Abstract
Background: Good Clinical Laboratory Practice (GCLP) is a standard that helps ensure the quality and reliability of research data through principles of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP). The implementation of GCLP includes careful documentation of procedures, competencies and safety measures. Implementation of GCLP is influenced by existing resources and quality systems, thus laboratories in low- and middle-income countries may face additional challenges.
Methods: This paper describes implementation of Good Clinical Laboratory Practice (GCLP) at the Kenya Medical Research Institute-Center for Microbiology Research (KEMRI-CMR) as part of a quality system to support medical research. This study employed assessment, twinning (institutional mentorship) model, conducting relevant training workshops and Kaizen 5S approaches to implement an effective quality management system using GCLP standard. This was achieved through a collaboration between the KEMRI/Wellcome Trust Research Programme (KWTRP) and KEMRI-CMR. The aim was compliance and continuous monitoring to meet international GCLP standards in a way that could be replicated in other research organizations.
Results: Following a baseline assessment in March 2017, training, mentorship and a cycle of quality audit and corrective action using a Kaizen 5S approach (sorting, setting in order, shining, standardizing and sustaining) was established. Laboratory personnel were trained in writing standard operating procedures and analytical plans, microbiological techniques, and good documentation practice. Mid-term and exit assessments demonstrated significant declines in non-conformances across all GCLP elements. KEMRI-CMR achieved GCLP accreditation in May 2018 by Qualogy Ltd (UK).
Conclusions: Involving all the laboratory personnel in implementation of quality management system processes is critical to success. An institutional mentorship (twinning) approach shows potential for future collaborations between accredited and non-accredited organizations to accelerate the implementation of high-quality management systems and continuous improvement.
Keywords: Good Clinical Laboratory Practice, Quality Assurance, Quality system, medical research, quality management system.
Introduction
Medical laboratories play an important role in disease diagnosis, treatment guidance, drug resistance monitoring and surveillance of diseases of public health interest (Gershy & Rotz, 2010; Martin et al., 2005; Wians, 2009). According to Nkengasong (2010), 20% of clinical trials in Africa have been suspended due to serious Good Clinical Practice (GCP) breaches, which mainly impact on participants’ safety and reliability of the data generated. This can be addressed by implementing integrated, tiered and harmonized operations, and a well-functioning laboratory quality system ( Nkengasong, 2010). Moreover, the emergence of the recent Ebola virus disease epidemic in West Africa in 2015 emphasized the need to rapidly develop better laboratory systems that will foster increased accuracy and reliability of the data generated ( Gostin et al., 2015; Heymann et al., 2015), which have often been the traditional meaning of quality in medical laboratories ( Harteloh, 2004). In medical research, it is imperative to note that generation of reproducible and re-constructible results can be achieved when the clinical laboratory operates under a robust and mature quality management system (QMS) that complies with the GCLP standards, thus providing an excellent path for the success of conducting medical research.
What is the GCLP standard?
GCLP is a standard that supports both the research and clinical aspects of Good Laboratory Practice ( Ezzelle et al., 2008). It was developed to support and strengthen research laboratories performing human clinical trials and provides a platform for monitoring the global conduct of clinical laboratory work performed under harmonized operations (Marcella et al., 2009). This standard was developed by merging the principles of Good Clinical Practice and Good Laboratory Practice in conjunction with the regulatory authorities and accrediting bodies, and was the same approach adopted by the British Association of Research Quality Assurance (BARQA) to develop the Good Clinical Laboratory Practice standard ( Stiles et al., 2003). The GCLP standard focusses on the building blocks of a quality system, which includes assessments, assay validation and verification, training of personnel involved in the research, organization and personnel, specimen management, laboratory equipment, reagents, records and reports, laboratory safety, quality control and proficiency testing programmes, laboratory information systems, and the overall quality management plan of the laboratory (Marcella et al., 2009). The expectation of implementing the GCLP quality system is that data of high quality will be generated when the laboratory complies to the GCLP guidelines. In addition, it provides guidance on the development of a quality system that ensures integrity, validity and reliability of clinical trials data.
The Kenya Medical Research Institute – Centre for Microbiology and Research (KEMRI-CMR)
The Kenya Medical Research Institute (KEMRI) is a Kenyan government parastatal that regulates and conducts research in human health with the aim of improving wellbeing, and the formulation and implementation of policy formulation, while collaborating with other global research organizations. It has its centers widely spread around the country that perform research focusing on different fields ( KEMRI, 2018). Even though KEMRI is the leading medical research organization in the country, some of its centers do not have up-to-date quality systems in place to support medical research. There is an urgent need to establish an effective quality management system using GCLP guidelines to support clinical trials and other studies.
KEMRI-CMR, based in Nairobi, is one of the oldest KEMRI research centers. Research has focused predominantly on traditional and molecular characterization of enteric pathogens in communities and in hospital attendees, in addition to their transmission, virulence and antimicrobial profiles. To promote and support its research activities, KEMRI-CMR engaged its sister organization KEMRI-Wellcome Trust Research Programme (KWTRP), to assist in the development of a quality system using GCLP guidelines. The KWTRP, based in Kilifi, has been actively undertaking microbiological research since 1992, predominantly on invasive bacterial infections in children, including surveillance and antimicrobial treatment trials. KWTRP has been GCLP-accredited since 2007. Here, we describe how the quality management system was implemented at KEMRI-CMR using GCLP guidelines to support medical microbiological research with the goal of gaining recognition of the quality of their management system by attaining GCLP accreditation.
Methods
Methodology used
This study employed assessment, twinning (institutional mentorship) model ( Makokha et al., 2014), and conducting training workshops to build a competent laboratory workforce and utilizing Kaizen 5S approaches to implement an effective quality management system using GCLP standards ( Stiles et al., 2003).
Baseline, mid-term and exit assessments
The QMS implementation progress was evaluated by performing assessments using a GCLP accreditation audit checklist, developed by Qualogy Ltd UK ( Qualogy, 2018) ( Table 1). This checklist consists of 12 sections of 15 questions, which covered the entire quality system elements defined by GCLP guidelines obtained from Qualogy, Ltd, and had a total score of 270 points. The audit checklist questions were asked by the mentors from KWTRP to the auditees (laboratory staff from KEMRI-CMR).
Table 1. Quality management system elements and their scores on the good clinical and laboratory practice (GCLP) accreditation checklist.
Source: Stiles et al., 2003.
| GCLP Elements | Total points |
|---|---|
| Section 1: Documents, records and reports | 30 |
| Section 2: Trial samples and management | 14 |
| Section 3: Organization and personnel | 22 |
| Section 4: Reporting of results | 10 |
| Section 5: Equipment, materials and reagents | 33 |
| Section 6: Internal audits and corrective action | 15 |
| Section 7: Retention and archiving of records | 32 |
| Section 8: Quality control and external quality assessment | 21 |
| Section 9: Planning of the work & sub-contracting | 17 |
| Section 10: Conduct of work | 24 |
| Section 11: Confidentiality, blinding and patient safety | 12 |
| Section 12: Facilities and safety | 40 |
| TOTAL | 270 |
In total, three assessments were performed throughout the process to establish the laboratory’s performance and progress towards GCLP accreditation, as well as determining any remaining gaps. In March 2017, a week after the initial engagement, a baseline assessment was conducted at KEMRI-CMR, using the GCLP accreditation checklist ( Qualogy, 2018). This assessment was performed by the laboratory quality officer (mentor, H.G.) from KWTRP and its results provided the basis for developing KEMRI-CMR-specific actions. A mid-term assessment was conducted 3 months (June 2017) after the baseline assessment, following a GCLP training workshop and corresponding GCLP assignment elements assessed using the GCLP accreditation checklist developed by the mentor laboratory (KWTRP). The exit assessment was performed three months (October 2017) after the mid-term assessment by an independent auditor from KWTRP (J.W. or R.M.), who was not involved in the training, and was the final assessment in readiness for the GCLP accreditation audit by Qualogy UK Ltd.
Twinning (institutional mentorship) model
The twinning (institutional mentorship) model was also employed to implement QMS ( Makokha et al., 2014). This was conducted during the period of May-June 2018. Using this model, a total of 24 laboratory staff from the mentee laboratory (KEMRI-CMR) were paired to the mentor laboratory (KWTRP) to learn and subsequently implement GCLP processes in their laboratory upon their return. A total of 12 laboratory staff from KEMRI-CMR were twinned with staff from KWTRP in the month of May 2017 and another 12 laboratory staff twinned in June 2017. To facilitate the twinning relationship, the laboratory quality officer (mentor) spent 1 week at the mentee laboratory to provide mentorship and coaching for the GCLP process.
Conducting KEMRI-CMR laboratory training
The QMS mandatory training and other relevant training workshops were identified with the aim of strengthening knowledge, skills and abilities, and changing attitudes. The training was mainly delivered through workshops, coaching, and visits to KWTRP for a period of 2 weeks. Training was delivered by the lead mentor (H.G.) and two co-mentors (J.W. or R.M.). Subjects of the training sessions, alongside the trainer and the dates of training, are listed in Table 2. Once these sessions were complete, staff were assigned a specific area to implement when they go back to their laboratory. Kaizen 5S ( Kobayashi, 2005) was implemented to establish the foundation for continuity of quality management system at KEMRI-CMR.
Table 2. Training sessions given to staff.
| Training | No. of staff
trained |
Trainers | Training dates |
|---|---|---|---|
| Microbiological techniques | 25 | Lead mentor & co-mentors | 25–28 April 2017 |
| KWTRP Exchange visits | 24 | Attached to section heads of
KWTRP |
1
st group: 2–12 May 2017
2 nd group: 19–30 June 2017 |
| Good documentation Practice | 25 | Lead mentor | 16 June 2017 |
| SOP writing training | 25 | Lead mentor | 7–9 June 2017 |
| Improvement projects and Quality Indicator training | 10 | Lead mentor | 6–7 July 2017 |
| Confidentiality, blinding and patient safety monitoring | 12 | Lead mentor (myself) & co-mentors
(Joseph & Robert) |
20–21 July 2017 |
| Method and equipment validation | 10 | Lead mentor | 24–25 August 2017 |
| Analytical plan writing training | 26 | Lead mentor | 6–8 September 2017 |
| Internal audits | 6 | Lead mentor | 8–11 August 2017 |
| Basic GCLP training | 30 | Lead mentor | 11–14 April 2017 |
KWTRP, KEMRI-Wellcome Trust Research Programme; SOP, standard operating practice; GCLP, good clinical and laboratory practice.
Data analysis
Data from the three assessments, training conducted were analyzed using Microsoft Excel and presented in tables and figures to extract their useful meaning.
Results
KEMRI-CMR performance
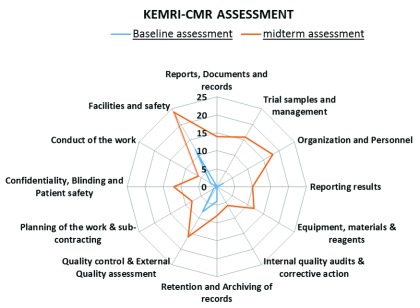
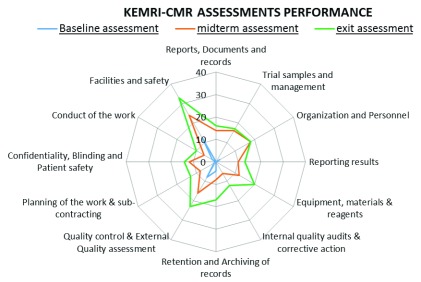
The KEMRI-CMR laboratory assessment performance is summarized in Figure 1 and Figure 2. All 12 elements in the GCLP accreditation checklist were improved at successive assessments ( Figure 1 and Figure 2). The most improved element was the facilities and safety element, followed by quality control, external quality assessment and equipment, reagents and materials elements. The laboratory performed less well in the reporting of results, conduct of the work, internal quality audits, corrective action, planning of the work and sub-contracting GCLP elements.
Figure 1. Comparison between baseline and mid-term assessments.
KEMRI-CMR, Kenya Medical Research Institute – Centre for Microbiology Research.
Figure 2. Kenya Medical Research Institute – Centre for Microbiology Research (KEMRI-CMR) performance comparison of the 12 good clinical and laboratory practice elements between the three assessments.
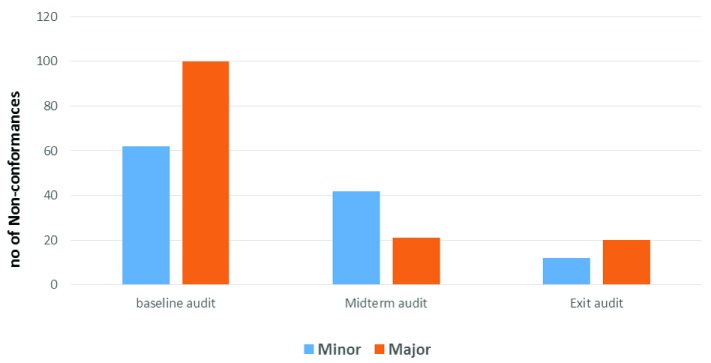
A total of 162 non-conformances arose from the baseline assessment (100 major findings and 62 minor findings); 62 non-conformances arose in the mid-term assessment (42 major findings & 20 minor findings); and 32 non-conformances arose in the final exit assessment (20 major findings & 12 minor findings). The decrease in major and minor non-conformities indicated progress in resolving queries and implementing corrective action ( Figure 3).
Figure 3. Non-conformance analysis at the baseline, midterm and exit assessments.
KEMRI-CMR laboratory training
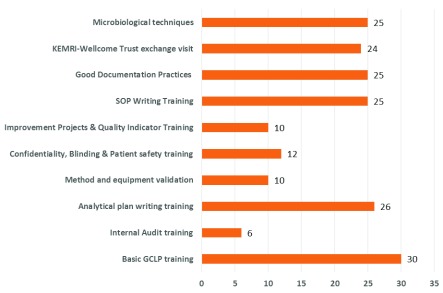
To build a competent and skilled laboratory workforce in the KEMRI-CMR laboratory, a total of 10 training sessions and workshops were conducted between April 2017 and September 2017. These trainings aimed to strengthen the quality of services and systems in KEMRI-CMR. GCLP training was provided ( Table 2) to twenty-five laboratory personnel. In total, 9 of the 10 conducted trainings were done onsite to allow more staff to attend and to reduce costs. All 25 (100%) laboratory personnel were trained in writing SOPs and analytical plans, microbiological techniques, and good documentation practice ( Figure 4).
Figure 4. Number of staff trained in the indicated area during the mentorship period.
SOP, standard operating practice; GCLP, good clinical and laboratory practice.
To decongest the laboratory and enhance efficient workflow for productivity management, principles of Kaizen 5S were adopted: equipment was rearranged for optimal workflow while removing obsolete and un-wanted materials from the laboratory. Equipment that was close to sinks was removed and placed separately as per the specimen workflow. The removal of obsolete equipment and old records that consumed considerable space enhanced the efficiency of the workflow. Documentation was done before and after the Kaizen 5S for comparison. KEMRI-CMR achieved a GCLP accreditation in May 2018 by Qualogy Ltd (UK).
Discussion
The results from the baseline to exit GCLP assessments showed the greatest improvements in facilities and safety element (21 points), followed by quality control and external quality assessment (15 points) and equipment, reagents and materials elements (14 points). The areas that were more challenging to improve were internal quality audits and corrective action (an improvement of 6 points), reporting of results and planning of the work (improvement of 3 points). There was also slower progress in reporting of the results, planning of the work and sub-contracting GCLP elements. This was attributed to unfamiliarity with the internal audit system by the laboratory staff, characterized by inadequate follow-up of the internal audit findings and insufficient documentation of corrective actions (management reviews as described by ISO 15189 standards) described by Maina et al. (2014) as Factor X. Despite the less strong performance in these three GCLP elements, the KEMRI-CMR laboratory QMS performance improved steadily from 10.7% at baseline assessment to successfully achieving 76.3% at the exit assessment.
The improvement of KEMRI-CMR’s laboratory QMS performance was in a large part due to staff’s positive attitude and commitment to work, and continued senior management support. Despite an initial opposition to change, there was a great enthusiasm to continue improving laboratory performance as observed in the final assessment results. Clearly identifying gaps and involving all staff in frank discussions about their solutions was key to achieving this. The foundation of best practice, and a ‘quality culture’ were established through the exchange visits, conducting trainings, mentors’ assistance coupled with managerial commitment. This reflects reports from other institutions implementing quality management process ( Andiric & Massambu, 2014).
The implementation of Kaizen 5S greatly improved the laboratory’s workflow and space. The results indicate that there is a strong foundation for continuity of the quality management system at KEMRI-CMR ( Khamis et al., 2009). The entire laboratory was physically re-organized by placing the equipment strategically to improve efficiency and enhance safety. The entire Kaizen 5S methodology for this study provided the best platform to accelerate the process of quality improvement process at KEMRI-CMR.
Engaging the management team of KEMRI-CMR through the leadership of the Centre Director was crucial in securing financial support for renovating the laboratory and providing adequate human resources for the quality implementation process. His open-door policy style of management and having frequent discussions with the laboratory staff and the mentors made him clearly understand the significance of implementing a quality management system. Moreover, the formation of the fortnightly laboratory meetings to provide reports, feedback and recommendations accelerated the implementation of the quality management system using GCLP guidelines.
Conducting training on-site has also been shown to be an improvement factor during QMS implementation ( Nkwawir et al., 2014). The trainings conducted at KEMRI-CMR laboratory coupled with twinning of the KEMRI-CMR laboratory staff to the KWTRP through exchange visits also accelerated the implementation of a QMS. Conducting mandatory and supplementary QMS training to cover best laboratory practices within the KEMRI-CMR laboratory led to more staff being trained ( Figure 4).
Using the twinning model or the institutional mentorship approach ( Makokha et al., 2014) helped the mentor to more fully understand the operational functionality of the mentee laboratory by participating in the laboratory activities, providing hands-on trainings and guidance regarding what aspects of the quality management system to be implemented.
In addition, the continued presence of the mentors at the KEMRI-CMR laboratory during the entire QMS implementation period helped to design specific activities tailored in their approach to assisting laboratory improvements, developing a working culture that emphasizes quality and a sustainable QMS as previously implemented by other organizations during their QMS journey ( Nkwawir et al., 2014). Only one training (GCLP training) was attended by staff drawn from other departments. This was to enhance their understanding of the GCLP concept so that they could support the laboratory’s journey of implementing the quality management system. The experience at KEMRI-CMR during the quality implementation process clearly reveals what other laboratories that fully commit their concerted effort can achieve in implementing a quality improvement process.
Conclusions
Implementing an efficient and effective quality management system requires a system-wise approach and strong teamwork to ensure that set goals and objectives are realized. Compliance with GCLP standards, coupled with periodic audits/assessments, will help ensure that clinical research and trials performed at KEMRI-CMR meets international standards. Involving all laboratory personnel in the implementation of a QMS process is critical to its success. The use of an institutional mentorship (twinning) approach also shows the potential for future collaborations between accredited and non-accredited organizations and can be used to accelerate the implementation of a good QMS and continuous improvement.
Data availability
Data generated in the present study are available on figshare, DOI: https://doi.org/10.6084/m9.figshare.7200707 ( Gumba, 2018).
Funding Statement
This study was funded by the Wellcome Trust (203077).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- Andiric LR, Massambu CG: One laboratory’s progress toward accreditation in Tanzania. Afr J Lab Med. 2014;3(2):202. 10.4102/ajlm.v3i2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzelle J, Rodriguez-Chavez IR, Darden JM, et al. : Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J Pharm Biomed Anal. 2008;46(1):18–29. 10.1016/j.jpba.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershy-Damet GM, Rotz P, Cross D, et al. : The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol. 2010;134(3):393–400. 10.1309/AJCPTUUC2V1WJQBM [DOI] [PubMed] [Google Scholar]
- Gostin LO, Waxman HA, Foege W: The president's national security agenda: curtailing Ebola, safeguarding the future. JAMA. 2015;313(1):27–8, Publication: Bulletin of the World Health Organization; Type: Research. 10.1001/jama.2014.16572 [DOI] [PubMed] [Google Scholar]
- Gumba H: KEMRI-CMR Data collected & analyzed.xls. figshare Dataset.2018. [Google Scholar]
- Harteloh PP: Understanding the quality concept in health care. Accred Qual Assu. 2004;9(1–2):92–5. 10.1007/s00769-003-0677-x [DOI] [Google Scholar]
- Heymann DL, Chen L, Takemi K, et al. : Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet. 2015;385(9980):1884–901. 10.1016/S0140-6736(15)60858-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenya Medical Research Institute (KEMRI) website. Accessed on 13 July 2018. Reference Source [Google Scholar]
- Khamis N, Abrahman MN, Jamaludin KR, et al. : Development of 5S practice checklist for manufacturing industry.In: Proceedings of the World Congress on Engineering,2009;1: ISBN: 978-988-17012-5-1. Reference Source [Google Scholar]
- Kobayashi K: What is 5S? A Content Analysis of Japanese Management Approach. Unpublished Master’s Thesis, Griffith University, Southport.2005. Reference Source [Google Scholar]
- Maina RN, Mengo DM, Mohamud AD, et al. : Progressing beyond SLMTA: Are internal audits and corrective action the key drivers of quality improvement? Afr J Lab Med. 2014;3(2):222. 10.4102/ajlm.v3i2.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makokha EP, Mwalili S, Basiye FL, et al. : Using standard and institutional mentorship models to implement SLMTA in Kenya. Afr J Lab Med. 2014;3(2):220. 10.4102/ajlm.v3i2.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Hearn TL, Ridderhof JC, et al. : Implementation of a quality systems approach for laboratory practice in resource-constrained countries. AIDS. 2005;19 Suppl 2:S59–S65. 10.1097/01.aids.0000172878.20628.a8 [DOI] [PubMed] [Google Scholar]
- Nkengasong JN: A shifting paradigm in strengthening laboratory health systems for global health: acting now, acting collectively, but acting differently. Am J Clin Pathol. 2010;134(3):359–360. 10.1309/AJCPY5ASUEJYQ5RK [DOI] [PubMed] [Google Scholar]
- Nkwawir SC, Batumani NN, Maruta T, et al. : From grass to grace: How SLMTA revolutionised the Bamenda Regional Hospital Laboratory in Cameroon. Afr J Lab Med. 2014;3(2):203. 10.4102/ajlm.v3i2.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualogy Good Clinical Laboratory practice accreditation. Accessed on 11 July 2018. Reference Source [Google Scholar]
- Sarzotti-Kelsoe M, Cox J, Cleland N, et al. : Evaluation and recommendations on good clinical laboratory practice guidelines for phase I-III clinical trials. PLoS Med. 2009;6(5):e1000067. 10.1371/journal.pmed.1000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles T, Grant V, Mawbey N: Good clinical laboratory practice (GCLP): A quality system for laboratories which undertake the analyses of samples from clinical trials.Ipswich (UK): British Association of Research Quality Assurance2003;1–17, ISBN 1-904610-00-5. Reference Source [Google Scholar]
- Sollecito WA, Johnson JK: Mclaughlin and Kaluzny's Continuous Quality Improvement in Health Care.Jones & Bartlett Learning, Sudbury, Mass.2012. [Google Scholar]
- Todd CA, Sanchez AM, Garcia A, et al. : Implementation of Good Clinical Laboratory Practice (GCLP) guidelines within the External Quality Assurance Program Oversight Laboratory (EQAPOL). J Immunol Methods. 2014;409:91–8. 10.1016/j.jim.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wians FH: Clinical laboratory tests: Which, why, and what do the results mean? Lab Medicine. 2009;40(2):105–113. 10.1309/LM4O4L0HHUTWWUDD [DOI] [Google Scholar]