The plant growth regulator diethyl aminoethyl hexanoate (DA-6) promotes the germination and seedling establishment of aged soybean seeds through enhancing the conversion from triacylglycerol to fatty acids and sugars.
Keywords: Aged soybean seed, DA-6, fatty acid, germination, glycometabolism, seedling establishment
Abstract
Soybean seeds contain higher concentrations of oil (triacylglycerol) and fatty acids than do cereal crop seeds, and the oxidation of these biomolecules during seed storage significantly shortens seed longevity and decreases germination ability. Here, we report that diethyl aminoethyl hexanoate (DA-6), a plant growth regulator, increases germination and seedling establishment from aged soybean seeds by increasing fatty acid metabolism and glycometabolism. Phenotypic analysis showed that DA-6 treatment markedly promoted germination and seedling establishment from naturally and artificially aged soybean seeds. Further analysis revealed that DA-6 increased the concentrations of soluble sugars during imbibition of aged soybean seeds. Consistently, the concentrations of several different fatty acids in DA-6-treated aged seeds were higher than those in untreated aged seeds. Subsequently, quantitative PCR analysis indicated that DA-6 induced the transcription of several key genes involved in the hydrolysis of triacylglycerol to sugars in aged soybean seeds. Furthermore, the activity of invertase in aged seeds, which catalyzes the hydrolysis of sucrose to form fructose and glucose, increased following DA-6 treatment. Taken together, DA-6 promotes germination and seedling establishment from aged soybean seeds by enhancing the hydrolysis of triacylglycerol and the conversion of fatty acids to sugars.
Introduction
The legume species soybean (Glycine max L.) originated in East Asia (Kuroda et al., 2006; Lam et al., 2010), and is now widely grown, being the primary oilseed crop in the world, with the USA, Brazil, Argentina, India, and China being the main soybean-growing countries (Zhou et al., 2015; Schulte et al., 2017). In China, where soybean production depends on maize–soybean intercropping, the area under which soybean is grown has increased significantly in recent years (Yang et al., 2014, 2015; Chen et al., 2017; Liu et al., 2017). However, it is noteworthy that, despite this, China is currently the major soybean-importing country globally; to meet the increasing demand for plant protein, oil, and food, further improvements in soybean production are essential.
Seed germination is one of the most important stages of the plant life cycle, contributing to the distribution of wild species, and to increased yield and quality of cultivated crop plants (Shu et al., 2013, 2015, 2016b, c; Kan et al., 2016; Rubio de Casas et al., 2017). Generally, the emergence of the radicle indicates the completion of seed germination (Bewley, 1997; Shu et al., 2016c). Immediately following seed germination, seedling establishment is another key developmental stage, whereby the seedling transitions from the heterotrophic to the autotrophic state (Eastmond, 2006; Quettier et al., 2008; Chen and Thelen, 2010; Theodoulou and Eastmond, 2012; Eastmond et al., 2015). Consequently, both seed germination and seedling establishment are essential for subsequent plant development. It is worth noting that both processes are powered by the energy that is stored in the seed itself (Eastmond, 2004, 2006; Quettier et al., 2008; Chen and Thelen, 2010).
The mitochondrial FAD-dependent glycerol-3-P dehydrogenase:ubiquinone oxidoreductase (FAD-GPDH) pathway has been proposed to be involved in the breakdown of fatty acids and glycerol in plant seeds (Huang, 1975). Several elegant studies demonstrated that the FAD-GPDH cascade is particularly important in oilseed plants, by which the hydrolysis of triacylglycerol releases free fatty acids and glycerol, with the fatty acids and glycerol then being converted to sugars, which support the seed germination and seedling establishment processes (Eastmond, 2004, 2006; Quettier et al., 2008; Theodoulou and Eastmond, 2012). The Arabidopsis SDP6 (Sugar-Dependent 6) gene encodes FAD-dependent glycerol-3-P dehydrogenase (FAD-G3P), and, although sdp6 mutant seeds are able to germinate, the seedlings exhibit a marked arrested growth phenotype in the absence of exogenous sucrose during the seedling establishment stage (Quettier et al., 2008). This means that the transition from glycerol and fatty acids to sucrose is significantly impaired in sdp6 seeds, with the exogenous supply of sucrose fully rescuing the arrested growth phenotype of the sdp6 mutant (Quettier et al., 2008).
Another key gene, SDP1 (Sugar-Dependent 1), encoding a patatin-like domain-containing triacylglycerol lipase, also plays a key role during post-germinative growth (Eastmond, 2006). In sdp1, the hydrolysis of triacylglycerol is blocked, resulting in sdp1 seeds also exhibiting an arrested growth phenotype in the absence of sucrose, mimicking the sdp6 phenotype (Eastmond, 2006). Altogether, both the hydrolysis of triacylglycerol and the conversion of fatty acids and glycerol to sugars are important for the successful powering of seed germination and seedling establishment, with the sugar supply allowing the young seedlings to achieve the photosynthetic autotrophic state (Graham, 2008; Kelly et al., 2011).
Numerous studies have demonstrated that, compared with the cereal crop seeds, including rice, wheat, and maize, soybean seeds contain much higher oil and fatty acid contents (Schmidt et al., 2011; Lestari et al., 2013; Dhakal et al., 2014; Liu et al., 2014; Li et al., 2017; Teng et al., 2017). During storage, seed respiration, a catabolic reaction, utilizes glucose and other biomolecules (principally oils and fatty acids), and, as a consequence, significantly shortens seed longevity and decreases the rates of seed germination and seedling establishment, even causing soybean seeds which had been stored for long periods to be incapable of germination (Barros et al., 2017; Munz et al., 2017). The ability of aged soybean seed to germinate decreases markedly as storage time increases; interestingly, the loss of germination potential also correlates with a decline in RNA integrity in soybean seeds following prolonged storage (Fleming et al., 2017). Another study demonstrated that, during natural aging processes, phospholipase Dα (PLDα) affected the soybean seed phospholipid and triacylglycerol profiles, suggesting that suppression of PLDα activity in soybean seed has the potential to improve seed quality during long-term storage (Lee et al., 2012). A decline in the germination ability of aged soybean seed significantly constrains soybean production, as it results in poor germination and seedling emergence from farm-saved seeds in the field. Therefore, it would be worthwhile developing an efficient method to promote the germination and seedling establishment of aged soybean seeds. Moreover, dissection of the precise physiological and molecular mechanisms underlying the reduced germination and seedling emergence capabilities of aged seeds will help us to better understand the soybean seed germination and seedling establishment processes.
Diethyl aminoethyl hexanoate (DA-6) is a novel artificial plant growth regulator, which can increase leaf chlorophyll content, and increase the photosynthetic rate and the rates of carbon and oxygen metabolism in plants (Yokoyama et al., 1982; Zhang et al., 2008; Jiang et al., 2012). In agriculture, DA-6 has been registered for use on a range of crops, including cabbage, pakchoi, cotton, tomato, soybean, peanut, and maize (Jiang et al., 2012). Furthermore, DA-6 also increased microalgal growth and simultaneously improved the quality and quantity of microalgal lipid for biodiesel production (Salama et al., 2014; Jiang et al., 2015), while treatment with DA-6, in combination with EDTA, appeared to be optimal for the remediation efficiency of Lolium perenne L. (perennial ryegrass) on lead-contaminated soil (He et al., 2013). However, to date, minimal information is available on the role of DA-6 in seed science research especially on seed germination and early seedling establishment from aged soybean seeds.
Here, we report that DA-6 promotes germination and seedling establishment in naturally and artificially aged soybean seeds by increasing triacylglycerol hydrolysis, fatty acid metabolism, and glycometabolism. Several types of physiological and biochemical analyses and quantitative PCR (qPCR) assays demonstrated that DA-6 increased the hydrolysis of triacylglycerol and the conversion of fatty acids to sugars during imbibition of aged soybean seeds, and, consequently, increased seed germination and seedling establishment from aged soybean seeds. We believe that this effective treatment will significantly expand the potential applications of DA-6 in agricultural systems, especially in countries where farm-saved seed is at risk of deterioration during storage.
Materials and methods
Plant materials and growth condition
The prevailing soybean cultivar in Southwestern China, Nandou-12 (ND-12), was employed in this study. The seeds were grown in the modern agricultural research and development base of Sichuan Agricultural University (Chengdu, China), and were harvested at the same time. The elite soybean seeds were used for dry storage for different periods as described in the Results section. All the soybean seeds in our lab are stored in a closed container box at room temperature, and the humidity is <5%. Silicon dioxide was added to the box to maintain the dry conditions.
Controlled deteriorate treatment assay
The assay of controlled deterioration treatment (CDT) was performed according to the protocol described elsewhere, with modifications (Chen et al., 2012, 2016). Those studies investigated the seed longevity in Arabidopsis (Chen et al., 2012, 2016), while this study focused on soybean seeds, thus some procedures are modified. Briefly, the soybean seeds were put into warm water (58 °C) for 20 min, and then the treated seeds were dried for 2 d at room temperature. Finally, the dried seeds were chosen for further germination analyses.
Seed germination and seedling establishment
Soybean seeds were incubated in 9 cm Petri dishes on two layers of medium-speed qualitative filter paper. Twenty-five seeds were placed in each Petri dish and 20 ml of sterile water or 200 μM DA-6 solution was added. The dishes were incubated in a box at 25 °C (Sanyo Versatile Environmental Test Chamber MLR-350H, made in Japan) under dark conditions.
The germination experiments were performed at 25 °C and 60% relative humidity under dark conditions; the germination rates under dark conditions were recorded using a green safety light, according to a previous assay (Barrero et al., 2014). Radicle emergence was scored at the indicated time points. For each germination test, ≥75 seeds per type of soybean seed were used, and three experimental replications were performed. Post-germination growth data including radicle length and fresh weight of germinated seeds were quantified 2–3 d after imbibition according to the particular experiment. For each germination test, the average germination percentage ±SE of experiments was calculated.
All the germinated and non-germinated soybean seeds (25 seeds per Petri dish) were transferred into soil and grown in greenhouses under 25 °C with 16 h light and 8 h dark conditions. Subsequently, after 2 weeks, the rates of seedling establishment, plant height, dry weight of seedlings, and total chlorophyll content were quantified according to the requirements of the experiments.
Gene expression analysis
Total RNA preparation, first-strand cDNA synthesis, and the qPCR assay were performed as in our previously described protocol (Shu et al., 2016a). In detail, DNase I-treated total RNA (2 μg) was denatured and then subjected to reverse transcription using Moloney murine leukemia virus reverse transcriptase (200 U per reaction; Promega Corporation). Gene expression was quantified in the logarithmic phase using the expression of the housekeeping GmTubulin RNA as an internal control. Three biological replicates were performed for each experiment. Quantitative PCR was performed on a QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific, USA), with a real-time detection system according to the manufacturer’s instructions with Vazyme™ AceQ qPCR SYBR Green Master mix, and data were calculated using the comparative CT method (Schmittgen and Livak, 2008). Primer sequence for qPCR are shown in Supplementary Table 1 at JXB online.
Quantification of total chlorophyll
About 1 cm2 of leaves (avoiding the thicker veins) were sampled with a puncher, and then were cut into filaments of ~5 mm in length and ~1 mm in width for further analysis. Subsequently, the filaments were placed in a graduated tube containing 5 ml of of 80% acetone, and the tubes were placed under dark conditions until the filaments had turned completely white (overnight). To compensate for the possible losses due to volatilization, the extraction can be made up to 5 ml with 80% acetone, and the solution in the tube was gently poured into the cuvette, and finally the level of total chlorophyll was analyzed according to the protocol described elsewhere (Xie et al., 2007). The SpectraMax i3x Multi-Mode microplate reader (Molecular Devices, LLC, USA) was employed.
Quantification of various sugars
Samples were taken at different time points (0, 12, 24, 36, and 48 h) during imbibition. After 15 min at 105 °C in an oven, they were dried at 75 °C until a constant weight was detected and were then ground in a clean mortar and put into an Eppendorf tube. A 50 mg aliquot of sample was put into a 10 ml graduated centrifuge tube with addition of 3 ml of 80% ethanol, and placed in a 80 °C water bath with constant stirring for 40 min. Next, the supernatant was collected after centrifugation at 5000 g for 10 min. The extracted solution was diluted to 50 ml with 80% ethanol after extracting three times for the sugar content analysis.
Sucrose content was measured by using the resorcinol method and estimated on the basis of the absorbance at a wavelength of 480 nm (Shi et al., 2016). Fructose content was quantified according to the method published elsewhere (Cai et al., 2016). Total soluble sugar analysis was performed by using the anthrone sulfuric acid method (Lei et al., 2014). The SpectraMax i3x Multi-Mode microplate reader (Molecular Devices, LLC, USA) was employed.
Fatty acid extraction and measurements
Soybean seeds were ground with liquid nitrogen and quantified by using a freeze drying system. Fatty acids were extracted from soybean seed powder according to our previously published protocol (Yang et al., 2017). Briefly, 2 ml of n-hexane was added to the ground soybean seeds (50 mg per tube), followed by 15 min ultrasonic extraction (40 kHz), and then the samples were kept at room temperature for 3 h. Subsequently, the solution was centrifuged at 10000 rpm and 4 °C for 10 min. Next, the supernatant was mixed and 3 ml of 0.4 M methanolic potassium hydroxide solution (Me-OH) was added, with vortex oscillation for 30 s, and then kept at room temperature for 1 h. Next, we transferred the upper liquid layer to a 5 ml capacity bottle and added n-hexane up to 5 ml, and further injected the extract into the GC-MS system through a 0.45 μm organic phase filter.
A total of 37 fatty acid methyl ester (FAME) standard mixtures including common fatty acids (C4–C24) were purchased from Nu-chek-prep Inc. (USA). Identification and quantification of each fatty acid were carried using the methods described previously (Yang et al., 2017). Three biological replications were performed. To explore the relationship among the contents of fatty acids in different samples, a heat map was created by the Illustrator software.
Measurement of soluble invertase activity
A 100 mg aliquot of soybean seeds was ground in a chilled mortar with 8 ml of ice-cold sterile water, and subsequently it was transferred into a 10 ml bottle. Next, it was put into a refrigerator at 4 °C for 3 h, and then the solution was centrifuged at 4000 rpm and 4 °C for 10 min. Next, the standard curve determination and quantification of soluble invertase activity in different types of soybean seeds under imbibition were measured according to a previously published procedure (Tang et al., 1996). Three biological replications were performed.
Statistical analysis
The data, including germination rates, fresh weight, and radicle length of germinated seeds, and fatty acid and sugar quantification results, were analyzed using Student’s t-test (SPSS 19.0). Image J software was used to measure the length of radicles.
Results
Natural aging significantly decreases soybean seed germination and seedling establishment
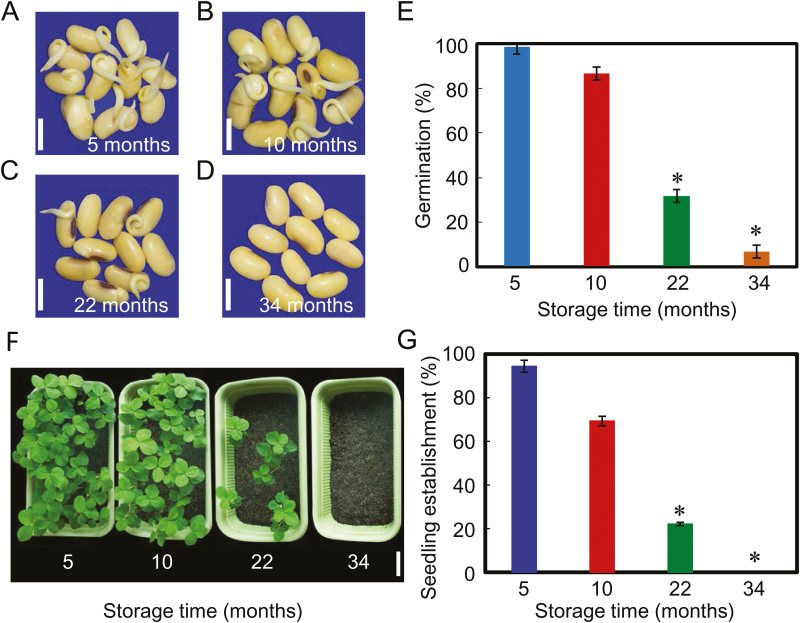
Soybean seed germination and seedling establishment were assessed in seeds subjected to different periods (i.e. 5, 10, 22, and 34 months) of dry storage. Seed germination analysis clearly showed that the germination rates of soybean seeds subjected to short-term storage (5 and 10 months) were significantly higher than those under long-term storage treatments (22 and 34 months) (Fig. 1A–E). The radicle length and fresh weight data of germinated seeds also supported the germination findings (Supplementary Fig. S1A, B). The final germination rate was nearly 100% and 85% for seeds stored for 5 and 10 months, respectively; however, the final germination rate of seeds stored for 22 months was only 30% (Fig. 1A–E), while almost all of the soybean seeds which had been stored for 34 months failed to germinate (Fig. 1D, E).
Fig. 1.
Natural aging significantly decreases soybean seed germination and seedling establishment abilities. (A–D) Representative photographs of naturally aged soybean seeds during the imbibition process (60 h after sowing). Soybean seeds were stored for 5, 10, 22, or 34 months after harvest and then subjected to analysis. Scale bar=10 mm. (E) The quantitative analysis of final germination rates of different samples (A–D) are shown (72 h after sowing). (F) The early seedling establishment phenotype of different samples (A–D) immediately following germination (2 weeks after sowing). Scale bar=100 mm. (G) The quantitative analysis of the seedling establishment rates of different samples are shown (2 weeks after sowing). Percentages are the average of three repeats ±SE. The germination experiments were performed under 25 °C and 60% relative humidity conditions, while the seedlings were grown under 25 °C and 16 h light with 8 h dark conditions. The asterisk (*) indicates a significant difference at P<0.0 by Student’s t-test analysis.
Next, we investigated the effect of soybean seed storage time on the early seedling establishment process. The same number of soybean seeds which were subjected to each of several different periods of storage were sown in soil, and then the percentage establishment of seedlings was scored. The results revealed that the seedling establishment rates from soybean seeds stored for short periods (5 or 10 months) were significantly higher than those from seeds stored for long periods (22 or 34 months) (Fig. 1F, G). Furthermore, it was noted that the seedling establishment rate after 14 d from seeds stored for 10 months (nearly 70%) was lower than their final germination rate (nearly 85%), and that a similar trend was also detected for seeds stored for 22 months (Fig. 1E, G). In particular, there was no seedling emergence from the seeds stored for 34 months, although their germination rate was ~10% (Fig. 1E, G). Taken together, these analyses confirmed the previous conclusion that the natural aging process significantly decreased the ability of soybean seeds to germinate and for seedlings to establish.
DA-6 increases germination and seedling establishment from aged soybean seeds but not from fresh seeds
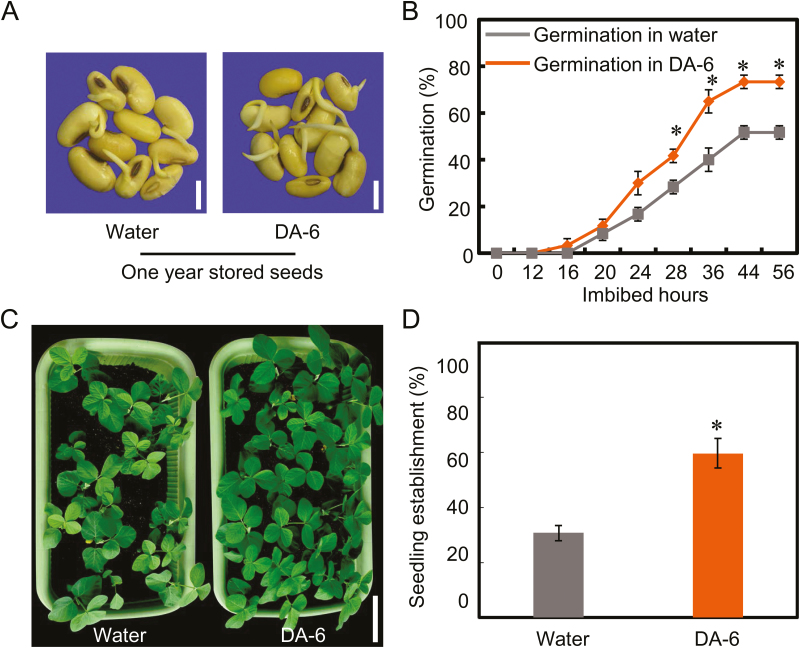
We had previously screened several plant growth regulators from those frequently employed in agriculture for their ability to promote germination and seedling establishment from aged soybean seeds (data not shown). Under our experimental conditions, we found that DA-6 increased germination and seedling establishment for both naturally and artificially aged soybean seeds. The results showed that DA-6 significantly increased the germination of naturally aged soybean seeds (stored for 12 months) (Fig. 2A, B). DA-6-treated naturally aged soybean seeds germinated more quickly than did the aged seeds without DA-6 treatment (Fig. 2A, B), and the data on the effects of DA-6 on radicle lengths and the fresh weights of germinated seedlings were consistent with the effects on germination (Supplementary Fig. S2A, B). Furthermore, DA-6 also promoted seedling establishment in naturally aged soybean seeds (Fig. 2C, D), with the seedling establishment rate of DA-6-treated aged seeds (60%) being twice that of seeds without DA-6 treatment (30%) (Fig. 2D).
Fig. 2.
DA-6 promotes germination and seedling establishment from naturally aged soybean seeds. (A) Representative photographs of naturally aged soybean seeds (stored for 12 months) during the imbibition process (60 h after sowing), with or without DA-6 treatment. Scale bar=10 mm. (B) The quantitative analysis of final germination rates of seeds stored for 12 months in the absence or presence of DA-6 treatment. (C) The early seedling establishment phenotype of seeds stored for 12 months with or without DA-6 application. Scale bar=100 mm. (D) The statistical data of early seedling establishment for (C) are shown (15 d after sowing). Percentages are the average of three repeats ±SE. The germination experiments were performed under 25 °C and 60% relative humidity conditions, while the seedlings were grown under 25 °C and 16 h light with 8 h dark conditions. The asterisk (*) indicates a significant difference at P<0.05 by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was used.
Given that DA-6 increased germination of naturally aged soybean seeds (Fig. 2), we then investigated the effects of DA-6 on the germination of fresh soybean seeds. Soybean seeds which had been stored for 1 month (‘fresh seeds’) were employed in this experiment. The results showed that no effect of DA-6 on the germination or seedling establishment from fresh soybean seeds was detected (Supplementary Fig. S3A–D). These findings demonstrated that DA-6 promotes the germination of only aged soybean seeds, but not fresh seeds (Fig. 2; Supplementary Fig. S3).
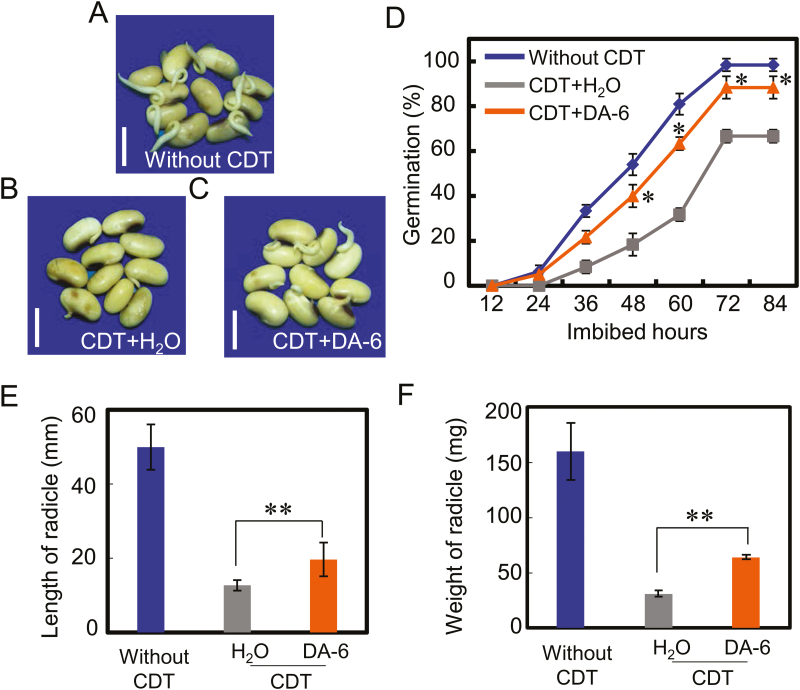
In order to control precisely the experimental conditions, we employed the CDT method, which is the prevailing artificial aging method used in seed longevity research (Bhattacharyya et al., 1985; Das and Sen-Mandi, 1992; Chen et al., 2012, 2016). Similar to the effect of DA-6 on germination and early seedling establishment from naturally aged soybean seeds (Fig. 2; Supplementary Fig. S2), the results showed that DA-6 also increased germination in artificially aged soybean seeds (Fig. 3A–D), while the radicle lengths and fresh weights of germinated seeds were also in line with the germination findings (Fig. 3E, F).
Fig. 3.
DA-6 enhances the germination ability of artificially aged soybean seeds. (A–C) Representative photographs of different types of soybean seeds (healthy seeds without CDT, CDT seeds with H2O, and CDT seeds with DA-6 treatment) during imbibition (48 h after sowing). Scale bar=10 mm. (D) The quantitative analysis of final germination rates of different samples (A–C) is shown. (E and F) The radical length (E) and fresh weight (F) of germinated soybean seeds were analyzed (48 h after sowing). The germination experiments were performed under 25 °C and 60% relative humidity with dark conditions; the germination rates under dark conditions were recorded using a green safety light, according to a previous assay (Barrero et al., 2014). The average percentages of three repeats ±SE are shown. The asterisk (*) indicates a significant difference at P<0.05 by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was used.
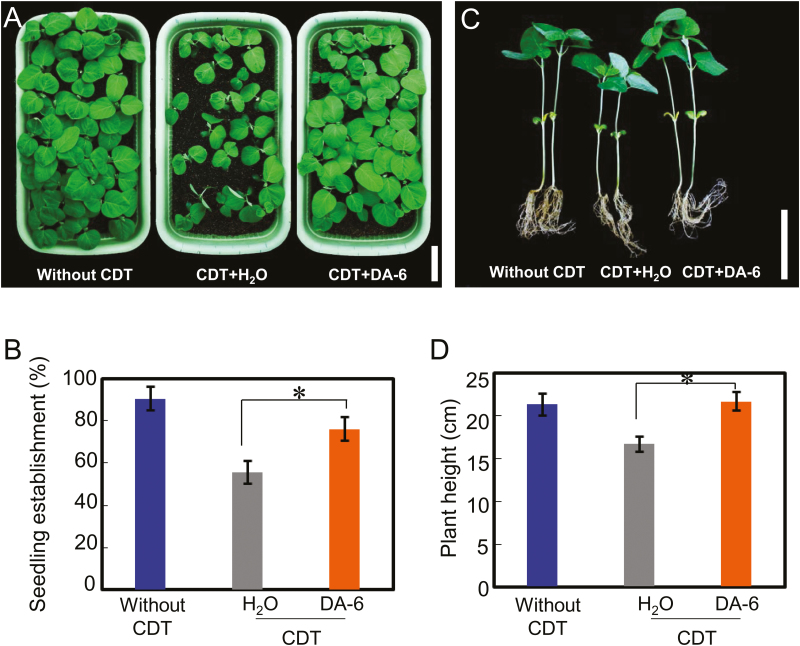
A positive effect of DA-6 on seedling establishment from artificially aged soybean seeds was also detected (Fig. 4A, B). The germination and seedling establishment rates of CDT-aged soybean seeds were lower than those from seeds without CDT, in both the presence and absence of DA-6 (Figs 3, 4). DA-6 also promoted the growth of seedlings from artificially aged soybean seeds, as illustrated by the corresponding values for the heights and dry weights of seedlings from aged seeds in the presence or absence of DA-6 (Fig. 4C, D; Supplementary Fig. S4A). DA-6 treatment also increased the total chlorophyll concentration in seedling true leaves (Supplementary Fig. S4B), a result which was consistent with findings from previous studies (Jiang et al., 2012; Jiang et al., 2015). Furthermore, given the finding that DA-6 resulted in similar positive effects on germination and seedling establishment from both naturally and artificially aged soybean seeds, subsequent experiments employed only artificially aged seeds.
Fig. 4.
DA-6 positively regulates the seedling establishment of artificially aged soybean seeds. (A) The early seedling establishment phenotype of different types of soybean seeds (healthy seeds without CDT, CDT seeds with H2O, and CDT seeds with DA-6 treatment). Scale bar=100 mm. (B) The statistical data of early seedling establishment for (A) are shown (15 d after sowing). (C) Representative images of the height of soybean seedlings. Scale bar=100 mm. (D) The quantitative analysis of plant height for (C). Percentages are the average of three repeats ±SE. The seedlings were grown under 25 °C and 16 h light with 8 h dark conditions. The asterisk (*) indicates a significant difference at P<0.05 by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was employed.
DA-6 treatment increases the concentration of soluble sugars in aged soybean seeds during imbibition
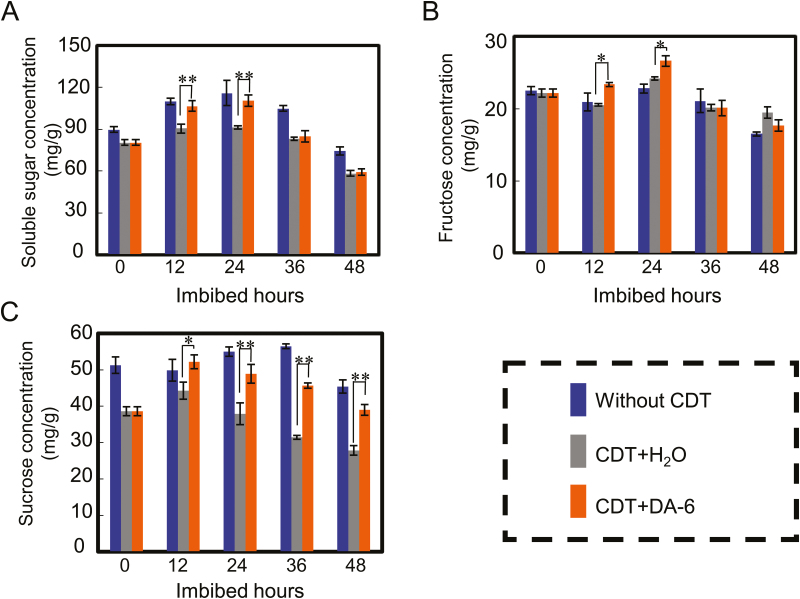
During seed germination and early seedling establishment, the energy supporting these biological processes comes primarily from carbon reserves stored in the seed itself, with sucrose and fructose being the main forms of these carbon reserves (Eastmond, 2004, 2006; Theodoulou and Eastmond, 2012). To investigate further the physiological and molecular mechanisms underlying the positive effects of DA-6 on germination and seedling establishment from aged soybean seeds, we next analyzed the concentrations of soluble sugars, namely sucrose and fructose, during imbibition of aged soybean seeds in the presence or absence of DA-6.
During soybean seed imbibition, the concentration of total soluble sugars increased initially, before decreasing (Fig. 5A). The concentration of total soluble sugars in DA-6-treated CDT-aged soybean seeds was higher than that of aged seeds that had not been DA-6 treated, especially after 12 h and 24 h imbibition (Fig. 5A). The soluble sugars in seeds are predominantly sucrose and fructose, so we then investigated the effect of DA-6 on these two sugars. The results revealed that, after exogenous DA-6 treatment, the concentrations of fructose and sucrose in CDT-aged soybean seeds were higher than those in CDT-aged seeds without DA-6 treatment (Fig. 5B, C). It is noted that the CDT+DA-6 seeds showed a higher fructose content than both control and CDT-aged seeds at some time points (Fig. 5B). These results suggested that DA-6 promoted the germination and seedling establishment of aged soybean seeds by increasing the concentrations of soluble sugars, principally sucrose.
Fig. 5.
DA-6 treatment increases the concentration of soluble sugars during imbibition by aged soybean seeds. Different types of soybean seeds (healthy seeds without CDT, CDT seeds with H2O, and CDT seeds with DA-6 treatment) were employed. (A) Soluble sugar quantification analysis. (B) Fructose concentration quantification. (C) Sucrose concentration analysis. The average percentages of four repeats ±SE are shown. Asterisks (*) and (**) indicate a significant difference at P<0.05 and P<0.01, respectively, by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was used.
DA-6 increases the concentrations of several fatty acids during imbibition of aged soybean seeds
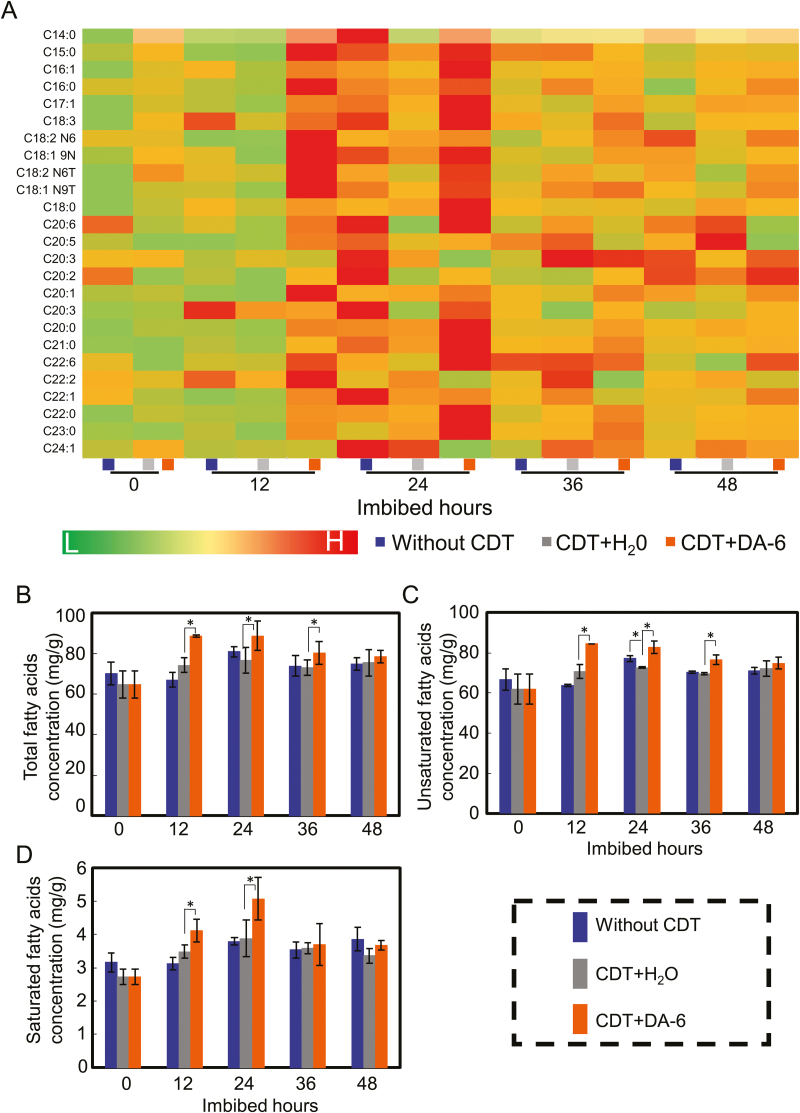
It is well known that, during the germination of and early seedling establishment from seeds of oilseed crops, hydrolysis of triacylglycerol (oil) releases fatty acids and glycerol, which, in turn, are converted by gluconeogenesis to produce different types of soluble sugar (Eastmond, 2004, 2006; Quettier and Eastmond, 2009; Theodoulou and Eastmond, 2012). To better understand the causes of the increase in soluble sugar concentrations in CDT-aged soybean seeds after DA-6 treatment, we quantified the levels of several fatty acids during soybean seed imbibition.
GC-MS analysis showed that, after 24 h imbibition, the concentrations of most of the fatty acids in untreated soybean seeds increased (Fig. 6A). In seeds artificially aged by CDT, this increase in fatty acid concentrations after 24 h imbibition was largely absent (Fig. 6A). However, exogenous DA-6 application to CDT-aged seeds fully restored the fatty acid profile, with the concentrations of most of the fatty acids in DA-6-treated CDT seeds being higher than those in seeds without DA-6 treatment after both 12 h and 24 h imbibition (Fig. 6A). The concentrations of total, unsaturated, and saturated fatty acids in the DA-6-treated CDT-aged soybean seeds were all higher than the corresponding levels in CDT seeds in the absence of DA-6 treatment at most of the time points (Fig. 6B–D). It is noted that the level of unsaturated fatty acids in CDT+H2O seeds is significantly lower than that in seeds without CDT after 24 h imbibition (Fig. 6C). Similar to this, the level of total fatty acids in CDT+H2O seeds also decreased compared with the seeds without CDT, although the difference was not significant (Fig. 6B). Finally, we investigated the effects of DA-6 treatment on the concentration of each of the primary types of fatty acids in soybean seeds, namely palmitic, stearic, oleic, linoleic, arachidic, and linolenic acids. The results showed that DA-6 treatment increased the concentration of each fatty acid in aged soybean seeds during imbibition (Supplementary Fig. S5). Taken together, these findings indicated that DA-6 positively regulates the conversion of triacylglycerol to fatty acids, which are important precursors for the production of soluble sugars.
Fig. 6.
DA-6 treatment increases the concentration of total fatty acids in aged soybean seeds during imbibition. Different types of soybean seeds (healthy seeds without CDT, CDT seeds with H2O, and CDT seeds with DA-6 treatment) were employed. (A) The heat map analysis of the contents of several types of fatty acids during the soybean seed imbibition process with the time-course. The heat map was created by the Illustrator software. The fatty acid level from low (L) to high (H) indicates the minimum and maximum in the entire database. (B) The total fatty acid concentration in different types of soybean seeds during imbibition. (C) The unsaturated fatty acid concentration in soybean seeds during imbibition. (D) The saturated fatty acid concentration in soybean seeds during imbibition. The average percentages of four repeats ±SE are shown. Asterisks (*) and (**) indicate a significant difference at P<0.05 and P<0.01, respectively, by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was employed.
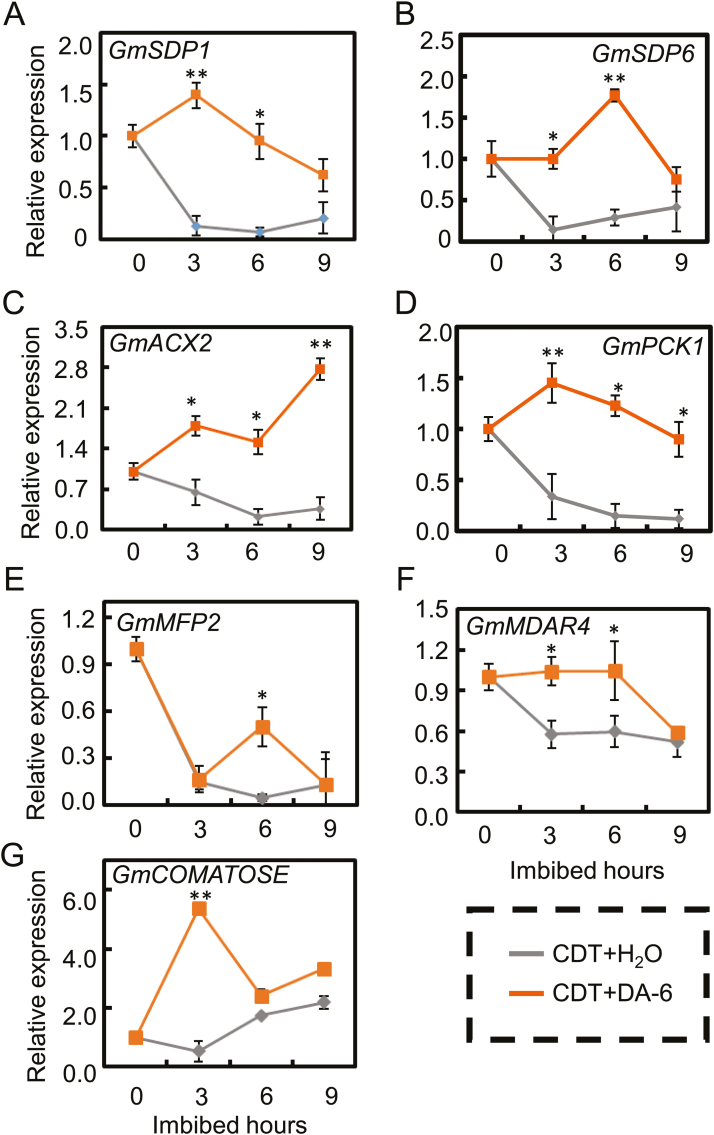
DA-6 increases the transcription of several key genes involved in the conversion of triacylglycerol to fatty acids and sugars in aged soybean seeds during imbibition
We had observed that DA-6 treatment increased the concentrations of several fatty acids and soluble sugars in aged soybean seeds during imbibition (Figs 5, 6). Subsequently, we further analyzed the effects of DA-6 on increasing the conversion of triacylglycerol to fatty acids and sugars in aged soybean seeds during imbibition by studying the effects on the transcription pattern of key genes involved in the conversion of triacylglycerol to fatty acids and sugars. The results of qPCR analysis showed that the transcript levels of the key genes involved in the triacylglycerol hydrolysis pathway, namely GmSDP1 (Fig. 7A), GmSDP6 (Fig. 7B), GmACX2 (Fig. 7C), GmPCK1 (Fig. 7D), GmMFP2 (Fig. 7E), GmMDAR4 (Fig. 7F), and GmCOMATOSE (Fig. 7G), were all up-regulated (to varying degrees) in DA-6-treated aged soybean seeds during imbibition, compared with aged seeds without DA-6 application.
Fig. 7.
Positive effect of DA-6 on the transcription of several key genes which are involved in hydrolysis of triacylglycerol in aged soybean seeds during imbibition. Gene expression was investigated by qPCR assay during the course of the imbibition process. Different types of soybean seeds (CDT seeds with H2O and CDT seeds with DA-6 treatment) were employed. These different types of soybean seeds were employed for mRNA extraction, and three replications were performed. GmSDP6 and GmSDP1 encode FAD-G3P dehydrogenase and patatin-like domain-containing triacylglycerol lipase, and GmACX2 and GmPCK1 encode acyl-CoA oxidase and phosphoenolpyruvate carboxykinase, respectively. Those genes are all involved in the pathways by which the triacylglycerol was transferred to fatty acids and sugars during imbibition. (A) GmSDP1; (B) GmSDP6; (C) GmACX2; (D) GmPCK1; (E) GmMFP2; (F) GmMDAR4; (G) GmCOMATOSE. Asterisks (*) and (**) indicate a significant difference at P<0.05 and P<0.01, respectively, by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was used.
GmSDP6 and GmSDP1 encode FAD-G3P dehydrogenase and patatin-like domain-containing triacylglycerol lipase (Eastmond, 2004, 2006; Quettier et al., 2008), respectively, which are the key enzymes in the hydrolysis of triacylglycerol. The expression levels of GmSDP1 and GmSDP6 in DA-6-treated aged soybean seeds were twice to three times those in seeds without DA-6 treatment (Fig. 7A, B). Similarly, the levels of GmACX2 and GmPCK1 transcripts detected in DA-6-treated aged soybean seeds were higher than in the corresponding aged seeds without DA-6 application (Fig. 7C, D). GmACX2 and GmPCK1 encode acyl-CoA oxidase and phosphoenolpyruvate carboxykinase, respectively, which are also involved in the process of β-oxidation of fatty acids (Li-Beisson et al., 2010; Penfield et al., 2012; Eastmond et al., 2015). GmPCK1 catalyzes the conversion of oxaloacetate (OAA) to phosphoenolpyruvate (PEP), the rate-limiting step in the metabolic pathway (Penfield et al., 2012).
The accumulation of the transcripts of GmMFP2, GmMDAR4, and GmCOMATOSE after DA-6 treatment was also detected (Fig. 7E–G). These three genes are also involved in β-oxidation pathways (Rylott et al., 2006; Eastmond, 2007; Kunz et al., 2009). The accumulation of transcripts of these genes induced by DA-6 treatment might be expected to cause an increase in the production of soluble sugars. Altogether, the qPCR results suggested that DA-6 treatment of aged seeds increased the hydrolysis of triacylglycerol, as well as promoting the conversion of fatty acids to sugars during seed imbibition, by up-regulating expression of key genes involved in the respective pathways. The end-products of triacylglycerol hydrolysis, namely sucrose and fructose, provide the energy required to support the DA-6-induced germination and early seedling establishment from aged soybean seeds.
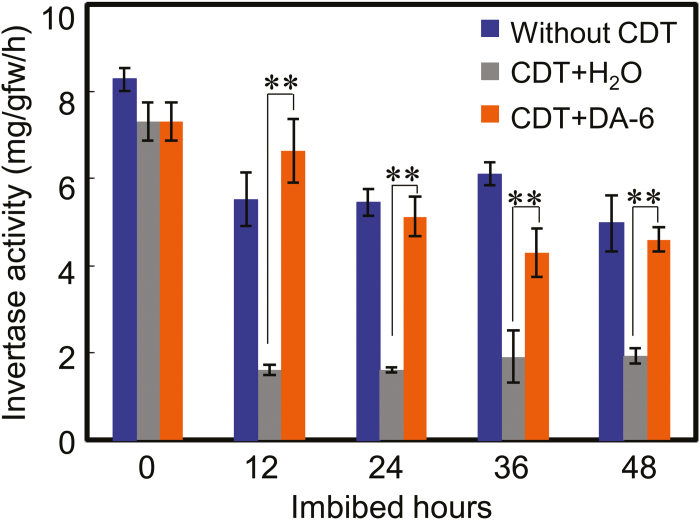
DA-6 increases invertase activity in aged soybean seeds during imbibition
Invertase is the key enzyme catalyzing the hydrolysis of sucrose to give equimolar amounts of glucose and fructose (Jameson et al., 2016; Wei et al., 2016). We showed that, during germination of aged soybean seeds, DA-6 treatment increased the concentration of soluble sugars, including sucrose and fructose (Fig. 5). A previous study had demonstrated that suppressed invertase activity was associated with a delayed seed germination phenotype in Arabidopsis (Su et al., 2016). To explore the relationship between DA-6 treatment, sucrose hydrolysis, and the germination ability of aged soybean seeds further, we quantified invertase activity during soybean seed germination. The results revealed that invertase activity was significantly repressed during imbibition of aged soybean seeds, compared with the activity in imbibed fresh seeds; while DA-6 treatment of aged seeds markedly increased invertase activity, compared with the seeds which had not been treated with DA-6 (Fig. 8). This finding was consistent with the positive effect of DA-6 on the germination and early seedling establishment of aged soybean seeds (Figs 2–4), and was in agreement with the increase in fructose concentration in DA-6-treated aged soybean seeds during imbibition (Fig. 5).
Fig. 8.
DA-6 treatment increases the invertase activity in aged soybean seeds during imbibition. Different types of soybean seeds (healthy seeds without CDT, CDT seeds with H2O, and CDT seeds with DA-6 treatment) were employed. The invertase enzyme activity in these distinct types of seeds was analyzed during imbibition. The average percentages of three repeats ±SE are shown. Asterisks (**) indicate a significant difference at P<0.01 by Student’s t-test analysis. A 200 μM concentration of exogenous DA-6 was used.
Discussion
In the present study, phenotypic analysis, quantification of fatty acid and sugar concentrations, and analysis of gene expression and enzyme activity have demonstrated that the plant growth regulator DA-6 reverses the reduced germination and early seedling establishment rates exhibited by aged soybean seeds. The physiological and molecular mechanisms responsible for this positive effect are proposed to be as follows: in the aged soybean seeds, DA-6 promotes the hydrolysis of triacylglycerol to fatty acids and glycerol, and also enhances the conversion of fatty acids and glycerol to soluble sugars, which supply the energy needed during the soybean seed germination and early seedling establishment processes. This study also demonstrated that aged soybean seeds actually contain sufficient energy for germination, stored in the seeds as different food reserves, namely triacylglycerol, fatty acids, glycerol, and sugars. However, the aged seeds cannot use the storage energy, except for soluble sugars, because the conversion of triacylglycerol to soluble sugars appears to be blocked, resulting in the failure of seed germination and early seedling establishment.
DA-6 has a positive effect on germination and early seedling establishment in aged soybean seeds
Numerous elegant studies have demonstrated that DA-6, an artificial plant growth regulator, has a range of biological functions which can impact on field crop production (Zhang et al., 2008; Jiang et al., 2012; He et al., 2013; Salama et al., 2014; Jiang et al., 2015). The present investigation extended the practical uses of DA-6, demonstrating that DA-6 can reverse the loss of germination and seedling establishment activities associated with aging in soybean seeds.
Seed germination and subsequent seedling establishment are vital steps during the plant life cycle, contributing to the determination of plant distribution in wild species, and of yield in crops. Research from the laboratory, including the current study, has demonstrated that the decrease in seed germination ability as the seed ages is positively associated with the duration of the storage period (Yin et al., 2014; Nguyen et al., 2015; Yin et al., 2015; Fleming et al., 2017). In tillage agriculture, farm-saved seeds are stored by the farmer for varying periods of time, often under less than ideal conditions, resulting in seed aging and a decline in germination characteristics. Consequently, enhancement of the germination and seedling establishment from aged seeds is a worthwhile target in both basic and applied aspects of plant biology and crop production research.
Interestingly, a previous study had reported that hydrated graphene ribbon treatment could promote the germination of aged wheat seeds by increasing the concentrations of carbohydrate, amino acids, and fatty acids, and ensuring cell membrane integrity (Hu and Zhou, 2014). Ultrasonic treatment also improved seedling growth from aged grass seeds, including Festuca arundinacea Schreb. (tall fescue) and Psathyrostachys juncea L. (Russian wildrye), but the underlying mechanisms need further investigation (Liu et al., 2016). A more recent study revealed that phytosynthesized silver nanoparticles increased the germination of aged rice seeds, with the mechanisms underlying this positive effect being suggested to include enhanced water uptake, rebooting of the reactive oxygen species (ROS) antioxidant systems in seeds, generation of hydroxyl radicals to achieve cell wall loosening, and nanocatalysis to accelerate starch hydrolysis (Mahakham et al., 2017). However, most of these studies focused only on germination but not seedling establishment from aged seeds, and identified that there were diverse mechanisms underlying the effects promoting germination of aged seeds.
DA-6 enhances the hydrolysis of triacylglycerol to sugars in aged soybean seeds
It is noted that the process of seed germination is distinct from that of seedling establishment, especially in aged soybean seeds. Germination analysis showed that percentage seedling establishment was generally lower than that of seed germination, especially in the soybean seeds stored for 10, 22, or 34 months (Fig. 1). For the soybean seeds stored for 34 months, the germination rate reached ~10%, but there was zero emergence (Fig. 1). These data suggested that the level of releasable energy stored in the seed itself is important for both processes (seed germination and seedling establishment); if the energy reserves were exhausted in the first stage (seed germination), then the germinated seed could not complete the second process (seedling establishment).
It is well known that the primary energy sources used by seeds during germination and seedling establishment are the soluble sugars, principally sucrose and fructose (Eastmond, 2004, 2006; Quettier et al., 2008; Theodoulou and Eastmond, 2012). In oilseed species, including soybean and Arabidopsis, the hydrolysis of triacylglycerol produces fatty acids and glycerol, with the fatty acids and glycerol both being further converted into sucrose (Theodoulou and Eastmond, 2012). Consequently, these biochemical reactions, converting triacylglycerol to fatty acids and glycerol, and fatty acids and glycerol to sucrose, are vital for generating the energy to complete the seed germination and seedling establishment processes.
In the present investigation, we found that the germination ability of aged soybean seeds (following long-term storage) was lower than that of seeds stored for a shorter period (Fig. 1; Supplementary Fig. S1), and that DA-6 treatment significantly increased the germination and seedling establishment from aged soybean seeds (Figs 2–4; Supplementary Figs S2, S4). Subsequent research indicated that the conversion of seed storage oil (triacylglycerol) into sucrose during imbibition was insufficient to power germination of the aged soybean seeds in the absence of exogenous DA-6 application. Several lines of evidence supported this conclusion. DA-6 treatment markedly increased the levels of different types of fatty acids and soluble sugars (Figs 5, 6; Supplementary Fig. S5). In line with this, qPCR analysis further showed that DA-6 treatment increased the transcription of several key genes involved in the triacylglycerol hydrolysis pathway (Fig. 7).
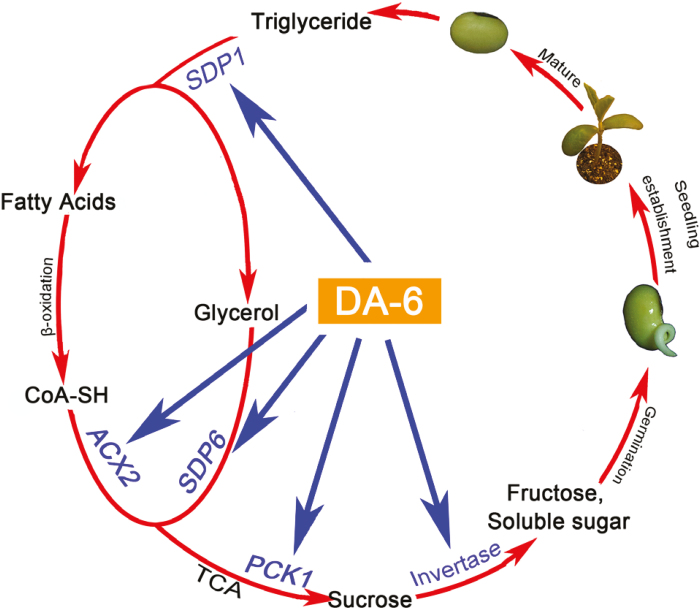
Taken together, the findings from the present investigation demonstrated that, during imbibition of aged soybean seeds, DA-6 increases the rate of hydrolysis of triacylglycerol to fatty acids and glycerol, and also the conversion of fatty acids and glycerol to sucrose and fructose by promoting the transcription of the key genes and the activity of the enzymes involved in those pathways (Fig. 9). On one hand, this study extended the range of agronomic and physiological crop processes identified as being affected by DA-6. On the other hand, the present investigation also demonstrated that the conversion of triacylglycerol to soluble sugars is important for the germination of aged seeds of oilseed crops.
Fig. 9.
The proposed working model through which DA-6 promotes germination and seedling establishment from aged soybean seeds. In the aged soybean seeds, the transition from triacylglycerol to fatty acid and glycerol and then to the soluble sugars was blocked in the absence of DA-6. The application of exogenous DA-6 treatment in aged soybean seeds promoted the transcription of several key genes and elevated the activity of invertase which is involved in this pathway. Taken together, this model revealed that DA-6 promotes the germination and seedling establishment from aged soybean seeds by increasing the hydrolysis of triacylglycerol and the conversion of fatty acids and glycerol to sugars.
Given the importance of the phytohormones abscisic acid (ABA) and gibberellins (GAs) in the regulation of seed germination (Shu et al., 2013, 2015, 2016c, 2018), the relationship between DA-6 and the biosynthesis and signaling pathways of ABA and GA during germination of aged soybean seeds needs further investigation. For instance, does DA-6 regulate the biosynthesis and/or signal transduction pathways of ABA and GA? DA-6 is an artificial plant hormone, so the dissection of its receptor(s) will help us better understand the action of DA-6. Furthermore, the relationship between the DA-6 receptor(s) and ABA and GA also needs further exploration. Future studies should address whether DA-6 is also involved in the regulation of germination and early seedling establishment in aged seeds of the cereal crops (such as wheat, rice, and maize).
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Natural aging significantly decreases soybean seed germination ability.
Fig. S2. DA-6 promotes germination ability of natural aged soybean seeds
Fig. S3. DA-6 has no effect on germination and seedling establishment of fresh soybean seeds.
Fig. S4. DA-6 treatment increases the total chlorophyll content and dry weight in seedlings which germinated from CDT-aged soybean seeds.
Fig. S5. DA-6 treatment increases the concentration of several types of fatty acids in aged soybean seeds during imbibition.
Table S1. Primer sequences used in this study.
Acknowledgements
We would like to thank Dr Huhui Chen (State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resource, School of Life Sciences, Sun Yat-sen University, Guangzhou) for critical reading and valuable comments. This work was supported by the National Natural Science Foundation of China (31872804, 31701064), the National Key Research and Development Program of China (2017YFD0201306), and Sichuan Science and Technology Program (2018JZ0060).
Author contributions
KS conceived and designed this study. W-GZ, FC, S-HZ, C-QY, Y-JM, H-WS, X-FL, Y-JD, HY, J-BD, JL, G-QF, and W-GL performed the experiments. KS and W-YY analyzed the data. KS wrote and revised the paper.
References
- Barrero JM, Downie AB, Xu Q, Gubler F. 2014. A role for barley CRYPTOCHROME1 in light regulation of grain dormancy and germination. The Plant Cell 26, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros JAS, Cavalcanti JHF, Medeiros DB, Nunes-Nesi A, Avin-Wittenberg T, Fernie AR, Araújo WL. 2017. Autophagy deficiency compromises alternative pathways of respiration following energy deprivation in Arabidopsis thaliana. Plant Physiology 175, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Hazra AK, Sen-Mandi SJ. 1985. Accelerated ageing of seeds in hot water: germination characteristics of aged wheat seeds. Seed Science & Technology 13, 683–690. [Google Scholar]
- Cai Y, Shao L, Li X, Liu G, Chen S. 2016. Gibberellin stimulates regrowth after defoliation of sheepgrass (Leymus chinensis) by regulating expression of fructan-related genes. Journal of Plant Research 129, 935–944. [DOI] [PubMed] [Google Scholar]
- Chen H, Chu P, Zhou Y, Li Y, Liu J, Ding Y, Tsang EW, Jiang L, Wu K, Huang S. 2012. Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. Journal of Experimental Botany 63, 4107–4121. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chu P, Zhou YL, Ding Y, Li Y, Liu J, Jiang LW, Huang SZ. 2016. Ectopic expression of NnPER1, a Nelumbo nucifera 1-cysteine peroxiredoxin antioxidant, enhances seed longevity and stress tolerance in Arabidopsis. The Plant Journal 88, 608–619. [DOI] [PubMed] [Google Scholar]
- Chen M, Thelen JJ. 2010. The plastid isoform of triose phosphate isomerase is required for the postgerminative transition from heterotrophic to autotrophic growth in Arabidopsis. The Plant Cell 22, 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Du Q, Liu X, et al. . 2017. Effects of reduced nitrogen inputs on crop yield and nitrogen use efficiency in a long-term maize–soybean relay strip intercropping system. PLoS One 12, e0184503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Sen-Mandi S. 1992. Scutellar amylase activity in naturally aged and accelerated aged wheat seeds. Annals of Botany 69, 497–501. [Google Scholar]
- Dhakal KH, Jung KH, Chae JH, Shannon JG, Lee JD. 2014. Variation of unsaturated fatty acids in soybean sprout of high oleic acid accessions. Food Chemistry 164, 70–73. [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. 2004. Glycerol-insensitive Arabidopsis mutants: gli1 seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. The Plant Journal 37, 617–625. [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. 2006. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. The Plant Cell 18, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ. 2007. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. The Plant Cell 19, 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Astley HM, Parsley K, Aubry S, Williams BP, Menard GN, Craddock CP, Nunes-Nesi A, Fernie AR, Hibberd JM. 2015. Arabidopsis uses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nature Communications 6, 6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MB, Richards CM, Walters C. 2017. Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. Journal of Experimental Botany 68, 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA. 2008. Seed storage oil mobilization. Annual Review of Plant Biology 59, 115–142. [DOI] [PubMed] [Google Scholar]
- He S, Wu Q, He Z. 2013. Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere 93, 2782–2788. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhou Q. 2014. Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Scientific Reports 4, 3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH. 1975. Enzymes of glycerol metabolism in the storage tissues of fatty seedlings. Plant Physiology 55, 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson PE, Dhandapani P, Novak O, Song J. 2016. Cytokinins and expression of SWEET, SUT, CWINV and AAP genes increase as pea seeds germinate. International Journal of Molecular Sciences 17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jiang Y, He S, Zhang H, Pan C. 2012. Dissipation of diethyl aminoethyl hexanoate (DA-6) residues in pakchoi, cotton crops and soil. Bulletin of Environmental Contamination and Toxicology 88, 533–537. [DOI] [PubMed] [Google Scholar]
- Jiang L, Pei H, Hu W, Han F, Zhang L, Hou Q. 2015. Effect of diethyl aminoethyl hexanoate on the accumulation of high-value biocompounds produced by two novel isolated microalgae. Bioresource Technology 197, 178–184. [DOI] [PubMed] [Google Scholar]
- Kan G, Ning L, Li Y, Hu Z, Zhang W, He X, Yu D. 2016. Identification of novel loci for salt stress at the seed germination stage in soybean. Breeding Science 66, 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, Quettier AL, Shaw E, Eastmond PJ. 2011. Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiology 157, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, Gierth M. 2009. The ABC transporter PXA1 and peroxisomal beta-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. The Plant Cell 21, 2733–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Kaga A, Tomooka N, Vaughan DA. 2006. Population genetic structure of Japanese wild soybean (Glycine soja) based on microsatellite variation. Molecular Ecology 15, 959–974. [DOI] [PubMed] [Google Scholar]
- Lam HM, Xu X, Liu X, et al. . 2010. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nature Genetics 42, 1053–1059. [DOI] [PubMed] [Google Scholar]
- Lee J, Welti R, Roth M, Schapaugh WT, Li J, Trick HN. 2012. Enhanced seed viability and lipid compositional changes during natural ageing by suppressing phospholipase Dα in soybean seed. Plant Biotechnology Journal 10, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Zeng B, Yuan Z, Su X. 2014. Changes in carbohydrate content and membrane stability of two ecotypes of Calamagrostis arundinacea growing at different elevations in the drawdown zone of the Three Gorges Reservoir. PLoS One 9, e91394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestari P, Van K, Lee J, Kang YJ, Lee SH. 2013. Gene divergence of homeologous regions associated with a major seed protein content QTL in soybean. Frontiers in Plant Science 4, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QT, Lu X, Song QX, et al. . 2017. Selection for a zinc-finger protein contributes to seed oil increase during soybean domestication. Plant Physiology 173, 2208–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, et al. . 2010. Acyl-lipid metabolism. Arabidopsis Book 8, e0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Q, Karagić Đ, Liu X, Cui J, Gui J, Gu M, Gao W. 2016. Effects of ultrasonication on increased germination and improved seedling growth of aged grass seeds of tall fescue and Russian wildrye. Scientific Reports 6, 22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rahman T, Yang F, Song C, Yong T, Liu J, Zhang C, Yang W. 2017. PAR interception and utilization in different maize and soybean intercropping patterns. PLoS One 12, e0169218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Li QT, Lu X, et al. . 2014. Soybean GmMYB73 promotes lipid accumulation in transgenic plants. BMC Plant Biology 14, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P. 2017. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Scientific Reports 7, 8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz E, Rolletschek H, Oeltze-Jafra S, Fuchs J, Guendel A, Neuberger T, Ortleb S, Jakob PM, Borisjuk L. 2017. A functional imaging study of germinating oilseed rape seed. New Phytologist 216, 1181–1190. [DOI] [PubMed] [Google Scholar]
- Nguyen TP, Cueff G, Hegedus DD, Rajjou L, Bentsink L. 2015. A role for seed storage proteins in Arabidopsis seed longevity. Journal of Experimental Botany 66, 6399–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Clements S, Bailey KJ, Gilday AD, Leegood RC, Gray JE, Graham IA. 2012. Expression and manipulation of phosphoenolpyruvate carboxykinase 1 identifies a role for malate metabolism in stomatal closure. The Plant Journal 69, 679–688. [DOI] [PubMed] [Google Scholar]
- Quettier AL, Eastmond PJ. 2009. Storage oil hydrolysis during early seedling growth. Plant Physiology and Biochemistry 47, 485–490. [DOI] [PubMed] [Google Scholar]
- Quettier AL, Shaw E, Eastmond PJ. 2008. SUGAR-DEPENDENT6 encodes a mitochondrial flavin adenine dinucleotide-dependent glycerol-3-P dehydrogenase, which is required for glycerol catabolism and post germinative seedling growth in Arabidopsis. Plant Physiology 148, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio de Casas R, Willis CG, Pearse WD, Baskin CC, Baskin JM, Cavender-Bares J. 2017. Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytologist 214, 1527–1536. [DOI] [PubMed] [Google Scholar]
- Rylott EL, Eastmond PJ, Gilday AD, Slocombe SP, Larson TR, Baker A, Graham IA. 2006. The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal beta-oxidation is essential for seedling establishment. The Plant Journal 45, 930–941. [DOI] [PubMed] [Google Scholar]
- Salama ES, Kabra AN, Ji MK, Kim JR, Min B, Jeon BH. 2014. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresource Technology 172, 97–103. [DOI] [PubMed] [Google Scholar]
- Schmidt MA, Barbazuk WB, Sandford M, May G, Song Z, Zhou W, Nikolau BJ, Herman EM. 2011. Silencing of soybean seed storage proteins results in a rebalanced protein composition preserving seed protein content without major collateral changes in the metabolome and transcriptome. Plant Physiology 156, 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schulte LA, Niemi J, Helmers MJ, et al. . 2017. Prairie strips improve biodiversity and the delivery of multiple ecosystem services from corn–soybean croplands. Proceedings of the National Academy of Sciences, USA 114, 11247–11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang B, Yang P, Li Y, Miao F. 2016. Differences in sugar accumulation and mobilization between sequential and non-sequential senescence wheat cultivars under natural and drought conditions. PLoS One 11, e0166155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Chen Q, Wu Y, et al. . 2016. a ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. The Plant Journal 85, 348–361. [DOI] [PubMed] [Google Scholar]
- Shu K, Chen Q, Wu Y, Liu R, Zhang H, Wang S, Tang S, Yang W, Xie Q. 2016b ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C transcription. Journal of Experimental Botany 67, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Liu XD, Xie Q, He ZH. 2016c Two faces of one seed: hormonal regulation of dormancy and germination. Molecular Plant 9, 34–45. [DOI] [PubMed] [Google Scholar]
- Shu K, Meng YJ, Shuai HW, Liu WG, Du JB, Liu J, Yang WY. 2015. Dormancy and germination: how does the crop seed decide?Plant Biology 17, 1104–1112. [DOI] [PubMed] [Google Scholar]
- Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S, Liu C, Feng Y, Cao X, Xie Q. 2013. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in arabidopsis. PLoS Genetics 9, e1003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Zhou W, Yang W. 2018. APETALA 2-domain-containing transcription factors: focusing on abscisic acid and gibberellins antagonism. New Phytologist 217, 977–983. [DOI] [PubMed] [Google Scholar]
- Su T, Wolf S, Han M, Zhao H, Wei H, Greiner S, Rausch T. 2016. Reassessment of an Arabidopsis cell wall invertase inhibitor AtCIF1 reveals its role in seed germination and early seedling growth. Plant Molecular Biology 90, 137–155. [DOI] [PubMed] [Google Scholar]
- Tang X, Ruffner HP, Scholes JD, Rolfe SA. 1996. Purification and characterisation of soluble invertases from leaves of Arabidopsis thaliana. Planta 198, 17–23. [DOI] [PubMed] [Google Scholar]
- Teng W, Li W, Zhang Q, Wu D, Zhao X, Li H, Han Y, Li W. 2017. Identification of quantitative trait loci underlying seed protein content of soybean including main, epistatic, and QTL×environment effects in different regions of Northeast China. Genome 60, 649–655. [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Eastmond PJ. 2012. Seed storage oil catabolism: a story of give and take. Current Opinion in Plant Biology 15, 322–328. [DOI] [PubMed] [Google Scholar]
- Wei H, Bausewein A, Steininger H, Su T, Zhao H, Harms K, Greiner S, Rausch T. 2016. Linking expression of fructan active enzymes, cell wall invertases and sucrose transporters with fructan profiles in growing taproot of chicory (Cichorium intybus): impact of hormonal and environmental cues. Frontiers in Plant Science 7, 1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Ying Y, Ying T. 2007. Quantification of chlorophyll content and classification of nontransgenic and transgenic tomato leaves using visible/near-infrared diffuse reflectance spectroscopy. Journal of Agricultural and Food Chemistry 55, 4645–4650. [DOI] [PubMed] [Google Scholar]
- Yang C, Iqbal N, Hu B, Zhang Q, Wu H, Liu X, Zhang J, Liu W, Yang W, Liu J. 2017. Targeted metabolomics analysis of fatty acids in soybean seeds using GC-MS to reveal the metabolic manipulation of shading in the intercropping system. Analytical Methods 9, 2144–2152. [Google Scholar]
- Yang F, Huang S, Gao R, Liu W, Yong T, Wang X, Wu X, Yang W. 2014. Growth of soybean seedlings in relay strip intercropping systems inrelation to light quantity and red far-red ratio. Field Crops Research 155, 245–253. [Google Scholar]
- Yang F, Wang XC, Liao DP, et al. . 2015. Yield response to different planting geometries in maize–soybean relay strip intercropping systems. Agronomy Journal 107, 296–304. [Google Scholar]
- Yin G, Xin X, Song C, Chen X, Zhang J, Wu S, Li R, Liu X, Lu X. 2014. Activity levels and expression of antioxidant enzymes in the ascorbate–glutathione cycle in artificially aged rice seed. Plant Physiology and Biochemistry 80, 1–9. [DOI] [PubMed] [Google Scholar]
- Yin X, He D, Gupta R, Yang P. 2015. Physiological and proteomic analyses on artificially aged Brassica napus seed. Frontiers in Plant Science 6, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Hsu WJ, Poling S, Hayman E. 1982. Bioregulation of pigment biosynthesis by onium compounds. ACS Symposium Series 181, 153–173. [Google Scholar]
- Zhang H, Xie L, Xu P, Jiang S. 2008. Dissipation of the plant growth regulator hexanoic acid 2-(diethylamino) ethyl ester in pakchoi and soil. International Journal of Environmental Analytical Chemistry 88, 561–569. [Google Scholar]
- Zhou Z, Jiang Y, Wang Z, et al. . 2015. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nature Biotechnology 33, 408–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.