Abstract
Background and Objectives:
The periodontal regenerative techniques using growth factors and stem cells are gaining momentum in periodontics. However, relatively little is known about the biological process and the potential role of direct transplantation of autologous periodontal ligament stem cells (PDLSC) niche in promoting periodontal regeneration.
Aim:
The aim of this is to clinically and to radiographically evaluate the effects of direct transplantation of autologous PDLSC niche (A-PDLSc Ni) in intrabony defects.
Materials and Methods:
Among 28 patients, 14 sites in test group were treated with open flap debridement (OFD) followed by direct transplantation of A-PDLSc Ni and other 14 sites in control group were treated with OFD. Clinical and radiographic assessment was done for each site before surgical therapy and at intervals of 3, 6, 9, and 12 months using radiovisiography. For clinical and radiographic parameters, intragroup comparison was made by paired t-test and unpaired t-test for intergroup comparisons.
Results:
The result showed that significant reduction (P < 0.05) of clinical parameters in both the OFD and A-PDLSc Ni groups. Radiographic parameters such as alveolar crest changes, defect area resolution were not statistically significant in both the OFD and A-PDLSc Ni groups whereas improvement in defect density was statistically significant (P < 0.05) only in the autologous periodontal ligament stem cell niche group.
Conclusion:
In the present study, treatment of intrabony defect by direct transplantation of autologous periodontal ligament stem cells niche in comparison with OFD showed a significant reduction in probing pocket depth (PPD), gain in clinical attachment level, and there was no gingival recession seen owing to thick gingiva. Radiographically, there was alveolar crest improvement, decrease in defect area, and increase in defect density in A-PDLSC Ni group.
Keywords: Intrabony defect, niche, open flap debridement, periodontal ligament stem cell
INTRODUCTION
It is a major challenge in regenerative medicine to reconstruct the osseous defects induced by periodontal disease and achieve periodontal regeneration. In the field of tissue regeneration, the use of tissue-resident stem cells as regenerative materials has gained considerable attention owing to their self-renewal property and multilineage differentiation capacity.
Stem cells are responsible for the growth, homeostasis, and repair of many tissues. The maintenance and survival of stem cells are regulated by inputs from their local microenvironment, often referred to as the “stem cell niche”. The stem cell niche hypothesis was developed in 1978 by Schofield, who proposed that stem cells reside within fixed compartments or niches which are conducive to the maintenance of definitive stem cell properties.[1]
Among the different dental stem cells, periodontal ligament stem cells (PDLSCs) can serve as a cell source, easily accessible from soft-tissue adherent to extracted tooth and provide excellent therapeutic effect in promoting periodontal regeneration. Ex vivo cultured PDLSCs isolated from soft-tissue adherent to extracted teeth have demonstrated the ability to regenerate periodontal tissues (cementum, periodontal ligament, and alveolar bone) in experimental animal models[2] and human studies.[3] A scaffold or a carrier material helps in the delivery of stem cells and various angiogenic factors to the site where periodontal regeneration is desired. Gelatin, a thermal-denatured bovine/porcine-derived collagen from skin is flexible, biocompatible, and biodegradable has the potential to be used as a scaffold to support osteoblasts and to promote bone regeneration in defective areas.[4] To complete tissue engineering concept, an addition of exogenous or endogenous growth factors present in the stem cell niche would fulfill the requirement. The soft-tissue adherent to the extracted root surface is an excellent source for PDLSCs along with its extracellular matrix (Niche) which contains endogenous growth factors to stimulate quiescent stem cells to develop the required cells to assist in regeneration.
These PDLSCs possess low mitotic activity which when called for, i.e., during physiologic turnover phenomenon derive the signals from extracellular matrix (ECM) and along with growth factors begins to execute its function of in situ replacing old cells by the new ones.
Based on growing research, on biological importance of niche/microenvironment on residing stem cells in physiologic and postinjury status is the inspiration for this study. Two human studies using ex vivo cultured PDLSCs along with bone graft materials have reported the periodontal regeneration.[3,5] Vandana et al. have reported successful clinical and radiographic improvement of periodontal osseous defects treatment based on autologous stem cell assistance in periodontal regeneration technique (SAI-PRT) which abides by tissue engineering concept, i.e., by utilizing PDLSCs (cells), niche (growth factors), and gelatin sponge (scaffold).[6,7,8,9,10] In our study, there was no bone graft used along with the stem cells for periodontal regeneration.
Considering the biologic rationale, preclinical animal,[11] and two human studies[3,5] based on ex vivo cultured PDLSCs along with the bone graft material; the present study was attempted to evaluate the regenerative ability of direct application of PDL tissue comprising of PDLSCs along with its niche without any bone graft material.
The current study also aims to evaluate the clinical and radiographic effectiveness of autologous PDLSC niche (A-PDLSc Ni) in the treatment of periodontal intrabony defects of periodontitis patients by the direct application of autologous PDLSCs along with its niche based on SAI-PRT.
MATERIALS AND METHODS
A human prospective, randomized single-blinded controlled trial with parallel study design was performed for 12 months. The research protocol was approved by the Institutional Ethical Committee (Ref. No. CODS/977/2015 = 2016) and was in accordance with RGUHS research protocol. The study duration was one and half a years which was conducted from November 2015 to April 2017. Based on the inclusion and exclusion criteria, patients who visited the Department of Periodontics were selected for the study. Both sexes of age group of 30–50 years diagnosed with periodontitis (AAP 1999) were included in this study.
Sample size selection
Sixteen patients, as determined by formula,[3] (n = zx 2 × 2 × s2)/d2 where, zx = 1.96, s = variance, d = minimum difference expected. The selected patients were grouped into two categories; control group was treated within only open flap debridement (OFD) and test group (A-PDLSc Ni) was treated with OFD followed by direct application of autologous PDLSCniche (A-PDLSCniche).
Systemically healthy periodontitis patients who had periodontal pocket depth of ≥5–8 mm following Phase I therapy-scaling and root planning in vital, asymptomatic tooth with radiographic evidence of angular bone loss, presence of at least one tooth that needs to be extracted due to impaction or nonfunctional reasons, and those who consented the tooth extraction were included in the study. Patient showing unacceptable oral hygiene during presurgical period, patients suffering from any known systemic diseases, for example, uncontrolled diabetes, anticoagulant therapy, immunosuppressive therapy mobile teeth and teeth with gingival recession, pregnant or lactating individuals, smokers, and alcoholics were excluded from the study.
Complete medical and dental histories were obtained along with comprehensive clinical and radiographic examinations. All patients were informed about the nature of the study procedures involved, as well as potential benefits associated with this surgery and written informed consent was obtained. In the current study, autologous PDLSCs niche was directly transplanted into the periodontal osseous defect without any manipulation and ex vivo culture.
Clinical and radiographic parameters were recorded at baseline and at 3, 6, 9, and 12 months postoperatively by the same calibrated examiner. Clinical measurements were assessed by using UNC 15 Probe (Hu-Friedy Mfg. Co., LLC) from a fixed reference point (stent) and rounded up to the nearest millimeter.[12] They included plaque index (PI) (Sillness and Loe, 1964), bleeding on probing (gingival bleeding index [GBI]), probing depth (PD), clinical attachment level (CAL), and gingival marginal position (GMP) and gingival thickness (GT) were recorded. GT was measured by transgingival probing method.[13]
Individually customized bite blocks and parallel angle technique were used to obtain standardized radiovisiography. Quantitative radiographic measurements at various intervals (baseline, 3 months, 6 months, 9 months, and 1 year) measured were linear and area measurements of the treated defect such as defect area resolution/bone fill. The qualitative measurement regarding bone-like tissue density assessment was done using histograms.[14]
Randomization
The intrabony defects were randomly assigned to one of the two procedures. The randomization was carried out by computer-generated random sequence allocation to one of the treatment employed, and the patients were allocated by operator to test and control group. Test group was treated with autologous periodontal ligament stem cells niche after OFD, and the control group was treated only with OFD.
Blinding
The clinical parameters were recorded by the single calibrated examiner throughout the study who was blinded to the treatment procedure and the blind was not broken until the clinical trial was completed.
Procedural steps
All surgeries were performed by one experienced periodontist. For the access flap, modified or simplified papilla preservation technique was selected on the basis of the width of the interdental papilla. The elevation of the flap was kept at a minimum to allow the exposure of the defect and the careful debridement of the root surface. Complete debridement of the intrabony defect was done and followed by extraction of designated tooth either due to impaction or nonfunctional reasons [Figure 1].
Figure 1.
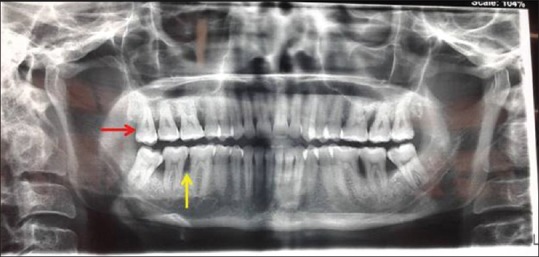
Orthopantomogram showing tooth indicated for extraction by red arrow and intrabony defect by yellow arrow
The presence of PDLSCs in soft-tissue scrapings of roots of extracted molar has been reported.[7] Based on this report, the PDL tissue adherent to tooth root and alveolar socket comprised of PDLSCs along with its niche (PDL tissue niche) components were gently removed using sterile curette and mixed with gelatin sponge (Abgel®©™) to form a transferable mass which was placed in the periodontal defect and was gently condensed. The presutured knot was tightened, and periodontal dressing was placed. Postoperative instructions were given, and suture removal was done after 10 days. One day before surgery, 500 mg amoxicillin 3 capsules per day was prescribed and continued for 5 days. Patients were asked to continue same medication for 5 days. Aceclofenac 100 mg, twice daily for 3–5 days was prescribed. Supragingival debridement was done once in 3 months during the 12 month follow-up period [Figures 2-4].
Figure 2.
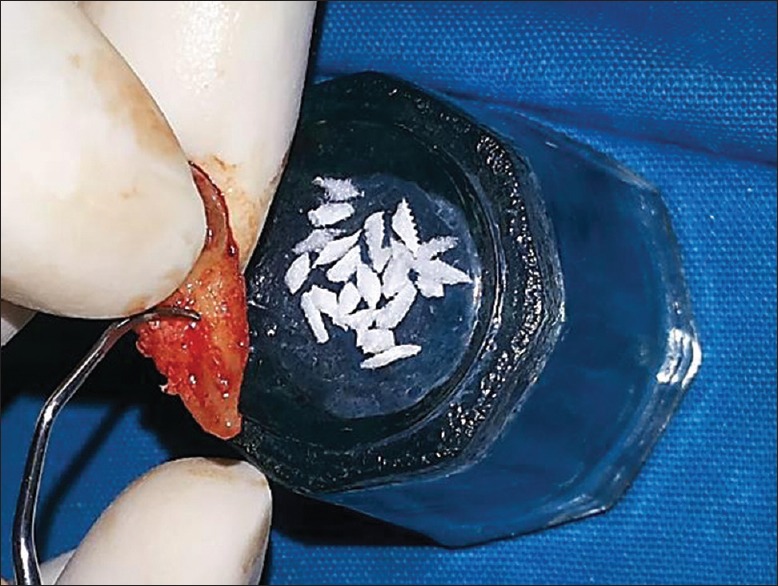
Soft-tissue adherent to root of an extracted third molar, and the extraction socket harboring the periodontal ligament stem cell niche and Abgel®©™ (1 mm × 1 mm)
Figure 4.
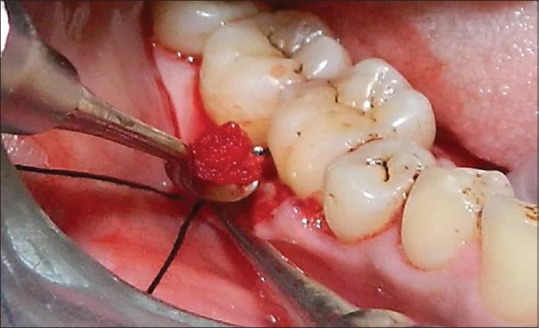
Transferring the harvested tissue into the defect
Figure 3.

Transferable mass
The data collected were subjected to SPSS software statistical analysis with 95% confidence interval. Results are expressed as mean ± standard deviation and percentages. For clinical and radiographic parameters, intragroup comparison was made by paired t-test and unpaired t-test for intergroup comparisons.
RESULTS
The results of this study are discussed as follows;
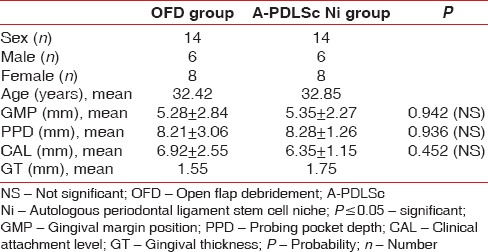
In the present, randomized controlled trial (RCT), age and gender were matched among 28 patients, 6 males and 8 females in each group, without any significant difference at baseline values for GMP, PPD, CAL, and GT between the control and test groups [Table 1].
Table 1.
Demographic data
The plaque and gingival bleeding reduction were significant from baseline to end of the study period within the OFD and (A-PDLSc Ni) groups [Graphs 1 and 2].
Graph 1.
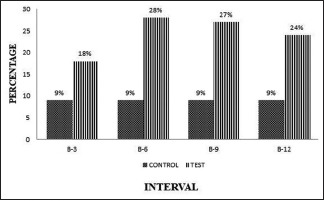
Intergroup comparison of plaque index at different intervals
Graph 2.
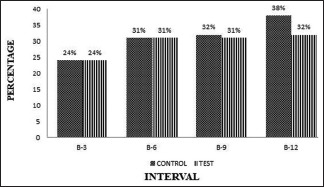
Intergroup comparison of the gingival bleeding index at different intervals
Within the groups, the gingival margin position did not show any significant change from baseline to different study intervals.
The PPD reduction was similar in OFD and (A-PDLSc Ni) groups at 3 months. The test group (A-PDLSc Ni) showed significant PPD reduction and CAL gain at 6, 9, and 12 months than OFD group. The PPD reduction ranges from 5.8% to 9.2%; 23.39% to 28.4% in OFD, and (A-PDLSc Ni) group, respectively, from baseline to 12 months [Graph 3].
Graph 3.
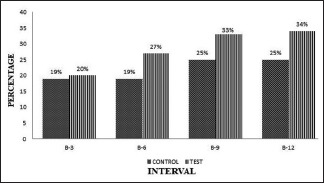
Intergroup comparison of probing pocket depth at different intervals
The A-PDLSC Ni showed that significant gain in CAL at 6, 9, and 12 months than OFD groups. The CAL measurement ranges from 16% to 25%, 29% to 45% in OFDk, and A-PDLSc Ni, respectively, from baseline to 12 months [Graph 4].
Graph 4.
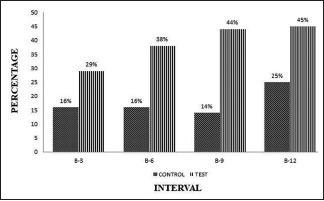
Intergroup comparison of clinical attachment level at different intervals
Radiographic measurements
The cementoenamel junction (CEJ) to alveolar bone crest measurement was showing higher in A-PDLSc Ni groups; enhancement occurred from 24% to 28% at different intervals whereas in OFD group, there was alveolar crest resorption from baseline to different intervals [Graph 5].
Graph 5.
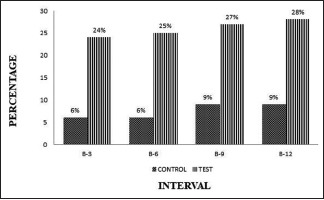
Intergroup comparison of the cementoenamel junction - ABC at different intervals
Within the groups, the defect area improvement ranged from 15% to 11% and 32% to 50% in OFD and A-PDLSc Ni groups, respectively, which was not statistically significant at 3 months. At 6, 9, and 12 months, A-PDLSc Ni group showed that significant improvement in defect area resolution from 30% to 51% from baseline to 12 months. Between the groups, the defect area was not significant at all intervals [Graph 6].
Graph 6.
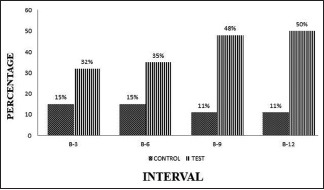
Intergroup comparison of defect area at different intervals
The density changes ranged from 19% to 13% and 30% to 51% in OFD and A-PDLSc Ni groups, respectively, which was not significant initially (3 months) showed significant improvement at 6, 9, and 12 months [Graph 7].
Graph 7.
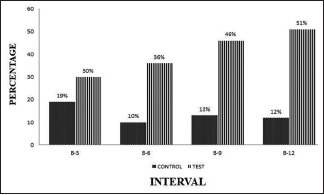
Intergroup comparison of density at different intervals
DISCUSSION
Numerous studies have indicated that ex vivo cultured stem cells obtained from either bone marrow or the periodontal ligament can be utilized along with different graft materials to regenerate periodontal tissue in vivo.[15] However, the major disadvantages of ex vivo cultured stem cells are difficulty in cell delivery, cell immunogenicity, tumorigenicity, difficulty in control of cell fates in vitro and in vivo, cost-effectiveness, and lengthy duration.[15]
Hence, to avoid this, in our study, we attempted to transplant the autologous A-PDLSC niche directly into the intrabony defect by passing the ex vivo culture method and without use of any other bone graft materials.
The results of the current study are discussed here; there was significant reduction in PI, GBI and gingival index within the A-PDLSc Ni and OFD groups as the patients were on maintenance phase. The gingival margin position showed stability (i.e., no gingival recession was found) within the A-PDLSc Ni and OFD group owing to the thick gingiva of more than 1 mm in both the groups. The GT has an influence on the postoperative position on GMP i.e., thicker gingiva of more than 1 mm showed minimal recession.[13] The pocket depth reduction was statistically significant within A-PDLSc Ni group and OFD group, but on comparison between the groups A-PDLSc Ni group showed significant PPD reduction of 27%, 33%, and 34% at 6, 9, and 12 months, respectively. There was a significant improvement in CAL in OFD group at 9 months and 12 months, but A-PDLSc Ni showed significant CAL improvement which was 38%, 44%, and 45% at 6, 9, and 12 months than OFD group. There are no comparative studies available in the literature based on use of A-PDLSc Ni.
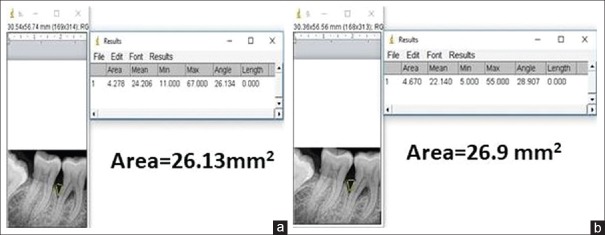
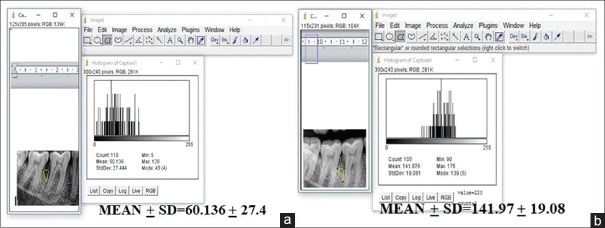
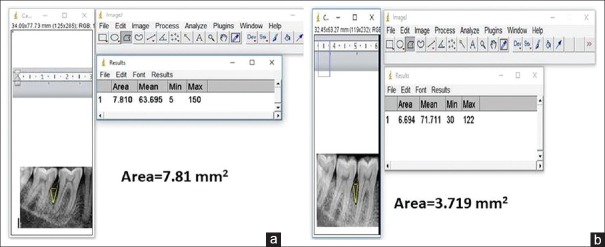
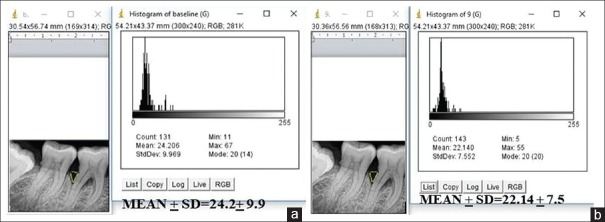
Radiographic examination is a complementing measure of great importance to obtain the diagnosis of periodontitis, even though it does not reveal the real state of cellular activity, but shows the consequences on dentoalveolar structures. In the present study, we evaluated the radiographic parameters such as alveolar bone crest changes, defect area, and bone density of the defect at baseline, 3, 6, 9, and 12 months interval using Image J analyser for the first time in the literature. Initially, the alveolar bone crest showed resorption in the OFD group with the bone enhancement of 9% at the end of the study. In the A-PDLSc Ni group, the initial alveolar bone crest loss up to 3 months was improved to crestal bone enhancement after 6 months till 12 months to the extent of 28%. The defect area was not statistically significant at 3 months in both OFD and A-PDLSc Ni group whereas A-PDLSc Ni group showed statistical significance at 6, 9 and 12 months with 35%, 48% and 50% of reduction in defect area, respectively. On intragroup comparison, A-PDLSc Ni group showed higher percentage of defect area resolution than OFD group. Using histogram, the defect density was statistically significant within the group at all intervals. On comparison, the defect density in A-PDLSC Ni group 36%, 46%, 51%, at 6, 9, and 12 months, respectively. The bone density changes were not significant between the group; however, higher percentage of bone density enhancement was found in A-PDLSC Ni group [Figures 5-8].
Figure 5.
(a) Area of the defect at baseline in open flap debridement group. (b) Area of the defect at baseline in periodontal ligament stem cell niche group
Figure 8.
(a) Density of the defect at 12 months in open flap debridement group. (b) Density of the defect at 12 months in autologous periodontal ligament stem cell niche group
Figure 6.
(a) Area of the defect at 12 months in open flap debridement group. (b) Area of the defect at 12 months in autologous periodontal ligament stem cell niche group
Figure 7.
(a) Density of the defect at baseline in open flap debridement group. (b) Density of the defect at baseline in autologous periodontal ligament stem cell niche group
The results obtained in the present study cannot be compared with the results of the previous study by Feng et al.2010 and Chen et al.2016 where in they have utilized ex vivo cultured PDLSCs along with the other bone graft materials.
In the current study, CAL improvement and radiographic measurements, such as alveolar bone crest enhancement (improvement in alveolar bone crest from CEJ) and defect area (bone fill) resolution, were statistically nonsignificant when compared to control group. However, these parameters showed higher improvement. This can be discussed with due consideration to clinical significance versus statistical significance.
Statistical significance only addresses a hypothesis about whether or not differences exist, statistically between groups. Statistically significant differences alone should not be the primary influence for clinical interpretation of a study's outcome for application to patient care. Statistically significant differences do not provide clinical insight into important variables such as treatment effect size, magnitude of change, or direction of the outcome. In addition, whether results achieve statistically significant differences are influenced by factors such as the number and variability of subjects, as well as the magnitude of effect. Therefore, P values should be considered along with effect size, sample size, and study design. Evidence-based practitioners should examine research outcomes for their clinical significance rather than just statistical significance. Several measures can be used to determine clinical relevance including clinical significance, effect sizes, confidence intervals, and magnitude-based inferences. Clinically relevant changes in outcomes are identified (sometimes interchangeably) by several similar terms including “minimal clinically important differences,” “clinically meaningful differences,” and “minimally important changes.” Changes in outcomes exceeding these minimal values are considered clinically relevant. It is important to consider that both harmful changes and beneficial changes may be outcomes of treatment; therefore, the term “clinically-important changes” should be used to identify both minimal and beneficial differences, but also to recognize harmful changes.[16]
The two human studies[3,5] using ex vivo cultured stem cells along with bone graft are elaborately presented below to delineate the current study methodology and measurements which makes those two human studies noncomparable.
In a retrospective pilot study conducted by Feng et al., three periodontitis patients aged 25–45 years were treated with ex vivo cultured transplantation of PDLSCs along with bone graft CALCITITE and subsequent follow-up done up to 72 months in 16 teeth. No control groups were included in the study. Clinical outcomes were measured regarding PD, gingival recession, and attachment gain for a duration of 32–72 months. Surgical reentry was done for two patients to assess periodontal regeneration. A total of 12 teeth were treated with the follow-up of 42 months, and three teeth were treated with the follow-up of 72 months. At 42 months, there was significantly decreased PDs and attachment loss compared with pretreatment level (P < 0.001). They have also noted significant gingival recession compared with the pretreatment level in both the groups, which would have been responsible for pocket depth reduction. After 42 months, there was no significant gain in attachment compared to that of 3 months in both the patients. At both these sites, surgical reentry was done at 72 months to assess periodontal regeneration. In one site clinical evaluation was done for 72 months and noted subsequent reduction in pocket depth, increase in CAL along with increase in gingival recession. However, there is no mention about recording of PI, GBI, and GI. In their study, the CAL improvement and PPD reduction brought about by graft material and ex vivo cultured PDLSCs cannot be discriminated.
The use of graft material masks the periodontal regeneration brought about by ex vivo cultured stem cells in their study. Overall, their study reported the combined effect of bone graft and ex vivo cultured PDLSCs at various intervals. The follow-up intervals were not consistent among the treated sites and there was no radiographic assessment.[3]
A study conducted by Chen et al. (2016) was a single-center randomized controlled trial involving 30 patients aged 18–65 years who were age matched without sex matching. In their study, 21 teeth under test group was treated with ex vivo cultured PDLSC sheets along with Bio-Oss + guided tissue regeneration (GTR) and control group was treated with only Bio-oss and GTR without PDLSC sheets. During a 12-month follow-up study, the frequency and extent of adverse events along with the efficacy of treatment was assessed by the magnitude of alveolar bone regeneration following the surgical procedure. At 12 month follow-up period, all the treated teeth in both the groups were adequately recovered following surgery. There was no loss of treated teeth during the trial. No statistical significant differences were found for the increased CAL, PD, or GR between the cell (PDLSC sheets along with Bio-Oss and GTR) and control groups (Bio-Oss and GTR) at 3 months postsurgery (P > 0.05). The magnitude of increase in alveolar bone height at 3, 6, and 12 months (bone fill over time) was determined as the decrease in the bone defect depth. Each group showed a significant increase in the alveolar bone height over time (P < 0.001). However, no statistical significant differences were found between the cell group and control group in their study. The limitation in their study is that the clinical follow-up of 3 months is too less to determine the regeneration clinically, i.e., PPD reduction and CAL improvement.[5]
The current study authors would like to present their possible explanation to the biologic basis for SAI-PRT as follows; the A-PDLSC niche utilization in the treatment of periodontal intrabony defect has been attempted based on knowledge of availability of natural PDL tissue harboring PDLSCs with its niche, so that their extracellular matrix effect on potential PDLSCs facilitated the differentiation of required cells to fulfill periodontal regeneration at the time of need. The discussion on this possible mechanisms appears speculative as the scientific knowledge perceived from supportive available literature was carried out in this study. To our surprise, the speculative supportive knowledge worked out constructively as clinical and radiographic improvement in the treatment of intrabony defects as a reality.
The existence of biologic rationale and preclinical animal studies justified the application of PDLSC niche in periodontal tissue regeneration. So far, the niche concept was never there in limelight. However, the stem cell niche concept in the year 1978 has gained experimental support and conceptual complexity since proposed by Schofield.[1] The niche comprises stem cells, nonstem cells, and ECM in the body.
The stem cell niche is the in vivo microenvironment where stem cells both reside and receive stimuli that determine their fate. Therefore, the niche should not be considered simply a physical location for stem cells, rather as the place where extrinsic signals interact and integrate to influence stem cell behavior. The crosstalk between different cell types intrinsic to the stem cell niche offers the opportunity to target these cell communication networks and tailor the dynamics of normal stem cells to boost their ability to respond to therapeutic need.
Stem cells are fundamental to tissue maintenance and regeneration/repair; the signals they receive from ECM and extrinsic environment play a critical role in tissue homeostasis. ECM proteins act as stem cell anchor and constitute mechanical scaffolding unit to transmit stem cell signaling.[17] In vivo, the situation for stem cell is totally different as compared to in vitro study. The restricted, proliferative potential observed in vitro may not be representative of stemness incapacity but more likely of the absence of physiologic niche allowing self-maintenance[18] i.e., the stem cells in vitro cultured may have restricted proliferative potential due to lack of physiologic niche. A common critique of ex vivo cultured adult stem cells-based experiments conducted in vivo is the disconnection between the physiological niches in which stem cells reside and the plastic or glass plates in which they are observed.
The PDLSCs niche is dealt in recent times. Periodontal ligament stem cells (PDLSCs) can serve as a cell source, easily accessible from soft tissue adherent to extracted tooth and provide excellent therapeutic effect in promoting periodontal regeneration. Viability and proliferation (population doubling time) ability of periodontal ligament stem cells isolated from the periodontium of healthy teeth were approximately 4 h.[19] These PDLSCs are located in the area 0–20 μm from the perimeter of blood vessels[20] possess low-mitotic activity which when called for, i.e., during physiologic turnover phenomenon, get the signals from ECM and along growth factors begins to execute its function in situ replacing old by new ones. Considering this physiologic phenomena, in SAI-PRT the stem cell-containing PDL tissue, i.e PDLSCs along with its niche adherent to extracted tooth root was transferred to the debrided site for periodontal regeneration which is the possible mechanism of successful periodontal clinical and radiographic improvements. As a basic requirement for any stem cell study to be conducted, the identification of MSC markers and characterization regarding evaluation of their potential to form different types of tissues is required. The evidence for these two scientific issues are reported by Feng et al., 2010 and Chen et al. 2016 who utilized autologous PDLSCs present in scrapings of tissue adherent to extracted third molar and relevant evidence for osteogenic, cementogenic and adipogenic potential are obtained. The presence of mesenchymal stem cell (MSC) markers such as CD34, CD45, CD73, CD105, CD146, CD166, SSEA4, 3G5, and STRO-1 are reported.[3]
MSCs have better curative potential than other type of adult stem cells. Dental MSCs, such as stem cells from human exfoliated deciduous teeth and PDLSCs, are gaining importance in recent times for the treatment of periodontal osseous defects as well as in the medical field to treat spinal cord injury and neuronal degenerative disorders such as Parkinson's disease, cerebral palsy, and Alzheimer's disease.[21] Considering the beneficial regenerative potential of PDLSCs, these cells are being utilized in the current study.
The role of stem cells during periodontal regeneration from the recent literature is presented here.
During periodontal wound healing, the extent of periodontal regeneration either complete or incomplete depends on the source of stem cell present in the healthy periodontal ligament apical to the debrided pocket. If the pocket is deep, the existing stem cell furnishes in complete or incomplete regeneration. The adjacent uninvolved sites, such as PDL apical to periodontal pocket area, are the source of stem cells to help in regeneration during periodontal regeneration after pocket elimination procedures. Till date, the use of graft or any other means as a treatment modality have been tried probably because the existing stem cells were not possible to complete regeneration. Hence, in the current study, the use of healthy PDLSCs niche from the extracted healthy tooth were used in the defect area after debridement to facilitate the additional regeneration from transplanted autologous PDLSCs to favor/assist existing PDLSCs to favor periodontal regeneration which may be partial or complete.
The role of stem cells during inflammation is discussed here; inflammation in periodontal tissue not only diminishes bacteria but also destroys periodontal supporting tissues. Growing evidence suggests that inflammation also hampers the regenerative ability of PDLSCs. PDLSCs derived from i-PDLSCs is inflammed Periodontal Ligament stem cells had greater proliferation and faster migration but had lower osteogenic capacity[22] and lower cementogenesis potential compared with PDLSCs obtained from h-PDLSCs is Healthy Periodontal Ligament stem cells (h-PDLSCs). These inhibitory effects were due to activation of nuclear factor-kappa B (NF- κB), upregulation of β-catenin, and activation of the canonical Wnt pathway.[22,23]
The deciduous PDLSCs are not an ideal source for periodontal regeneration. Jin's group discovered that PDLSCs derived from resorbed primary teeth expressed increased RUNX2, which upregulated RANKL and downregulated osteoprotegerin (OPG) at both the mRNA and protein levels. These imbalances between RANKL and OPG finally led to osteoclast differentiation and root absorption. Thus, d-PDLSCs from resorbed primary teeth may cause unexpected activation of osteoclasts when used in periodontal regeneration.[24]
The PDLSCs in periodontal disease and healthy sites differ due to inflammation. Growing evidence suggests that inflammation hampers the regenerative ability of PDLSCs. PDLSCs derived from inflamed periodontal tissues (i-PDLSCs) had greater proliferation and faster migration but had lower osteogenic capacity and lower cementogenesis potential compared with PDLSCs obtained from healthy periodontal tissue (h-PDLSCs). These inhibitory effects were due to activation of NF-κB, upregulation of β-catenin, and activation of the canonical Wnt pathway.[22,23]
Viability and proliferation ability of cells isolated from the periodontium of healthy teeth were significantly greater than those of cells isolated from the periodontitis-affected teeth.[19]
In the SAIPRT, no attempt was made to isolate PDLSCs present in the tissue scrapings adherent to extracted root of impacted molars. The recent concept of tissue niche effect on stem cell activity prompted the author to extrapolate it to periodontal wound healing. This is the first attempt to utilize autologous stem cells in PDL tissue niche directly to periodontal diseased treated site. The extracted tooth roots with its adherent soft tissue (which is PDLSC niche in scientific terms) from its socket which would have been discarded into the bucket as biologic waste got transplanted into periodontal osseous defect. Nature'sesponse to best of the waste is evident at recipient sites of the treated sites. There was no adverse reaction following the treatment.
The novel thought process of utilizing this soft tissue has proved to be a simple innovative approach in treatment of periodontal osseous defects. Since the soft tissue adherent to extracted tooth root is removed, whether it be referred as PDLSC niche autograft is thought-provoking. The word graft may be suitable as both donor site, and recipient site exists in a given subject.
The niche is a point of intervention still underexplored that offers a uniquely drug-able opportunity to affect regenerative medicine and anticancer treatments[12] Various niches, such as bone marrow, skin, central nervous system, GIT, and muscles, have been explained since last decade. Based on this, there is a need to explore the underexplored PDL niche.
The preliminary evidence of PDLSCs along with its niche/microenvironment has proved clinical and radiographic success. As of now, the innocent and immature scientific minds questions the successful clinical and radiographic outcome of SAI-PRT due to inadequate knowledge and scientific acumen. None the less, the benefits obtained from this biologic waste is a scientific satisfaction to those who venture to benefit the periodontitis patients. The procedure is nonexpensive as compared to various unpredictable graft and GTR materials used for periodontal regeneration.
The major disadvantage of ex vivo cultured stem cells is difficult in cell delivery, cell immunogenicity, tumorigenicity, and difficulty in control of fates in vitro and in vivo situation.[15] Ex vivo culture which is expensive adds to the disadvantages.
The pluripotent stem cells carry additional risk due to their inherent property of ability to differentiate into cells of all three germ layers. These include ability to acquire mutations when maintained for prolonged periods in culture, to grow and differentiate into inappropriate cellular phenotypes to form benign teratoma or malignant outgrowths and fail to mature. These confer additional risks to the patients/individuals.[25] Based on the plausible explanations, the RCT was carried out using autologous PDLSCs without ex vivo cell culture based on the “best of waste” concept, i.e., periodontal tissue adherent to the extracted tooth root was utilized before disposing the extracted tooth.
Due to inherent disadvantages as presented above, there was no attempt made in the present study to manipulate the stem cells present in the PDL tissue scraped from the extracted healthy tooth root. No time the stem cells have been isolated and cultured. The whole tissue, containing PDLSCs along with the niche was utilized. Hence, all the risks or disadvantages associated with ex vivo cultured stem cell therapy are not applicable in this study.
The autologous tissue that is PDL tissue adherent to extracted root containing PDLSCs along with its niche was the regenerative material, which is obtained with ease and making them an attractive source of autologous PDLSCs niche that would have been otherwise discarded in the bucket. This technique based on “best of waste” concept for periodontal regeneration was without any biological hazard as the patient's own tissue was used. It could be simply comparable to a soft-tissue palatal autograft where in patients own tissue is used for the treatment of gingival recession defects regarding safety.
Earlier studies by the author on SAIPRT[6,7,8,9,10] (best of waste concept) lead to current RCT to confirm and generate evidence for periodontal regeneration.
CONCLUSION
The clinical reduction of PPD and improvement in CAL along with radiographic enhancement of alveolar crest, improvement in defect area and density are valuable outcomes of A-PDLSC niche application in the treatment of periodontal intrabony defects. This procedure is most economical as person's own PDLSCs are used for periodontal regeneration without any adverse effect. It is recommended clinically on a regular basis, provided there is a healthy tooth for extraction on scientific reason along with periodontal osseous defect. The small size of periodontal defects allowed us to investigate the PDLSC and its niche concept successfully without the stem cell isolation and ex vivo stem cell identification/culture. However, ex vivo cell expansion is essential to treat large size defects.
The only limitation is the need of a tooth to be extracted (to procure PDLSC niche) along with the periodontal osseous defect to be treated. SAIPRT principle is recommended on regular clinical basis as the complex tissue engineering is made simple at chairside. The radiographic measurement using Image J software is an asset for a clinical trial. The defect area (defect fill) and measurement of defect density using histogram for the first time in the literature provided the best of radiographic information to support clinical success. To confirm the clinical and radiographic success of SAIPRT, human histologic studies have to be conducted which is on its way. Due to ethical reasons, reentry is restricted in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Yan XZ, Yang F, Jansen JA, de Vries RB, van den Beucken JJ. Cell-based approaches in periodontal regeneration: A systematic review and meta-analysis of periodontal defect models in animal experimental work. Tissue Eng Part B Rev. 2015;21:411–26. doi: 10.1089/ten.TEB.2015.0049. [DOI] [PubMed] [Google Scholar]
- 3.Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, et al. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis. 2010;16:20–8. doi: 10.1111/j.1601-0825.2009.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med. 2008;19:1173–82. doi: 10.1007/s10856-007-3154-y. [DOI] [PubMed] [Google Scholar]
- 5.Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res Ther. 2016;7:33. doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandana KL, Desai R, Dalvi PJ. Autologous stem cell application in periodontal regeneration technique (SAI-PRT) using PDLSCs directly from an extracted tooth. An insight. Int J Stem Cells. 2015;8:235–7. doi: 10.15283/ijsc.2015.8.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandana KL, Praveen NC, Chauhan G, Mittal D. Autologous direct stem cell application in periodontal regeneration technique in the treatment of periodontal intrabony defects: An 1-year follow-up study. Int J Oral Health Sci. 2016;6:83–7. [Google Scholar]
- 8.Vandana KL, Ryana H, Dalvi PJ. Autologous periodontal stem cell assistance in periodontal regeneration technique (SAI-PRT) in the treatment of periodontal intrabony defects: A case report with one-year follow-up. J Dent Res Dent Clin Dent Prospects. 2017;11:123–6. doi: 10.15171/joddd.2017.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandana KL, Dalvi PJ. Autologous stem cell assistance in periodontal regeneration technique (SAI-PRT) in treatment of grade II furcation defect. Int J Res Rev. 2017;4:5–9. doi: 10.15171/joddd.2017.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laxman VK, Desai RG. Tooth for a tooth: Tissue engineering made easy at dental chairside. J Indian Soc Periodontol. 2017;21:169–71. doi: 10.4103/jisp.jisp_32_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 12.Srinivas M, Chethana KC, Padma R, Suragimath G, Anil M, Pai BS, et al. A study to assess and compare the peripheral blood neutrophil chemotaxis in smokers and non smokers with healthy periodontium, gingivitis, and chronic periodontitis. J Indian Soc Periodontol. 2012;16:54–8. doi: 10.4103/0972-124X.94605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandana KL, Gupta I. The relation of gingival thickness to dynamics of gingival margin position pre- and post-surgically. J Indian Soc Periodontol. 2016;20:167–73. doi: 10.4103/0972-124X.175173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalvi P, Vandana KL. Growing bone assessment made easy at chairside. J Dent Res Rev. 2016;3:79–81. [Google Scholar]
- 15.Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 2012;59:203–27. doi: 10.1111/j.1600-0757.2012.00443.x. [DOI] [PubMed] [Google Scholar]
- 16.Page P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther. 2014;9:726–36. [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraro F, Celso CL, Scadden D. Adult stem cels and their niches. Adv Exp Med Biol. 2010;695:155–68. doi: 10.1007/978-1-4419-7037-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370:82–96. doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soheilifar S, Amiri I, Bidgoli M, Hedayatipanah M. Comparison of periodontal ligament stem cells isolated from the periodontium of healthy teeth and periodontitis-affected teeth. J Dent (Tehran) 2016;13:271–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodontal Res. 2006;41:547–53. doi: 10.1111/j.1600-0765.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 21.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu N, Shi S, Deng M, Tang L, Zhang G, Liu N, et al. High levels of β-catenin signalling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J Bone Miner Res. 2011;26:2082–95. doi: 10.1002/jbmr.440. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Konermann A, Guo T, Jäger A, Zhang L, Jin Y, et al. Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions. Biochim Biophys Acta. 2014;1840:1125–34. doi: 10.1016/j.bbagen.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Zhang Y, Wang Q, Dong Z, Shang L, Wu L, et al. Periodontal ligament stem cells modulate root resorption of human primary teeth via runx2 regulating RANKL/OPG system. Stem Cells Dev. 2014;23:2524–34. doi: 10.1089/scd.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. [Last accessed on 2018 Jul 27]. Available from: https://icmr.nic.in/guidelines/Guidelines_for_stem_cell_research_2017.pdf .