Abstract
The skin commensal Propionibacterium acnes, recently renamed Cutibacterium acnes, along with the other major pathophysiological factors of increased seborrhea, hyperkeratinization of the pilosebaceous unit, and inflammation, has long been implicated in the pathogenesis of acne. Recent advances have contributed to our understanding of the role of P. acnes in acne. Although there are no quantitative differences in P. acnes of the skin of patients with acne compared with controls, the P. acnes phylogenic groups display distinct genetic and phenotypic characteristics, P. acnes biofilms are more frequent in acne, and different phylotypes may induce distinct immune responses in acne. P. acnes plays a further important role in the homeostasis of the skin’s microbiome, interacting with other cutaneous commensal or pathogenic microorganisms such as Staphylococcus epidermidis, Streptococcus pyogenes, and Pseudomonas species. In the era of increasing antimicrobial resistance, the selection of acne treatment targeting P. acnes and the prevention of antibiotic resistance play a key role in improving outcomes in acne patients and public health.
Keywords: Priopionibacterium acnes, biofilm, phylotypes, acne, antimicrobial resistance
Introduction
The cutaneous microbiome exists in a finely tuned equilibrium in healthy skin that when perturbed may lead to various inflammatory skin diseases. The three most commonly observed cutaneous genera are Corynebacteria, Propionibacteria, and Staphylococci 1.
Propionibacterium acnes has been implicated in the pathophysiology of prostate cancer 2, sarcoidosis 3, infective endocarditis 4, infections involving prosthetic devices (such as prosthetic joints, central nervous system ventricular shunts, and cardiac implantable devices) 5, and acne, the last of which is the focus of this review. P. acnes is a Gram-positive, non-spore-forming human skin commensal that prefers anaerobic growth conditions 6, 7. It is a member of the normal skin microbiota along with P. avidum, P. granulosum, and P. humerusii 8. The P. acnes genome is 2.5 Mb in size and has been completely sequenced. It has genes encoding metabolic enzymes, enabling it to survive in microaerophilic conditions, but also lipases that degrade the lipids of the pilosebaceous follicle, providing the bacterium with the energy it needs 9. Recently, a taxonomic reclassification was proposed in which P. acnes was renamed Cutibacterium acnes to account for genomic adaptive changes and to differentiate it from other Propionibacteria species. In particular, specific lipase genes were identified encoding for triacylglycerol lipase and lysophospholipase able to degrade sebum lipids 10. However, it has been proposed that it is taxonomically valid to continue to use the genus name Propionibacterium for the cutaneous group within dermatology specialties for a range of different reasons, including to avoid confusion with the previous name, Corynebacterium acnes 11.
In this review, we describe the characteristics of P. acnes concerning taxonomy, the role of different phylotypes and P. acnes biofilm in acne pathophysiology, and the targeting of P. acnes with appropriate acne treatments and the respective implications in the homeostasis of the skin’s microbiome and the emergence of antimicrobial resistance.
P. acnes taxonomy
With regard to taxonomy, P. acnes has been classified into three phylotypes (phylogroups) based on gene sequences or biological characteristics (lipase activity): I, II, and III 12. These phylogroups in turn have been split into distinct subspecies known as P. acnes subsp. acnes, P. acnes subsp. defendens, and P. acnes subsp. elongatum, respectively, to denote distinct phylogenetic, genomic, and phenotypic characteristics as well as their association with different clinical diseases, including acne and progressive macular hypomelanosis 13, 14.
The subspecies P. acnes subsp. acnes has been described. Extracellular enzymes include RNase, neuraminidase, hyaluronidase, acid phosphatase, lecithinase, and lipase. The bacterial cells ferment glucose, and lactic acid is produced from fermentable carbohydrates in variable quantities. The major long-chain fatty acid produced is 13-methyltetradecanoic acid 14. Gene sequence analysis of P. acnes on the basis of the genes recA and tly revealed further phylogenetic subdivisions within the type I clade: the types IA, IB, and IC. Higher-resolution methods provided additional differentiation of IA strains into types IA1 and IA2 13. So P. acnes is subdivided into six phylotypes: IA1, IA2, IB, IC, II, and III 15. Multi-locus sequence typing and single-locus sequence typing (SLST) identified further subgroups among phylotypes, called clonal complexes.
The P. acnes phylogroups have been associated with specific diseases and distinct virulence, biochemical, and immunological characteristics that will be discussed in the following section.
P. acnes in acne: the role of distinct phylotypes
P. acnes has been regarded as an important member of the cutaneous microbiota. It has been linked to the inflammatory skin condition acne vulgaris for more than 100 years. The four major pathophysiological factors implicated in the pathogenesis of acne include the role of P. acnes, increased seborrhea, hyperkeratinization of the pilosebaceous unit, and inflammation 16.
P. acnes colonization of the skin is necessary but not sufficient for the establishment of acne pathology. P. acnes dominates the microbiota of pilosebaceous units and accounts for 87% of clones in patients with acne and in individuals without acne 17. P. acnes has been reported to represent more than 30% of the facial microbiota in patients with acne 1, but another study of 55 patients with facial acne reported lower rates (less than 2%) of sampled bacteria 18. These results should be interpreted with caution given the role of the sampling methodologies used. Different sampling methods, such as swab, scrape, cyanoacrylate gel biopsy, and needle biopsy, are used to collect skin bacteria for testing. Each technique targets different skin structures and anatomical sites. The sampling of superficial and intra-stratum corneum bacterial populations is considered quite straightforward. However, the sampling of hair follicle populations has proven more difficult and a skin biopsy may be required. The use of tape-stripping for hair follicle sampling in acne can be misleading, as multiple superficial and intra-stratum corneum microbial populations are sampled but bacteria may reside in a deeper part of the hair follicle 19. This area is inaccessible with the above-mentioned sampling methodologies, providing very little material from inside the hair follicle and making it difficult to standardize 8. P. acnes sampling with bacterial culture may not reliably distinguish between P. acnes populations with possibly variable pathogenic potential 20.
Although there is no quantitative difference of P. acnes in the skin of patients with acne compared with controls 17, its phylogenic groups display distinct genetic and phenotypic characteristics in acne 10 and different phylotypes are known to induce distinct immune responses in acne 12. Different P. acnes types have been isolated from acne vulgaris, and the type III strains have been associated with progressive macular hypomelanosis, underscoring the importance of genetic division of P. acnes and suggesting the involvement of specific P. acnes phylotypes in the pathophysiology of acne 21.
Focusing on acne, the typing of P. acnes isolates has revealed distinct profiles in patients with acne ( Table 1) 15, 22, 23. A case-control study reported loss of P. acnes phylotype diversity in patients with severe inflammatory acne, and there was a predominance of phylotype IA1 compared with healthy controls. With additional molecular typing methods, the SLST type A1 was predominant in the acne group 15. On the other hand, a small study in 29 patients with mild acne compared with 34 patients with severe acne did not reveal the association of a specific P. acnes phylotype with the severity of acne, and phylotype IA1 and SLST type A1 were the predominant types in both groups 22.
Table 1. The potential role of distinct Propionibacterium acnes types in patients with acne.
| Study | P. acnes phylotypes | SLST types | Acne patients
studied |
Proposed roles |
|---|---|---|---|---|
| Dagnelie et al. 15 (2018) | Predominance of phylotypes
IA1 (84.4%) and II |
A1 | 24 patients with
severe acne of face and back versus 12 controls |
- Decrease of phylotype
diversity may be due to hyperseborrhea and qualitative sebum modifications in acne - Loss of diversity may activate innate immunity and trigger inflammatory acne |
| Nakase et al. 23 (2017) | - Isolates of clade A
(60.3%) predominated - Strains of clade F more frequent in severe acne (40%) compared with mild acne (23.3%) - Phylogenetic type A5 most frequent (29.4%) |
113 patients with
acne |
||
| Paugam et al. 22 (2017) | - Phylotype IA1 the most
frequent in mild acne (55.2%) and in severe acne (67.6%) - No difference of phylotypes between mild and severe acne groups |
A1 predominance with no
difference between acne groups |
29 patients with
mild acne and 34 patients with severe acne |
- In a small number of
patients, the severity of acne was not associated with a specific P. acnes group |
SLST, single-locus sequence type.
The role of the P. acnes biofilm
Bacteria may exist as biofilms in their natural habitat. A biofilm is defined as a microbial aggregate embedded in extracellular matrix which protects cells from harmful conditions in the environment and facilitates escaping from host surveillance mechanisms. Burkhart and Burkhart (2007) suggested that P. acnes biofilm may penetrate into the sebum and act like an adhesive, leading to the increased cohesiveness of corneocytes and the formation of microcomedones 24. Additionally, a high availability of sebum, a nutritional substrate for P. acnes, may result in an increased proportion of metabolically active bacteria and contribute to a pro-inflammatory phenotype of the P. acnes biofilm. This may explain the acne flares in adolescence, when increased hormone and sebum production are dominant 25.
In vitro growth of P. acnes biofilms demonstrated the composition of the extracellular polymeric substance (EPS) matrix of P. acnes biofilm with extracellular DNA, proteins, and glycosyl residues as well as upregulated mRNA expression of Christie–Atkins–Munch-Peterson (CAMP) factor 1 26.
Only one study has investigated P. acnes biofilm in acne patients compared with controls. A case-control study in facial biopsies showed that follicular P. acnes was more frequently demonstrated in samples from acne patients compared with matching controls. Furthermore, P. acnes from acne samples more frequently formed biofilms in the sebaceous follicles compared with control samples 20. Although similar biofilms have also been observed in skin diseases other than acne, such as folliculitis, folliculitis decalvans, and hidradenitis suppurativa, these were seen in terminal hair follicles 27– 29.
Target activities of P. acnes in acne
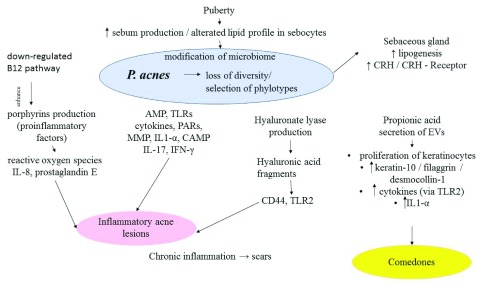
As P. acnes modulates the differentiation of keratinocytes and increases local inflammation, it is regarded as an etiological agent of both the microcomedone (a structure invisible to the naked eye) in the early stages of acne and of the inflammatory acne lesions 30. The different target activities of P. acnes in acne are summarized in Figure 1.
Figure 1. Propionibacterium acnes: loss of diversity, selection of phylotypes, and its different target activities in acne.
P. acnes induces the production of AMP, TLRs, cytokines, PARs, MMP, IL-1a, CAMP, hyaluronate lyase, and porphyrins, resulting in the formation of inflammatory acne lesions. It modulates the differentiation of keratinocytes by inducing keratin 10, filaggrin, and desmocollin 1 expression. It stimulates the sebaceous glands and sebum synthesis via the CRH/CRH receptor pathway. AMP, antimicrobial peptide; CAMP, Christie–Atkins–Munch-Peterson; CRH, corticotropin-releasing hormone; EV, extracellular vesicle; IFNγ, interferon-gamma; IL, interleukin; MMP, matrix metalloproteinase; PAR, protease-activated receptor; TLR, Toll-like receptor.
P. acnes shows complex interactions with key events implicated in the pathogenesis of acne. It interacts with the innate immunity, including Toll-like receptors (TLRs), antimicrobial peptides (AMPs), protease-activated receptors (PARs), and matrix metalloproteinase (MMP), and upregulates the secretion of pro-inflammatory cytokines, including interleukin-1a (IL-1a), IL-1β, IL-6, IL-8, IL-12, tumor necrosis factor-alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF), by human keratinocytes, sebocytes, and macrophages 16, 31. Moreover, the production of AMP (LL-37, β-defensin 2), cytokines (IL-1α), and MMP was associated with the increased expression of the G-protein-coupled receptor PAR-2 in keratinocytes from acne-affected skin 32. P. acnes extracts are directly able to modulate the differentiation of keratinocytes by inducing b1, a3, a6s, aVb6 integrin expression, and filaggrin expression on keratinocytes, changes seen in the development of acne lesions 33. Interplay between P. acnes and macrophages in the perifollicular dermis can induce IL-1β 32, which in turn may further activate the NLRP3-inflammasome pathway in antigen-presenting cells and myeloid cells 19. Recent in vitro studies have revealed that P. acnes can induce IL-17 production by T cells (Th1/Th17) 31. Clusters of CD3 + cells have been demonstrated in the vicinity of the P. acnes-positive comedones, cells that were absent from the surrounding inflamed lesions. These findings in early acne stage further support the role of P. acnes in the initiation of inflammation 34. P. acnes releases extracellular vesicles (EVs) which also induce cellular responses via TLR2 signal cascades. These P. acnes-derived EVs induce IL-8 and GM-CSF and decrease epidermal keratin-10 and desmocollin, contributing to the development of acne lesions 35.
Yu et al. showed that acne-associated P. acnes phylotypes induced distinct cytokine patterns in vitro in peripheral blood mononuclear cells from healthy individuals, including higher levels of inflammatory interferon-gamma (IFN-γ) and IL-17, suggesting a mechanism of inducing acne via both Th1 and Th17 pathways 12. On the other hand, P. acnes phylotypes associated with healthy skin induced higher levels of IL-10. Moreover, there were different expression patterns between phylotypes; acne-associated phylotypes showed higher expression of an adhesion protein, whereas phylotypes associated with healthy skin showed higher expression of a cell surface hydrolase. These identified immune responses and proteomes of different P. acnes strains provided deeper insight into how specific P. acnes phylotypes influence the pathogenesis of acne 12. In a follow-up study, Agak et al. reported differential effects of acne-affected skin- and healthy skin-associated lineages of P. acnes on CD4 + T-cell and Th17 cell responses and suggested that P. acnes strains express different antigenic components on their surface structure, possibly explaining the higher IL-17 levels induced in acne-affected skin-associated P. acnes strains 36.
Furthermore, P. acnes has been implicated in lipogenesis and sebum production, as it stimulates the sebaceous glands and sebum synthesis via the corticotropin-releasing hormone (CRH)/CRH receptor pathway 37. Expression of the complete CRH system has been described in acne; a study in biopsies from the facial skin of patients with acne reported a stronger expression of CRH in sebocytes of acne-involved skin compared with non-involved and normal skin 38. In particular, CRH augments the synthesis of sebaceous lipids and induces IL-6 and IL-8 release by sebocytes, mediated by the CRH receptor 39.
A recent study reported that a secretory CAMP factor of P. acnes has a role in its cytotoxicity, as mutations of CAMP diminished P. acnes colonization and inflammation in mice 40. P. acnes CAMP factor can induce cell death of sebocytes in sebaceous glands, resulting in amplification of the inflammation response 41. In addition, a study reported that the P. acnes surface protein CAMP factor 1 stimulated keratinocytes in vitro by interacting directly with TLR2 42.
Porphyrins are secreted by P. acnes and can generate reactive oxygen species that induce inflammation in keratinocytes and result in acne lesions. Johnson et al. showed that acne-associated P. acnes strains produced more porphyrins than health-associated strains isolated from individuals and that vitamin B 12 supplementation significantly increased porphyrin production in the acne-associated strains only 43. Another study showed that the P. acnes vitamin B 12 biosynthesis pathway was downregulated in acne patients compared with healthy individuals. Furthermore, intramuscular vitamin B 12 supplementation repressed its own biosynthesis in P. acnes and promoted increased porphyrin production in healthy subjects 44.
Hyaluronic acid (HA) lyase is a ubiquitous enzyme with two distinct variants in the P. acnes population that differ in their ability to degrade HA and could be involved in the pro-inflammatory responses seen in acne. One variant is present in P. acnes type IA strains and is associated with acne, and the other one is in type IB and II strains and is associated mainly with soft and deep tissue infections. HA fragments interact with cell surface receptors such as CD44 and TLR2 and induce the inflammatory response 45.
Apart from its target activities in acne, P. acnes has an intriguing role in the homeostasis of the skin’s microbiome, interacting with other cutaneous microorganisms such as Staphylococcus epidermidis, Streptococcus pyogenes, and Pseudomonas species . In the microbiome of healthy skin, S. epidermidis may limit the overcolonization with P. acnes strains and reduce P. acnes-induced IL-6 and TNF-α production by keratinocytes. On the other hand, P. acnes may limit the proliferation of S. aureus and S. pyogenes by promoting triglyceride hydrolysis and propionic acid secretion. As a result, an acidic pH is maintained in the pilosebaceous follicle. A change of the microbiome composition may lead to a disturbed skin barrier and inflammation. In acne, a modified profile of P. acnes is noticed; different phylotypes differ between patients with and without acne 46. Hall et al. showed in cutaneous samples that when P. acnes was present, Pseudomonas species typically were not, and vice versa 47. Interestingly, antibiotic treatment for acne that decreases P. acnes colonization on the skin may also result in Gram-negative folliculitis caused by Pseudomonas 48. Megyeri et al. recently proposed that P. acnes strains may be implicated in antimicrobial defense pathways by triggering a local increase in the autophagic activity of keratinocytes 49.
P. acnes resistance to antibiotics
The antibiotic resistance of P. acnes is a worldwide problem, and rates of resistance increased from 20% in 1979 to 64% in 2000; rates for tetracyclines were lower compared with rates for clindamycin and erythromycin 50. A study of 664 patients in the UK, Spain, Italy, Greece, Sweden, and Hungary reported that the prevalence of P. acnes resistance rates ranged from 50.8% to 93.6% to any antibiotic (tetracycline, macrolide, lincosamide, and streptogramin B) and that all included dermatologists who specialized in treating acne were colonized with resistant Propionibacteria 51.
A difference in the in vitro antibiotic susceptibility patterns of P. acnes among different countries is recognized 52– 56. A possible explanation is the fact that there are different antibiotic-prescribing habits among the countries and even different concomitant topical agents used. In studies from Korea, the UK, Colombia, Mexico, Hong Kong, Hungary, and Spain, P. acnes antibiotic resistance was noted in 36.7%, 55.5%, 40%, 75.5%, 54.7%, 51%, and 94% of patients with acne, respectively ( Table 2) 57.
Table 2. Different rates of Propionibacterium acnes antibiotic resistance in acne patients in different countries.
| Study | Country, date | Patients with
acne, number |
Any antibiotic
resistance, n (%) |
Clindamycin
resistance, n (%) |
Erythromycin
resistance, n (%) |
Azithromycin
resistance, n (%) |
Oxytetracycline
resistance, n (%) |
Doxycyline
resistance, n (%) |
Minocycline
resistance, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Moon 59 | Korea, 2011 | 100
(30 P. acnes strains isolated) |
11 (36.7) | 9 (30) | 8 (26.7) | NS | 1 (3.3) | 2 (6.7) | 3 (10) |
| Coates 50 | UK, 1991–2000 | 4,274 | 34.5% in 1991
55.5% in 2000 |
1997: ~48% | 1997: 57.6% | NR | 1991: 12.5%
1998: 29.9% |
NR | NR |
| 1997 | 72 | 72 (100) | 65 (90.3) | 68 (94.4) | NR | 38 (52.8) | NR | NR | |
| Mendoza 52 | Colombia, 2005,
2006 |
100 | 40% | 15% | 35% | NS | 8% | 9% | 1% |
| Gonzalez 53 | Northern Mexico,
2010 |
49 | 37 (75.5) | 36% | 46% | 82% | 14% | 20% | 0 |
| Luk 54 | Hong Kong, 2009 | 111
( P. acnes isolated from 86 patients) |
47 (54.7) | (53.5) | 18 (20.9) | NS | 14 (16.3) | 14 (16.3) | 14 (16.3) |
| Abdel-Fattah 55 | Egypt, 2011–2012 | 115
( P. acnes isolated from 98 patients) |
NR | 65 (66.3) | 48 (49) | 5 (5.1) | 18 (18.4) | 6 (6.1) | NS |
| Ross 51 | 1999–2001 | 622 | |||||||
| UK | NR | 50% | 50% | NS | 26% | NR | 0 | ||
| Greece | NR | 75% | 75% | NS | 7% | NR | 0 | ||
| Hungary | 51% | 45% | 45% | NS | 0 | NR | 0 | ||
| Italy | NR | 58% | 58% | NS | 0 | NR | 0 | ||
| Spain | 94% | 91% | 91% | NS | 5% | NR | 0 | ||
| Sweden | NR | 45% | 45% | NS | 15% | NR | 0 | ||
| Dumont 56 | France, 2010 | 273 | NR | NS | 205 (75.1) | NS | 26 (9.5) | 26 a | NS |
Sampling only from closed comedones. aOnly the strains resistant to tetracycline (26 patients) were tested with doxycycline. NR, not reported; NS, not studied. From Dessinioti and Katsambas 52. Reprinted with permission from Elsevier.
Macrolide-resistant P. acnes is frequently isolated from patients with acne vulgaris, and the majority of resistant isolates have the 23S rRNA mutation 58. Long-term, low-concentration exposure to macrolides increased the resistance of P. acnes 59.
Implications of antimicrobial resistance
The effect of acne treatments may be influenced by the presence of antibiotic‐resistant P. acnes 34. The widespread use of antibiotics to treat acne may result in the development of P. acnes strains with cross-resistance to different antibiotics and have possible implications in acne and other diseases where P. acnes may be the causative pathogen 51.
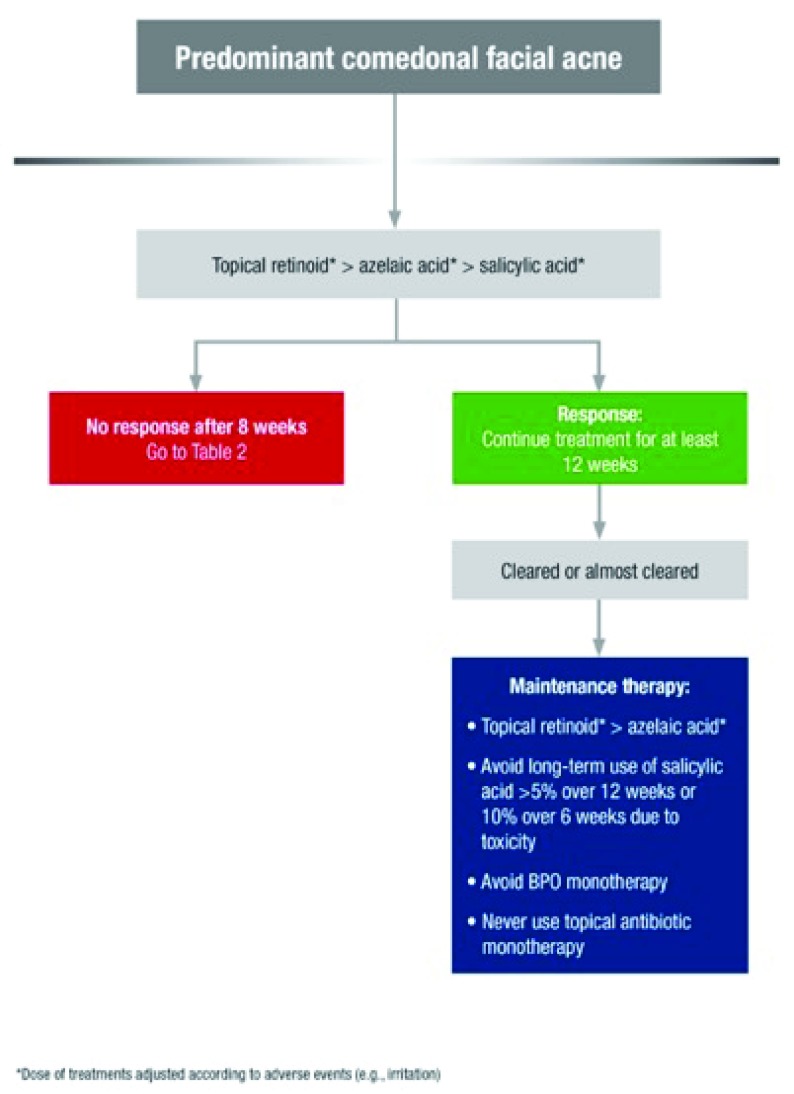
Given the frequent use of antibiotics for acne treatment, recommendations on acne treatments aim to limit the risk of antimicrobial resistance of P. acnes and other bacteria 60– 62. As a general rule, the long-term use of topical antibiotics in monotherapies should be avoided, as they may lead to an increase in antibiotic-resistant P. acnes 63. Antibiotics are not indicated for predominantly comedonal facial acne ( Figure 2). Topical antibiotics, especially as a fixed combination with benzyl peroxide (BPO) or retinoid, may be indicated for predominantly papulopustular inflammatory facial acne. Topical fixed-dose combination treatments present the advantage of a quicker onset of action and may limit the risk of antimicrobial resistance associated with antibiotic monotherapy. If the use of topical antibiotics is indicated, BPO or a topical retinoid should be added with the aim to reduce the risk of antimicrobial resistance 64, 65. Topical antibiotics are not suitable for maintenance acne therapy; instead, topical retinoids are preferred, and BPO is added for an antimicrobial effect if needed 60. BPO further exhibits antimicrobial activity against P. acnes. Azelaic acid inhibits the synthesis of cellular protein in aerobic and anaerobic microorganisms, such as P. acnes, and does not induce bacterial resistance 61.
Figure 2. Treatment algorithm for predominant comedonal facial acne.
*Dose of treatments adjusted according to adverse events (for example, irritation). BPO, benzyl peroxide. From Gollnick et al. 61. Reprinted with permission from John Wiley & Sons.
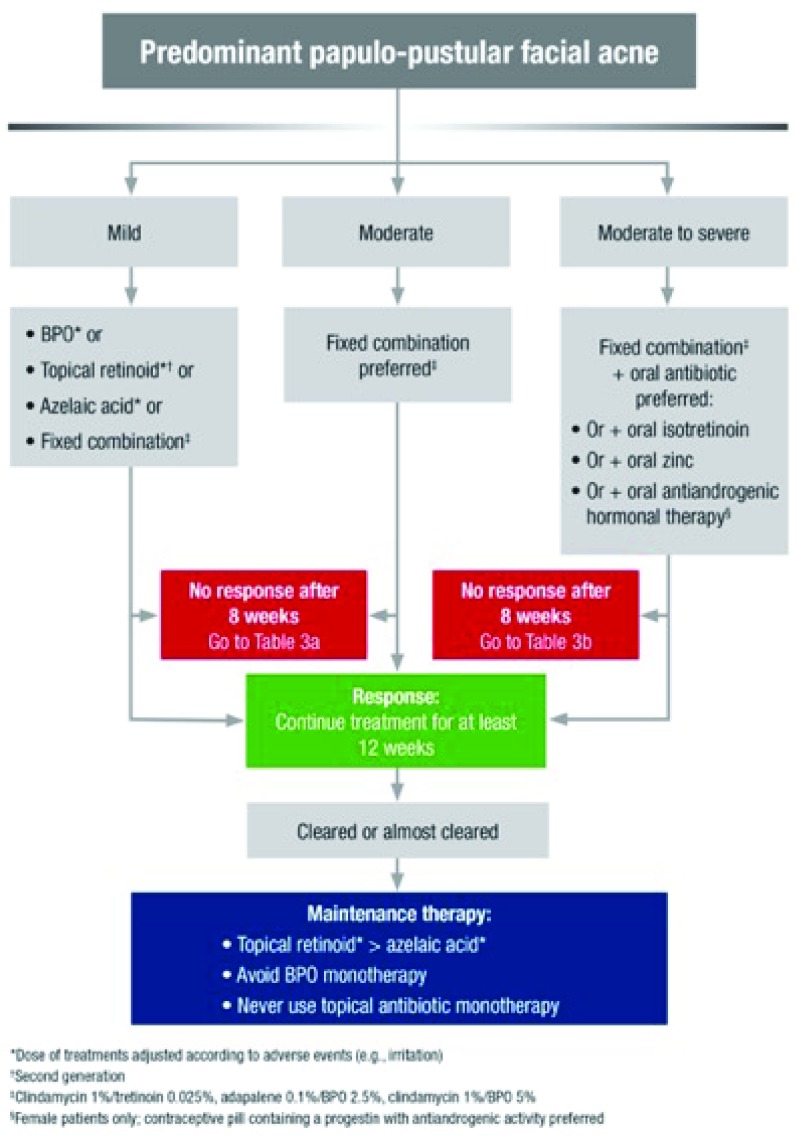
Systemic antibiotics for acne, in combination with a topical agent (BPO, retinoid, or azelaic acid), are indicated for moderate to severe inflammatory papulopustular acne and acne affecting the trunk ( Figure 3). The duration of the oral antibiotic regimen should not exceed 3 months. Oral tetracyclines (doxycycline or lymecycline) are the antibiotic of first choice for acne when a systemic antibiotic is considered 61, 62, 66. Treatment with oral macrolides should be avoided because of high rates of antimicrobial resistance reported for P. acnes worldwide 51.
Figure 3. Treatment algorithm for predominant papulopustular facial acne.
*Dose of treatments adjusted according to adverse events (for example, irritation). †Second generation. ‡Clindamycin 1%/tretinoin 0.025% (not with oral antibiotic), adapalene 0.1%/BPO 2.5%, clindamycin 1%/BPO 5% (not with oral antibiotic). §Female patients only; contraceptive pill containing a progestin with anti-androgenic activity preferred. BPO, benzyl peroxide. From Gollnick et al. 61. Reprinted with permission from John Wiley & Sons.
A study of 56 patients with cystic or severe acne vulgaris treated with oral isotretinoin (1 mg/kg per day) reported that the colonization of the skin with P. acnes was modified; oral isotretinoin, though not an antibiotic, correlated with a reduction in the numbers of P. acnes, including isolates resistant to antibiotics, that were cultured from the cheeks, but there was no effect in P. acnes sampled from other anatomic sites 67.
Emerging off-label therapeutic modalities for acne, such as topical photodynamic therapy (PDT) with photoactivation of aminolaevulinic acid (ALA) or methyl aminolaevulinic acid (MAL), target P . acnes, underlying its role in the pathogenesis of acne 68. The mode of action of PDT includes not only photodynamic damage of the sebaceous gland but also the photodestruction of P. acnes 69, 70.
The potential for vaccination against P. acnes was investigated, and relevant studies initially stopped in 2011, as effectiveness in humans with acne was not shown 71. Interestingly, a recent study reported the efficacy of CAMP factor antibodies in the neutralization of the acne inflammatory response in ex vivo acne models; the incubation of ex vivo acne skin explants from acne patients with monoclonal antibodies (mAbs) to the P. acnes-secreted CAMP factor diminished the amounts of pro-inflammatory cytokines IL-8 and IL-1β 40. The authors also proposed that the injection of the mAb to CAMP factor directly into acne lesions may prove to be beneficial 40.
Conclusions
Significant progress has been made in understanding the role of P. acnes in the pathogenesis of acne. Although there is no quantitative difference of P. acnes among patients with acne and healthy individuals, P. acnes phylogenic groups may display distinct genetic and phenotypic characteristics. Different phylotypes may induce distinct immune responses, and the P. acnes biofilm has been reported more frequently in patients with acne. Furthermore, P. acnes plays important roles in the homeostasis of the skin’s microbiome, interacting with other cutaneous microorganisms such as S. epidermidis, S. pyogenes, and Pseudomonas species. Non-antibiotic approaches targeting P. acnes without inducing antibiotic resistance may improve patient outcomes in acne while avoiding public health issues.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Andrew McDowell, Northern Ireland Centre for Stratified Medicine, Biomedical Sciences Research Institute, Altnagelvin Area Hospital, University of Ulster, Londonderry, UK
Christine Roques, Laboratoire de Génie Chimique, Faculty of Pharmacy, Université de Toulouse, Université Paul Sabatier, Toulouse, France
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Grice EA, Kong HH, Conlan S, et al. : Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–2. 10.1126/science.1171700 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Davidsson S, Mölling P, Rider JR, et al. : Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect Agents Cancer. 2016;11:26. 10.1186/s13027-016-0074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schupp JC, Tchaptchet S, Lützen N, et al. : Immune response to Propionibacterium acnes in patients with sarcoidosis-- in vivo and in vitro. BMC Pulm Med. 2015;15:75. 10.1186/s12890-015-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamamoto R, Miyagawa S, Hagiya H, et al. : Silent Native-valve Endocarditis Caused by Propionibacterium acnes. Intern Med. 2018;57(16):2417–20. 10.2169/internalmedicine.9833-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Achermann Y, Goldstein EJC, Coenye T, et al. : Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–40. 10.1128/CMR.00092-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thiboutot DM, Layton AM, Anne Eady E: IL-17: a key player in the P. acnes inflammatory cascade? J Invest Dermatol. 2014;134(2):307–10. 10.1038/jid.2013.400 [DOI] [PubMed] [Google Scholar]
- 7. Kalis C, Gumenscheimer M, Freudenberg N, et al. : Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J Immunol. 2005;174(7):4295–300. 10.4049/jimmunol.174.7.4295 [DOI] [PubMed] [Google Scholar]
- 8. Omer H, McDowell A, Alexeyev OA: Understanding the role of Propionibacterium acnes in acne vulgaris: The critical importance of skin sampling methodologies. Clin Dermatol. 2017;35(2):118–29. 10.1016/j.clindermatol.2016.10.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Liu J, Cheng A, Bangayan NJ, et al. : Draft Genome Sequences of Propionibacterium acnes Type Strain ATCC6919 and Antibiotic-Resistant Strain HL411PA1. Genome Announc. 2014;2(4): pii: e00740-14. 10.1128/genomeA.00740-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dréno B, Pécastaings S, Corvec S, et al. : Cutibacterium acnes ( Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32(Suppl 2):5–14. 10.1111/jdv.15043 [DOI] [PubMed] [Google Scholar]
- 11. Alexeyev OA, Dekio I, Layton AM, et al. : Why we continue to use the name Propionibacterium acnes. Br J Dermatol. 2018;179(5):1227. 10.1111/bjd.17085 [DOI] [PubMed] [Google Scholar]
- 12. Yu Y, Champer J, Agak GW, et al. : Different Propionibacterium acnes Phylotypes Induce Distinct Immune Responses and Express Unique Surface and Secreted Proteomes. J Invest Dermatol. 2016;136(11):2221–8. 10.1016/j.jid.2016.06.615 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. McDowell A: Over a Decade of recA and tly Gene Sequence Typing of the Skin Bacterium Propionibacterium acnes: What Have We Learnt? Microorganisms. 2017;6(1): pii: E1. 10.3390/microorganisms6010001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Dekio I, Culak R, Misra R, et al. : Dissecting the taxonomic heterogeneity within Propionibacterium acnes: proposal for Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes subsp. elongatum subsp. nov. Int J Syst Evol Microbiol. 2015;65(12):4776–87. 10.1099/ijsem.0.000648 [DOI] [PubMed] [Google Scholar]
- 15. Dagnelie MA, Corvec S, Saint-Jean M, et al. : Decrease in Diversity of Propionibacterium acnes Phylotypes in Patients with Severe Acne on the Back. Acta Derm Venereol. 2018;98(2):262–7. 10.2340/00015555-2847 [DOI] [PubMed] [Google Scholar]
- 16. Dessinioti C, Katsambas AD: The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):2–7. 10.1016/j.clindermatol.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 17. Fitz-Gibbon S, Tomida S, Chiu BH, et al. : Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133(9):2152–60. 10.1038/jid.2013.21 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Dreno B, Martin R, Moyal D, et al. : Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp Dermatol. 2017;26(9):798–803. 10.1111/exd.13296 [DOI] [PubMed] [Google Scholar]
- 19. Alexeyev OA: Bacterial landscape of human skin: seeing the forest for the trees. Exp Dermatol. 2013;22(7):443–6. 10.1111/exd.12160 [DOI] [PubMed] [Google Scholar]
- 20. Jahns AC, Lundskog B, Ganceviciene R, et al. : An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol. 2012;167(1):50–8. 10.1111/j.1365-2133.2012.10897.x [DOI] [PubMed] [Google Scholar]
- 21. Barnard E, Liu J, Yankova E, et al. : Strains of the Propionibacterium acnes type III lineage are associated with the skin condition progressive macular hypomelanosis. Sci Rep. 2016;6: 31968. 10.1038/srep31968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paugam C, Corvec S, Saint-Jean M, et al. : Propionibacterium acnes phylotypes and acne severity: an observational prospective study. J Eur Acad Dermatol Venereol. 2017;31(9):e398–e399. 10.1111/jdv.14206 [DOI] [PubMed] [Google Scholar]
- 23. Nakase K, Hayashi N, Akiyama Y, et al. : Antimicrobial susceptibility and phylogenetic analysis of Propionibacterium acnes isolated from acne patients in Japan between 2013 and 2015. J Dermatol. 2017;44(11):1248–54. 10.1111/1346-8138.13913 [DOI] [PubMed] [Google Scholar]
- 24. Burkhart CG, Burkhart CN: Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57(4):722–4. 10.1016/j.jaad.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 25. Sardana K, Gupta T, Garg VK, et al. : Antibiotic resistance to Propionobacterium acnes: worldwide scenario, diagnosis and management. Expert Rev Anti Infect Ther. 2015;13(7):883–96. 10.1586/14787210.2015.1040765 [DOI] [PubMed] [Google Scholar]
- 26. Jahns AC, Eilers H, Alexeyev OA: Transcriptomic analysis of Propionibacterium acnes biofilms in vitro. Anaerobe. 2016;42:111–8. 10.1016/j.anaerobe.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 27. Jahns AC, Lundskog B, Berg J, et al. : Microbiology of folliculitis: a histological study of 39 cases. APMIS. 2014;122(1):25–32. 10.1111/apm.12103 [DOI] [PubMed] [Google Scholar]
- 28. Jahns AC, Lundskog B, Nosek D, et al. : Microbiology of folliculitis decalvans: a histological study of 37 patients. J Eur Acad Dermatol Venereol. 2015;29(5):1025–6. 10.1111/jdv.12448 [DOI] [PubMed] [Google Scholar]
- 29. Jahns AC, Killasli H, Nosek D, et al. : Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122(9):804–9. 10.1111/apm.12220 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Isard O, Knol AC, Ariès MF, et al. : Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J Invest Dermatol. 2011;131(1):59–66. 10.1038/jid.2010.281 [DOI] [PubMed] [Google Scholar]
- 31. Kistowska M, Gehrke S, Jankovic D, et al. : IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134(3):677–85. 10.1038/jid.2013.438 [DOI] [PubMed] [Google Scholar]
- 32. Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, et al. : Acne vulgaris. Nat Rev Dis Primers. 2015;1: 15029. 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- 33. Jarrousse V, Castex-Rizzi N, Khammari A, et al. : Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytes. Arch Dermatol Res. 2007;299(9):441–7. 10.1007/s00403-007-0774-5 [DOI] [PubMed] [Google Scholar]
- 34. Alexeyev OA, Lundskog B, Ganceviciene R, et al. : Pattern of tissue invasion by Propionibacterium acnes in acne vulgaris. J Dermatol Sci. 2012;67(1):63–6. 10.1016/j.jdermsci.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 35. Choi EJ, Lee HG, Bae IH, et al. : Propionibacterium acnes-Derived Extracellular Vesicles Promote Acne-Like Phenotypes in Human Epidermis. J Invest Dermatol. 2018;138(6):1371–9. 10.1016/j.jid.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 36. Agak GW, Kao S, Ouyang K, et al. : Phenotype and Antimicrobial Activity of Th17 Cells Induced by Propionibacterium acnes Strains Associated with Healthy and Acne Skin. J Invest Dermatol. 2018;138(2):316–24. 10.1016/j.jid.2017.07.842 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Zouboulis CC: Propionibacterium acnes and sebaceous lipogenesis: a love-hate relationship? J Invest Dermatol. 2009;129(9):2093–6. 10.1038/jid.2009.190 [DOI] [PubMed] [Google Scholar]
- 38. Ganceviciene R, Graziene V, Fimmel S, et al. : Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160(2):345–52. 10.1111/j.1365-2133.2008.08959.x [DOI] [PubMed] [Google Scholar]
- 39. Krause K, Schnitger A, Fimmel S, et al. : Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm Metab Res. 2007;39(2):166–70. 10.1055/s-2007-961811 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Hata TR, Tong YL, et al. : The Anti-Inflammatory Activities of Propionibacterium acnes CAMP Factor-Targeted Acne Vaccines. J Invest Dermatol. 2018;138(11):2355–64. 10.1016/j.jid.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 41. Liu PF, Nakatsuji T, Zhu W, et al. : Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine. 2011;29(17):3230–8. 10.1016/j.vaccine.2011.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lheure C, Grange PA, Ollagnier G, et al. : TLR-2 Recognizes Propionibacterium acnes CAMP Factor 1 from Highly Inflammatory Strains. PLoS One. 2016;11(11):e0167237. 10.1371/journal.pone.0167237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson T, Kang D, Barnard E, et al. : Strain-Level Differences in Porphyrin Production and Regulation in Propionibacterium acnes Elucidate Disease Associations. mSphere. 2016;1(1): pii: e00023-15. 10.1128/mSphere.00023-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang D, Shi B, Erfe MC, et al. : Vitamin B 12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci Transl Med. 2015;7(293):293ra103. 10.1126/scitranslmed.aab2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nazipi S, Stødkilde-Jørgensen K, Scavenius C, et al. : The Skin Bacterium Propionibacterium acnes Employs Two Variants of Hyaluronate Lyase with Distinct Properties. Microorganisms. 2017;5(3): pii: E57. 10.3390/microorganisms5030057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dréno B: What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31 Suppl 5:8–12. 10.1111/jdv.14374 [DOI] [PubMed] [Google Scholar]
- 47. Hall JB, Cong Z, Imamura-Kawasawa Y, et al. : Isolation and Identification of the Follicular Microbiome: Implications for Acne Research. J Invest Dermatol. 2018;138(9):2033–40. 10.1016/j.jid.2018.02.038 [DOI] [PubMed] [Google Scholar]
- 48. Böni R, Nehrhoff B: Treatment of gram-negative folliculitis in patients with acne. Am J Clin Dermatol. 2003;4(4):273–6. 10.2165/00128071-200304040-00005 [DOI] [PubMed] [Google Scholar]
- 49. Megyeri K, Orosz L, Bolla S, et al. : Propionibacterium acnes Induces Autophagy in Keratinocytes: Involvement of Multiple Mechanisms. J Invest Dermatol. 2018;138(4):750–9. 10.1016/j.jid.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 50. Coates P, Vyakrnam S, Eady EA, et al. : Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol. 2002;146(5):840–8. 10.1046/j.1365-2133.2002.04690.x [DOI] [PubMed] [Google Scholar]
- 51. Ross JI, Snelling AM, Carnegie E, et al. : Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467–78. 10.1046/j.1365-2133.2003.05067.x [DOI] [PubMed] [Google Scholar]
- 52. Mendoza N, Hernandez PO, Tyring SK, et al. : Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52(6):688–92. 10.1111/j.1365-4632.2011.05403.x [DOI] [PubMed] [Google Scholar]
- 53. González R, Welsh O, Ocampo J, et al. : In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol. 2010;49(9):1003–7. 10.1111/j.1365-4632.2010.04506.x [DOI] [PubMed] [Google Scholar]
- 54. Luk NM, Hui M, Lee HC, et al. : Antibiotic-resistant Propionibacterium acnes among acne patients in a regional skin centre in Hong Kong. J Eur Acad Dermatol Venereol. 2013;27(1):31–6. 10.1111/j.1468-3083.2011.04351.x [DOI] [PubMed] [Google Scholar]
- 55. Abdel Fattah NS, Darwish YW: In vitro antibiotic susceptibility patterns of Propionibacterium acnes isolated from acne patients: an Egyptian university hospital-based study. J Eur Acad Dermatol Venereol. 2013;27(12):1546–51. 10.1111/jdv.12057 [DOI] [PubMed] [Google Scholar]
- 56. Dumont-Wallon G, Moyse D, Blouin E, et al. : Bacterial resistance in French acne patients. Int J Dermatol. 2010;49(3):283–8. 10.1111/j.1365-4632.2009.04270.x [DOI] [PubMed] [Google Scholar]
- 57. Dessinioti C, Katsambas A: Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 2017;35(2):163–7. 10.1016/j.clindermatol.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 58. Nakase K, Okamoto Y, Aoki S, et al. : Long-term administration of oral macrolides for acne treatment increases macrolide-resistant Propionibacterium acnes. J Dermatol. 2018;45(3):340–3. 10.1111/1346-8138.14178 [DOI] [PubMed] [Google Scholar]
- 59. Moon SH, Roh HS, Kim YH, et al. : Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012;39(10):833–7. 10.1111/j.1346-8138.2012.01626.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Leccia MT, Auffret N, Poli F, et al. : Topical acne treatments in Europe and the issue of antimicrobial resistance. J Eur Acad Dermatol Venereol. 2015;29(8):1485–92. 10.1111/jdv.12989 [DOI] [PubMed] [Google Scholar]
- 61. Gollnick HP, Bettoli V, Lambert J, et al. : A consensus-based practical and daily guide for the treatment of acne patients J Eur Acad Dermatol Venereol. 2016;30(9):1480–90. 10.1111/jdv.13675 [DOI] [PubMed] [Google Scholar]
- 62. Nast A, Dréno B, Bettoli V, et al. : European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–8. 10.1111/jdv.13776 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Leyden JJ, Wortzman M, Baldwin EK: Antibiotic-resistant Propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis. 2008;82(6):417–21. [PubMed] [Google Scholar]
- 64. Feneran AN, Kaufman WS, Dabade TS, et al. : Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79–92. 10.2147/CCID.S13873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dréno B, Lambert J, Bettoli V: Are retinoid/antibiotic fixed-dose combination acne treatments associated with antibiotic resistance? Eur J Dermatol. 2016;26(1):90–1. 10.1684/ejd.2015.2654 [DOI] [PubMed] [Google Scholar]
- 66. Thiboutot D, Gollnick H, Bettoli V, et al. : New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–50. 10.1016/j.jaad.2009.01.019 [DOI] [PubMed] [Google Scholar]
- 67. Ryan-Kewley AE, Williams DR, Hepburn N, et al. : Non-antibiotic Isotretinoin Treatment Differentially Controls Propionibacterium acnes on Skin of Acne Patients. Front Microbiol. 2017;8:1381. 10.3389/fmicb.2017.01381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dessinioti C, Masouri S, Drakaki E, et al. : Short-contact, low-dose methyl aminolaevulinate photodynamic therapy for acne vulgaris. Br J Dermatol. 2016;175(1):215. 10.1111/bjd.14460 [DOI] [PubMed] [Google Scholar]
- 69. Sakamoto FH, Lopes JD, Anderson RR: Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part I. Acne vulgaris: when and why consider photodynamic therapy? J Am Acad Dermatol. 2010;63(2):183–93; quiz 193-4. 10.1016/j.jaad.2009.09.056 [DOI] [PubMed] [Google Scholar]
- 70. Hongcharu W, Taylor CR, Chang Y, et al. : Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000;115(2):183–92. 10.1046/j.1523-1747.2000.00046.x [DOI] [PubMed] [Google Scholar]
- 71. Zouboulis CC, Dessinioti C, Tsatsou F, et al. : Anti-acne drugs in phase 1 and 2 clinical trials. Expert Opin Investig Drugs. 2017;26(7):813–23. 10.1080/13543784.2017.1337745 [DOI] [PubMed] [Google Scholar]