Abstract
Background: Diabetes caused by insulin production disturbance is considered as the most common metabolic disorder all over the world. Diabetes may outbreak because of low insulin secretion by Islets of Langerhans β-cells, insulin resistance or both of them. In this way, using stem cells, which have the capability to differentiate into pancreatic β-cells, is one of novel methods in this field. MSCs are the most important candidates for cellular therapy.
Materials and Methods: Insulin level was examined using ELIZA method. In order to examine the morphology of differentiated cells, they were stained by Dithizone. Insulin-producer cells are cells which turn into red as a result of staining. Specific gene involving insulin-producing cells was evaluated by Real Time-PCR method.
Results: The ELISA results showed that the treated cells secreted more insulin than the control group. Moreover, we found differentiation of MSCs toward insulin-secreting cells. In order to evaluate insulin production in clusters on day 21 of differentiation, we used dithizone (DTZ) staining. PDX-1 gene was confirmed by RT- PCR analysis.
Conclusion: In this study, we differentiated MSCs into insulin-producing cells in vitro. It is concluded that MSCs may be considered as an excellent candidate in β-cell therapy in diabetes patients.
Key Words: Mesenchymal stem cells, Wharton’s jelly, Differentiation, Insulin producing cells
Introduction
Diabetes, caused by insulin production disturbance, is considered as the most common metabolic disorder all over the world. Diabetes may outbreak because of low insulin secretion by Islets of Langerhans β-cells, insulin resistance or both of them1. Currently more than 387 million people suffer from diabetes mellitus around the world. According to estimates, this figure will reach 500 million people by 2030. Generally, diabetes is divided into two types: Type I is a disorder which caused by auto immune destruction of Pancreas β-cells , and in Type II diabetes caused mainly because of progressive body resistance against insulin which results in β-cells destruction and insufficient insulin production.2
Although the exact source of this disorder is unknown, there are some evidence which reveal that genetic and environmental factors affect the process of auto immune excitation3-5. on the other hand, no conclusive treatment has been found for diabetes, while taking oral drugs and insulin injection are among common treatments. Also, Pancreas transplantation and Islets langerhans cells are also practiced. But, Pancreas transplantation is limited because of Diabetes spread and limited number of donors.
In this way, using stem cells, which have the capability to differentiate into Pancreas β-cells, is one of novel methods in this field6. MSCs are the most important candidates for cellular therapy. There is fundamental indication from in vitro7, preclinical 8-10 and clinical 11-14 studies. Stem cells are unspecialized cells with high proliferation and have the following characteristics: regeneration, differentiation, self-renewal 15 if induced by a specific driver. Stem cells can divide several times and maintain multipotency, and can differentiate into specialized cells 16.
There are two kinds of stem cells in the bone marrow: 1) hematopoietic stem cells, and 2) Mesenchymal Stem Cells (MSCs), which differentiation into fat cells, cartilage and bone in specific circumstances17. Meanwhile genesis, Pancreas produces factors which cause all stem cells to differentiate into insulin-producer cells 18.
MSCs express surface indices MHC-I , CD25 , CD44 , CD73 , CD90 , CD105 , CD166 and is negative in terms of surface markers MHC-II , CD14,CD31 , CD34 , CD45,HLA-DR. Three main condition for approving the identity of mesenchymal are:1) in the culture, cell adherence to flask bottom, 2) expression of some markers and lacking expression of other markers, and 3) the capability to differentiate into fat, bone and cartilage cells.
Given that providing MSCs from bone marrow is laborious, and in some cases observing ethical codes isn’t possible, researchers are looking for alternative resources, including umbilical cord blood, Wharton’s Jelly, and placenta19-21. Because these tissues are discarded after delivery, so they don’t put the newborn and the mother at risk.
Researchers insist that Pancreas genesis and Neural System genesis have some similarities, although these two tissues were formed by different resources22-24.
In this way, Pancreas endocrine cells are produced from embryonic stem cells using the a novel method called “neuron production” as follow:: 1) genesis of embryonic body(EB) which includes the cells differentiating the three layer 2) differentiation of cells which express nestin against fetal bovine serum reduction and culturing in ITSFN(Insulin-Transferrin-Selenium-Fibronectin), and 3) proliferation and maintaining precursor cells in the presence of alkaline fibroblastic growth factor(bFGF/FGF-2) in a medium free of serum, with N2, and B27 and 4) Induction of differentiation and maintaining positive insulin cells through adding Nicotine Amide and removing bFGF 25-27.
Some researchers have shown that fibroblastic growth factors can play a significant role in differentiation of pancreatic B-cells in various dilutions. In this way, many FGFs, including 22 items have been examined. These factors are secreted from various tissues and affect the target tissue28.
bFGF can stimulate angiogenesis in vitro and in vivo, and help diabetes treatment by angiogenesis. FGF10 play its role in signaling mesenchymal- epithelial interaction and cell proliferation.
Other researchers have argued that the presence of neuro-epithelial protein nestin in human and rat islets Langerhans may by an indicator for endocrine precursor cells. Also, they showed that nestin is present in endothelial and mesenchymal cells of vessels inside and outside the islets of Langerhans, and showed that the cells proliferated in mature islets Langerhans are primarily endocrine29-32.
Wang and Phuc et al. (2011) could create pancreatic β-cells from mesenchymal stem cells of umbilical cord blood, which, after injection, could lower blood glucose of diabetic rat.
They found that mesenchymal stem cells of umbilical cord vein wall have less capacity in terms of differentiation, compared to Wharton’s Jelly mesenchymal cells and umbilical cord blood. Lower differentiation capacity is obvious in bone and fat differentiation33,34.
Another research (2010) showed that growth factors, including retinoic acid, FGF and activin are used for differentiation of stem cells into cells which express PDX-135.
Until now, many researches have tried to produce mesenchymal stem cells and their differentiation into pancreatic β-cells using various molecules; unfortunately, they couldn’t produce prefect pancreatic β- cells.
This research aims at isolation mesenchymal stem cells from Wharton’s Jelly. The differentiation of MSCs to pancreatic cells was evaluated in presence of ActivinA, Retinoic Acid, Fibroblast Growth Factor10, Nestin and Forskolin in culture medium. The concentration of the components, mentioned above, was also optimized in the culture. Thus, a novel method for producing pancreatic cells may be explained.
MATERIALS AND METHODS
Collection of human umbilical cord
In an experimental study, human umbilical cord samples were collected in fully sterilized condition from six mothers after delivery at the Obstetrical Department of Imam Educational and Therapeutic Hospital, Sari, North of Iran. Then samples were transported to the Medical University.
Preparation and culture of Wharton’s jelly
First, in a 10 mm-plate, all clots and arteries of umbilical cord were isolated under laminar hood. Then, the gelatinous substance within the umbilical cord, Wharton jelly, was cut into small pieces mechanically, and was rinsed several times with Phosphorous buffered saline (PBS) at 1250 rpm for 5 min. For lysing the remaining RBC, 2 ml Hypertonic Chloride, Ammonium (USA, Pharmin Gen) was added to the sample and was rinsed after 10 min. Cell deposition, which included cells that separated from the placenta tissue, was transferred to T75 Flask and was cultured in High Glucose-Dulbecco's Modified Eagle Medium- F12 (HG- DMEM-F12) (USA, Gibco) supplemented with 10 % fetal bovine serum (FBS) and 50 u/ml Penicillin-Streptomycin. The plate was incubated in 5% CO2 at 37 ºC.
Isolation and characterization of Mesenchymal Stem Cells (MSCs) from other umbilical cord cells
The medium was changed after 24 hours; the suspended particles (non-mesenchymal cells) migrated from the medium and the mesenchymal cells adhered to flask, and turned into spindle-like shapes. When the confluency of the cells reached 80-90%, the mesenchymal cells were harvested using 0.25% trypsine including 1ml/M EDTA (USA, Sigma).
The suspension was centrifuged at 1250 rpm for 5 min. The cell deposition was rinsed two times and centrifuged. Finally, it was filtered using grade 70 micron filter.
To approve MSCs identity, positive markers of stem cell surface such as CD105, CD90, CD44 and negative markers, CD34 and HLA-DR were evaluated by flow cytometry. Conjugating related antibodies, Fluor chromes FITC, PE and PerCP, isotype controls of PerCP, PE and IgG1-FITC were used.
The WJ-MSCs were induced to differentiate into adipocytes, osteocytes and chondrocytes. The differentiation protocol has been described previously26. In this way, WJ-MSCs at 1.6 × 105 cells/ml were cultured in HG-DMEM-F12 complemented with 15% FBS and 2mMol/ L- glutamine and Penicillin-Streptomycin for 6 days. The cells were then fixed with 0.4% paraformaldehyde (PFA) and stained with oil-red-o (sigma) to confirm the adipocyte. For osteoblastic differentiation, the WJ-MSCs were cultured in HG-DMEM-F12 complemented with 10 nMol/L b-glycerol phosphate (sigma) and 50mg/ml ascorbic acid-2 phosphate (sigma). After fixation, the differentiated cells were stained with Alizarin red, and then the calcium deposition was confirmed. For chondrogenesis differentiation, WJ-MSCs were seeded in a HG-DMEM-F12 complete culture media at 1.6 × 105 cells/ml with 5 μl of chondrogenesis culture media for 14 days. The micromass cultures were then stained with Alcian blue.
Differentiation of MSCs into insulin-producing cells
After isolation and confirmation of MSCs, optimization of Activin A, Retinoic Acid, Fibroblast Growth Factor10, Nestin and Forskolin in various dilutions were checked in the culture, and the optimum dilution was obtained. Then induction treatment was conducted in 3-5 days. Stimulating cell proliferation, various additives, including (Gibco) B27 and (Gibco) N2 were added to the culture.
Dithizone staining
To examine the morphology of differentiated cells, they were stained with Dithizone by completely dissolving 50 mg of DTZ (Sigma) in 5 ml of dimethyl sulfoxide (DMSO, Sigma). The solution was stored in -20 ᵒC in the dark. At the time of staining, the working solution was prepared by diluting the stock solution (pH 7.8) to a ratio of 1:100 in the culture medium. We added 3 ml of the working DTZ solution to each well and the plates were incubated for 30 min at 37ᵒC. The plates were then rinsed three times with PBS and crimson-red differentiated clusters were examined with an inverted phase contrast microscope.
Enzyme-linked immunosorbent assay (ELISA)
For evaluation of insulin secretion, the supernatant of differentiated cells was collected and insulin content was examined by ELISA method using an Insulin ELISA kit (Demeditec, Germany) according to the protocol.
The evaluation of the PDX-1 expression in mRNA level which confirms the insulin-producing cells was done by reveres transcription polymerase chain reaction (RT-PCR).
RNA extraction and cDNA synthesis
Extraction of total RNA from differentiated cells was used by AccuzolTM, a ready-to-use reagent (Bioneer, Korea) according to the manufacturer’s protocol. After evaluating the quality and quantity of RNA by electrophoresis and spectrophotometer, respectively, cDNA synthesis was done by AccuPower® CycleScript RT Premix (dN6) (Bioneer, Korea). The concentration of RNA used for cDNA synthesis was 1µg. The thermal profile for cDNA synthesis was: primer annealing at 25℃ for 30 s, cDNA synthesis at 45 ℃ for 4 min and melting secondary structure & cDNA synthesis at 55℃ for 30 s and repeated 12 times as well as heat inactivation at 95℃ for 5 min.
Reverse transcription polymerase chain reaction (RT-PCR)
For assessment of insulin-producing cells, we evaluated expression of PDX-1 gene, which was related to insulin production. First, to set the primers, we performed conventional PCR. PCR amplification was done for PDX-1 gene and EF-1 as reference gene, in a Thermocycler (Eppendorf, UK) by using 2μl of CDNA, 1μM of each primer, and 12.5μl of Taq DNA Polymerase 2x Master Mix, Red (Ampliqon, Denmark) in a 20 µl total volume reaction.
The primer sequences were as follows: EF1 (Forward): 5´-CTGAACCATCCAGGCCAAAT-3´ and EF1 (Reverse): 5´-GCCGTGTGGCAATCCAAT-3´ which amplified a 59 bp fragment and for PDX1 gene we used GGATGAAGTCTACCAAAGC-3´ as a forward and 5´-CGTGAGATGTACTTGTTGAA-3´ as reverse to amplify a 157 bp fragment. The thermal profile for amplification were 95ᵒC for 2 min for initial denaturation, followed by 40 cycles denaturation at 95ᵒC for 30s, annealing at 45ᵒC for EF-1 and 56ᵒC for PDX-1 for 30s, extension at 72ᵒC for 30S, and final extension at 72ᵒC for 2 min.
PCR products were electrophoresed on 2% agarose gel. Electrophoresis was performed at 100v for 40 min. Fragments with 59 bp and 157 bp were scored as EF1 and PDX1 gene, respectively.
Real-time polymerase chain reaction (RT-PCR)
Evaluation of PDX1 expression at mRNA level was performed using the real-time PCR system (IQ5, Biorad, USA). The EF1 gene was used as an endogenous reference gene allowing normalization of the expression level of the target gene to normalize the expression levels of target gene. Primer sequence was mentioned in RT-PCR part. We used SYBR Premix EX TaqII (2X) (Takara, Cat. #: RR820L) master mix for amplification. The thermal profile for PDX-1 and EF-1 were as follows: The thermal profile was as follows: Initial denaturation at 95°C for 2 min, followed by 45 cycles of denaturation at 95°C for 30 s, primer annealing for PDX-1 at 56°C and for EF-1 at 45°C for 30 s, and extension at 72°C for 30 s. This cycle was followed by a melting curve analysis, ranging from 45°C for EF-1 and 56°C for PDX-1 to 95°C, with increasing steps in temperature ( 0.5°C every 10 s).
Statistical analysis
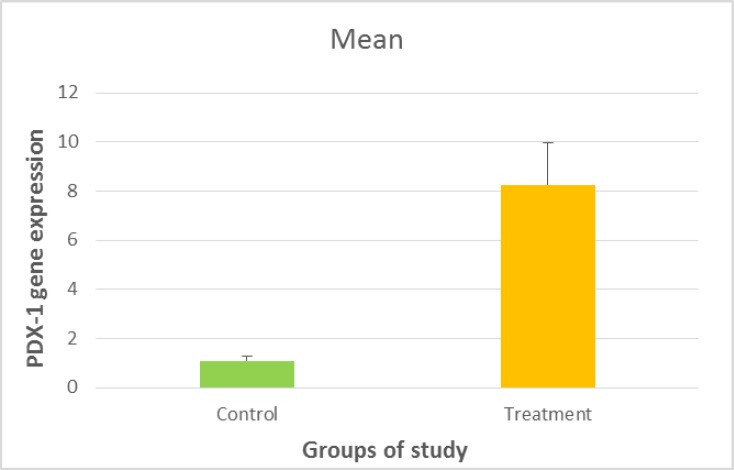
The relative expression of PDX-1 was calculated by 2-ΔΔCt, as previously described by Livak36.The data were presented as means ± SD. Data were assessed by one-way ANOVA followed by Tukey’s test for comparison of the experimental group. The statistical analysis was performed using SAS software. P-values <0.05 were considered statistically significant (Figure 6).
Figure 6.
PD X-1 gene expression in MSCs and IPCs P-value = 0.001
Results
In our study, we differentiated Wharton jelly´s mesenchymal stem cells (WJ-MSCs) toward insulin-producing cells (IPCs). For this purpose, after isolation and differentiation of WJ-MSCs toward IPCs, we performed the morphological and molecular analysis.
Morphological and Phenotypical Characterization of WJ-MSCs
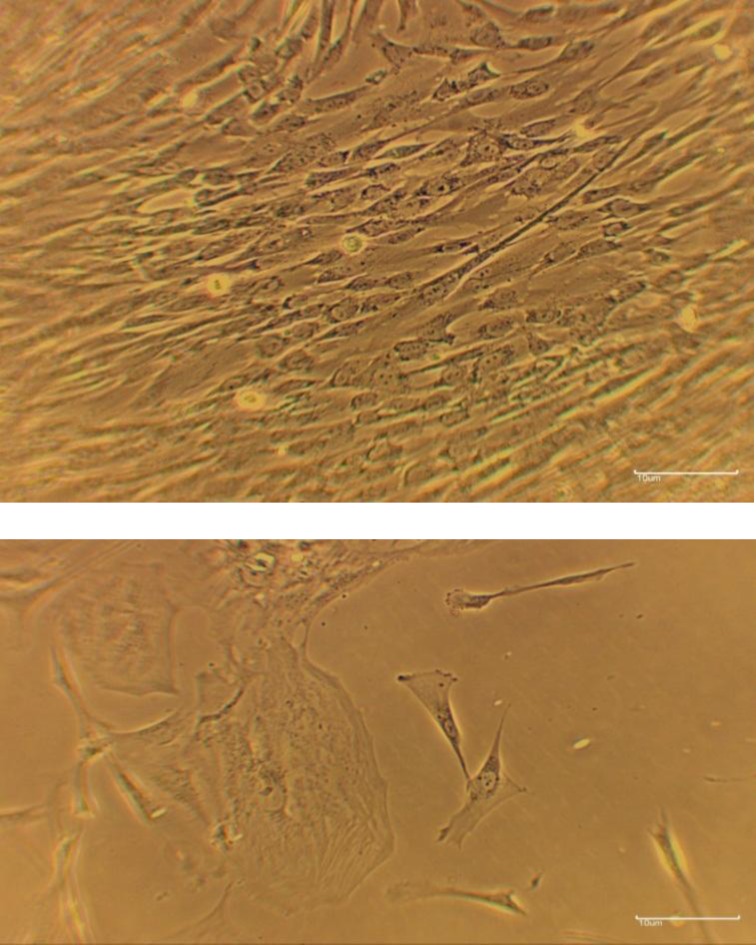
At the end of the expansion phase, the cultured WJ-MSCs become homogenous, spindle shaped, and fibroblast-like to arrange in monolayers (Figure 1).
Figure1.
The Wharton`s Jelly mesenchymal cells were grown from
Flow cytometric analysis showed that these cells expressed high levels of CD90, CD105 and CD44, but negligible levels of CD34, and HLA-DR, which are surface markers for hematopoietic stem cells. Moreover, their multilineage differentiation potential was confirmed. The cells have the potential to differentiate into adipocytes, chondrocytes, and osteocytes when they were exposed the appropriate growth factors. These data indicate that MSCs were the majority of the WJ-derived cells.
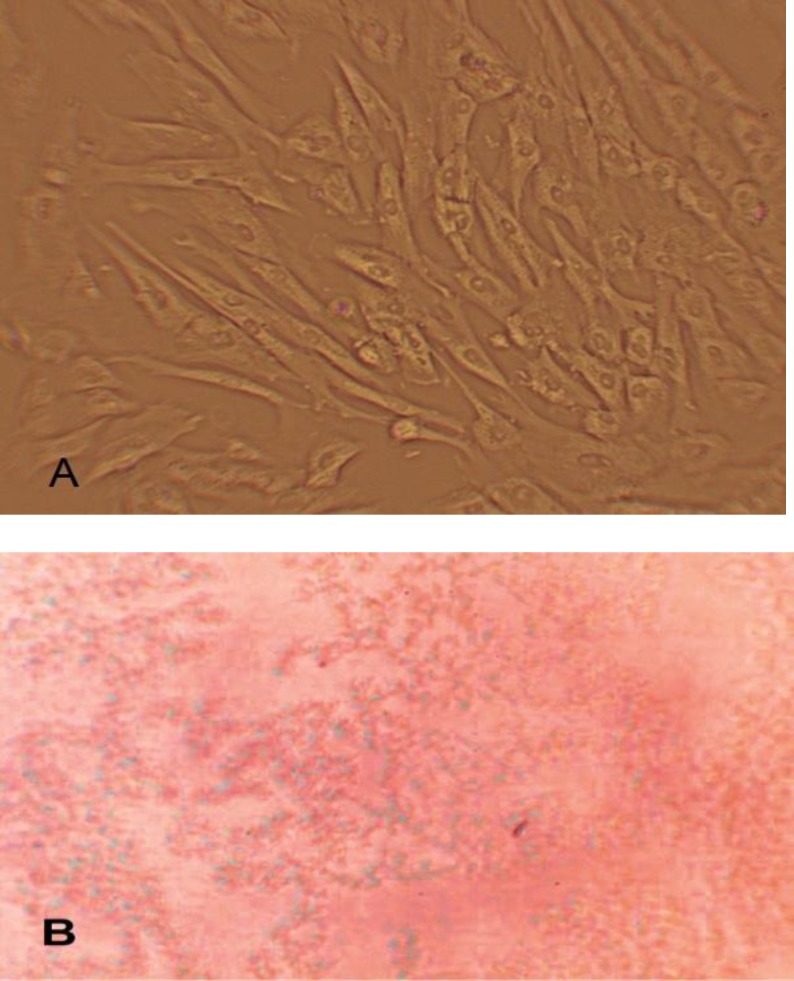
Dithizone (DTZ) staining
In order to evaluate insulin production in clusters at day 21 of differentiation, we used dithizone (DTZ) staining. The stock solution was prepared as previously described37. Insulin-producing cells are the cells which turn into red in result of staining (Figure 2).
Figure2.
DTZ (Dithizone) staining; observed with inverted microscope and magnified ×100 A: Untreated cells B: In cells treated with dithizone, insulin granules come in red brick.
ELISA test
In order to determine the amount of insulin secreted by the differentiated cells after the day 28, the cell culture medium was tested by using ELISA technique. The results showed that the differentiated cells compared to the undifferentiated cells, secrete insulin. We could be able to differentiate MSCs toward the insulin producing cells.
PDX-1 expression in IPCs
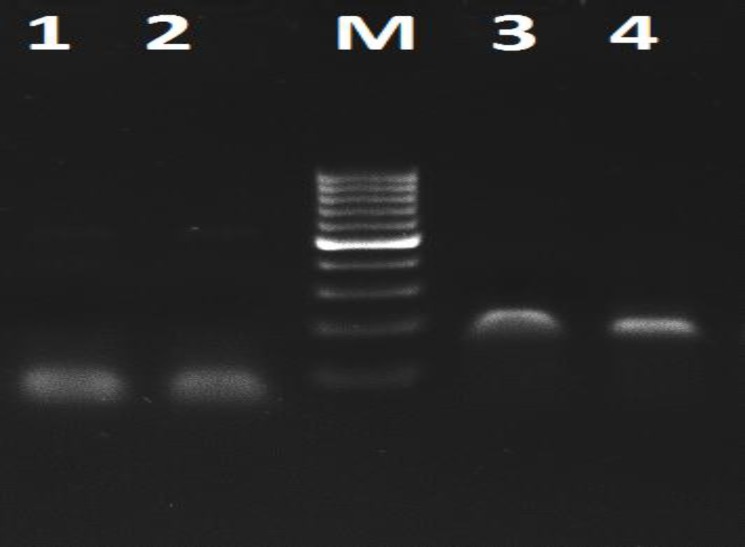
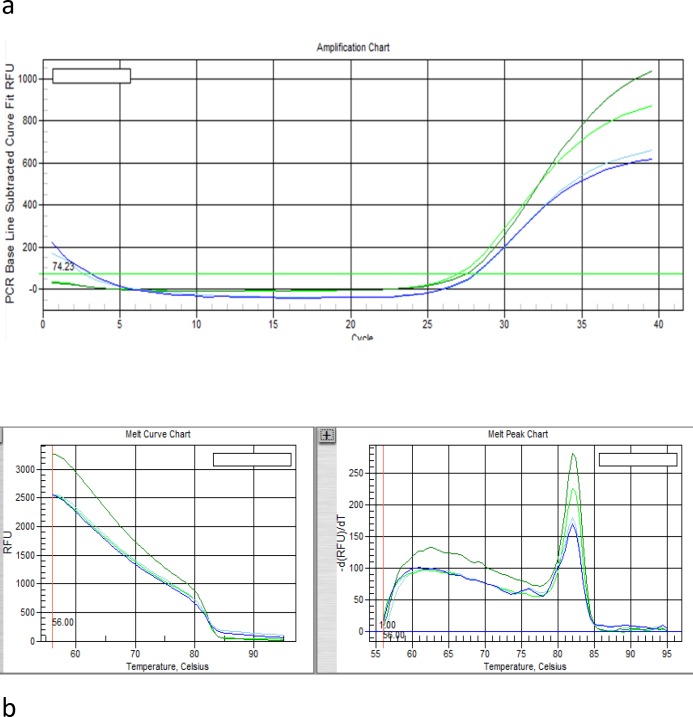
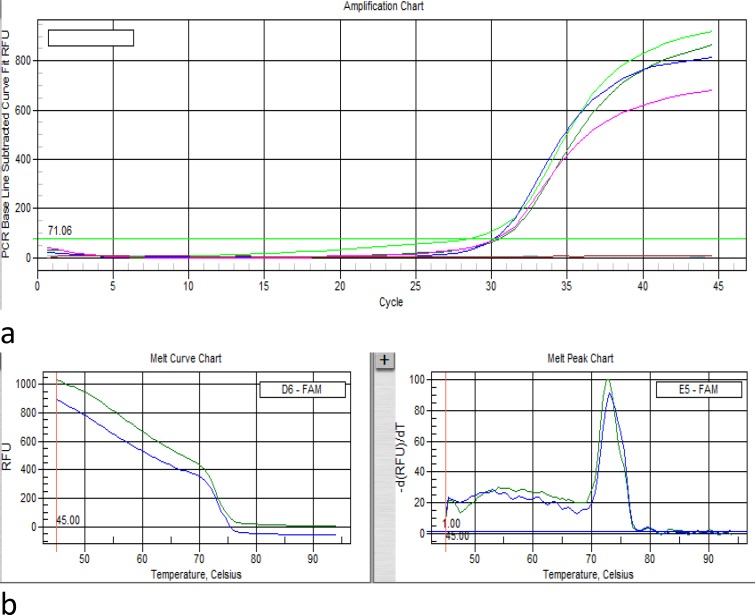
After differentiation, the expression of β-cell specific gene such as PDX-1 was assessed in Wharton jelly mesenchymal stem cell and insulin-producing cells by qRT-PCR. The results of RT-PCR for primers assessment are shown in Figure3. The amplification plot and melt curve of PDX-1 and EF-1 represent in Figures 4-a,b and 5-a,b respectively. The expression levels of PDX1 versus EF1 in IPCs were7.55 fold-changes higher than WJ-MSCs, and it was statistically significant (p= 0.001) (Figure 5).
Figure 3.
Electrophoresis of PDX-1 and EF-1 on 2% agarose gel; M shows marker (100bp); Lane 1, 2 represent PCR product of the EF-1 amplification in 45ᵒC; Lane 3, 4 represent PCR product of the PDX-1 amplification in 56ᵒC
Figure 4.
Amplification plot (a) and melt curve (b) of EF1- Reference Gene
Figure5.
Amplification plot (a) and melt curve (b) of PDX-1Gene
Discussion
MSCs are self-renewing cells with multilineage differentiation potential38. The ease of isolation and expansion has rendered any potentially significant source of stem cells as it could be used in the tissue engineering and regenerative medicine, whereas it represents a therapeutic potential for DM33. Previously, some publications demonstrated the differentiation capability of adult stem cells toward the pancreatic cell lineage39-43. Recent studies have revealed the potential of MSCs from different sources for the treatment of the diabetes mellitus 44,45 and the diabetic complications, including diabetic cardiomyopathy46, diabetic retinopathy47, diabetic polyneuropathy48, diabetic nephropathy49 and diabetic wounds50. MSCs can be isolated from several organs and tissues, e.g. bone marrow, dental pulp, adipose tissues, and umbilical cords51. Wharton`s Jelly method was used to derive MSCs, by which several significant advantages have been reflected such as the high number and the relatively easy isolation52. MSCs are capable to differentiate into islet-like cells and it has immunomodulatory abilities subject to the possibility to reduce the risk of immune rejection53. Insulin Producing Cells (IPCs) can be obtained by two methods: indirect and direct differentiation. The indirect differentiation application for the chemicals, for example, nicotinamide and growth factors are shown as inductors. In this method, the application of the high glucose concentration in the medium is critical, since it is a potent inducer of differentiation. Another method is direct differentiation, which is based on the modification of the genetic material, for example, by using the viral vectors54.
Raikwar et al. (2013) reported the ectopic expression of Pancreatic and duodenal home box Factor-1(PDX-1) which is a critical transcription factor for pancreas, and increased embryonic stem cells differentiation into insulin producing cells.
Sipione and Hanson and Rajagopal et al. (2003-2004) tried to show that transplanted stem cells promote treatment of diabetes. They, in their research, reported that neurons and neural precursors are the main producers of insulin/proinsulin 55-57.
Moreover, in a study which yielded 7.3u/ml insulin, endodermal layer cells were sustained using Activin A and then adding FGF10 and retinoic acid led to PDX-1 cells expression as the main marker of the insulin producing cells58-59.
In other study, researchers showed that endodermal cells can generate pancreatic precursors in exposure of Retinoic acid and cyclopamin60, 61.
One of the important signs of the beta pancreatic cells is the insulin secretion. Thereby, in the present study, the secretion rate of insulin in the differentiated MSCs was studied.
In this research, after the successful isolation of mesenchymal stem cells (MSCs) and its confirmation via three approaches, including adhesion of fibroblast spindle-shaped cells at the bottom of the flask and the differentiation into the fat, bone and cartilage cell lines and the examination of the positive and negative CD markers, in the next phase, we conducted the differentiation into the pancreatic β cells with Activin A (100 ng/ml), Retinoic Acid (0.2 nM/ml), Fibroblast Growth Factor10 (20 ng/ml), Nestin (50 ng/ml) and Forskolin (250 ng/ml) and optimized their concentration in the various dilutions in the culture medium, where we could produce the insulin producing cells.
In this study, mesenchymal stem cells (MSCs) were differentiated into the pancreatic β cells with Activin A, Retinoic Acid, Fibroblast Growth Factor10, Nestin and Forskolin and optimized their concentration in the various dilutions in the culture medium, where we could produce adequate the insulin secreting cells. In our study, the best molecular combination to produce insulin was Forskolin+Activin A+ Fibroblast Growth Factor10 and Retinoic Acid (211/7u/ml), as it was determined by ELISA.
It is required to study the combination of the differentiate factors mentioned above in the animal model. In respect to the efficient role of the in vivo compared to the in vitro on the differentiation, the study of this subject seems indispensable.
CONCLUSION
Mesenchymal stem cells were differentiated into the pancreatic β cells with Activin A, Retinoic Acid, Fibroblast Growth Factor10, Nestin and Forskolin and optimized their concentration in the various dilutions in the culture medium. We optimized the best molecular combination to produce adequate insulin level. Therefore, it is concluded that MSCs may be considered as an excellent candidate in β- cell therapy in diabetes patients.
Acknowledgment
This study was supported by a study Grant (no. 93.1462) from Immunogenetics Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. We also acknowledge the support of Imam Hospital, Sari, for their providing umbilical cord samples.
CONFILCT OF INTEREST
The authors report no conflicts of interest.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Rmeileh NM, Husseini A, Capewell S, et al. Preventing type 2 diabetes among Palestinians: comparing five future policy scenarios. BMJ open. 2013;3(12):e003558. doi: 10.1136/bmjopen-2013-003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abediankenari S, Eslami MB, Sarrafnejad A, et al. Dendritic cells bearing HLA-G inhibit T-Cell activation in type 1 diabetes. Allergy Asthma Immunol. 2007;6(1):1–7. [PubMed] [Google Scholar]
- 4.Abediankenari S, Ghasemi M. Generation of immune inhibitory dendritic cells and CD4+T regulatory cells inducing by TGF-beta. Iran J Allergy Asthma Immunol. 2009;8(1):25–30. [PubMed] [Google Scholar]
- 5.Li G, Abediankenari S, Kim YJ, et al. TGF-beta combined with M-CSF and IL-4 induces generation of immune inhibitory cord blood dendritic cells capable of enhancing cytokine-induced ex vivo xpansion of myeloid progenitors. Blood. 2007;110(8):2872–9. doi: 10.1182/blood-2006-10-050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang DQ, Cao LZ, Burkhardt BR, et al. In vivo and in vitro characterization of insulin- producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–32. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Med. 2005;11(4):367–80. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 8.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischemic cardiomyopathy. Eur Heart J. 2009;30(22):2722–32. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amado LC, Schuleri KH, Saliaris AP, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48(10):2116–24. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 11.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302–10. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldman AW, DiFede DL, Fishman JE, et al. Tran’s endocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare JM, DiFede DL, Castellanos AM, et al. Randomized Comparison of Allogeneic Vs. Autologous Mesenchymal Stem Cells for Non-ischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69(5):526–37. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287(5457):1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 16.Becker AJ, McCuloch EA, Tili JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–4. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 17.Mishra PJ, Mishra PJ, Glod JW, et al. Mesenchymal stem cell: flip side of the coin. Cancer Res. 2009;69(4):1255–8. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 18.Vaca P, Martin F, Vegara-Meseguer JM, et al. Induction of differentiation of embryonic stem cells into insulin-secreting cells by fetal soluble factors. Stem cells. 2006;24(2):258–65. doi: 10.1634/stemcells.2005-0058. [DOI] [PubMed] [Google Scholar]
- 19.Mafi R, Hindocha S, Mafi P, et al. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications-a systematic review of the literature. Open Orthop J. 2011;5(Suppl 2):242–8. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–75. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 21.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 20011;7(2):463–77. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292(5520):1389–94. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 23.Kim SK, Hebrok M, Melton DA. Melton,Notochord to endoderm signaling is required for pancreas development. Development. 1997;124(21):4243–52. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 24.Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveal an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–42. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki S, Yamato E, Miyazaki J. Regulated expression of PDX-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53(4):1030–7. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- 26.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 27.Zulewski H, Abraham EJ, Gerlach MJ, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiation ex vivo into pancreatic endocrine, and hepatic phenotypes. Diabetes. 2001;50(3):521–33. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 28.Crossley JA, Jennifer A, Berry E, et al. Insulin-dependent diabetes mellitus and prenatal screening results: current experience from a regional screening programme. Prenatal Diagnosis. 1996;16(11):1039–42. doi: 10.1002/(SICI)1097-0223(199611)16:11<1039::AID-PD985>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Abraham EJ, Leech CA, Lin JC, et al. Insulintropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producin cells. Endocrinology. 2002;143(8):3152–61. doi: 10.1210/endo.143.8.8973. [DOI] [PubMed] [Google Scholar]
- 30.Selander L, Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev. 2002;113(2):189–92. doi: 10.1016/s0925-4773(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 31.Treutelaar MK, Skidmore JM, Dias-Leme CL, et al. Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes. 2003;52(10):2503–12. doi: 10.2337/diabetes.52.10.2503. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey RK, Bucay N, Beattie GM, et al. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52(10):2519–25. doi: 10.2337/diabetes.52.10.2519. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 34.Chandra V, G S, Phadnis S, Nair PD, et al. Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue derived stem cells. Stem Cells. 2009;27(8):1941–53. doi: 10.1002/stem.117. [DOI] [PubMed] [Google Scholar]
- 35.Aguayo-Mazzucato C, Bonner-Weir S. Stem Cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):139–48. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Shiroi A, Yoshikawa M, Yokota H, et al. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem cells. 2002;20(4):284–92. doi: 10.1634/stemcells.20-4-284. [DOI] [PubMed] [Google Scholar]
- 38.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 39.Govindasamy V, Ronald VS, Abdullah AN, et al. Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res. 2011;90(5):646–52. doi: 10.1177/0022034510396879. [DOI] [PubMed] [Google Scholar]
- 40.Koblas T, Zacharovová K, Berková Z, et al. In vivo differentiation of human umbilical cord blood-derived cells into insulin-producing beta cells. Folia Biol (Praha) 2009;55(6):224–32. [PubMed] [Google Scholar]
- 41.Tang DQ, Cao LZ, Burkhardt BR, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–32. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang LJ. Liver stem cell-derived beta-cell surrogates for treatment of type 1 diabetes. Autoimmun Rev. 2006;5(6):409–13. doi: 10.1016/j.autrev.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang DQ, Lu S, Sun YP, et al. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006;86(1):83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao F, Wu DQ, Hu YH, et al. In vitro cultivation of islet-like cell clusters from human umbilical cord blood-derived mesenchymal stem cells. Transl Res. 2008;151(6):293–302. doi: 10.1016/j.trsl.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Chandra V, Swetha G, Muthyala S, et al. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS ONE. 2011;6(6):e20615. doi: 10.1371/journal.pone.0020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang N, Li J, Luo R, et al. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinal Diabetes. 2008;116(2):104–11. doi: 10.1055/s-2007-985154. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Li K, Yan X, et al. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1415–22. doi: 10.1007/s00417-010-1384-z. [DOI] [PubMed] [Google Scholar]
- 48.Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57(11):3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezquer FE, Ezquer ME, Parrau DB, et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl. 2008;14(6):631–40. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 51.Kuo , CY , Lin CH. Stem cell therapy: differentiation potential of insulin producing cells from human adipose derived stem cells and umbilical cord MSCs. International Journal of Clinical Medicine Research. 2014;1(1):21–25. [Google Scholar]
- 52.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Domínguez-Bendala J, Lanzoni G, Inverardi L, et al. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med. 2012;1(1):59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahmati S, Alijani N, Kadivar M. In vitro generation of glucose-responsive insulin producing cells using lentiviral based PDX-1 gene transduction of mouse (C57BL/6) mesenchymal stem cells. Biochem Biophys Res Commun. 2013;437(3):413–9. doi: 10.1016/j.bbrc.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 55.Sipione S, Eshpeter A, Lyon JG. Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia. 2004;47(3):499–508. doi: 10.1007/s00125-004-1349-z. [DOI] [PubMed] [Google Scholar]
- 56.Hansson M, Tonning A, Frandsen U, et al. Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53(10):2603–9. doi: 10.2337/diabetes.53.10.2603. [DOI] [PubMed] [Google Scholar]
- 57.Rajagopal J, Anderson WJ, Kume S, et al. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299(5605) doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- 58.D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone – expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 59.Shim JH, Kim SE, Woo DH, et al. Directed differentiation of human embryonic stem cells towards pancreatic cell fate. Diabetologia. 2007;50(6):1228–38. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 60.Hu YH, Wu DQ, Gao F, et al. Notch signaling : a novel regulating differentiation mechanism of human umbilical cord blood – derived mesenchymal stem cells into insulin _producing cells in vitro. Chin Med J (Engl) 2010;123(5):606–14. [PubMed] [Google Scholar]
- 61.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):139–48. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]