Abstract
Background
Metastatic breast cancer (MBC) treatment has changed substantially over time, but we do not know whether survival post-metastasis has improved at the population level.
Methods
We searched for studies of MBC patients that reported survival after metastasis in at least two time periods between 1970 and the present. We used meta-regression models to test for survival improvement over time in four disease groups: recurrent, recurrent estrogen (ER)-positive, recurrent ER-negative, and de novo stage IV. We performed sensitivity analyses based on bias in some studies that could lead earlier cohorts to include more aggressive cancers.
Results
There were 15 studies of recurrent MBC (N = 18 678 patients; 3073 ER-positive and 1239 ER-negative); meta-regression showed no survival improvement among patients recurring between 1980 and 1990, but median survival increased from 21 (95% confidence interval [CI] = 18 to 25) months to 38 (95% CI = 31 to 47) months from 1990 to 2010. For ER-positive MBC patients, median survival increased during 1990–2010 from 32 (95% CI = 23 to 43) to 57 (95% CI = 37 to 87) months, and for ER-negative MBC patients from 14 (95% CI = 11 to 19) to 33 (95% CI = 21 to 51) months. Among eight studies (N = 35 831) of de novo stage IV MBC, median survival increased during 1990–2010 from 20 (95% CI = 16 to 24) to 31 (95% CI = 24 to 39) months. Results did not change in sensitivity analyses.
Conclusion
By bridging studies over time, we demonstrated improvements in survival for recurrent and de novo stage IV MBC overall and across ER-defined subtypes since 1990. These results can inform patient-doctor discussions about MBC prognosis and therapy.
Breast cancer is the leading cause of cancer death among women globally and the second leading cause in the United States (1,2). Treatment paradigms for this disease are changing rapidly and mortality is steadily declining, largely due to advances in population screening and early-stage treatments (3,4) that decrease risk of distant recurrence. Women with metastatic breast cancer (MBC)—an estimated three-quarters of whom were diagnosed with stage I–III disease and later developed distant recurrence, and an estimated one-quarter of whom were diagnosed with de novo distant spread (stage IV) (5)—continue to have incurable disease. To treat MBC, there are 23 drugs that are recommended in the 2018 guidelines of the National Comprehensive Cancer Network or approved as of 2018 by the Food and Drug Administration (6–9). Most of these drugs demonstrated a survival benefit in randomized controlled trials (RCTs) (10–24). However, in part because metastatic recurrence is not recorded in US population registries, we do not know whether RCT results have translated into improved survival at the population level for patients experiencing recurrence. Because new treatments are often toxic and costly, it is essential to assess the impact of these treatment changes on MBC survival for the average patient in the general population.
To assess the population-level impact of MBC treatment, several prior studies have examined the difference in survival between time periods (25–45); in recurrent disease, the majority of these studies are within a single institution or network. However, no study has incorporated data across multiple decades and treatment settings, and studies have reached differing conclusions. Strikingly, because of these discrepancies, we do not currently know whether the changes made in the treatment of MBC have led to improved outcomes for patients.
To fill this important gap, we conducted meta-regression analyses of published results of changes in survival to examine trends over several decades and determine whether population-level survival after diagnosis of MBC has improved as new treatments were introduced. We also identified studies with biases that may obscure conclusions about population-based survival trends and conducted sensitivity analyses excluding these studies. The results are intended to inform clinical practice and future data collection protocols to facilitate unbiased estimation of the population impact of MBC treatment advances.
Methods
This study used aggregate data from published studies and was exempt from Institutional Review Board approval.
Study Selection
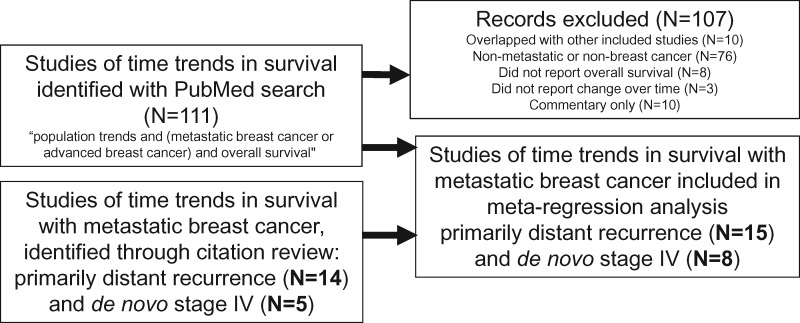
Two investigators (JLC and AWK) searched for published, population-level studies of survival trends over time among patients with distant recurrence or de novo stage IV breast cancer (Figure 1). For inclusion, studies had to fulfill the following criteria: 1) assessed overall or relative survival after metastasis for at least two distinct time intervals in a similar population; 2) time intervals reported covered some subset of the time period between 1970 and the present; and 3) reported median overall survival in months or 5-year overall or relative survival percentages, or provided a Kaplan-Meier curve. Only studies in English were included. We included only studies with at least two time intervals such that heterogeneity across time periods would be relatively reduced. We selected 1970 as the lower bound on the time period to study because all of the 23 drugs used today were introduced subsequent to 1970 (Figure 2). Multiple study types met our inclusion criteria: retrospective cohorts from single or multiple institutions, retrospective cohorts identified from cancer registries, and prospective cohorts of patients enrolled on successive RCTs from the same study group. Notably, we included RCT follow-up data from within the same institution or research network over time: in these reports, both arms of the RCT were included together, and the consistency of the institution or research network over time allowed for some between-timepoint internal consistency. We searched PubMed October 18, 2017 and again June 28, 2018 with the following terms: “population trends AND (metastatic breast cancer or advanced breast cancer or distant recurrence) AND overall survival.” We augmented this search by reference tracking of identified articles, in particular of one landmark study of recurrent disease (25) and another of de novo stage IV disease (43), to find additional relevant studies.
Figure 1.
Study selection.
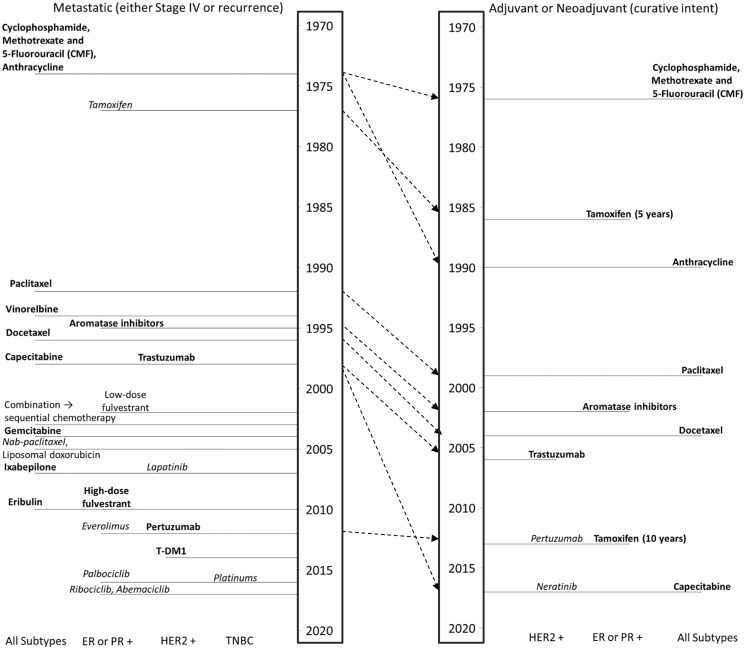
Figure 2.
Timeline diagram of breast cancer therapies. Bold font indicates that an overall survival benefit was reported in a randomized clinical trial or meta-analysis; italic font indicates that a progression-free survival (but no overall survival) benefit was reported in a randomized clinical trial or meta-analysis; and plain font indicates that no overall survival benefit nor progression-free survival benefit has been reported. Year of introduction is date of Food and Drug Administration (FDA) approval or, for drugs that are not FDA approved to treat breast cancer (vinorelbine, liposomal doxorubicin, platinums), the date of the first major publication that led to widespread use. Dashed arrows show the transition of each therapy from the metastatic setting, where nearly all were originally introduced, to the early-stage setting.
Data Collection
Data extraction and review of inclusion and exclusion criteria were performed separately by two investigators (JLC and AWK). For each study, we extracted at least one of the following estimates for each cohort in given time interval: median overall survival (reported in days, months, or years), 5-year overall or relative survival percentages, or a Kaplan-Meier curve from which the aforementioned values could be inferred. We extracted the sample size of each time cohort and the starting and ending time of the interval defining the cohort. When available, we extracted the same information by estrogen receptor (ER) status. Where median overall survival was not reported, we converted the 5-year survival probability or Kaplan-Meier curve to the median overall survival time under the exponential distribution assumption. The assumption of exponential distribution for overall survival time was reasonable based on visual examination of the Kaplan-Meier curves.
In addition to this collection of data for meta-analysis, we categorized studies based on the types of metastatic disease included: only de novo stage IV disease, or at least 80% recurrent disease, with no more than 20% de novo stage IV disease (“primarily recurrent disease”). For studies of primarily recurrent disease, we categorized studies based on the types of recurrence included: at least 80% distant recurrence, with no more than 20% locoregional recurrence (“primarily distant recurrence”), or distant and locoregional recurrences, with greater than 20% locoregional recurrences. For studies of only recurrent or primarily recurrent disease, we noted whether there was a cap on year of diagnosis. We also noted for each study what prognostic factors (such as recurrence-free interval, ER positivity, age at diagnosis, and site of metastasis) were measured in different time intervals and whether they changed over time, and results of multivariable analysis, if performed.
Statistical Methods
We analyzed primarily recurrent disease, ER-positive primarily recurrent disease, ER-negative primarily recurrent disease, and de novo stage IV disease separately. There was only one study of de novo stage IV disease that included results by ER status separately. For each case, we performed random-effects meta-regression analysis (46) assuming an underlying mixed effect model that predicted median overall survival based on the year of recurrence (Supplemental Methods, available online). We included a study-specific random effect to account for within-study correlations and weighted the analysis by the cohort size. We included a quadratic term of time in the regression to assess potential nonlinearity. The statistically significant quadratic terms (P = .036 for recurrent disease, <.001 for ER-positive, .046 ER-negative, and .044 for de novo stage IV disease) suggested the inadequacy of the simple linear model for describing the time trend, and so these terms were retained. We tested for improvement over each decade (1980–1990, 1990–2000, and 2000–2010) by calculating the median survival ratio from the fitted function over that decade; reported P values are two-sided and those less than .05 were considered statistically significant.
For analysis of de novo stage IV disease, three studies reported 5-year relative survival rather than overall survival (40,47,48), which we converted to median overall survival in months under the exponential distribution assumption. Relative survival in these studies was defined as the ratio of observed survival for people with stage IV breast cancer to expected survival for people from the reference population matched on demographic characteristics. Relative survival is expected to be close to overall survival in these instances, because the mortality of the reference population is almost negligible relative to the mortality of the stage IV cancer patients. To allow incorporation of the results of these three studies into the meta-analysis, we included an additional covariate indicating whether relative or overall survival was used, in order to adjust for a small but potential elevation in relative survival compared with overall survival.
We performed two sensitivity analyses in studies of primarily recurrent disease to explore the effects of study biases on our results. A subset of studies capped year of diagnosis either in the same year as, or shortly before, the year of recurrence, which could lead to overselection of aggressive disease in the earliest cohorts studied (Supplementary Figure 1, available online). Another subset of studies included over 20% locoregional recurrences in their definition of metastatic recurrence. We repeated the meta-regression excluding each of these sets of studies in turn to assess impact on our results.
Results
Recurrent Breast Cancer
We identified 15 studies that included 18 678 breast cancer patients with primarily recurrent disease: these studies included analyses of overall survival trends in a series of adjuvant RCTs (N = 2) (25,27) and metastatic RCTs (N = 2) (34,37) conducted within a specific research network or institution over time; registry-based studies (N = 3) (33,35,36), and single-institution studies (N = 8) (26,28–32,38,49–51) (Table 1). Individually, 12 of the 15 studies showed improvements in survival over time.
Table 1.
Studies of change in survival over time for patients with metastatic breast cancer, either distant recurrence or de novo Stage IV disease
| Diagnosis period | Recurrence time periods compared | Disease status | Source population | N | Median survival (months) | Statistical significance | References |
|---|---|---|---|---|---|---|---|
| 1974–1994 | 1974–1979 to 1995–2000 | Recurrent | Adjuvant clinical trials, MD Anderson Cancer Center | 834 | 15 to 58 | UV only | Giordano et al. (25) |
| Any year | 1976–1982 to 1996–2006 | Recurrent | Single center, US | 382 | 26 to 33 | UV (no MV done) | Vogel et al., Zeichner et al. (49,50) |
| 1978–2002 | 1978–1983 to 2004–2010 | Recurrent | Adjuvant trials, US (ECOG) | 3447 | 20 | UV only | Tevaarwerk et al. (27) |
| 1979–2013 | 1979–2000 to 2001–2013 | Recurrent | Single center, Japan | 813 | 26 to 45 | UV only | Nakano et al. (28) |
| 1975–2004 | 1980–1984 to 2000–2004 | Recurrent | Single center, France | 1038 | 16 to 31 | UV only | Largillier et al. (29) |
| 1980–2005 | 1980–1994 to 1995–2008 | Recurrent | Single center, Japan | 252 | 32 to 60 | UV & MV | Tsuji et al. (30) |
| 1983–2003 | 1990–1996 to 1997–2003 | Recurrent | Single center, Japan | 321 | 25 to 34 | UV only | Anan et al. (31) |
| Any year | 1992–2000 to 2001–2008 | Recurrent | Single center, Japan | 407 | 20 to 50 | UV & MV | Shigematsu et al. (32) |
| 1976–2004 | 1979–1984 to 2000–2004 | Recurrent (90%) & stage IV | Stockholm region, Sweden | 5463 | 16 to 15 | No improvement | Foukakis et al. (33) |
| Any year | 1983–1986 to 1998–2001 | Recurrent & stage IV | Metastatic trials, Italy | 640 | 18 to 24 | UV only | Gennari et al. (34) |
| Any year | 1985–1989 to 2010–2014 | Recurrent (85%) & stage IV | Kalmar county, Sweden | 784 | 13 to 33 | UV (no MV done) | Sundquist et al. (35) |
| 1989–2001 | 1991–1992 to 1999–2001 | Recurrent (80%) & stage IV | British Columbia | 2150 | 15 to 22 | UV & MV | Chia et al. (36) |
| Any year | 1991–1994 to 2003–2006 | Recurrent & stage IV | Metastatic trials, Greece | 1361 | 15 to 31 | UV only | Dafni et al. (37) |
| Any year | 1995–2000 to 2008–2013 | Recurrent (80%) & stage IV | Single center, Germany | 716 | 33 to 35 | No improvement | Weide et al. (38) |
| Any year | 2001–2004 to 2005–2008 | Recurrent (85%) & stage IV | Single center, Italy | 70 | 35 to 30 | No improvement | Giuliani et al. (51) |
| Diagnosis period | Disease status | Source population | N | Median survival (months) | Statistical significance | References | |
| 1975–1977 to 2006–2012 | Stage IV | Multiple registries, US | NR* | 5 yr†: 19% to 34% | UV (no MV) | Jemal et al. (47), Di Meglio et al. (52) ‡ | |
| 1975–1984 to 1995–2002 | Stage IV | Eindhoven Registry, Netherlands | 1089§ | 18 to 21 | UV & MV** | Ernst et al. (41) | |
| 1985–1994 to 1995–2004 | Stage IV | City of Hope Cancer Center | 274 | 30 to 36 | No improvement | Pal et al. (42) | |
| 1987–1993 to 1994–2000 | Stage IV | Three centers, France | 724 | 23 to 29 | UV (no MV) | Andre et al. (43) | |
| 1988–1993 to 1999–2003 | Stage IV | SEER Registry, US | 15 438 | 16 to 20 | UV & MV | Dawood et al. (44), Di Meglio et al. (75) ‡ | |
| 1992–1995 to 2004–2007 | Stage IV | Saarland Registry, Germany | 984 | 5 yr†: 20% to 21% | No improvement | Holleczek et al. (40) | |
| 1995–1999 to 2005–2008 | Stage IV | Registry, Netherlands | 8031 | 17 to 23 | UV & MV†† | Ruiterkamp et al. (45) | |
| 2000–2004 to 2005–2008 | Stage IV | Registry, Czech Republic | 3766 | 5 yr†: 22% to 20% | No improvement | Pavlik et al. (48) | |
For weighting within meta-analysis, estimated N from reported confidence intervals to be approximately 6079. ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio of death; MV = multivariable analysis; NR = not reported; SEER = Surveillance Epidemiology and End Results; US = United States; UV = univariate analysis.
Reported relative rather than overall survival.
In meta-regression, excluded the final time interval of this study (N = 554) as overlapped with time intervals in study (45).
Multivariable was statistically significant only comparing 1995–2002 with 1985–1994 if data on metastasis site and number were available (these prognostic factors worsened over time).
Multivariable was statistically significant only comparing 2004–2007 with 1995–1999 (trend for 2000–2003 vs 1995–1999, P = .08).
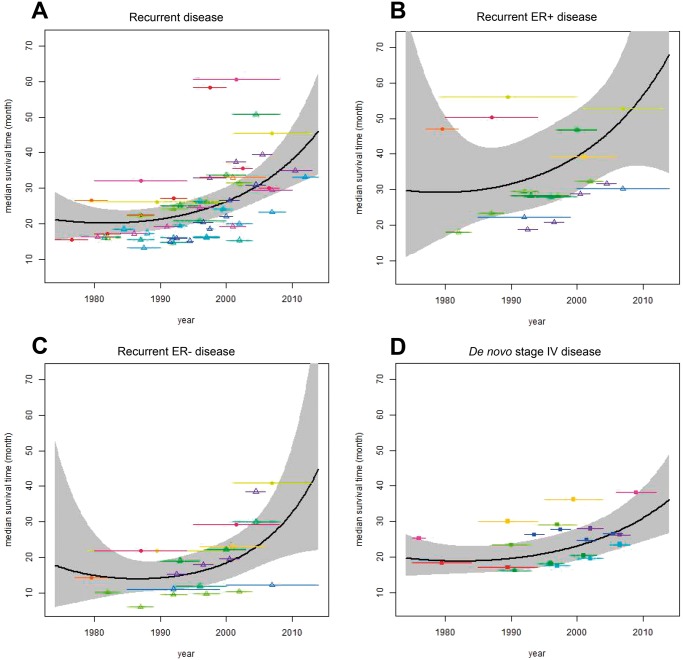
Meta-regression of the 15 studies demonstrated no statistically significant improvement in median unadjusted survival from 1980 to 1990 (median survival ratio 1.05; 95% confidence interval [CI] = 0.91 to 1.22; P = .50) but improvement from 1990 to 2000 (median survival ratio = 1.24; 95% CI = 1.16 to 1.32; P < .001) and from 2000 to 2010 (median survival ratio = 1.45; 95% CI = 1.21 to 1.73; P < .001) (Table 2; Figure 3A). We estimated that median unadjusted survival across all breast cancer subtypes was 20 (95% CI = 17 to 25) months in 1980, 21 (95% CI = 18 to 25) months in 1990, 26 (95% CI = 23 to 30) months in 2000, and 38 (95% CI = 31 to 47) months in 2010 (Table 2).
Table 2.
Median survival estimates and survival ratios from the model
| Year of detection of metastatic disease |
||||
|---|---|---|---|---|
| Outcome | 1980 | 1990 | 2000 | 2010 |
| Median survival after recurrence (95% CI) (all), mo | 20.2 (16.5 to 24.6) | 21.2 (18.2 to 24.7) | 26.2 (22.7 to 30.4) | 38.0 (30.5 to 47.3) |
| Survival ratio (95% CI) relative to previous decade | NA | 1.05 (0.91 to 1.22) | 1.24 (1.16 to 1.32) | 1.45 (1.21 to 1.73) |
| Median survival after recurrence (95% CI) (ER+), mo | 29.1 (16.7 to 50.7) | 31.6 (23.3 to 42.7) | 39.4 (29.5 to 52.5) | 56.5 (36.6 to 87.3) |
| Survival ratio (95% CI) relative to previous decade | NA | 1.08 (0.66 to 1.79) | 1.25 (1.05 to 1.49) | 1.44 (0.93 to 2.21) |
| Median survival after recurrence (95% CI) (ER–), mo | 14.8 (8.2 to 26.8) | 14.1 (10.5 to 19.0) | 18.4 (13.6 to 24.8) | 32.6 (20.7 to 51.4) |
| Survival ratio (95% CI) relative to previous decade | NA | 0.95 (0.55 to 1.66) | 1.30 (1.08 to 1.57) | 1.77 (1.13 to 2.79) |
| Median survival after de novo stage IV (95% CI) (all), mo | 18.9 (15.1 to 23.5) | 19.5 (16.1 to 23.8) | 23.1 (19.0 to 28.0) | 31.0 (24.3 to 39.4) |
| Survival ratio (95% CI) relative to previous decade | NA | 1.04 (0.92 to 1.17) | 1.18 (1.13 to 1.23) | 1.34 (1.16 to 1.55) |
*CI = confidence interval.
Figure 3.
Meta-regression of the improvement in median survival after breast cancer metastasis over time in (A) recurrent disease, (B) estrogen receptor (ER)-positive recurrent disease, (C) ER-negative recurrent disease, and (D) de novo stage IV disease. In A–C), open triangles indicate studies that included only distant recurrence, and closed circles indicate studies that included locoregional recurrence. Each study is represented by a different color.
We repeated this analysis for the eight studies (28–32,35,37,49,50) that collected data on unadjusted survival after recurrence separately for ER-positive (3073 patients) and ER-negative disease (1239 patients) (Table 2, Figure 3, B and C). Although numbers were smaller than for recurrent disease as a whole, we observed similar trends in survival improvement over time in ER-positive and ER-negative recurrent disease: from 1980 to 1990, the median survival ratio was 1.08 (95% CI = 0.66 to 1.79; P = 0.75) for ER-positive disease and 0.95 (95% CI = 0.55 to 1.66; P = 0.87) for ER-negative disease; from 1990 to 2000, the median survival ratio was 1.25 (95% CI = 1.05 to 1.49; P = .014) for ER-positive disease and 1.30 (95% CI = 1.08 to 1.57; P = .007) for ER-negative disease; and from 2000 to 2010, the median survival ratio was 1.44 (95% CI = 0.93 to 2.21; P = .10) for ER-positive disease and 1.77 (95% CI = 1.13 to 2.79; P = .01) for ER-negative disease. We estimated median unadjusted survival in ER-positive disease to have been 29 (95% CI = 17 to 51) months in 1980, 32 (95% CI = 23 to 43) months in 1990, 39 (95% CI = 30 to 53) months in 2000, and 57 (95% CI = 37 to 87) months in 2010. In ER-negative disease, we estimate median unadjusted survival to have been 15 (95% CI = 8 to 27) months in 1980, 14 (95% CI = 11 to 19) months in 1990, 18 (95% CI = 14 to 25) months in 2000, and 33 (95% CI = 21 to 51) months in 2010.
Among the 12 studies that showed survival improvement over the interval studied, 10 included multivariable analysis incorporating variables associated with disease aggressiveness and survival after recurrence (Table 1). In 7 of these 10 multivariable analyses, the association between recurrence year and survival disappeared with adjustment for potential confounding variables related to disease aggressiveness (Table 1). In other words, disease aggressiveness appeared to decrease over time, and this decrease appeared to explain much or all of the observed survival improvement. However, the variables associated with disease aggressiveness differed between studies (Supplementary Table 1, available online). For example, 12 studies assessed ER status over time, with five showing an increase in ER-positive disease and seven showing no change. Similarly, nine studies assessed metastasis site: one showed a decrease in visceral disease, two showed an increase, and six showed no change. The most consistent change was an increase in recurrence-free interval, assessed in 13 studies and observed in 12 (Supplementary Table 1, available online).
Sensitivity Analyses to Account for Potential Study Biases
In eight studies, there was truncation of diagnosis ascertainment in the earliest cohorts of MBC patients with recurrent disease (hereafter called “left-truncation”) that could introduce an ascertainment bias, where earlier cases would include more aggressive disease that was more likely to recur early (Supplementary Figure 1, available online). In four studies, diagnosis and recurrence were truncated in the same year (25–28,30), and in the other four, diagnosis was truncated 2–7 years before recurrence (29,31,33,36). Thus, in the earliest cohorts, only recently diagnosed patients from the time period were included. By definition, such patients had a shorter recurrence-free interval than in later cohorts, and because shorter recurrence-free interval is associated with shorter survival after distant recurrence (53,54), earlier patients were selected for more aggressive disease. In sensitivity analysis, we excluded studies with this potential ascertainment bias and concluded there was no change in the finding that survival improved over time: 20 months (95% CI = 17 to 25 months) in 1990 and 41 months (95% CI = 32 to 53 months) in 2010.
Second, several studies (25,28,30,49,51) included over 20% locoregional recurrences (Figure 3A–C), which are treated very differently from distant recurrences and have longer survival (55). When we repeated the analysis without these five studies, we concluded there was no change in the finding that survival improved, though survival was modestly lower when only distant recurrences were included (vs distant and local recurrences): 19 months (95% CI = 17 to 23 months) in 1990 and 34 months (95% CI = 27 to 43 months) in 2010.
De Novo Stage IV Breast Cancer
We identified eight studies that assessed survival improvement in exclusively de novo stage IV disease (n = 35 831 patients). These included registry-based studies (N = 6, including two with nonoverlapping data from the Surveillance, Epidemiology, and End Results [SEER] Program of the National Cancer Institute) (5,40,41,44,45,47,48,56) and single- or multi-institution studies (N = 2) (42,43) (Table 1). Meta-regression found no statistically significant improvement from 1980 to 1990 (median survival ratio = 1.04; 95% CI = 0.92 to 1.17; P = 0.55) but statistically significant improvement thereafter (median survival ratio = 1.18 from 1990 to 2000 [95% CI = 1.13 to 1.23; P < .001] and 1.34 from 2000 to 2010 [95% CI = 1.16 to 1.55; P < .001]) (Table 2). We estimated median unadjusted survival to have been 19 (95% CI = 15 to 23) months in 1980, 20 (95% CI = 16 to 24) months in 1990, 23 (95% CI = 19 to 28) months in 2000, and 31 (95% CI = 24 to 39) months in 2010 (Table 2;Figure 3D). Only one of these studies reported survival separately for ER-positive and ER-negative disease (43) and showed improvement for ER-positive disease but not ER-negative disease from 1987 to 2000. Multivariable analysis was performed in four of the six studies: the association between time period of diagnosis and survival persisted after multivariable analysis in three of the four studies (Table 1).
Discussion
This study examined population trends in overall survival of breast cancer patients with distant recurrence or de novo metastatic disease from studies spanning the 40-year interval from 1970 to 2010. We found no evidence for an improvement in survival between 1980 and 1990, consistent with the slow pace of new drug introduction in that time period. However, we found a substantial improvement beginning in 1990, consistent with the steady flow of new treatments that began with paclitaxel in 1992. The pattern and magnitude of survival improvement was similar between primarly recurrent and de novo stage IV disease and across ER-defined subtypes. Even for ER-negative disease, which has the worst prognosis, survival more than doubled between 1990 and 2010. These results support the impact of newer treatments on MBC survival at the population level and may inform patient-doctor discussions about the prognosis and treatment of MBC.
We performed this study to address a gap in the data on survival of MBC patients in the general population. This gap exists in part because US population-based cancer registries do not continuously follow early-stage cancer patients for distant recurrence, which is how most breast cancer patients develop MBC (vs presenting with de novo stage IV disease). Thus, US registries cannot measure the interval between distant cancer recurrence and death. The studies we identified of MBC survival over time were heterogeneous in their time frames and patient populations. These between-study differences may account for their variable findings, with some concluding that there was a survival improvement over time and others not.
We note that some studies concluded that the survival improvement over time they observed was more likely to have resulted from a reduction in disease aggressiveness over time rather than from new treatments (27,29). This conclusion was based on the discrepancy between the univariate analyses, where a statistically significant association between calendar year and survival after recurrence was found, and the multivariable analyses including recurrence-free survival, where it often was not. We propose three possible explanations for why survival after recurrence would appear to improve over time and this association would disappear when adjusting for or stratifying by recurrence-free interval. The first is that, as proposed previously, disease aggressiveness has decreased over time. The second is that improved adjuvant therapy over time might lengthen recurrence-free interval and also survival after metastasis (eg, by selecting for more indolent micro-metastases, which then would grow more slowly after recurrence). The third is that the same treatments used in the adjuvant setting, where they lengthen recurrence-free interval, are also used in the metastatic setting, where they independently lengthen survival after metastasis.
It appears unlikely that a reduction in disease aggressiveness over time would entirely explain our results of an improvement in survival over time. Notably, the surrogates for disease aggressiveness assessed in the different studies changed variably (or not at all) over time, whereas the lengthening of recurrence-free interval was nearly universal. This discordance suggests that the lengthening of the recurrence-free interval may have occurred in these studies because of factors other than those captured by the markers of disease aggressiveness. In particular, recurrence-free interval may have lengthened because of improved adjuvant therapy and/or biased ascertainment in studies that left-truncated ascertainment of the earliest cohorts, such that the limited time-window between diagnosis and recurrence would select for shorter survival in earlier years. We addressed this bias in two ways. First, because the years of the earliest time period (the one most sensitive to this bias) varied between studies, we mitigated bias by including all studies together in a meta-regression. Second, we performed a sensitivity analysis excluding studies with this ascertainment bias, which did not change the finding of survival improvement over time. Furthermore, we observed survival improvement in both ER-positive and ER-negative MBC, and thus the survival improvement over time cannot be fully explained by a potential increase in the prevalence of ER-positive relative to ER-negative disease (57).
It also appears unlikely that improved adjuvant therapy would account entirely for the improvement in survival after distant recurrence seen here, given that we find a remarkably similar improvement in survival after metastasis in de novo stage IV disease, where no adjuvant therapy is given. Previous studies have hypothesized the reverse, that the administration of adjuvant therapy prior to recurrence might decrease survival after metastasis by selecting for resistant clones (58). We do not see shorter survival after primarily distant recurrence compared with de novo stage IV disease, but we also do not see longer survival as might have been expected if adjuvant therapy selected for more indolent recurrent disease.
We suspect that the most likely explanation for the disappearance of the effect of year of recurrence on survival after recurrence after adjusting for or stratifying by recurrence-free interval is that successful treatments for metastatic disease are often shifted to earlier-stage breast cancer. In other words, the accelerated pace of new drug development after 1990 may have improved both recurrence-free interval and survival with metastatic disease in the same patients. For example, HER2-targeted therapies offer a longer recurrence-free interval and also longer survival after recurrence (18,59), and other drug classes including endocrine therapy and chemotherapy may function similarly. Thus, adjustment for recurrence-free interval could obscure a true effect of improved treatment on survival after recurrence because the advances that explain improved recurrence-free interval could also explain improved survival after recurrence.
We note that there have been other changes in the diagnosis and management of breast cancer over time that could lead to changes in recurrence-free interval or survival after recurrence, independent of the effects of improvements in treatment. Earlier detection of distant recurrence [eg, by more use of advanced imaging after primary treatment (60)] over time could cause lead-time bias resulting in longer survival, but this change should also lead to shorter recurrence-free interval, which was not observed. Conversely, increased population screening (eg, mammography) for early-stage disease over time could cause lead-time bias resulting in longer recurrence-free interval, but likely unchanged survival. It is difficult to estimate how these effects in concert might lead to changes on the population level, but improvements in treatment remains the simplest explanation to account for lengthening of recurrence-free survival, lengthening of survival after recurrence, and the interdependence of these two time intervals.
Our study has limitations. First, only three of the studies we analyzed reported data on HER2 status, so we cannot determine how much the success of HER2-targeted therapies may have contributed to our findings for ER-positive and ER-negative breast cancer. However, because HER2-positive cancers were the minority in studies that reported HER2 (<30%) (28,50,61), they are unlikely to account for all of the observed survival improvement, suggesting that improvement has been made in triple-negative disease as well. Similarly, only one-quarter of the patients analyzed for recurrent disease had ER status available, decreasing the precision of estimates by ER status. Second, we are unable to link improvement in survival to the introduction of specific therapies, instead focusing on decade-by-decade trends; going forward, data sets that allow more granular estimates of survival after metastasis (by year instead of interval of diagnosis) and include subtype information (ER and HER2 status and, as therapies are increasingly targeted, other factors such as BRCA1/2 status) will be essential to evaluate the impact of specific therapies on survival. Third, we cannot rule out that a shift in disease biology contributed to our findings. Changes in practice beyond new treatments, such as earlier diagnosis of metastatic recurrence due to more sensitive imaging modalities, may also have contributed to observed survival trends. Fourth, we may have missed certain reports with our search strategy, such as those not in English. Publication bias might have biased our studies toward those showing an improvement; the result that survival did not improve over time would be provocative with adequate power (27), but smaller studies not showing an improvement may not have pursued publication. Fifth, although we collected studies across the entire period from 1970 to 2018, data were too sparse between 1970 and 1980 and between 2010 and 2018 to inform estimates. Further studies will be needed to assess additional improvement after 2010, in particular of newer therapies such as the CDK4/6 inhibitors, where no overall survival benefit has been seen yet in randomized clinical trials. Finally, the existing evidence comes from a subset of the population, because some of the studies came from single institutions (albeit different ones).
There are several implications of our results for filling data gaps and biases in determining true population trends in MBC survival. The ascertainment bias we identified with left-truncated data could be resolved by including any distant recurrence, without a cap on diagnosis year. Although costly, the most straightforward way to bridge the data gap in collection of recurrence information by US population-based cancer registries would be to mandate reporting of distant recurrence. Alternative indirect approaches have been developed such as linkage of registry information to claims data in SEER-Medicare and other settings, with some success but also limitations (62–68). Promising new approaches include a broader data linkage initiative encompassing pharmacy, pathology, imaging, electronic medical records and other data sources, and using natural language processing or other methods to capture events (69–74). Simulation models may also help to fill the evidence gap by simulating cancer outcomes by incorporating the best available data and testing assumptions through sensitivity analyses (3,75).
The question of whether MBC patients are living longer with newer treatments is clinically relevant. Systemic therapies, including novel targeted agents, have clinically significant side effects and costs that must be weighed against their potential benefits. Evidence of progress can inform patient-doctor conversations about MBC prognosis and the likely benefit of existing therapies.
In conclusion, our meta-regression of existing studies demonstrated an improvement in survival over time for MBC, whether recurrent or de novo and across ER subtypes. This study synthesized evidence across a 40-year horizon and supported the hypothesis that advances tested in RCTs have translated to improved survival in the population. The gaps and biases we identified offer a road map for improvement in observational data collection to support future measurement of the real-world benefit of emerging therapies that show promise in RCTs.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (U01CA12958); and the Breast Cancer Research Foundation (GWS and AWK). JLC is supported by a Damon Runyon Physician-Scientist Training Award.
Notes
Affiliations of authors: Department of Medicine, Stanford University School of Medicine, Stanford, CA (JLC, GWS, AWK); Department of Biomedical Data Science (SKP, CX) and Department of Radiology (SKP, CX), Stanford University School of Medicine, Stanford, CA; Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (LT, AWK); Department of Oncology, Georgetown University Medical Center, Lombardi Comprehensive Cancer Center, Cancer Prevention and Control Program, Washington, DC (CJC, JSM); Department of Population Health, Harvard Pilgrim Health Care, Boston, MA (NKS).
Supplementary Material
References
- 1. Torre LA, Islami F, Siegel RL, et al. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;264:444–457. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;671:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;35317:1784–1792. [DOI] [PubMed] [Google Scholar]
- 4. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA. 2018;3192:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;266:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN guidelines insights: breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;154:433–451. [DOI] [PubMed] [Google Scholar]
- 7. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;3525:2875–2884. [DOI] [PubMed] [Google Scholar]
- 8. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;3532:3638–3646. [DOI] [PubMed] [Google Scholar]
- 9. Hortobagyi GN. Ribociclib for HR-positive, advanced breast cancer. N Engl J Med. 2017;3763:289.. [DOI] [PubMed] [Google Scholar]
- 10. Canellos GP, Pocock SJ, Taylor SG 3rd, et al. Combination chemotherapy for metastatic breast carcinoma. Prospective comparison of multiple drug therapy with L-phenylalanine mustard. Cancer. 1976;385:1882–1886. [DOI] [PubMed] [Google Scholar]
- 11. Jones S, Winer E, Vogel C, et al. Randomized comparison of vinorelbine and melphalan in anthracycline-refractory advanced breast cancer. J Clin Oncol. 1995;1310:2567–2574. [DOI] [PubMed] [Google Scholar]
- 12. Nabholtz JM, Senn HJ, Bezwoda WR, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol. 1999;175:1413–1424. [DOI] [PubMed] [Google Scholar]
- 13. Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;2324:5542–5551. [DOI] [PubMed] [Google Scholar]
- 14. O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;2012:2812–2823. [DOI] [PubMed] [Google Scholar]
- 15. Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;3779769:914–923. [DOI] [PubMed] [Google Scholar]
- 16. Dieras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;186:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;146:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;34411:783–792. [DOI] [PubMed] [Google Scholar]
- 19. Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;2934:4498–4504. [DOI] [PubMed] [Google Scholar]
- 20. Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;2624:3950–3957. [DOI] [PubMed] [Google Scholar]
- 21. Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;3332:3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mauri D, Polyzos NP, Salanti G, et al. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;10024:1780–1791. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Ren J, Sun W.. Systematic review of ixabepilone for treating metastatic breast cancer. Breast Cancer. 2017;242:171–179. [DOI] [PubMed] [Google Scholar]
- 24. Gibson L, Lawrence D, Dawson C, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;4:CD003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;1001:44–52. [DOI] [PubMed] [Google Scholar]
- 26. Rogoz B, Houze de l'Aulnoit A, Duhamel A, et al. Thirty-year trends of survival and time-varying effects of prognostic factors in patients with metastatic breast cancer-a single institution experience. Clin Breast Cancer. 2017; doi:10.1016/j.clbc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 27. Tevaarwerk AJ, Gray RJ, Schneider BP, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;1196:1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakano M, Fujisue M, Tashima R, et al. Survival time according to the year of recurrence and subtype in recurrent breast cancer. Breast. 2015;245:588–593. [DOI] [PubMed] [Google Scholar]
- 29. Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;1912:2012–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuji W, Teramukai S, Ueno M, et al. Prognostic factors for survival after first recurrence in breast cancer: a retrospective analysis of 252 recurrent cases at a single institution. Breast Cancer. 2014;211:86–95. [DOI] [PubMed] [Google Scholar]
- 31. Anan K, Mitsuyama S, Koga K, et al. Disparities in the survival improvement of recurrent breast cancer. Breast Cancer. 2010;171:48–55. [DOI] [PubMed] [Google Scholar]
- 32. Shigematsu H, Kawaguchi H, Nakamura Y, et al. Significant survival improvement of patients with recurrent breast cancer in the periods 2001-2008 vs. 1992-2000. BMC Cancer. 2011;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foukakis T, Fornander T, Lekberg T, et al. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat. 2011;1302:553–560. [DOI] [PubMed] [Google Scholar]
- 34. Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;1048:1742–1750. [DOI] [PubMed] [Google Scholar]
- 35. Sundquist M, Brudin L, Tejler G.. Improved survival in metastatic breast cancer 1985-2016. Breast. 2017;31:46–50. [DOI] [PubMed] [Google Scholar]
- 36. Chia SK, Speers CH, D'Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;1105:973–979. [DOI] [PubMed] [Google Scholar]
- 37. Dafni U, Grimani I, Xyrafas A, et al. Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res Treat. 2010;1193:621–631. [DOI] [PubMed] [Google Scholar]
- 38. Weide R, Feiten S, Friesenhahn V, et al. Metastatic breast cancer: prolongation of survival in routine care is restricted to hormone-receptor- and Her2-positive tumors. Springerplus. 2014;31:535.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giuliani J, Bonetti A.. Trends in survival for patients with metastatic breast cancer: is survival improving? Tumori. 2015;1014:347–352. [DOI] [PubMed] [Google Scholar]
- 40. Holleczek B, Arndt V, Stegmaier C, et al. Trends in breast cancer survival in Germany from 1976 to 2008—a period analysis by age and stage. Cancer Epidemiol. 2011;355:399–406. [DOI] [PubMed] [Google Scholar]
- 41. Ernst MF, van de Poll-Franse LV, Roukema JA, et al. Trends in the prognosis of patients with primary metastatic breast cancer diagnosed between 1975 and 2002. Breast. 2007;164:344–351. [DOI] [PubMed] [Google Scholar]
- 42. Pal SK, Dehaven M, Nelson RA, et al. Impact of modern chemotherapy on the survival of women presenting with de novo metastatic breast cancer. BMC Cancer. 2012;12:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;2216:3302–3308. [DOI] [PubMed] [Google Scholar]
- 44. Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;2630:4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruiterkamp J, Ernst MF, de Munck L, et al. Improved survival of patients with primary distant metastatic breast cancer in the period of 1995-2008. A nationwide population-based study in the Netherlands. Breast Cancer Res Treat. 2011;1282:495–503. [DOI] [PubMed] [Google Scholar]
- 46. Knapp G, Hartung J.. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;2217:2693–2710. [DOI] [PubMed] [Google Scholar]
- 47. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;1099:djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pavlik T, Majek O, Buchler T, et al. Trends in stage-specific population-based survival of cancer patients in the Czech Republic in the period 2000-2008. Cancer Epidemiol. 2014;381:28–34. [DOI] [PubMed] [Google Scholar]
- 49. Vogel CL, Azevedo S, Hilsenbeck S, et al. Survival after first recurrence of breast cancer. The Miami experience. Cancer. 1992;701:129–135. [DOI] [PubMed] [Google Scholar]
- 50. Zeichner SB, Ambros T, Zaravinos J, et al. Defining the survival benchmark for breast cancer patients with systemic relapse. Breast Cancer (Auckl.) 2015;9:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giuliani J, Bonetti A.. Trends in survival for patients with metastatic breast cancer: is survival improving? Tumori. 2015;1014:347–352. [DOI] [PubMed] [Google Scholar]
- 52. Di Meglio A, Freedman RA, Lin NU, et al. Time trends in incidence rates and survival of newly diagnosed stage IV breast cancer by tumor histology: a population-based analysis. Breast Cancer Res Treat. 2016;1573:587–596. [DOI] [PubMed] [Google Scholar]
- 53. Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;561:67–78. [DOI] [PubMed] [Google Scholar]
- 54. Chang J, Clark GM, Allred DC, et al. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;973:545–553. [DOI] [PubMed] [Google Scholar]
- 55. Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;152:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;2418:2743–2749. [DOI] [PubMed] [Google Scholar]
- 57. Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;35616:1670–1674. [DOI] [PubMed] [Google Scholar]
- 58. Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;2111:2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;2925:3366–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rocque G, Blayney DW, Jahanzeb M, et al. Choosing wisely in oncology: are we ready for value-based care? J Oncol Pract. 2017; doi:10.1200/JOP.2016.019281. [DOI] [PubMed] [Google Scholar]
- 61. Tao L, Chu L, Wang LI, et al. Occurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse population. Cancer Causes Control. 2016;279:1127–1138. [DOI] [PubMed] [Google Scholar]
- 62. Whyte JL, Engel-Nitz NM, Teitelbaum A, et al. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care. 2015;537:e49–e57. [DOI] [PubMed] [Google Scholar]
- 63. Nordstrom BL, Whyte JL, Stolar M, et al. Identification of metastatic cancer in claims data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 2:21–28. [DOI] [PubMed] [Google Scholar]
- 64. Hassett MJ, Ritzwoller DP, Taback N, et al. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med Care. 2014;5210:e65–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Earle CC, Nattinger AB, Potosky AL, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40(8 Suppl):IV-75–IV-81. [DOI] [PubMed] [Google Scholar]
- 66. Chubak J, Yu O, Pocobelli G, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst. 2012;10412:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chawla N, Yabroff KR, Mariotto A, et al. Limited validity of diagnosis codes in Medicare claims for identifying cancer metastases and inferring stage. Ann Epidemiol. 2014;249:666–672.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Warren JL, Mariotto A, Melbert D, et al. Sensitivity of medicare claims to identify cancer recurrence in elderly colorectal and breast cancer patients. Med Care. 2013; doi:10.1097/MLR.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. J Registry Manag. 2014;412:57–64. [PubMed] [Google Scholar]
- 70. Sledge GW Jr, Miller RS, Hauser R.. CancerLinQ and the future of cancer care. Am Soc Clin Oncol Educ Book 2013;33430:430–434. [DOI] [PubMed] [Google Scholar]
- 71.Colwell J. FDA Partners with ASCO's CancerLinQ. Cancer Discov 2017;78:OF2. [DOI] [PubMed] [Google Scholar]
- 72. Lau EC, Mowat FS, Kelsh MA, et al. Use of electronic medical records (EMR) for oncology outcomes research: assessing the comparability of EMR information to patient registry and health claims data. Clin Epidemiol. 2011;3:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Warren JL, Yabroff KR.. Challenges and opportunities in measuring cancer recurrence in the United States. J Natl Cancer Inst. 2015;1078:djv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Penberthy L. Research grade data from cancer registries—the SEER perspective American Society of Clinical Oncology annual meeting, 2017; Chicago, IL. [Google Scholar]
- 75. Munoz D, Near AM, van Ravesteyn NT, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;10611:dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.