | 181 (73.0%) | 12 (75.0%) | 156(71.6%) | 10(71.4%) |
| Widowed | 62 (25.0%) | 3 (18.8%) | 57(26.1%) | 2(14.3%) | |
| Single | 5 (92.0%) | 1(6.2%) | 2(0.9%) | 2(14.3%) | |
| Number of pregnancies | 0 1- | 4 (1.6%) | 1(6.3%) | 2(0.9%) | 1(7.1%) |
| 4 | 56 (22.6%) | 11(68.8%) | 40(18.3%) | 5(3.6%) | |
| ≥5 | 188 (75.8%) | 4(25.0%) | 176(80.7%) | 8(5.7%) | |
| Level of Education | None | 91 (36.7%) | 5(31.3%) | 85939.0%) | 1(7.1%) |
| Primary | 74 (29.8%) | 4(25.0%) | 68(31.2%) | 2(14.3%) | |
| Secondary | 51 (20.6%) | 5(25.0%) | 42(19.3%) | 4(28.6%) | |
| Tertiary | 32 (12.9%) | 2(6.3%) | 23(10.6%) | 7(50.0%) | |
Table 2.
Clinical Stages at Presentation
| Clinical Stages at Presentation | Total (N=248) | HIV Positive (n=16) | HIV Negative (n=218) | HIV Decline (n=14) | |
|---|---|---|---|---|---|
| Early | 1b 2a |
23(9.3%) 49(19.8%) |
0(0.0%) 1(6.25%) |
23(10.6%) 46(21.1%) |
0 2(14.3%) |
| Total | 72(29.1%) | 1(6.2%) | 69(31.7%) | 2(14.3%) | |
| Advanced | 2b | 31(12.5%) | 3(18.8%) | 25(11.5%) | 3(21.4%) |
| 3a | 88(35.5%) | 6(37.5%) | 76(34.9%) | 6(42.9%) | |
| 3b | 50(20.2%) | 4(25.0%) | 44(20.2%) | 2(14.3%) | |
| 4a | 5(2.0%) | 1(6.3%) | 3(1.4%) | 1(7.1%) | |
| 4b | 2(0.8%) | 1(6.3%) | 1(0.5%) | 0(0.0%) | |
| Total | 176(70.6%) | 15(93.8%) | 149(68.3%) | 12(85.7%) | |
Table 3.
Histological Types
| Histological Types | Total | HIV Status | ||
|---|---|---|---|---|
| N = 248 (%) | HIV positive N = 16 (%) | HIV negative N = 218 (%) | HIV Decline N = 13 | |
| Squamous cell carcinoma | 220 (88.7) | 12 (75.0) | 198 (90.8) | 10 (76.9) |
| Adenocarcinoma | 18 (7.3) | 3 (18.8) | 13 (6.0) | 2 (15.4) |
| Adenosquamous carcinoma | 6 (2.4) | 1(6.2) | 4(1.8) | 1(7.7) |
| Others * | 4 (1.6) | 0 | 3 (1.4) | 1 (7.7) |
Others - Endometrial adenocarcinoma with cervix extension; small cell carcinoma (neuroendocrine
Discussion
Cervical cancer is the fourth most common cancer in women globally with an estimated 528,000 new cases in 2012 and 266,000 deaths with 85% and 87% of cases in less developed countries, respectively. In these countries, ICC is the second commonest after breast cancer. In sub-Saharan Africa, Invasive cervical cancer (ICC) is the second cancer-related causes of deaths in women after breast cancer (Ferlay et al., 2012). Invasive cervical cancer is strongly associated with HIV infection (Tanon et al., 2012). A case-control study in Cote d’Ivoire in the early antiretroviral (ART) era showed a prevalence 16.7% in patients with ICC compared to 8.3% in the control group. However, other studies in other parts of Africa found no or weak association between HIV and ICC (Tanon et al. 2012). Our study showed a prevalence of 6.8%. While this rate is lower than the value from Cote d’Ivoire it is 1.7 - 2.5 times higher than the value from studies among patients with ICC presenting for chemoradiation. (Abdus-Salam et al., 2008; Abdullai et al. 2018). While the lower prevalence compared with Cote d’Ivoire study cannot be explained by the wider availability of ART as majority [12/16; 75%] of our patients in this study were unaware of their HIV status, the higher prevalence compared with the studies in radiation oncology units might be explained by possibility of many of these patients been lost to follow-up and associated possibility of AIDS-death related between the time of clinical evaluation in the Gynaecology clinics and the Radiation Oncology units of the respective hospitals.
In this study, a total of 248 cases of histologically confirmed cervical cancer were diagnosed over 26 months’ period. Of these cases, 70.6% presented in advanced stages showing the problem of late presentation of ICCs in most of our health care facilities. This is similar to findings from other studies in Nigeria (Anorlu et al., 2004; Adewuyi, et al., 2008; Ikechebelu, et al. 2010; Oguntayo, et al., 2011; Awolude et al., 2016). More obvious is the higher proportion of HIV positive women (93.80%) presenting in advanced stages. This is related with the increased proliferative activities of cervical squamous cancer in women with HIV infection (Lytvynenko et al., 2017). Also, the low coverage of cervical screening programme in developing countries is an important factor (Denny, 2011). In this study 90.3% of the patients had no prior history of cervical cancer screening. (Lytvynenko et al., 2017). Peculiarities of proliferative activity of cervical squamous cancer in HIV infection. Georgian Med News. 270:10-15]. Also, the low coverage of cervical screening programme in developing countries is an important factor (Denny, 2011). In this study (90.3%) of the patients had no prior history of cervical cancer screening. These patients diagnosed at advanced stages have few treatment options and are often limited to either chemo-radiation or palliative care in the phase of dearth of skill for surgical interventions (Jonah et al., 2016). However, establishment of organised, routine cervical cancer screening will lead to reversal of this late presentation even in HIV population. In a HIV clinic in Kenya where cervical cancer screening was routinely made available, (93.1%) of the ICC were in early stage of IA1 (Chemtai et al., 2013).
The HIV-positive patients in this study were younger than the HIV-negative patients. This finding is consistent with similar published works from other parts of Africa (Sasco et al., 2010; Chemtai et al., 2013; Abdullahi et al., 2018). It has been documented that HIV and other immunosuppressive conditions induce shorter precancer phase and more rapid progression of invasive cervical cancer (Gizaw et al., 2017). HIV infection is linked with multiple, persistent and carcinogenic HPV infection (Travassos et al., 2017). This is related to their poor clinical state making them more at risk of co-infections noted for promoting the activities of the HPV that is the causative organism for cervical cancer (Wilen et al., 2012). The mechanisms of this aggressive course of HPV associated cervical cancer include HIV infection lowering the immunity through the destruction of CD4 lymphocytes. This level of destruction, which reduces the ability of the body to fight infective agents, is related to the patient’s HIV viral load (Wilen et al., 2012). This leads to occurrence of opportunistic infections and reactivation of the dormant ones in HIV infected individuals. It then becomes imperative that screening HIV-positive women for cervical cancer should be initiated at the time of diagnosis of HIV infection and follow the recommended guideline. This younger age at presentation of HIV-positive patients with ICC can also, be explained by earlier sexual debut (18 vs 22 vs 24.5) years and higher modal life-time sexual partners (4 vs 1 vs 2) among this group of patients compared with HIV-negative patients and those that declined HIV screening. Early age of sexual debut and a large number of sexual partners are associated with increased risk of cervical cancer due to increased risk of transmission of HPV infection and other sexually transmitted infections (STIs) (Chemtai et al., 2013), higher risk of multiple HPV infection and higher likelihood of HPV persistence (Travassos et al., 2017)
The distribution of histological types of ICC from this study showed that squamous cell carcinoma is the most common and was seen in 220 of the 248 cases (88.7%). This is almost similar to findings from other studies. Denny et al. in their multi-centre study involving 2 centres in Nigeria and a centre in South Africa found that 476 of the 570 (83.5%) histologically confirmed ICC were of squamous cell variant (Denny et al., 2014), while Ikechebelu et al. in their study from Eastern part of Nigeria showed that 89.0% of the cases were of Squamous cell carcinoma variant (Ikechebelu et al., 2010) and Oguntayo et al. in the Northern Nigeria city of Zaria showed SCC accounting for 95% of the histological variants (Oguntayo et al., 2011). This predominant squamous cell pattern was irrespective of HIV status of the patients as seen in previous studies (Dismas et al., 2012). But what was revealing in this study is the relatively more cases of adenocarcinoma seen among the HIV positive group of patients. Also, HIV seropositivity may be associated with advanced presentation and, possibly, poor tumour differentiation (Matovelo et al., 2012).
An important limitation of this study is the opportunistic nature of the HIV screening among these patients. Additionally, the study protocol did not include follow up of the patients to determine the outcome of the treatment especially for those referred for radiation treatment. Finally, clinical staging was based on pelvic examination by a trained gynaecologist and residents without the anxilliary procedures like imaging, anoscopy and cystoscopy.
The present study examined the clinic-pathological characteristics and HIV status among patients presenting for routine care for ICC in a tertiary health institution in Nigeria. Though, the prevalence of 6.8% in this study is higher than that previously documented in this same institution, the rate might even be higher if routine HIV screening is established for patients with ICC. The documentation of HIV status prior to treatment is an important prognostic factor in these patients. It will facilitate the early commencement of treatment for HIV positive cases which is associated with less cancer-related treatment toxicity and better treatment compliance (Ntekim et al., 2015) and the associated better outcome. Also, the documented relatively younger ages of presentation among HIV positive patients makes it important for every HIV service programme to have organised routine cervical cancer screening services as part of the comprehensive HIV treatment, care and support programme. This will avail the HIV positive population access to cervical precancer screening services, prompt detection of precancer cervical lesions and early ICC which are amenable to curative treatments. This will avert the known accelerated progression from HPV infection to CIN to ICC among HIV infected women (Moodley et al., 2005; Liu et al., 2018) and late presentation of cases. The relatively higher proportion of the adenocarcinoma histological type might not be unrelated with the HIV status (Denny et al., 2014), the effects of mixed HPV infection and the relatively common non-HPV-16 types in HIV positive patients (Kelly et al., 2017).
In conclusion, this study suggests that HIV is relatively common among patients with ICC with more lower age of presentation and more predisposing factors of earlier onset of sexual intercourse and more lifetime sexual partners. The proportion of adenocarcinoma histological types was slightly higher among HIV positive patients compared with the seronegative ICC patients and those with unknown HIV status. Larger studies in this and similar settings with high HIV prevalence and high burden of cervical cancer are required to substantiate these findings and the possibility or otherwise of effects of HIV on the initial and progression of this strongly HPV associated, but preventable, cervical cancer cases.
Abbreviations:
- AC
– Adenocarcinoma,
- ACS
– American Cancer Society,
- AIDS
– Acquired Immunodeficiency Syndrome,
- ART
– Antiretroviral Therapy,
- CDC
– Centre for Disease Control,
- FIGO
– International Federation for Gynaecology and Obstetrics,
- FMOH
– Federal Ministry of Health,
- HIV
– Human Immunodeficiency Virus,
- HPV
– Human Papilloma Virus,
- HTS
– HIV Testing Services,
- ICC
– Invasive Cervical Cancer,
- KS
– Kaposi’s Sarcoma,
- NHL
– Non- Hodgkin’s Lymphoma,
- SCC
– Squamous Cell Carcinoma,
- UNAIDS
– United Nations Programme on HIV and AIDS,
- WHO
– World Health Organization.
Footnotes
Competing Interests: No conflicting interests exist either financially or otherwise.
References
- 1.Abdus-Salam A, Ogunnorin O, Abdus-Salam R. HIV Seroprevalence in Patients with Carcinoma of the Cervix in Ibadan, Nigeria. Ghana Medical Journal. 2008;42(4):141–3. [PMC free article] [PubMed] [Google Scholar]
- 2.Adewuyi SA, Shittu SO, Rafindadi A.H. Sociodemographic and clinicopathologic characterization of cervical cancers in northern Nigeria. European Journal of Gynaecological Oncology. 2008;29(1):61–4. [PubMed] [Google Scholar]
- 3.Anorlu RI, Orakwue CO, Oyeneyin L, Abudu O.O. Late presentation of patients with cervical cancer to a tertiary hospital in Lagos: what is responsible? European Journal of Gynaecological Oncology. 2004;25(6):729–32. [PubMed] [Google Scholar]
- 4.Awolude OA, Akinyemi JO, Oyerinde SO, Adewole I.F. Time of symptoms to health-seeking among cervical cancer patients in Ibadan, Nigeria. Tropical Journal of Obstetrics and Gynaecology. 2016;33(1):99–107. [Google Scholar]
- 5.Bosch F. X, Lorincz A, Munoz N, Meijer CJ, Shah K.V. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chemtai M, Craig R, Cohenb MM, Bukusi EA, Megan J, Huchkob M.J. Prevalence, characteristics, and outcomes of HIV-positive women diagnosed with invasive cancer of the cervix in Kenya. International Journal of Gynecology and Obstetrics. 2013;123(3):231–235. doi: 10.1016/j.ijgo.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denny L. Cervical cancer treatment in Africa. Curr Opin Oncol. 2011;23(5):469–74. doi: 10.1097/CCO.0b013e3283495a3f. [DOI] [PubMed] [Google Scholar]
- 8.Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, Snyman L, Wiredu E, Molijn A, Quint W, Ramakrishnan G, Schmidt J. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. International Journal of Cancer. 2014;134:1389–1398. doi: 10.1002/ijc.28425. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ. Cancer risk in people infected with human immunodeficiency virus in the United States. International Journal of Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 10.Federal Ministry of Health. National Guidelines for HIV Prevention Treatment and Care. National AIDS and STIs Control Programme. Abuja, Nigeria: Federal Ministry of Health, Nigeria (FMOH); 2016. [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2012. 2013. [accessed on 15th July 2017]. Available from: http://globocan.iarc.fr . [Google Scholar]
- 12.Gizaw M, Addissie A, Getachew S, Ayele W, Mitiku I, Moelle U, Yusuf T, Begoihn M, Assefa M, Jemal A, Kantelhardt E.J. Cervical cancer patient presentation and survival in the only oncology referral hospital, Ethiopia: a retrospective cohort study. Infectious Agents and Cancer. 2017;29(12):61. doi: 10.1186/s13027-017-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikechebelu JI, Onyiaorah IV, Ugboaja JO, Anyiam DC, Eleje G.U. Clinicopathological analysis of cervical cancer seen in a tertiary health facility in Nnewi, south-east Nigeria. Journal of Obstetrics and Gynaecology. 2010;30(3):299–301. doi: 10.3109/01443610903531394. [DOI] [PubMed] [Google Scholar]
- 14.Kay P, Soeters R, Nevin J, Denny L, Dehaeck CM, Williamson A.L. High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. Journal of Medical Virology. 2003;71:265–73. doi: 10.1002/jmv.10479. [DOI] [PubMed] [Google Scholar]
- 15.Kelly HA, Ngou J, Chikandiwa A, Sawadogo B, Gilham C, Omar T, Lompo O, Doutre S, Meda N, Weiss HA, Delany-Moretlwe S, Segondy M, Mayaud P, HARP Study Group Associations of Human Papillomavirus (HPV) genotypes with high-grade cervical neoplasia (CIN2+) in a cohort of women living with HIV in Burkina Faso and South Africa. PLoS One. 2017;12(3):e0174117. doi: 10.1371/journal.pone.0174117. doi:10.1371/journal.pone.0174117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32(6):795–808. doi: 10.1097/QAD.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lytvynenko M, Shkolnikov V, Bocharova T, Sychova L, Gargin V. Peculiarities of proliferative activity of cervical squamous cancer in HIV infection. Georgian Med News. 2017 Sep;2017(270):10–15. [PubMed] [Google Scholar]
- 18.Matovelo D, Magoma M, Rambau P, Massinde A, Masalu N. HIV serostatus and tumor differentiation among patients with cervical cancer at Bugando Medical Centre. BMC Research Notes. 2012;5:406. doi: 10.1186/1756-0500-5-406. doi:10.1186/1756-0500-5-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moodley M, Mould S. Invasive cervical cancer and human immunodeficiency virus (HIV) infection in KwaZulu-Natal, South Africa. Journal of Obstetrics and Gynaecology. 2005;25(7):706–10. doi: 10.1080/01443610500294599. [DOI] [PubMed] [Google Scholar]
- 20.Ntekim A, Campbel O, Rothenbacher D. Optimal management of cervical cancer in HIV- positive patients: a systematic review. Cancer Medicine. 2015;4(9):1381–1393. doi: 10.1002/cam4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nygard J. F, Nygard M, Skare GB, Thoresen S.O. Screening histories of women with CIN 2/3 compared with women diagnosed with invasive cervical cancer: a retrospective analysis of the Norwegian Coordinated Cervical Cancer Screening Program. Cancer Causes Control. 2005;16:463–474. doi: 10.1007/s10552-004-6295-z. [DOI] [PubMed] [Google Scholar]
- 22.Oguntayo OA, Zayyan M, Kolawole A.O, D;Adewuyi SA, Ismail H, Koledade K. Cancer of the cervix in Zaria, Northern Nigeria. ecancer. 2011;11(5):219. doi: 10.3332/ecancer.2011.219. DOI:10.3332/ecancer.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn M. A, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. International Journal of Gynecology and Obstetrics. 2006;95(Suppl. 1):S43–S103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 24.Robbins H. A, Shiels M. S, Pfeiffer R. M, Engels E. A. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881–890. doi: 10.1097/QAD.0000000000000163. http://doi.org/10.1097/QAD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rostad B, Schei B, da Costa F. Risk factors for cervical cancer in Mozambican women. International Journal of Gynecology and Obstetrics. 2003;200380:63–65. doi: 10.1016/s0020-7292(02)00376-4. [DOI] [PubMed] [Google Scholar]
- 26.Sasco AJ, Jaquet A, Boidin E, Ekouevi DK, Thouillot F, Lemabec T, Forstin MA, Renaudier P, N'dom P, Malvy D, Dabis F. The challenge of AIDS-related malignancies in sub-Saharan Africa. PLoS One. 2010;5:e8621. doi: 10.1371/journal.pone.0008621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. Journal of Acquired Immune Deficiency Syndromes. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanon A, Jaquet A, Ekouevi DK, Akakpo J, Adoubi I, Diomande I, Houngbe F, Zannou MD, Sasco AJ, Eholie SP, Dabis F, Bissagnene E. IeDEA West Africa Collaboration. The Spectrum of Cancers in West Africa: Associations with Human Immunodeficiency Virus. PLoS ONE. 2012;7(10):e48108. doi: 10.1371/journal.pone.0048108. doi:10.1371/journal.pone.0048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, Diakite N, Karcher S, Grundmann C, Marlink R, Dabis F, Anglaret X, Aconda Study Group Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire:2year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travassos AG, Netto E, Xavier-Souza E, Nóbrega I, Adami K, Timbó M, Abbehusen K, Fernandes S, Duran C, Haguihara T, Ferreira F, Brites C. Predictors of HPV incidence and clearance in a cohort of Brazilian HIV-infected women. PLoS One. 2017;12(10):e0185423. doi: 10.1371/journal.pone.0185423. doi:10.1371/journal.pone.0185423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNAIDS Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report. Geneva: UNAIDS; 2011. [Accessed 2017 Jan 15]. Available: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf . [Google Scholar]
- 32.Wilen CB, Tilton JC, Doms R.W. HIV: cell binding and entry. Cold Spring Harb. Perspectives in Medicine. 2012;2012(2):a00686610. doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Cervical cancer screening. In: Use of screening for cervical cancer. Lyon: IARC Press; 2005. pp. 117–162. [Google Scholar]


