Abstract
Royal jelly (RJ) from honeybee has been widely used as a health promotion supplement. The major royal jelly proteins (MRJPs) have been identified as the functional component of RJ. However, the question of whether MRJPs have anti-senescence activity for human cells remains. Human embryonic lung fibroblast (HFL-I) cells were cultured in media containing no MRJPs (A), MRJPs at 0.1 mg/ml (B), 0.2 mg/ml (C), or 0.3 mg/ml (D), or bovine serum albumin (BSA) at 0.2 mg/ml (E). The mean population doubling levels of cells in media B, C, D, and E were increased by 12.4%, 31.2%, 24.0%, and 10.4%, respectively, compared with that in medium A. The cells in medium C also exhibited the highest relative proliferation activity, the lowest senescence, and the longest telomeres. Moreover, MRJPs up-regulated the expression of superoxide dismutase-1 (SOD1) and down-regulated the expression of mammalian target of rapamycin (MTOR), catenin beta like-1 (CTNNB1), and tumor protein p53 (TP53). Raman spectra analysis showed that there were two unique bands related to DNA synthesis materials, amide carbonyl group vibrations and aromatic hydrogens. These results suggest that MRJPs possess anti-senescence activity for the HFL-I cell line, and provide new knowledge illustrating the molecular mechanism of MRJPs as anti-senescence factors.
Keywords: Major royal jelly protein, Human embryonic lung fibroblast (HFL-I) cell line, Anti-senescence, Relative proliferation activity, Telomere length, Molecular mechanism
1. Introduction
Royal jelly (RJ), secreted from hypopharyngeal and mandibular glands of nurse honeybees, contains a considerable amount of bioactive substances and has been widely used as a food (Buttstedt et al., 2014). About 50% of the dry mass of RJ is protein (Malecová et al., 2003), of which 80% belongs to the major royal jelly proteins (MRJPs) (Schmitzová et al., 1998). RJ is the principal food for honeybee queens (Shen et al., 2010). It is believed that exclusive feeding on RJ induces a honeybee larva to develop into a queen, whose lifespan can reach a maximum of eight years much longer than that of worker bees (Remolina and Hughes, 2008). For its beneficial effect on honeybee queens, RJ has become a well-known healthcare product in many countries in Asia and Europe. Consumers anticipate that RJ could exert a similar health benefit in humans.
Previous studies have shown that RJ has several kinds of bioactivity, including anti-oxidation (Jamnik et al., 2007; Cihan et al., 2013), neuronal function protection (Hashimoto et al., 2005; Narita et al., 2009; Hattori et al., 2010; Zamani et al., 2012), and immunoregulatory effects (Šver et al., 1996; Oka et al., 2001; Okamoto et al., 2003; Majtán et al., 2006; Mannoor et al., 2009). Moreover, RJ enhances the reproductivity of many animals such as female sheep, rabbit (Husein and Kridli, 2002), rat, quail, and hen (Shen et al., 2009). Many studies have also found that RJ is effective in extending the lifespans of mice (Inoue et al., 2003) and worms (Honda et al., 2011).
Ten proteins with a molecular weight of 49‒87 kDa have been reported in the MRJP family (Schmitzová et al., 1998; Drapeau et al., 2006). MRJP1, the most abundant component in the MRJP family, is the primary active substance in RJ (Kamakura, 2011). MRJPs can stimulate DNA synthesis and increase albumin production in hepatocytes (Kamakura et al., 2001). They also protect cells from apoptosis by the action of several important intracellular signaling factors (Kamakura, 2002). MRJPs extracted from RJ can stimulate the proliferation of several cell lines, including hepatocytes (Kamakura et al., 2001), human myeloid cell line U-937 (Watanabe et al., 1998), human monocytes (Kimura et al., 2003), and Tn-5B1-4 insect cells (Salazar-Olivo and Paz-González, 2005). Our recent study suggests that MRJPs can increase mean lifespan and reproductivity in Drosophila (Xin et al., 2016) and partially replace fetal bovine serum (FBS) for culturing several human cell lines and increase the cell viability (Chen et al., 2016).
The question of whether MRJPs have anti-senescence activity for human cells such as human embryonic lung fibroblast (HFL-I) cells remains. Therefore, in this study we examined the effects of MRJPs on the proliferation activity, population doubling level (PDL), senescence-associated β-galactosidase (SA-β-gal) activity, telomere length, expression of age-related genes, and Raman spectra of HFL-I cells.
2. Materials and methods
2.1. Cell line and reagents
The HFL-I cell line was obtained from Shanghai Sixin Biotechnology Co., Ltd. (Shanghai, China). Primers were supplied by TaKaRa Co., Ltd. (Beijing, China). Mammalian DNA extraction and SA-β-gal activity kits were purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Reverse transcription polymerase chain reaction (RT-PCR) and real-time quantitative PCR (qPCR) kits were supplied by TaKaRa Co., Ltd. (Beijing, China). A 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay kit was purchased from Nanjing Keygen Biotech. Co., Ltd. (Nanjing, China). FBS was purchased from HyClone Laboratories, Inc. (Logan, USA). F12K media were supplied by Gino Biotechnology Co., Ltd. (Hangzhou, China). All biochemical reagents were of the highest purity commercially available.
2.2. Preparation of MRJPs
Fresh RJ was provided by the Hangzhou Biyuntian Heath Food Co., Ltd. (Hangzhou, China) and stored at −80 °C. For extraction, RJ was diluted in phosphate buffered saline (PBS) and extracted at 4 °C for 12 h. Then the extraction was centrifuged at 12 000g for 30 min at 4 °C. MRJPs were obtained from the upper layer of the extraction, which was dialyzed against 500 volumes of double-distilled water (ddH2O) using a 1000 Da cutoff dialysis membrane at 4 °C (Salazar-Olivo and Paz-González, 2005). The concentration of MRJPs was measured by the Bradford method using bovine serum albumin (BSA) as a standard (Bradford, 1976).
2.3. HFL-I cultivation
The HFL-I cell line was cultured in F12K medium supplied with 10% (v/v) FBS at 37 °C in a humid environment with 5% (v/v) CO2. MRJPs and BSA solution were diluted with F12K medium by 5 mg/ml, and added to media as calculated. Five treatments were prepared as follows: (A) no MRJPs, (B) 0.1 mg/ml MRJPs, (C) 0.2 mg/ml MRJPs, (D) 0.3 mg/ml MRJPs, and (E) 0.2 mg/ml BSA. Cells were exposed to the various concentrations of MRJPs or BSA and cultured until they reached cellular senescence.
2.4. Morphological observations
Cells were observed every day and cell images were taken using an inverted microscope. The shape, size, and refractivity of HFL-I cells were considered as indicators to evaluate the condition of the cells.
2.5. Cumulative population doubling level (PDL) measurements
Cell passage cultivations were taken when the cell confluence level reached 80%–90%. The population doubling (PD) index was calculated according to the equation: PD=(lgH−lgO)/0.301, where H means the number of harvested cells and O means the original number of cells. An increase in PD was added to the previous PD to determine the cumulative PDL. Cells were defined as senescent when cell confluence failed to reach 90% in four weeks. The amounts of harvested and original cells were counted using a cell counter plate.
2.6. Cellular proliferation activity
The growth-promoting activity was measured by MTT assay (Yang et al., 2013). Logarithmic growth phase cells (2×103 cells/ml) suspended in 200 μl media were inoculated into 96-well plates and cultured consecutively for 4 d. The absorbance of each well at the designated time point was measured every day at 490 nm wavelength after the MTT assay. The proliferation activity was shown by the absorbance.
2.7. SA-β-gal activity
Logarithmic growth phase cells (2×103 cells/ml) suspended in 200 μl media were inoculated into 96-well plates. The SA-β-gal activity kit was used after the cells were completely adherent. SA-β-gal positive (blue-stained) cells were manually counted using an inverted microscope. SA-β-gal activity was expressed as the percentage of stained cells in the total cell population.
2.8. Relative telomere length
qPCR was used to measure the telomere length of HFL-I cells. After the indicated treatments, total DNA was extracted from cells using the mammalian DNA extraction kit. qPCR was performed using SYBR green PCR core reagents. The following primers were used: 5'-CGTTTGTTTGGGTTTGGGTT TGGGTTTGGGTTTGGGTT-3' (forward) and 5'-GC TTGCCTTACCCTTACCCTTACCCTTACCCTTAC CCT-3' (reverse); RPLP0: 5'-CAGCAAGTGGGAA GGTGTAATCC-3' (forward) and 5'-CCCATTCTA TCATCAACGGGTACAA-3' (reverse). The PCR products were obtained at 95 °C (30 s), followed by 40 cycles of 95 °C (5 s) and 60 °C (30 s). The ΔΔcycle threshold (ΔΔC T) method was used to quantify the data. RPLP0 was used as a reference gene. The relative expression (RE) of telomeres was expressed as fold change and calculated according to the equation: RE=2−ΔΔ C T.
2.9. Relative expression of age-related genes
The expression of four age-related genes was measured using qPCR. After the indicated treatments, total RNA was extracted from cells using the TRIzol method, and reverse translated to cDNA using the RT-PCR kit. qPCR was performed using SYBR green PCR core reagents. The primers were those listed in Table 1. The PCR products were obtained at 95 °C (30 s), followed by 40 cycles of 95 °C (5 s) and 60 °C (30 s). The ΔΔC T method was used to quantify the data and β-actin (ACTB) was used as a normalization gene. The RE of the four genes was also calculated according to the equation: RE=2−ΔΔ C T.
Table 1.
Primers used in RT-PCR
| Gene symbol | GenBank accession No. | Direction | PCR primer sequence (5' to 3') | Primer size (bp) |
| mTOR | NM_004958.3 | Forward | AAACTGCACGTCAGCACCATC | 21 |
| Reverse | AGCCGTCTCAGCCATTCCA | 19 | ||
| SOD1 | NM_000454.4 | Forward | AGTGCAGGGCATCATCAATTTC | 22 |
| Reverse | CCATGCAGGCCTTCAGTCAG | 20 | ||
| CTNNB1 | NM_001098209.1 | Forward | TTAGCTGGTGGGCTGCAGAA | 20 |
| Reverse | GGGTCCACCACTAGCCAGTATG | 22 | ||
| TP53 | NM_001126112.2 | Forward | TCGAGATGTTCCGAGAGCTGAAT | 23 |
| Reverse | GTCTGAGTCAGGCCCTTCTGTCTT | 24 | ||
| ACTB | NM_001101.3 | Forward | TGGCACCCAGCACAATGAA | 19 |
| Reverse | CTAAGTCATAGTCCGCCTAGAAGCA | 25 |
2.10. Raman spectrum analysis
With different frequencies of incident light, Raman spectroscopy can detect and analyze scattering spectra, molecular vibration, and rotation information from live cells. This method is often used as a diagnostic tool to study cancer cell lines or a single cancer cell (Yan et al., 2005; Li et al., 2013). It has been used to analyze the senescence of cells in vivo (Bai et al., 2015). Here we used Raman spectroscopy to estimate the biochemical changes in HFL-I cells in media treated with different concentrations of MRJPs.
After the culture media were removed, the cells were washed twice with PBS gently. Raman spectral data were obtained using a laser Raman microscope. An excitation laser of 785 nm with power of 50 mW was focused on a single cell each time through a 10×objective lens. Raman scattering light from the sample was collected and detected by a liquid nitrogen supplied charge coupled device (CCD) with a grating (600 gr/mm) under the visible region. Each cell was exposed to the laser for 3 s, and the spectrum of this targeted cell was drawn and analyzed using Microsoft Excel. The spectral resolution error was less than 5 cm−1, and the diameter of the laser spot at the focus point was less than 1 μm.
2.11. Statistical analysis
The data reported represent average values from at least three independent experiments and were analyzed using Statistical Product and Service Solutions (SPSS) software. One-way analysis of variance (ANOVA) was used to evaluate differences between each group. If there was a significant difference (P<0.05) between groups, Duncan analysis was used to compare all the groups in pairs. The values are shown as mean±standard deviation (SD), and P<0.05 was considered statistically significant.
3. Results
3.1. Morphological phenotypes of senescent cells
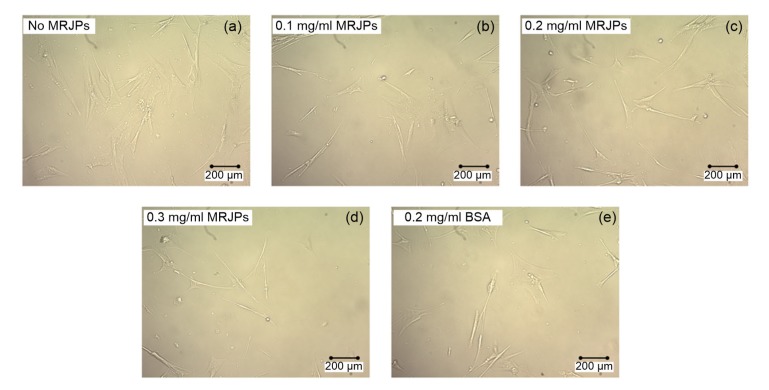
Cellular morphological observations showed that the manifestation of HFL-I cells was influenced by MRJPs. As shown in Fig. 1a, most of the cells in medium A (no MRJPs) became flat, and the cellular boundary was obscure, a cellular phenotype typical of senescent cells. Many HFL-I cells displayed the unattached state, losing their spindle shapes. However, most cells in medium C (0.2 mg/ml MRJPs; Fig. 1c) and medium D (0.3 mg/ml MRJPs; Fig. 1d) had a healthy shape and normal size. The cell boundaries were distinct. The condition of the cells in medium B (0.1 mg/ml MRJPs; Fig. 1b) and medium E (0.2 mg/ml BSA; Fig. 1e) was similar, and some of the cells in both media were in a senescent state.
Fig. 1.
Cellular morphology of HFL-I cells supplied with different concentrations of MRJPs or BSA at Day 59
3.2. Lifespan-extending effect of MRJPs
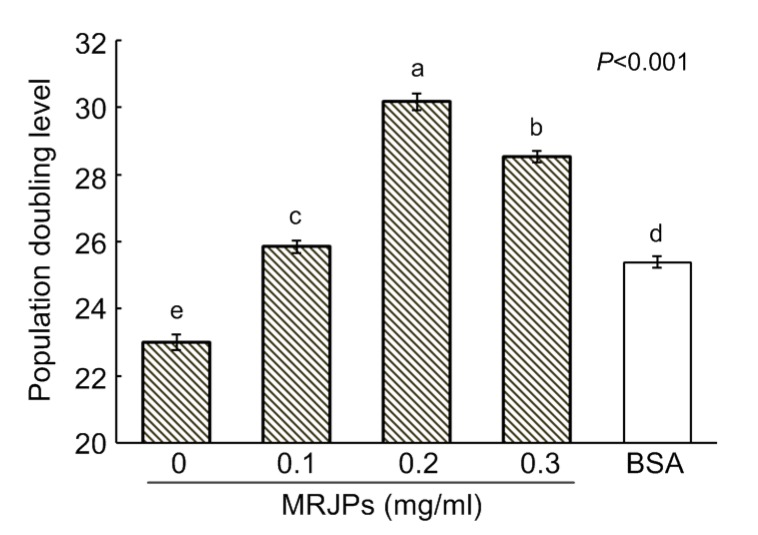
Fig. 2 shows that the average PDL of cells differed significantly among the treatments (P<0.001). Compared with the cells in medium A, the PDL of the cells in media B, C, D, and E increased by 12.4%, 31.2%, 24.0%, and 10.4%, respectively. Compared with the cells in medium E, the PDL of the cells in media B, C, and D increased by 1.8%, 18.8%, and 12.4%, respectively. The cells in medium C had the highest PDL, followed by the cells in media D, A, and E. Thus, a concentration of 0.2 mg/ml MRJPs (medium C) was the optimal dose for lifespan-extension of HFL-I cells.
Fig. 2.
Lifespans of HFL-I cells supplied with different concentrations of MRJPs or BSA
The PDL represents the lifespan of HFL-I. Data are expressed as mean±SD (n=3). Different lowercase letters (a–e) above bars indicate a significant difference from each other at P<0.05 by Duncan’s multiple range tests
3.3. Proliferation effect of MRJPs
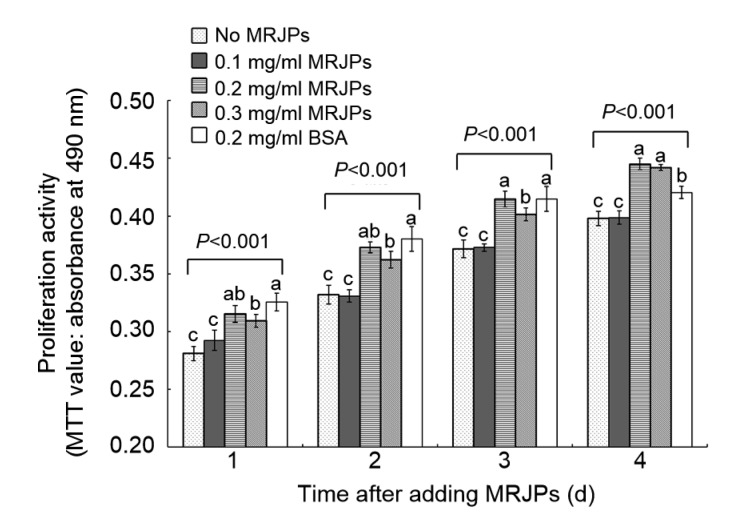
On the fourth day, the cells in media A, B, C, D, and E displayed differential cellular proliferation activity (absorbance): 0.39, 0.40, 0.45, 0.44, and 0.42, respectively (Fig. 3). Compared with the negative control (medium A), the relative proliferation activity (RPA) of the cells in media B, C, D, and E had increased by 1.0%, 11.9%, 11.1%, and 7.7%, respectively. In addition, the cells in medium B showed no significant difference (P>0.05) when compared with those in medium A after 4 d. However, the RPA of the cells in media C and D had increased significantly (P<0.001) compared with that of cells in medium A after 4 d.
Fig. 3.
Proliferation effect of MRJPs after 4 d
The proliferation activity represents the relative proliferation of HFL-I. Data are expressed as mean±SD (n=8). Different lowercase letters (a–c) above bars within the same day indicate a significant difference from each other at P<0.05 by Duncan’s multiple range test
3.4. SA-β-gal activity measurement
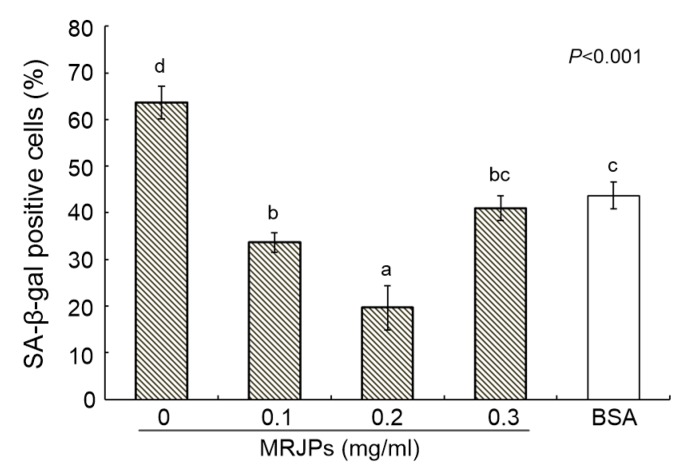
The percentage of positive cells measured by SA-β-gal activity assay (Fig. 4) showed the extent of the senescence of HFL-I cells. The cells in medium C had the lowest percentage of positive cells (19.7%), followed by the cells in media D (41.0%) and B (33.7%). Cells in medium A had the highest percentage (63.7%), followed by those in medium E (43.7%). The percentage of positive cells in media B, C, D, and E had decreased by 47.1%, 69.1%, 35.6%, and 31.4% respectively, compared with that in medium A.
Fig. 4.
Percentages of senescent cells after consecutive culturing for 59 d
Cells stained blue were defined as senescent. Data are expressed as mean±SD (n=3). Different lowercase letters (a–d) above bars indicate a significant difference from each other at P<0.05 by Duncan’s multiple range test
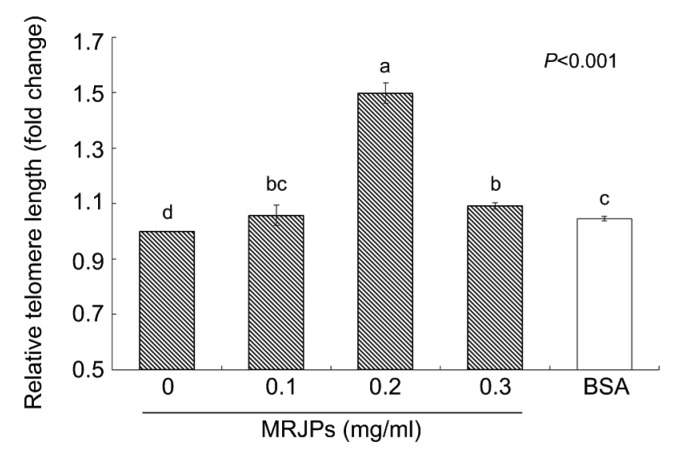
3.5. Effect of MRJPs on telomere length
Fig. 5 shows the effects of supplemental MRJPs of different concentrations in media on the relative telomere length in HFL-I cells. The cells in medium A clearly exhibited the lowest relative length (RL). The RL of HFL-I cells in medium C was significantly different from the RLs of cells in the other media. The RLs of cells in all the media containing MRJPs were higher than those of media E and A. Compared with the RL of cells in medium A, the RLs of the cells in media B, C, D, and E increased by 6%, 50%, 10%, and 4%, respectively.
Fig. 5.
Telomere length using qPCR
Data are shown as fold-changes of the negative control group (medium A). Data are expressed as mean±SD (n=3). Different lowercase letters (a–d) above bars indicate a significant difference from each other at P<0.05 by Duncan’s multiple range test
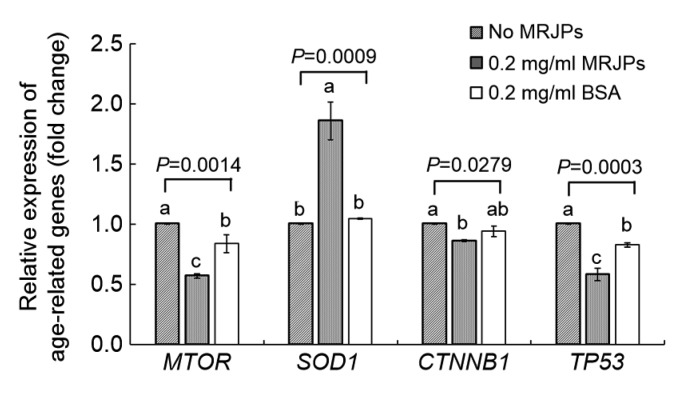
3.6. Effect of MRJPs on age-related gene expression
To understand the molecular mechanism underlying the anti-senescence effect of MRJPs on HFL-I cells, we measured the RE of mammalian target of rapamycin (MTOR), superoxide dismutase-1 (SOD1), catenin beta like-1 (CTNNB1), and tumor protein p53 (TP53) in HFL-I cells (Fig. 6).
Fig. 6.
Age-related gene expression using qPCR
Data are shown as fold-changes of the negative control group (medium A). Data are expressed as mean±SD (n=3). Different lowercase letters (a–c) above bars within the same gene indicate a significant difference from each other at P<0.05 by Duncan’s multiple range test
Cells in medium C (0.02 mg/ml MRJPs) were used to evaluate the effect on gene expression exerted by MRJPs as they showed the longest lifespan. Cells in media A (no MRJPs) and E (0.02 mg/ml BSA) were used as negative and positive controls, respectively. Significant differences (P<0.05) in RE of four age-related genes were observed between cells in media C and A (negative control). Cells in medium C showed lower RE of MTOR, CTNNB1, and TP53 than those in medium A, decreasing significantly (P<0.05) by 43%, 14%, and 42%, respectively. A significant increase (P=0.0009) of about 86% in SOD1 expression was observed in cells in medium C compared with that in medium A.
On the other hand, cells in medium C had a higher RE level of SOD1, and lower RE levels of MTOR and TP53 than those in medium E (positive control), while there was no significant difference in the RE of CTNNB1 (P>0.05). Compared with medium E, media supplemented with MRJPs showed stronger bioactivity than BSA to some extent.
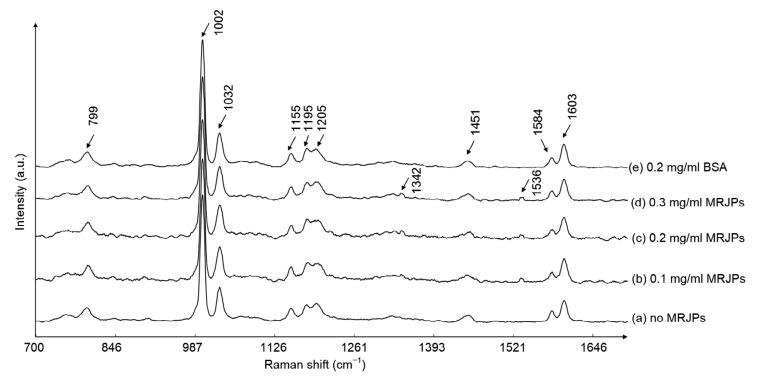
3.7. Raman spectrum analysis
To estimate the biochemical changes induced in HFL-I cells in media with different concentrations of MRJPs, we measured the mean spectra of treated cells normalized by the Raman peak of phenylalanine at 1002 cm−1. The contributions of peaks, as identified by Li et al. (2013) and Movasaghi et al. (2007), are listed in Table 2. The mean spectra from the different treatments were roughly similar (Fig. 7). However, bands at 1342 and 1536 cm−1 were detected only on the spectra of MRJP-treated groups and were assigned to purine (guanine and adenine) and amide carbonyl group vibrations and aromatic hydrogens, respectively.
Table 2.
Band positions and contributions
| Raman shift (cm−1) | Contribution |
| 799 | Backbone geometry and phosphate ion interactions |
| 1002 | Phenylalanine |
| 1032 | C–H/C–C stretching of phenylalanine |
| 1155 | C–C and C–N stretching of proteins |
| 1195 | Anti-symmetric phosphate vibrations |
| 1205 | Differences in collagen content |
| 1342 | Guanine and adenine |
| 1451 | CH2 bending and CH2CH3 deformation of proteins and collagens |
| 1536 | Amide carbonyl group vibrations and aromatic hydrogens |
| 1584 | C=C bending of phenylalanine |
| 1603 | C=C vibration of phenylalanine and tyrosine |
Fig. 7.
Mean Raman spectra of HFL-I cells
Substance changes as shown through Raman spectra. Each number in this figure represents one position of a band, as explained in Table 2. a.u.: arbitrary unit
4. Discussion
In this study, we discovered that MRJPs may exert an anti-senescence effect on HFL-I cells. The cells in MRJP media experienced no or delayed typical cell aging, whereas cells developed senescence in MRJP-free media. The PDL assay showed that MRJPs enabled extension of the lifespan of HFL-I cells. The cells in MRJP media also had higher RPA, lower SA-β-gal activity, and longer telomere length than cells in MRJP-free media. In MRJP-supplied media, the expression of SOD1 increased and the expression of MTOR, CNNB1, and TP53 decreased. In addition, Raman spectra analysis showed that peaks assigned to purine and amide carbonyl groups changed when MRJPs were added to the media.
The HFL-I cell line has been widely used in studies investigating cell senescence, apoptosis, proliferation, growth factors, and toxicological tests. Matsuo (2004) revealed that the lifespan of HFL-I cells is perturbed under oxidative stress. HFL-I is also used as a model organism in cell apoptosis tests (Xu et al., 2012; Ren et al., 2013; Chen et al., 2014; Ershova et al., 2016) and proliferation tests (Vietti et al., 2013; Zeng et al., 2015). We previously found that FBS culture media partially replaced by MRJPs could exert a proliferation effect on several cell lines, including the HFL-I cell line (Chen et al., 2016). Cell senescence is followed by a series of phenomena, including morphological changes, the loss of proliferation ability, and an increase in SA-β-gal activity (Cristofalo and Pignolo, 1993; Dimri et al., 1995; Toussaint et al., 2000; Liu et al., 2007; Debacq-Chainiaux et al., 2008; Hwang et al., 2009). Morphological changes are widely used to identify senescent cells. One of the most apparent morphological hall-marks of senescent human diploid cells is that they usually have irregular flat and enlarged shapes (Cristofalo, 1988; Cho et al., 2004). Moreover, some senescent fibroblasts retract their branches resulting in a higher number of cells per unit area devoid of cell–cell contacts (Foyer et al., 2009). In the morphological assay, senescence-related cellular morphologies partially disappeared after adding 0.2 or 0.3 mg/ml MRJPs. Thus, we discovered that MRJPs might have an anti-senescence effect on HFL-I cells. As for proliferation ability, the results of PDLs in this study imply that supplementation with MRJPs could significantly extend the mean lifespan of HFL-I cells. MTT assay after consecutive cultivation of HFL-I cells with or without different MRJPs concentrations also suggested that MRJPs might affect the proliferation of HFL-I cells.
Cellular senescence occurs following cell proliferation failure or exposure to a variety of cellular stresses, e.g. expression of activated oncogenes, oxidation, or DNA damaging agents. Studies have pointed out that proliferation rate is an essential parameter to judge the senescence level of cells (Toussaint et al., 2000) and that the SA-β-gal assay is a robust method to distinguish senescent cells from non-senescent cells in vitro (Itahana et al., 2004, 2007; Krishnamurthy et al., 2004; Liu et al., 2007; Debacq-Chainiaux et al., 2009). Our results showed that four-day incubation with addition of 0.2 or 0.3 mg/ml MRJPs significantly delayed the increase in senescent HFL-I cells, implying that MRJPs were likely to have promoted proliferation activity. From the results of SA-β-gal activity measurement, we found that the experimental MRJPs concentrations reduced the proportion of stained (senescent) cells and the best MRJP concentration was 0.2 mg/ml. Thus, MRJPs may play an important role in inhibiting the overexpression of β-galactosidase in HFL-I cells, and seem to exhibit an anti-senescence effect on HFL-I cells.
Telomeres are specialized functional complexes located at the ends of eukaryotic chromosomes (Blackburn, 2001). The shortening of telomeres could induce the DNA damage stress response and cell apoptosis. Without telomeres, terminal attrition of chromosomal DNA would lead to loss of genetic information and prevent cells from multiplying (Smogorzewska and de Lange, 2002; D'Adda di Fagagna et al., 2003; Takai et al., 2003). Numerous studies have proved that telomeres shorten with the aging of cells and tissues (Allsopp et al., 1992; Kitada et al., 1995; Jeanclos et al., 1998; Okuda et al., 2000). These phenomena all support the hypothesis that telomere length limits cell mitosis (Harley, 1991; Chan and Blackburn, 2004; Debacq-Chainiaux et al., 2008) and indicates the replicative potential and age of cells (Harley et al., 1990; Toussaint et al., 2000; von Zglinicki et al., 2005). It has been reported that telomere length is positively associated with proliferative potential in fibroblasts (Allsopp et al., 1992; Murillo-Ortiz et al., 2013). The finding that cells in MRJP media have longer telomeres shows that MRJPs have the ability to inhibit the shortening of telomeres in HFL-I cells and proves the anti-senescence effect of MRJPs on HFL-I cells.
Based on gene expression analysis of age-related genes and Raman spectroscopy analysis, we speculate that the regulation of age-related gene expression and the promotion of DNA and protein syntheses by MRJPs might be responsible for our observed results. MTOR, SOD1, CTNNB1, and TP53 are four age-related genes. MTOR plays a vital role in the mTOR/ S6K pathway. The mTOR/S6K signal pathway can be regulated by age-related factors such as stress, growth factors, nutritional conditions, and energy supplements (Sarbassov et al., 2005; Kapahi et al., 2010). Previous studies have indicated that the lifespan of yeasts, worms, fruit flies, and mice can be extended by inhibiting mTOR/S6K signal pathway through gene knockout, rapamycin treatment, or dietary restriction (Kapahi et al., 2010). MRJPs may exert the anti-senescence effect through inhibiting the expression of mTOR and signal transduction in the mTOR/S6K pathway. The oxidative stress theory claims that the accumulation of oxidative damage induced by reactive oxygen species (ROS) will lead to senescence and apoptosis (Salmon et al., 2010). However, overexpression of SOD1, an enzyme located mainly in the cytosol to remove highly toxic superoxide radicals, extends the lifespan of Drosophila, while sod1 knockdown causes a reduction of lifespan in mice and flies (Phillips et al., 1989; Elchuri et al., 2005; Landis and Tower, 2005). Previous reports revealed that many age-related genes are involved in the oxidative stress response (Zahn and Kim, 2007; Zhan et al., 2007; Salmon et al., 2010). Our results showed an overexpression of the SOD1 gene in the cells supplied with MRJP media. This finding supports the idea that MRJPs may protect HFL-I cells from oxidative damage through promoting the expression of the SOD1 gene and scavenging the ROS in vivo.
β-Catenin encoded by the CTNNB1 gene is a signal molecule in the Wnt/β-catenin pathway. This pathway is one of the most essential signal transduction pathways in the growth process of vertebrate creatures. It has been proposed that the transduction level of the Wnt/β-catenin pathway increases with age (Brack et al., 2007; Liu et al., 2007; Xu et al., 2008). Moreover, overexpression of the p53 protein is associated with overexpression of β-catenin (Damalas et al., 2001) and the activation of the Wnt/β-catenin pathway (Mao et al., 2001; Hiyama et al., 2010). MRJPs might inhibit β-catenin through the down-regulation of CTNNB1 gene expression, and the Wnt/β-catenin pathway may be suppressed as a result of the lack of the signal molecule (β-catenin). p53, encoded by the TP53 gene, plays a vital role in cell cycle control, cell stress reaction, and cell gene protection (May and May, 1999). p53 can be activated by cell stress factors such as genotoxicity, shortening of telomeres, hypoxia, and tumor gene activation (Kern et al., 1991). Senescence is induced by the accumulation of ROS oxidative damage, which leads to cell cycle arrest caused by p21 and E2F (the target genes of p53) (Gewirtz et al., 2008). Our findings suggest that MRJPs down-regulate the expression of the TP53 gene, which results in inhibition of p21 and lifespan extension of HFL-I cells. These results are consistent with the results above, showing that MRJPs induce a longer lifespan in HFL-I cells.
Raman spectroscopy is a rapid and non-invasive technique, which enables us to analyze cells and tissues in vivo without additional labeling or staining (Buschman et al., 2000; Swain et al., 2008). Guanine and adenine are the materials of DNA replication. Amide carbonyl group and aromatic hydrogens are compounds of protein synthesis. From changes in peaks representing purine bases and amide carbonyl groups in the Raman spectroscopy assay, we inferred that MRJPs might promote the syntheses of DNA and proteins in HFL-I cells. Our findings are coincident with previous studies which suggested that MRJPs stimulate hepatocyte DNA synthesis, prolong the proliferation of hepatocytes, and increase albumin production (Kamakura et al., 2001; Kamakura, 2002). However, since only small peaks representing purine bases and amide carbonyl groups were observed, they might also represent products from catabolism. So in the future, more studies are needed to prove whether these changes are related to DNA and protein syntheses. In general, we successfully used Raman spectroscopy to explore differences between cells growing in media with or without MRJPs. Our results might provide a new method to characterize cellular senescence.
5. Conclusions
We found that MRJPs could extend the lifespan of HFL-I cells when supplied in media. The anti-senescence effects of MRJPs were associated with up-regulation of SOD1 and down-regulations of MTOR, CTNNB1, and TP53, and had a stimulatory effect on DNA and protein syntheses. The anti-senescence effect of MRJPs on the HFL-I cell line may indicate a beneficial effect of MRJPs on human health.
Footnotes
Project supported by the Science and Technology Project of Zhejiang Province (No. 2017C32033) and the National Natural Science Foundation of China (No. 3127848)
Compliance with ethics guidelines: Chen-min JIANG, Xin LIU, Chun-xue LI, Hao-cheng QIAN, Di CHEN, Chao-qiang LAI, and Li-rong SHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Allsopp RC, Vaziri H, Patterson C, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai H, Li HY, Han ZB, et al. Label-free assessment of replicative senescence in mesenchymal stem cells by Raman microspectroscopy. Biomed Opt Express. 2015;6(11):4493–4500. doi: 10.1364/BOE.6.004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106(6):661–673. doi: 10.1016/S0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 4.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Buschman HP, Marple ET, Wach ML, et al. In vivo determination of the molecular composition of artery wall by intravascular Raman spectroscopy. Anal Chem. 2000;72(16):3771–3775. doi: 10.1021/ac000298b. [DOI] [PubMed] [Google Scholar]
- 7.Buttstedt A, Moritz RFA, Erler S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol Rev. 2014;89(2):255–269. doi: 10.1111/brv.12052. [DOI] [PubMed] [Google Scholar]
- 8.Chan SRWL, Blackburn EH. Telomeres and telomerase. Philos Trans Roy Soc B Biol Sci. 2004;359(1441):109–122. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D, Xin XX, Qian HC, et al. Evaluation of the major royal jelly proteins as an alternative to fetal bovine serum in culturing human cell lines. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(6):476–483. doi: 10.1631/jzus.B1500295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JP, Xu DG, Yu XY, et al. Discrepancy between the effects of morronside on apoptosis in human embryonic lung fibroblast cells and lung cancer A549 cells. Oncol Lett. 2014;7(4):927–932. doi: 10.3892/ol.2014.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho KA, Ryu SJ, Oh YS, et al. Morphological adjustment of senescent cells by modulating caveolin-1 status. J Biol Chem. 2004;279(40):42270–42278. doi: 10.1074/jbc.M402352200. [DOI] [PubMed] [Google Scholar]
- 12.Cihan YB, Ozturk A, Gokalp SS. Protective role of royal jelly against radiation-induced oxidative stress in rats. UHOD-Uluslar Hematol. 2013;23(2):79–87. doi: 10.4999/uhod.11016. [DOI] [Google Scholar]
- 13.Cristofalo VJ. Cellular biomarkers of aging. Exp Gerontol. 1988;23(4-5):297–305. doi: 10.1016/0531-5565(88)90032-0. [DOI] [PubMed] [Google Scholar]
- 14.Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993;73(3):617–638. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- 15.D'Adda di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 16.Damalas A, Kahan S, Shtutman M, et al. Deregulated β-catenin induces a p53-and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 2001;20(17):4912–4922. doi: 10.1093/emboj/20.17.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debacq-Chainiaux F, Pascal T, Boilan E, et al. Screening of senescence-associated genes with specific DNA array reveals the role of IGFBP-3 in premature senescence of human diploid fibroblasts. Free Radic Biol Med. 2008;44(10):1817–1832. doi: 10.1016/j.freeradbiomed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, et al. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo . Nat Protoc. 2009;4(12):1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 19.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc Natl Acad Sci USA. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drapeau MD, Albert S, Kucharski R, et al. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006;16(11):1385–1394. doi: 10.1101/gr.5012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elchuri S, Oberley TD, Qi WB, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24(3):367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 22.Ershova ES, Sergeeva VA, Chausheva AI, et al. Toxic and DNA damaging efforts of a functionalized fullerene in human embryonic lung fibroblasts. Mutat Res-Genet Toxicol Environ Mutagen. 2016;805:46–57. doi: 10.1016/j.mrgentox.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Foyer CH, Faragher R, Thornalley P. Redox Metabolism and Longevity Relationships in Animals and Plants. Taylor & Francis Group, New York, USA; 2009. [PubMed] [Google Scholar]
- 24.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76(8):947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 26.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto M, Kanda M, Ikeno K, et al. Oral administration of royal jelly facilitates mRNA expression of glial cell line-derived neurotrophic factor and neurofilament H in the hippocampus of the adult mouse brain. Biosci Biotechnol Biochem. 2005;69(4):800–805. doi: 10.1271/bbb.69.800. [DOI] [PubMed] [Google Scholar]
- 28.Hattori N, Ohta S, Sakamoto T, et al. Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid-Based Complement Alternat Med, 2011:165968. 2010 doi: 10.1093/ecam/nep029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiyama A, Sakai D, Risbud MV, et al. Enhancement of intervertebral disc cell senescence by Wnt/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62(10):3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda Y, Fujita Y, Maruyama H, et al. Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans . PLoS ONE. 2011;6(8):e23527. doi: 10.1371/journal.pone.0023527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husein MQ, Kridli RT. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim Reprod Sci. 2002;74(1-2):45–53. doi: 10.1016/S0378-4320(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 32.Hwang ES, Yoon G, Kang HT. A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci. 2009;66(15):2503–2524. doi: 10.1007/s00018-009-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue SI, Koya-Miyata S, Ushio S, et al. Royal jelly prolongs the life span of C3H/HeJ mice: correlation with reduced DNA damage. Exp Gerontol. 2003;38(9):965–969. doi: 10.1016/S0531-5565(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 34.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5(1):1–10. doi: 10.1023/B:BGEN.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 35.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence. In: Tollefsbol TO (Ed.), Biological Aging: Methods and Protocols. Humana Press, Totowa; 2007. pp. 21–31. [DOI] [PubMed] [Google Scholar]
- 36.Jamnik P, Goranovič D, Raspor P. Antioxidative action of royal jelly in the yeast cell. Exp Gerontol. 2007;42(7):594–600. doi: 10.1016/j.exger.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Jeanclos E, Krolewski A, Skurnick J, et al. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47(3):482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- 38.Kamakura M. Signal transduction mechanism leading to enhanced proliferation of primary cultured adult rat hepatocytes treated with royal jelly 57-kDa protein. J Biochem. 2002;132(6):911–919. doi: 10.1093/oxfordjournals.jbchem.a003304. [DOI] [PubMed] [Google Scholar]
- 39.Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473(7348):478–483. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- 40.Kamakura M, Suenobu N, Fukushima M. Fifty-seven-kDa protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum. Biochem Biophys Res Commun. 2001;282(4):865–874. doi: 10.1006/bbrc.2001.4656. [DOI] [PubMed] [Google Scholar]
- 41.Kapahi P, Chen D, Rogers AN, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11(6):453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kern SE, Kinzler KW, Bruskin A, et al. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- 43.Kimura M, Kimura Y, Tsumura K, et al. 350-kDa royal jelly glycoprotein (apisin), which stimulates proliferation of human monocytes, bears the β1-3galactosylated N-glycan: analysis of the N-glycosylation site. Biosci Biotechnol Biochem. 2003;67(9):2055–2058. doi: 10.1271/bbb.67.2055. [DOI] [PubMed] [Google Scholar]
- 44.Kitada T, Seki S, Kawakita N, et al. Telomere shortening in chronic liver diseases. Biochem Biophys Res Commun. 1995;211(1):33–39. doi: 10.1006/bbrc.1995.1774. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126(3):365–379. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Li ZF, Chen Y, Li YZ, et al. Raman microspectroscopy as a diagnostic tool to study single living nasopharyngeal carcinoma cell lines. Biochem Cell Biol. 2013;91(3):182–186. doi: 10.1139/bcb-2012-0024. [DOI] [PubMed] [Google Scholar]
- 48.Liu HJ, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 49.Majtán J, Kováčová E, Bíliková K, et al. The immunostimulatory effect of the recombinant apalbumin 1–major honeybee royal jelly protein–on TNFα release. Int Immunopharmacol. 2006;6(2):269–278. doi: 10.1016/j.intimp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Malecová B, Ramser J, O'Brien JK, et al. Honeybee (Apis mellifera L.) mrjp gene family: computational analysis of putative promoters and genomic structure of mrjp1, the gene coding for the most abundant protein of larval food. Gene. 2003;303:165–175. doi: 10.1016/S0378-1119(02)01174-5. [DOI] [PubMed] [Google Scholar]
- 51.Mannoor MK, Shimabukuro I, Tsukamotoa M, et al. Honeybee royal jelly inhibits autoimmunity in SLE-prone NZB×NZW F1 mice. Lupus. 2009;18(1):44–52. doi: 10.1177/0961203308094765. [DOI] [PubMed] [Google Scholar]
- 52.Mao CD, Hoang P, DiCorleto PE. Lithium inhibits cell cycle progression and induces stabilization of p53 in bovine aortic endothelial cells. J Biol Chem. 2001;276(28):26180–26188. doi: 10.1074/jbc.M101188200. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo M. Aging and oxidative stress resistance in human fibroblasts. J Clin Biochem Nutr. 2004;35(2):63–70. doi: 10.3164/jcbn.35.63. [DOI] [Google Scholar]
- 54.May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18(53):7621–7636. doi: 10.1038/sj.onc.1203285. [DOI] [PubMed] [Google Scholar]
- 55.Movasaghi Z, Rehman S, Rehman IU. Raman spectroscopy of biological tissues. Appl Spectrosc Rev. 2007;42(5):493–541. doi: 10.1080/05704920701551530. [DOI] [Google Scholar]
- 56.Murillo-Ortiz B, Albarrán-Tamayo F, López-Briones S, et al. Increased telomere length and proliferative potential in peripheral blood mononuclear cells of adults of different ages stimulated with concanavalin A. BMC Geriatr, 13:99. 2013 doi: 10.1186/1471-2318-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narita Y, Ohta S, Suzuki KM, et al. Effects of long-term administration of royal jelly on pituitary weight and gene expression in middle-aged female rats. Biosci Biotechnol Biochem. 2009;73(2):431–433. doi: 10.1271/bbb.80556. [DOI] [PubMed] [Google Scholar]
- 58.Oka H, Emori Y, Kobayashi N, et al. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 cell responses. Int Immunopharmacol. 2001;1(3):521–532. doi: 10.1016/S1567-5769(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 59.Okamoto I, Taniguchi Y, Kunikata T, et al. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003;73(16):2029–2045. doi: 10.1016/S0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- 60.Okuda K, Khan MY, Skurnick J, et al. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152(2):391–398. doi: 10.1016/S0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 61.Phillips JP, Campbell SD, Michaud D, et al. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci USA. 1989;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Remolina SC, Hughes KA. Evolution and mechanisms of long life and high fertility in queen honey bees. Age. 2008;30(2-3):177–185. doi: 10.1007/s11357-008-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren Q, Zeng HS, Zeng XF. Leflunomide inhibits the apoptosis of human embryonic lung fibroblasts infected by human cytomegalovirus. Eur J Med Res. 2013;18(1):3. doi: 10.1186/2047-783X-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salazar-Olivo LA, Paz-González V. Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol In Vitro. 2005;19(5):645–651. doi: 10.1016/j.tiv.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48(5):642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Schmitzová J, Klaudiny J, Albert Š, et al. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell Mol Life Sci. 1998;54(9):1020–1030. doi: 10.1007/s000180050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen LR, Zhang LW, Ding MH, et al. Research progress on nutritional and health-care functions and Molecular mechanism of royal Jelly. J Agric Sci Technol. 2009;11(4):41–47. doi: 10.3969/j.issn.1008-0864.2009.04.007. (in Chinese) [DOI] [Google Scholar]
- 69.Shen LR, Zhang WG, Jin F, et al. Expression of recombinant AccMRJP1 protein from royal jelly of Chinese Honeybee in Pichia pastoris and its proliferation activity in an insect cell line. J Agric Food Chem. 2010;58(16):9190–9197. doi: 10.1021/jf1007133. [DOI] [PubMed] [Google Scholar]
- 70.Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002;21(16):4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Šver L, Oršolić N, Tadić Z, et al. A royal jelly as a new potential immunomodulator in rats and mice. Comp Immunol Microbiol Infect Dis. 1996;19(1):31–38. doi: 10.1016/0147-9571(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 72.Swain RJ, Jell G, Stevens MM. Non-invasive analysis of cell cycle dynamics in single living cells with Raman micro-spectroscopy. J Cell Biochem. 2008;104(4):1427–1438. doi: 10.1002/jcb.21720. [DOI] [PubMed] [Google Scholar]
- 73.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13(17):1549–1556. doi: 10.1016/S0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 74.Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35(8):927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 75.Vietti G, Ibouraadaten S, Palmai-Pallag M, et al. Towards predicting the lung fibrogenic activity of nomaterials: experimental validation of an in vitro fibroblast proliferation assay. Part Fibre Toxicol, 10:52. 2013 doi: 10.1186/1743-8977-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Zglinicki T, Saretzki G, Ladhoff J, et al. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126(1):111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 77.Watanabe K, Shinmoto H, Kobori M, et al. Stimulation of cell growth in the U-937 human myeloid cell line by honey royal jelly protein. Cytotechnology. 1998;26(1):23–27. doi: 10.1023/A:1007928408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xin XX, Chen Y, Chen D, et al. Supplementation with major royal-jelly proteins increases lifespan, feeding, and fecundity in Drosophila . J Agric Food Chem. 2016;64(29):5803–5812. doi: 10.1021/acs.jafc.6b00514. [DOI] [PubMed] [Google Scholar]
- 79.Xu M, Yu Q, Subrahmanyam R, et al. β-Catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo. Mol Cell Biol. 2008;28(5):1713–1723. doi: 10.1128/MCB.01360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu ZL, Chou LS, Sun J. Effects of SiO2 nanoparticles on HFL-I activating ROS-mediated apoptosis via p53 pathway. J Appl Toxicol. 2012;32(5):358–364. doi: 10.1002/jat.1710. [DOI] [PubMed] [Google Scholar]
- 81.Yan XL, Dong RX, Zhang L, et al. Raman spectra of single cell from gastrointestinal cancer patients. World J Gastroenterol. 2005;11(21):3290–3292. doi: 10.3748/wjg.v11.i21.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang FF, Huang W, Li YF, et al. Anti-tumor effects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials. 2013;34(22):5689–5699. doi: 10.1016/j.biomaterials.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 83.Zahn JM, Kim SK. Systems biology of aging in four species. Curr Opin Biotechnol. 2007;18(4):355–359. doi: 10.1016/j.copbio.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zamani Z, Reisi P, Alaei H, et al. Effect of royal jelly on spatial learning and memory in rat model of streptozotocin-induced sporadic Alzheimer's disease. Adv Biomed Res, 1:26. 2012 doi: 10.4103/2277-9175.98150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng AH, Ou YY, Guo MM, et al. Human embryonic lung fibroblasts treated with artesunate exhibit reduced rates of proliferation and human cytomegalovirus infection in vitro . J Thorac Dis. 2015;7(7):1151–1157. doi: 10.3978/j.issn.2072-1439.2015.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhan M, Yamaza H, Sun Y, et al. Temporal and spatial transcriptional profiles of aging in Drosophila melanogaster . Genome Res. 2007;17(8):1236–1243. doi: 10.1101/gr.6216607. [DOI] [PMC free article] [PubMed] [Google Scholar]