Abstract
Background:
Guided tissue regeneration-based root coverage has emerged as a promising treatment modality in the treatment of gingival recession. A variety of nonresorbable and bioresorbable membranes have been successfully used. Among resorbable membranes, collagen has been extensively studied. Recently, a third generation barrier membrane derived from placenta has been introduced for periodontal regeneration.
Aim:
The objective of the present study is to clinically compare the efficacy of placental membrane (Amnion) and collagen membrane (Healiguide) for the treatment of gingival recession.
Materials and Methods:
Twelve patients having isolated bilateral gingival recession defects were included in the study and were divided into two groups randomly. Group I were treated by coronally positioned flap and amnion membrane and Group II were treated by coronally positioned flap and collagen membrane (Healiguide)™. Clinical parameters, including dental plaque index (PI), gingival index (GI), gingival recession depth, probing pocket depth, clinical attachment level, and gingival biotype, were recorded before surgery at baseline and then reevaluated at 3 and 6 months postoperatively.
Statistical Analysis:
Nonparametric test, i.e., Wilcoxon Signed-Ranks Test was used in the present study. Significance was reported at 95% confidence level.
Results:
The results of the present study revealed statistically no significant difference (P > 0.05) in dental PI improved, GI and probing pocket depth in both groups. Significant reduction in gingival recession defects and gain in clinical attachment level was observed in both the groups. Intergroup comparison of gingival recession defects and clinical attachment level yielded nonsignificant differences. However, a statistically significant increase (P < 0.05) in gingival tissue thickness was observed in Group II as compared to Group I.
Conclusion:
Both membranes are equally efficacious in the treatment of gingival recession. More gingival tissue thickness (gingival biotype) enhancement was observed in sites treated with collagen membrane.
Keywords: Coronally advanced flap, gingival biotype, growth factors, third generation membrane
INTRODUCTION
Gingival recession is defined as the apical displacement of gingival margin from the cementoenamel junction (CEJ).[1] Several etiological factors were attributed for its causation such as injudicious tooth brushing, disparaging periodontal disease, misaligned teeth, alveolar bone dehiscence, thin gingival biotype of gingiva covering a nonvascularized surface of root, and abnormal attachment of muscle and occlusal trauma.[2] It results in exposure of root, thus causing clinical problems such as root sensitivity, caries, and cervical abrasions on root, plaque control difficult.[3]
Various treatments, such as lateral positioned flap, free gingival autograft, free connective tissue autograft, and coronally positioned flap, have been used in achieving root coverage.[4] The coronally advanced flap (CAF) is one of the most commonly employed perioplastic surgical procedure for root coverage that offers advantages on comparison to other root coverage procedures, which includes better esthetics and procedure does not require donor site. However, CAF is employed to marginal tissue recession with adequate width and thickness of keratinized gingiva (KG).[5] Inspite of having low morbidity, it is proved to be unstable on long term because here healing occurs by repair (formation of long junctional epithelium). Guided tissue regeneration (GTR)-based root coverage has demonstrated histological new attachment formation along with clinical results of traditional root coverage procedures.[5]
Nonresorbable as well as bioresorbable membranes have been used for root coverage using principles of GTR. The major problem with the nonresorbable membrane is that it requires a second surgical procedure is required which may interfere with healing and clinical outcome.[6] To overcome these, equally efficacious bioresorbable membranes were developed. Again among absorbable membranes, collagen is the one which has been extensively studied in the root coverage procedure and proven to be highly efficacious in the treatment of gingival recession and is the second generation GTR membrane which has no growth factors.
Recently, amnion membrane, a third generation membrane which is a placental derived tissue containing collagen, has been introduced.[7] Amnion has been successfully used in the treatment of recession defect, vestibuloplasty, periodontal intrabony defects and ridge augmentation.[8,9,10,11] Amnion has shown favorable recession coverage results in many reported cases, literature showing comparison of this recently introduced amnion membrane with collagen membrane, which has been already extensively studied, is deficient.
Thus, the present study was planned to clinically compare the efficacy of this placental membrane (amnion membrane) and Healiguide™ in GTR for the treatment of gingival recession.
MATERIALS AND METHODS
The plan of the study was submitted to the Research Board cum Thesis Committee and Research Ethics Committee of the Institute for evaluation. The clearance was granted by these bodies to carry out this invasive study.
Twelve patients, seven females, and five males, between the age of 18 and 40 years, mean age 29, having isolated bilateral Miller's Class I or Class II gingival recession were selected for the study from among those reported to the Outpatient Department of Periodontology on the basis of following criteria: Inclusion criteria are as follows:
Patients aging between 18 and 40 years
Both male and female patients
Presence of bilateral Miller's Class I or Class II gingival recession
Patients with adequate width of keratinized gingival measured as the distance between the mucogingival junction and the projection on the external surface of the bottom of the gingival sulcus and it should be sufficient to cover the defect
Sufficient vestibular depth
Patients with acceptable oral hygiene
Patients willing to be a part of study and sign the informed consent form.
Exclusion criteria are as follows:
History of any systemic disease/compromising medical condition
History of use of systemic antibiotics in last 6 month
Patients with poor oral hygiene
Pregnant and lactating females
Smokers and alcoholic
Patients undergoing orthodontic therapy
Patients with a history of allergy to chlorhexidine mouthwash and tetracycline
Teeth with pulpal symptoms at the site of study
Any abrasion or carries on the exposed root surface
Teeth with buccal or interproximal restoration at the study site.
Patients enrolled in the study were informed about the study design, and a written informed signed consent was obtained from them before periodontal surgery.
Materials
(1) Amnion membrane allograft (Tata Memorial Hospital, Mumbai): It is a bioresorbable freeze dried irradiated membrane derived from human amnion tissue which is the innermost layer of fetal membrane and is composed of a single epithelial layer, a thick basement membrane, and an avascular stroma. (2) Collagen membrane allograft (Healiguide™) (Advance Biotec Products, Chennai, India): It is a thin sheet made of high-purity Type-I collagen derived from selected animal tissues and purified using a patented American technology.
Method – preoperative
Intraoral periapical radiograph using paralleling angle technique was taken for every patient to visualize any bone loss around the teeth that had to undergo periodontal surgery. Patients having any interdental bone loss on the desired teeth were excluded from the study. Routine laboratory investigations i.e., hemoglobin, bleeding time, clotting time, total leukocyte count, differential leukocyte count, and postprandial blood sugar level of patients were carried out. Patients were screened for HIV, HBsAg, and hepatitis C. The selected patients were put on Phase-I therapy, which included complete oral prophylaxis (scaling and root planing) and oral hygiene instructions. Following Phase I therapy, the patients were reevaluated after 3–4 weeks. Patients fulfilling these selection criteria were selected for the study.
A customized acrylic occlusal stent was fabricated with cold cure acrylic resin. Vertical groove was made on the midbuccal aspect. The periodontal probe was placed in the stent groove to record clinical parameter. This ensured accuracy and reproducibility of reading. A clinical pro forma was designed to have a systematic and methodical reading of all the information and parameters made during the study.
Following clinical parameters were recorded – (i) Dental plaque index (PI) improved (Gill et al. 2009), (2) gingival index (GI) (Loe and Silness 1963), (3) Gingival recession depth (Miller 1985): recession depth was measured by a graduated UNC-15 probe as the distance from the CEJ to free gingival margin at the middle of buccal surface using a customized acrylic stent, (4) Probing pocket depth: probing depth was recorded using graduated UNC-15 probe; from margin of free gingiva to periodontal pocket base using a customized acrylic stent, (5) Clinical attachment level: measured again using graduated UNC-15 probe; from CEJ to periodontal pocket base using a customized acrylic stent, and (6) Gingival biotype: gingival thickness was measured 3 mm below the free gingival margin at the attached gingiva by piercing #15 endodontic spreader with a stopper on attached gingiva. After removal of the reamer, the distance between the tip of the spreader and inner border of stopper was measured using a digital vernier caliper. All the parameters were recorded at baseline and postoperatively at 3 and 6 months.
Study design
Before the surgery, the selected teeth in each patient were divided into two groups randomly. Group I: Gingival recession defects treated with a combination of CAF with amnion membrane as GTR membrane. Group II: Gingival recession defects were treated with a combination of CAF with Heliguide™ as GTR membrane.
Surgical protocol
All cases were performed under local anesthetic solution of 2% lignocaine hydrochloride with 1:200,000 adrenaline (Xylocaine)®. Anesthetic solution was administered by either nerve block or local infiltration to adequately anesthetize the surgical site.
Surgical procedure
Both groups (Group I and II) underwent an identical CAF procedure. An intrasulcular incision was made using no. 15 Bard Parker blade on the buccal aspects of the involved teeth this incision was extended horizontally to involve the buccal aspect of the adjacent papillae, avoiding the gingival margin of the adjacent teeth. Two obliquely releasing incisions were made from the mesial and distal extremities of the horizontal incisions beyond the mucogingival junction. The vertical incisions were made at the line angles of the adjacent teeth or same teeth. A trapezoidal full-thickness flap was raised with Ward's periosteal elevator to the level of the mucogingival junction, and a partial thickness incision was made in the area apical to the mucogingival junction until the flap could be passively repositioned coronally from the cement-enamel junction.
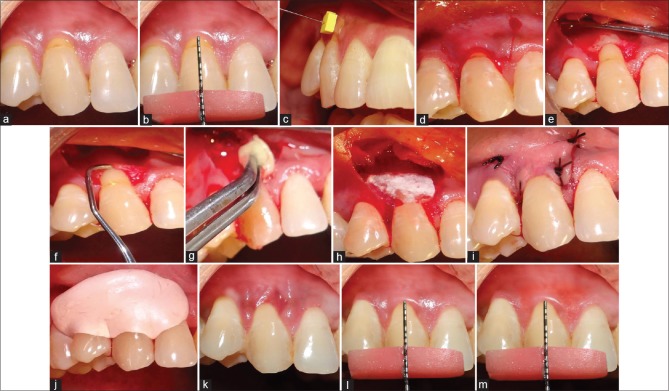
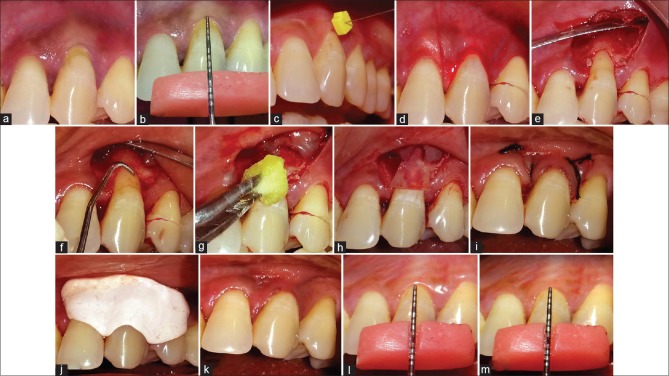
In Group I, after full, partial thickness flap was raised, root planing was done with Gracey curettes. Immediately after root planing, root biomodification was done with tetracycline HCl solution (100 mg/ml). It was burnished over root for 3–5 min. The area was rinsed thoroughly. Amnion (Placental membrane) was trimmed of appropriate size and positioned over the root coronal to the cement-enamel junction and apically 2–3 mm beyond the bony margin. The flap was then positioned coronally to cover the GTR membrane. Sling suture was given with 5-0 black braided silk suture (TRUSILK) to secure it coronally. Oblique releasing incisions were closed using simple interrupted sutures. Gentle pressure was applied against the flap for 2–3 min to secure a good adaptation. A periodontal dressing was applied to protect the surgical area for 14 days [Figure 1a-m] In Group II, the same procedure was done; however, the membrane used was collagen (Healiguide)™ and the membrane was sutured [Figure 2a-m].
Figure 1.
(a) Isolated gingival recession. (b) Preoperative measurement of gingival recession defect using UNC-15 Probe. (c) Gingival tissue thickness measurement using #15 endodontic spreader. (d) Sulcular and vertical incisions. (e) Flap elevation. (f) Root planing with Gracey curette. (g) Root biomodification with tetracycline hydrochloride. (h) Placement of amnion membrane. (i) After suturing. (j) Placement of periodontal dressing over treated site. (k) Fourteen days postoperative. (l) Three months postoperative. (m) Six months postoperative
Figure 2.
(a) Isolated gingival recession defect. (b) Preoperative measurement of gingival recession using UNC-15 Probe. (c) Gingival tissue thickness measurement using #15 endodontic spreader. (d) Sulcular and vertical incisions. (e) Flap elevation. (f) Root planing with Gracey curette. (g) Root biomodification with tetracycline hydrochloride. (h) Placement of collagen membrane (Healiguide). (i) After suturing. (j) Placement of periodontal dressing. (k) Fourteen days postoperative. (l) Three months postoperative. (m) Six months postoperative
Postoperative instructions were given. Patients were instructed for the care of surgical site and to report back in case of any bleeding or any adverse event. The patients were kept on supportive periodontal therapy/maintenance phase for next 6 months, and clinical parameters were reevaluated at the end of 3 and 6 months postoperatively. The data thus collected were compiled and put to statistical analysis.
Statistical analysis
The collected data were put to statistical analysis using Statistical Package for Social Sciences software (version 21.0 for Windows, SPSS Inc., Chicago, IL, USA). Analysis of data recorded in the present study showed that it was not distributed normally; tested by Kolmogorov–Smirnov and Shapiro–Walk test, so nonparametric test i.e., Wilcoxon signed-ranks test was used in the present study. Significance was reported at 95% confidence level. The results of this analysis were tabulated and plotted as graphs.
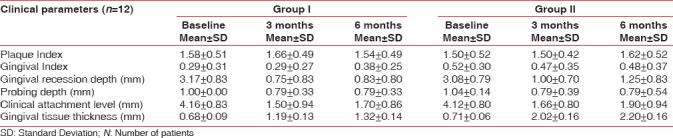
Measurements were recorded by single examiner, and surgical procedure was done by the same operator. All patients completed the study and fully complied with the recall program. The preoperative and postoperative parameters at 3 and 6 months, ascertained clinically, for Group I and Group II is presented in Table 1.
Table 1.
Summary of values of mean and standard deviation of dental plaque index, gingival index, gingival recession depth, probing pocket depth, clinical attachment level and gingival tissue thickness at different study intervals for Group I and Group II
Dental plaque index improved
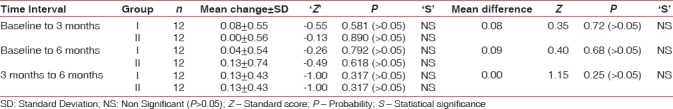
Changes in mean PI scores at different time intervals in both groups are shown in Table 2. Nonsignificant (P > 0.05) differences in PI score was observed from baseline to 6 months, in both the groups. Intergroup comparison of mean changes in PI scores in Group I and Group II at different time intervals also yielded statistically nonsignificant results (P > 0.05) [Table 2].
Table 2.
Mean change and standard deviation of dental plaque index scores in Group I and Group II and their comparison at different time intervals
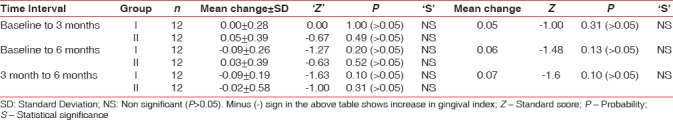
Gingival index
Mean change in GI scores at different time intervals in both groups is depicted in Table 3. Statistically nonsignificant differences were observed for both the groups at different study intervals. When mean scores of both the groups were compared with each other at different periods of observation, mean differences of 0.05, 0.06, and 0.07 were observed at baseline, 3, and 6 months postoperatively, respectively, which were statistically nonsignificant [Table 3]. Differences in mean scores were slightly higher in Group II as compared to Group I; however, results were statistically nonsignificant at different time intervals.
Table 3.
Mean gingival index score in Group I and Group II and their comparison at different time intervals
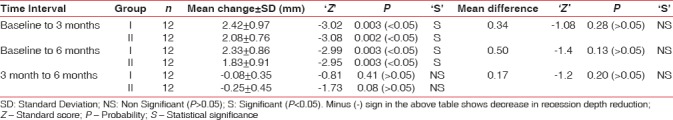
Gingival recession depth
Mean change in recession depth at different time intervals in both groups is represented in Table 4. Intergroup comparison of mean change in gingival recession depth of Group I and Group II at different time intervals yielded statistically nonsignificant differences when values were compared from baseline to different study intervals (P > 0.05) [Table 4].
Table 4.
Mean change in recession depth in Group I and Group II and their comparison at different time intervals
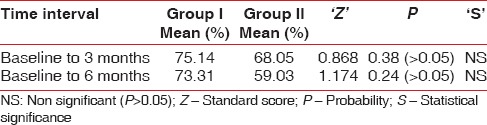
Root coverage percentage of recession depth of Group I and Group II at different time intervals are depicted in Table 5. When 3 and 6 months results were compared to baseline, there was statistically significant reduction of gingival recession depth for both the groups. Root coverage of 75.14% and 68.05% was recorded for Group I and Group II, respectively, from baseline to 3 months and was 73.31% and 59.03% from baseline to 6 months for both groups, respectively. However, no statistically significant difference was observed between two treatments. In Group I five sites attained 100% root coverage, and in Group II, only two sites achieved 100% root coverage.
Table 5.
Mean root coverage percentage (%) of recession depth of Group I and Group II at different time intervals
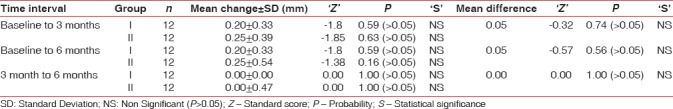
Probing pocket depth
Changes in probing pocket depth at different study intervals are illustrated in Table 6. Findings of intergroup comparison of probing pocket depth at different time intervals showed nonsignificant results [Table 6].
Table 6.
Mean change in probing depth in mm in Group I and Group II and their comparison at different time interval
Clinical attachment level
Changes in clinical attachment level at different time intervals in both groups are shown in Table 7. In Group I, gain in mean clinical attachment level from baseline and 3 months was 2.58 ± 0.99 mm (statistically significant, P < 0.05), from baseline to 6 months was 2.45 ± 0.89 mm (statistically significant, P < 0.05), and a loss of 0.12 ± 0.31 mm from 3 to 6 month which was statistically nonsignificant (P > 0.05). Gain of 2.40 ± 0.86 mm in mean clinical attachment level was recorded in Group II from baseline to 3 months (statistically significant, P < 0.05). A gain of 2.20 ± 1.19 mm (statistically significant, P < 0.05) from 3 to 6 months and a loss of 0.25 ± 0.72 mm (statistically nonsignificant P > 0.05) from 3 to 6 month was recorded.
Table 7.
Mean change in clinical attachment level in mm in Group I and Group II at different time intervals
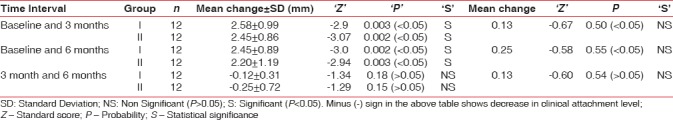
Intergroup comparison of the mean change in clinical attachment level between Group I and Group II at different time intervals yielded statistically nonsignificant differences [Table 7].
Gingival tissue thickness (gingival biotype)
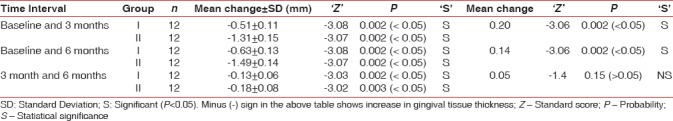
Intergroup comparison yielded statistically significant difference in gingival thickness (P < 0.05) from baseline to 3 months and from baseline to 6 months. Nonsignificant differences were observed from 3 to 6 months [Table 8].
Table 8.
Mean change in gingival tissue thickness in mm in Group I and Group II at different time intervals
DISCUSSION
Several perioplastic surgical techniques have been introduced to treat gingival recession defect that includes free gingival graft, CAF, CAF with connective tissue graft, CAF with and without vertical releasing incisions and various regenerative techniques, including the use of non resorbable membrane, resorbable, enamel matrix derivative, and a platelet-containing gel with a coronally displaced flap. These techniques have been used with varying degree of success.
Treating gingival recession defect with free gingival graft is a painful procedure for patients because of denudation of the palate, and unpredictable results have been seen with respect to harmony with the adjacent tissue. Subepithelial connective tissue graft has an excellent prognosis with good esthetic results. It is considered standard approach compared to other root coverage techniques; however, it has some disadvantages. It is traumatic and time-consuming for the patients. In addition, patient prone to gingival recession usually has thin palatal musosa and are unable to provide connective tissue of adequate thickness.[12] The coronally positioned flap has long been used as a method of gaining root coverage ever since it was introduced by “Norberg” (1926). It has been shown to be a predictable and reliable technique to promote root coverage and enhance the clinical outcome of the other procedures (e.g., GTR-based root coverage). Avoidance of second surgical site, significant root coverage, the good color blending of the treated area with respect to adjacent soft tissues can be predictably accomplished using this surgical approach.[13]
However, many surgical-treated defects regenerate incompletely be due to invasion of the defects by tissues by epithelial cells which form long junctional epithelium that plays a role in repair and not regeneration.[14]
Melchers concept of compartmentalization with GTR has been applied for treatment of recession defects. Bioresorbable membranes have replaced nonresorbable membrane as the latter require second surgery.
In this study, among resoborbable GTR-guided tissue regeneration membranes, collagen (Healiguide™; Advance Biotec Products, Chennai, India) a second generation membrane which has been extensively studied and a recently introduced amnion membrane (Freeze dried-irradiated -a product of Tata Memorial Hospital, Mumbai) have been compared.
Healiguide™
It is a bio-resorbable type-I bovine collagen membrane available in the form of a thin sheet enclosed in a box and available as three sizes (15 mm × 20 mm, 20 mm × 30 mm, and 30 mm × 40 mm). Collagen membrane as GTR material was used based on the following facts: (1) Type I collagen present in this membrane is also the main constituent of periodontal tissue and seems to be an appropriate barrier in the GTR technique. (2) It is either absorbed into the healing connective tissues or is resorbed by residents cells in 6–8 weeks. (3) Exogenous collagen is chemotactic for periodontal ligament fibroblasts.[15,16] The charge modifications and slight calcification of collagen are done additionally to enhance GTR as these modifications control the rate of biodegradation of collagen and to improve its tensile strength.
One of the main shortcomings of all second-generation GTR membranes is low predictability of regeneration. The condition for predictable tissue regeneration is stimulation of precursor cells with necessary messenger molecules. As the concept of tissue engineering has developed, third-generation membranes have come out in the market, which act both as barriers and also as delivery devices to release specific agents such as antibiotics, growth factors, and adhesion factors, at the wound site and direct natural wound healing.[17]
Amnion membrane is one such example. It was first used by Davis 1910.[18] It facilitates epithelization, preserves the normal epithelial phenotype, decreases inflammation, promotes angiogenesis, and decreases scar formation.[19]
Amnion contains a variety of specialized proteins such as fibronectin, laminin, proteoglycans and collagen type IV, V, and VII. It not only provides matrix for cellular migration and proliferation but also enhances the wound healing process. It has been reported to be nonimmunogenic to reduce inflammation, reduce scar tissue, has antibacterial properties, reduces pain at the site of application and act as a natural biological barrier.[20] Amnion membrane has revealed the presence of various growth factors in the membrane epithelium. Amnion membrane is also readily obtainable in large amounts, and its preparation and storage are relatively low in cost.[21]
Some reports have documented the use of amnion membrane with CAF for treatment of gingival recession.[22,23] To the best of our knowledge, none of the studies have compared the efficacy of GTR capability of amnion membrane to that of collagen membrane in the treatment of Miller's Class I and II gingival recession.
Systemically healthy patients were selected for this study to avoid the altered host response caused by various systemic illnesses. Smokers and tobacco chewers were excluded as they have an altered tissue response and smoking also interferes with the initial healing. No untoward reaction was reported in any of the patients. Soft tissue showed excellent healing regarding achievement of color, contour, and texture integration as that of clinically healthy gingiva. The tissue merging with the adjacent areas was satisfactory at the sites of vertical incisions in both the groups. The results of the study showed mean root coverage of 73% in Group I. Group II showed 59% mean root coverage.
Dental plaque index improved
There was statistically nonsignificant difference in the dental PI. These results may be attributed to reinforcement of oral hygiene and regular monitoring of the patient at different study intervals and also elucidates successful patient motivation and compliance to the instructions rendered. Results of the present study are comparable to the study done by Shieh and Zucchelli et al.[4,24]
Gingival index
Both groups showed statistically nonsignificant difference in GI. GI remained low throughout the experimental period. Results may be attributed to oral hygiene instructions rendered to the patient and patient compliance to them. These results also suggest that both the membranes did not enhance gingival inflammation and are well tolerated by host tissue. Results of the present study are in agreement to the findings of Shieh and Zucchelli et al.[4,24]
Gingival recession depth
In Group I, statistically significant achievement in mean gingival recession reduction by 2.42 mm and 2.33 mm was reported from baseline to 3 month and baseline to 6 months, respectively. Statistically significant reduction in mean gingival recession by 2.08 mm and 1.83 mm was reported from baseline to 3 month and baseline to 6 months, respectively, in Group II. The results are parallel to the studies conducted by Tinti et al.,[25] Rachlin et al.,[26] Trombelli et al. (1997),[27] Amarante et al.,[28] Leknes et al.,[29]. In both the groups, statistically significant reduction in gingival recession could be attributed to periodontal regeneration.
Although a mild reduction in recession coverage of 0.08 mm and 0.25 mm was reported from 3 to 6 months, respectively, in both groups, the difference was statistically nonsignificant. Similar results had been reported in the previous studies by Romagna-Genon, 2001[30] (used porcine collagen), Shieh[4] (used Bovine Achilles type 1 collagen), Borghetti et al. (1990).[31] This finding might be because of reversal of patient's behavior toward injudicious tooth brushing technique even under strict supervision. Results are discordant to the findings of Ghahroudi et al., 2013, who reported a significant reduction in gingival recession depth from 3 to 6 months in group where amnion membrane was placed.[12]
Intergroup comparison showed that mean recession reduction values were higher in Group I (statistically nonsignificant). This might be due to growth factors present in this membrane (Amnion) which might have played vital role in periodontal regeneration. Both groups showed maximum reduction in gingival recession from baseline to 3 months. In Group I, further reduction in gingival recession was observed from 3 to 6 months in two patients, which suggested an improved capacity of amnion membrane to induce creeping attachment.[31,32] Induction of fibroblast proliferation and presence of vascular growth factor in amnion membrane could accelerate angiogenesis and tissue maturation; these may be responsible for preventing necrosis of the coronal portion of the flap, resulting in better healing and more creeping attachment.[12]
Group I showed mean root coverage of 73% at the end of 6 months, and the results are comparable to study done by Sharma and Yadav.[33] Results are contrary to Gurinsky (case series), who reported 97% defect coverage, which might be because of smaller sample size in his study.[9] Group II revealed mean recession coverage of 59% at 6 months, and these findings are contradictory to Wang et al. 2001,[34] who reported 73% (Healiguide)™ and Babu et al. 2011[35] reported 84%. Shieh et al. 1997 using a purified cross-linked Achilles type 1 collagen membrane in the correction of recession defects reported 51.6% root coverage after 6 months of healing.[4]
Probing pocket depth
Statistically nonsignificant changes were recorded in both groups at different study intervals, and these findings are in accordance to Müller et al. (1999),[36] Amarante et al.,[28] Banihashemrad et al.,[37] and Jankovic et al.[38]
Clinical attachment level
There was a statistically significant gain in clinical attachment level by 2.58 mm and 2.45 mm in Group I from baseline to 3 months and from baseline to 6 months, respectively. Group II revealed statistically significant gain in clinical attachment level by 2.40 mm and 2.20 mm from baseline to 3 months and from baseline to 6 months, respectively. Similar results were recorded by Tinti et al.,[25] Pini Prato et al.,[39] Roccuzzo et al.,[40] Shieh et al.,[4] Waterman,[41] Huang et al. (2005),[42] Ghahroudi et al., and[12] Chakraborthy et al.[43]
Gain in clinical attachment level may suggest new attachment, reattachment, or periodontal regeneration. Actual phenomenon behind gain in clinical attachment level cannot be explained as histological evidence was not taken in consideration in this study.
On intergroup comparison, the mean gain in clinical attachment level was slightly higher in Group I, but the difference was statistically nonsignificant at different study intervals. Amnion membrane strongly resembles the oral mucosa basement membrane and contain different types of laminins, especially laminin-5, which plays an important role in the adhesion of gingival cells.[8] Laminins can promote regeneration, accelerate tissue adhesions, and preserve tissue, all of which are key factors in improved healing and might resulted in clinical attachment level improvements. Furthermore, the antimicrobial agents that are present in amnion membrane, especially secretory leukocyte proteinase inhibitor I, lactoferrin, defensin, and elafin might improve wound healing especially in patients with poor oral hygiene.[44]
Gingival tissue thickness (gingival biotype)
In the present study, it was planned to assess the alteration in gingival biotype after gingival recession treatment. Gingival phenotype is an important factor that could be relevant in deciding the risk for gingival recession and can affect outcome of treatment. A significant increase in the gingival tissue thickness was observed from baseline to end of the study period in both groups. Similar results were observed in the findings of Cardaropoli and Cardaropoli,[45] whereas a study by Paolantonio revealved nonsignificant increase in gingival tissue thickness.[46]
Intragroup comparison revealed increase in gingival tissue thickness in favor of Group II (statistically significant) from baseline to 3 months and baseline to 6 months. Thickness of the amnion membrane is around 300 nm in cross-section, unlike collagen membrane which averages around 750 nm.[19] Collagen membrane is thicker, and it degrades in 6–8 weeks. Increase in gingival biotype thickness may be attributed to the space created by the barrier membranes with clot formation, which subsequently leads to cellular proliferation and migration. Latter on secondary space is created as the barrier is degraded by the host enzymes. This secondary space may cause an increase in the thickness of the gingival tissue.
Thus, results of the study revealed statistically nonsignificant differences in Group I and Group II. These findings suggest that both root coverage modalities are almost equally efficacious in the management of Miller's Class I and Class II gingival recession. Results of the present study also demonstrated a statistically significant increase in the gingival tissue thickness (gingival biotype) in Group II.
A few advantages of amnion membrane over collagen membrane observed during the course of the study were better-handling properties and a thin diameter enabling it to mold according to the defect anatomy and root surfaces easily.
CONCLUSION
Both amnion and collagen membranes proved their efficacy as guided tissue regenerative materials in the treatment of Miller's Class I and II gingival recession. Significant improvements were observed in gingival recession reduction, gain in clinical attachment level, and increase in gingival tissue thickness (gingival biotype) in both groups from baseline to 3 months and baseline to 6 months. However, intergroup comparison of these parameters yielded nonsignificant differences. A significant increase in gingival thickness was observed in Group II in comparison to Group I.
During the course of the study, it was observed that amnion membrane has better handling properties than collagen due to its thickness which makes it easier to manipulate. The ability of amnion allograft to self-adhere eliminates the need for sutures, making the procedure less technically demanding, and decreasing surgical time which makes it suitable option for recession coverage in particularly hard to reach areas such as the molar region; however, the results of gingival tissue thickness enhancement were more favorable for sites treated with collagen membrane.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Akram Z, Rashid H, Vohra F. Autogenous connective tissue graft for the treatment of localized gingival recession: A case report. Int Dent J Students Res. 2015;3:105–8. [Google Scholar]
- 2.Chrysanthakopoulos NA. Occurrence, extension and severity of the gingival recession in a Greek adult population sample. J Periodontol Implant Dent. 2010;2:37–42. [Google Scholar]
- 3.Trabulsi M, Oh TJ, Eber R, Weber D, Wang HL. Effect of enamel matrix derivative on collagen guided tissue regeneration-based root coverage procedure. J Periodontol. 2004;75:1446–57. doi: 10.1902/jop.2004.75.11.1446. [DOI] [PubMed] [Google Scholar]
- 4.Shieh AT, Wang HL, O’Neal R, Glickman GN, MacNeil RL. Development and clinical evaluation of a root coverage procedure using a collagen barrier membrane. J Periodontol. 1997;68:770–8. doi: 10.1902/jop.1997.68.8.770. [DOI] [PubMed] [Google Scholar]
- 5.Lee EJ, Meraw SJ, Oh TJ, Giannobile WV, Wang HL. Comparative histologic analysis of coronally advanced flap with and without collagen membrane for root coverage. J Periodontol. 2002;73:779–88. doi: 10.1902/jop.2002.73.7.779. [DOI] [PubMed] [Google Scholar]
- 6.Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: A pilot study. J Periodontol. 2010;81:1797–804. doi: 10.1902/jop.2010.100060. [DOI] [PubMed] [Google Scholar]
- 7.Holtzclaw DJ, Toscano NJ. Amnion – Chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: A retrospective observational report. Clin Adv Periodontics. 2013;3:131–7. [Google Scholar]
- 8.Gurinsky B. A novel dehydrated amnion allograft for use in the treatment of gingival recession: A observational case series. J Implant Adv Clin Dent. 2009;1:11–6. [Google Scholar]
- 9.Kothiwale SV, Anuroopa P, Gajiwala AL. A clinical and radiological evaluation of DFDBA with amniotic membrane versus bovine derived xenograft with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009;10:317–26. doi: 10.1007/s10561-009-9126-3. [DOI] [PubMed] [Google Scholar]
- 10.Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS. Use of amnion as a graft material in vestibuloplasty: A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:574–8. doi: 10.1016/S107921040400006X. [DOI] [PubMed] [Google Scholar]
- 11.Suresh DK, Gupta A. Gingival biotype enhancement and root coverage using human placental chorion membrane. Clin Adv Periodontics. 2013;3:237–42. [Google Scholar]
- 12.Ghahroudi AA, Khorsand A, Rokn AR, Sabounchi SS, Shayesteh YS, Soolari A, et al. Comparison of amnion allograft with connective tissue graft for root coverage procedures: A double-blind, randomized, controlled clinical trial. J Int Acad Periodontol. 2013;15:101–12. [PubMed] [Google Scholar]
- 13.Zucchelli G, Mele M, Mazzotti C, Marzadori M, Montebugnoli L, De Sanctis M, et al. Coronally advanced flap with and without vertical releasing incisions for the treatment of multiple gingival recessions: A comparative controlled randomized clinical trial. J Periodontol. 2009;80:1083–94. doi: 10.1902/jop.2009.090041. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal NM. The use of collagen membranes to guide regeneration of new connective tissue attachment in dogs. J Periodontol. 1988;59:830–6. doi: 10.1902/jop.1988.59.12.830. [DOI] [PubMed] [Google Scholar]
- 15.Bunyaratavej P, Wang HL. Collagen membranes: A review. J Periodontol. 2001;72:215–29. doi: 10.1902/jop.2001.72.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Sowmya NK, Tarun Kumar AB, Mehta DS. Clinical evaluation of regenerative potential of type I collagen membrane along with xenogenic bone graft in the treatment of periodontal intrabony defects assessed with surgical re-entry and radiographic linear and densitometric analysis. J Indian Soc Periodontol. 2010;14:23–9. doi: 10.4103/0972-124X.65432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auer A, Srdjak JJ. Membranes for periodontal regeneration. Acta Stomatol Croatica. 2005;39:107–12. [Google Scholar]
- 18.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 19.Chopra A, Thomas BS. Amniotic membrane: A novel material for regeneration and repair. J Biomim Biomater Tissue Eng. 2013;18:106. [Google Scholar]
- 20.Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24:299–307. [PubMed] [Google Scholar]
- 21.Haberal M, Oner Z, Bayraktar U, Bilgin N. The use of silver nitrate-incorporated amniotic membrane as a temporary dressing. Burns Incl Therm Inj. 1987;13:159–63. doi: 10.1016/0305-4179(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 22.Singh H, Singh H. Bioactive amnion as a guided tissue regeneration (GTR) membrane for treatment of isolated gingival recession. A case report. Indian J Dent. 2013;4:110–3. [Google Scholar]
- 23.Shetty SS, Chatterjee A, Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J Indian Soc Periodontol. 2014;18:102–6. doi: 10.4103/0972-124X.128261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucchelli G, Clauser C, De Sanctis M, Calandriello M. Mucogingival versus guided tissue regeneration procedures in the treatment of deep recession type defects. J Periodontol. 1998;69:138–45. doi: 10.1902/jop.1998.69.2.138. [DOI] [PubMed] [Google Scholar]
- 25.Tinti C, Vincenzi G, Cortellini P, Pini Prato G, Clauser C. Guided tissue regeneration in the treatment of human facial recession. A 12-case report. J Periodontol. 1992;63:554–60. doi: 10.1902/jop.1992.63.6.554. [DOI] [PubMed] [Google Scholar]
- 26.Rachlin G, Koubi G, Dejou J, Franquin JC. The use of a resorbable membrane in mucogingival surgery. Case series. J Periodontol. 1996;67:621–6. doi: 10.1902/jop.1996.67.6.621. [DOI] [PubMed] [Google Scholar]
- 27.Trombelli L, Tatakis DN, Scabbia A, Zimmerman GJ. Comparison of mucogingival changes following treatment with coronally positioned flap and guided tissue regeneration procedures. Int J Periodontics Restorative Dent. 1997;17:448–55. [PubMed] [Google Scholar]
- 28.Amarante ES, Leknes KN, Skavland J, Lie T. Coronally positioned flap procedures with or without a bioabsorbable membrane in the treatment of human gingival recession. J Periodontol. 2000;71:989–98. doi: 10.1902/jop.2000.71.6.989. [DOI] [PubMed] [Google Scholar]
- 29.Leknes KN, Amarante ES, Price DE, Bøe OE, Skavland RJ, Lie T, et al. Coronally positioned flap procedures with or without a biodegradable membrane in the treatment of human gingival recession. A 6-year follow-up study. J Clin Periodontol. 2005;32:518–29. doi: 10.1111/j.1600-051X.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 30.Romagna-Genon C. Comparative clinical study of guided tissue regeneration with a bioabsorbable bilayer collagen membrane and subepithelial connective tissue graft. J Periodontol. 2001;72:1258–64. doi: 10.1902/jop.2000.72.9.1258. [DOI] [PubMed] [Google Scholar]
- 31.Borghetti A, Gardella JP. Thick gingival autograft for the coverage of gingival recession: A clinical evaluation. Int J Periodontics Restorative Dent. 1990;10:216–29. [PubMed] [Google Scholar]
- 32.Goldman H, Schluger S, Fox L, Cohen DW. 3rd ed. The C.V. Mosby Company; 1964. Periodontal Therapy; p. 3. [Google Scholar]
- 33.Sharma A, Yadav K. Amniotic membrane – A novel material for the root coverage: A case series. J Indian Soc Periodontol. 2015;19:444–8. doi: 10.4103/0972-124X.154166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HL, Al-Shammari KF. Guided tissue regeneration-based root coverage utilizing collagen membranes: Technique and case reports. Quintessence Int. 2002;33:715–21. [PubMed] [Google Scholar]
- 35.Babu HM, Gujjari SK, Prasad D, Sehgal PK, Srinivasan A. Comparative evaluation of a bioabsorbable collagen membrane and connective tissue graft in the treatment of localized gingival recession: A clinical study. J Indian Soc Periodontol. 2011;15:353–8. doi: 10.4103/0972-124X.92569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller HP, Stahl M, Eger T. Root coverage employing an envelope technique or guided tissue regeneration with a bioabsorbable membrane. J Periodontol. 1999;70:743–51. doi: 10.1902/jop.1999.70.7.743. [DOI] [PubMed] [Google Scholar]
- 37.Banihashemrad A, Aghassizadeh E, Radvar M. Treatment of gingival recessions by guided tissue regeneration and coronally advanced flap. N Y State Dent J. 2009;75:54–8. [PubMed] [Google Scholar]
- 38.Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int J Periodontics Restorative Dent. 2012;32:e41–50. [PubMed] [Google Scholar]
- 39.Pini Prato G, Tinti C, Vincenzi G, Magnani C, Cortellini P, Clauser C, et al. Guided tissue regeneration versus mucogingival surgery in the treatment of human buccal gingival recession. J Periodontol. 1992;63:919–28. doi: 10.1902/jop.1992.63.11.919. [DOI] [PubMed] [Google Scholar]
- 40.Roccuzzo M, Lungo M, Corrente G, Gandolfo S. Comparative study of a bioresorbable and a non-resorbable membrane in the treatment of human buccal gingival recessions. J Periodontol. 1996;67:7–14. doi: 10.1902/jop.1996.67.1.7. [DOI] [PubMed] [Google Scholar]
- 41.Waterman CA. Guided tissue regeneration using a bioabsorbable membrane in the treatment of human buccal recession. A re-entry study. J Periodontol. 1997;68:982–9. doi: 10.1902/jop.1997.68.10.982. [DOI] [PubMed] [Google Scholar]
- 42.Huang LH, Neiva RE, Wang HL. Factors affecting the outcomes of coronally advanced flap root coverage procedure. J Periodontol. 2005;76:1729–34. doi: 10.1902/jop.2005.76.10.1729. [DOI] [PubMed] [Google Scholar]
- 43.Chakraborthy S, Sambashivaiah S, Kulal R, Bilchodmath S. Amnion and chorion allografts in combination with coronally advanced flap in the treatment of gingival recession: A clinical study. J Clin Diagn Res. 2015;9:ZC98–101. doi: 10.7860/JCDR/2015/12971.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–40. doi: 10.1097/01.icu.0000172827.31985.3a. [DOI] [PubMed] [Google Scholar]
- 45.Cardaropoli D, Cardaropoli G. Healing of gingival recessions using a collagen membrane with a hemineralized xenograft: A randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2009;29:59–67. [PubMed] [Google Scholar]
- 46.Paolantonio M. Treatment of gingival recessions by combined periodontal regenerative technique, guided tissue regeneration, and subpedicle connective tissue graft. A comparative clinical study. J Periodontol. 2002;73:53–62. doi: 10.1902/jop.2002.73.1.53. [DOI] [PubMed] [Google Scholar]