Abstract
Cell-free translation systems based on cellular lysates optimized for in vitro protein synthesis have multiple applications both in basic and applied science, ranging from studies of translational regulation to cell-free production of proteins and ribosome-nascent chain complexes. In order to achieve both high activity and reproducibility in a translation system, it is essential that the ribosomes in the cellular lysate are enzymatically active. Here we demonstrate that genomic disruption of genes encoding ribosome inactivating factors – HPF in Bacillus subtilis and Stm1 in Saccharomyces cerevisiae – robustly improve the activities of bacterial and yeast translation systems. Importantly, the elimination of B. subtilis HPF results in a complete loss of 100S ribosomes, which otherwise interfere with disome-based approaches for preparation of stalled ribosomal complexes for cryo-electron microscopy studies.
Keywords: HPF, Stm1, Bacillus subtilis, Saccharomyces cerevisiae, cell-free translation system
Introduction
Cell-free translation systems based on cellular lysates optimized for in vitro protein synthesis have multiple applications both in basic and applied science, ranging from studies of translational regulation (Wu et al., 2007) to cell-free production of recombinant proteins (Pedersen et al., 2011) and ribosome-nascent chain complexes (Rutkowska et al., 2009). The preparation of cell-free translation systems is a compromise between, on one hand, the desired properties, such as high synthetic activity and reproducibility of the system and, on the other hand, simplicity of generating robust extracts as well as economic considerations. In laboratory settings, the most convenient and readily accessible method of producing biomass is by growing cells in a batch format in flasks. In this case, large-scale production of highly translationally active exponentially growing cells can be challenging due to relatively low yields. To maximize extract yields, one can harvest cultures in late exponential/early stationary phase. While this provides more biomass, there is a drawback in that cells often reduce their translational capacity during slow growth (Dai et al., 2016). Importantly, the translational activity decreases upon exiting rapid exponential growth – and an important mechanism at play is the reduction of the concentration of active ribosomes via ribosomal sequestration into inactive complexes by dedicated regulatory protein factors.
Bacteria reduce their translational capacity either by directly inactivating 70S ribosomes (Agafonov et al., 1999) or forming inactive ribosome dimers, so-called 100S ribosomes (Ueta et al., 2010; Gohara and Yap, 2018). In Escherichia coli, formation of inactive 70S is mediated by protein Y (YfiA) that in response to cold shock associates with vacant ribosomes and precludes tRNA and mRNA binding (Vila-Sanjurjo et al., 2004). 100S ribosome formation in Gammaproteobacteria is mediated by two cooperating factors: the Hibernation Promoting Factor (HPF; homologous to YfiA (Ueta et al., 2008)) and the Ribosome Modulation Factor (RMF) (Wada et al., 1990). In E. coli the 100S is highly unstable, and therefore formation of the 100S in the stationary phase does not significantly reduce the efficiency of translational lysates (Failmezger et al., 2017). In the majority of bacterial species, 100S formation is mediated by one factor – HPF – the long version of the “short” HPF present in the Gammaproteobacteria (Akanuma et al., 2016; Beckert et al., 2017; Khusainov et al., 2017). Neither the Staphylococcus aureus nor the Bacillus subtilis genomes encode YfiA. S. aureus and B. subtilis 100S are considerably more stable, therefore 100S formation can potentially have a significant impact on translation efficiency in lysates (Ueta et al., 2010; Akanuma et al., 2016; Basu and Yap, 2016).
In budding yeast, the Stm1 protein acts as a translational repressor (Balagopal and Parker, 2011) and is recruited to 80S ribosomes upon nutrient limitation (Ben-Shem et al., 2011; van den Elzen et al., 2014). Rather than causing dimerization, Stm1 and its metazoan ortholog SERBP1 occlude the mRNA-binding channel in both the A- and P-site sites thus forming stable inactive 80S particles (Ben-Shem et al., 2011; Anger et al., 2013). As expected for a ribosome inactivation factor, when Stm1 is added to yeast translational extracts, their activity is strongly inhibited (Balagopal and Parker, 2011).
We reasoned that disrupting the genes encoding for ribosome inactivating factors would yield more reproducible and active bacterial (B. subtilis Δhpf) and yeast (Saccharomyces cerevisiae stm1Δ) cell-free translation systems. Addition of either HPF (Ueta et al., 2010) or Stm1 (Balagopal and Parker, 2011) purified proteins to cell-free translation systems in trans is known to inhibit the efficiency of protein production. The use of a Δhpf strain could be an especially promising strategy for improving the translation efficiency of B. subtilis lysates. Since this bacterium already expresses its HPF during exponential growth (although at significantly lower levels than in stationary phase) (Akanuma et al., 2016), it is expected that cell-free translation systems prepared from the Δhpf strain would be more active than those prepared from the wild type strain regardless of growth phase.
Another motivation for examining translational efficiency of lysates prepared from the Δhpf B. subtilis strain is the potential utility of Δhpf lysates for generating stalled ribosomal complexes. A popular strategy for preparation of stalled ribosomal complexes utilizes cell-free translation of dicistronic 2Xerm-mRNA encoding two identical Erm-stalling leader peptides (Arenz et al., 2014; Crowe-McAuliffe et al., 2018). Stalled ribosomal dimers formed in the presence of the antibiotic erythromycin are readily separated from 70S monosomes – but not from 100S particles – on sucrose gradients. Use of the Δhpf strain lacking 100S ribosomes to generate extracts avoids this problem.
Results
Elimination of HPF Improves the Efficiency of B. subtilis Coupled Transcription-Translation System
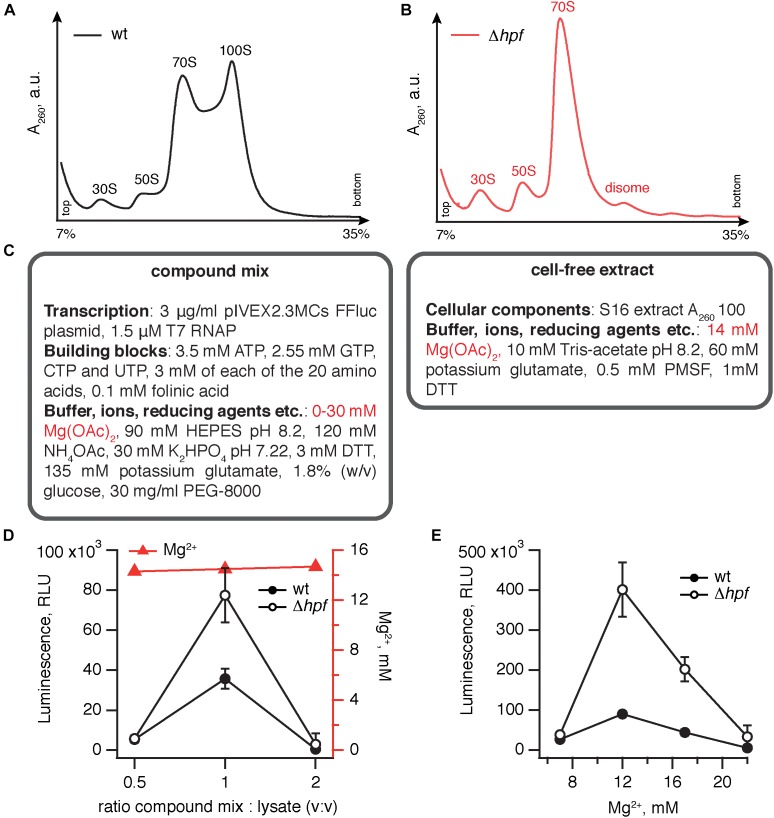
We opted for a coupled transcription-translation system utilizing the pIVEX2.3MCs FFluc plasmid (Starosta et al., 2010) that encodes the firefly luciferase ORF preceded by B. subtilis optimized ribosome binding site (RBS). Transcription of the mRNA is driven by recombinant T7 RNA polymerase added to the lysate (Antoun et al., 2004) and the efficiency of protein synthesis was quantified by measuring the luminescence of the translated luciferase protein. For preparing cell-free extracts we used the wild type 168 B. subtilis strain and an isogenic Δhpf mutant that displays no growth defect, except for a moderate increase in the lag phase (Akanuma et al., 2016). The cells were collected at OD600 1.8–2.2 and the lysates prepared as described in the Materials and Methods section. The polysome profile analyses show that in the case of the wild type strain, the 100S peak is dominant and stable (Figure 1A), while in the Δhpf mutant it is lacking altogether (Figure 1B). Importantly, the situation is dramatically different in the case of E. coli: when lysates are prepared from the stationary phase E. coli cells, no 100S peak is observed (Failmezger et al., 2017).
FIGURE 1.
Elimination of HPF improves the efficiency of B. subtilis coupled transcription-translation system. Polysome profile analysis of translational lysates prepared from wild type (A) and Δhpf (B) lysates demonstrates strictly HPF-dependent 100S ribosomes formation. (C) A cell-free translation system was assembled by combining the compound mix with the cell-free extract. The efficiency of translation was quantified by the activity of the firefly luciferase using the Steady-Glo Luciferase Assay (Promega). Titrations of the compound mix to cell-free extract ratio (D) and magnesium ion concentration (E) in the cell-free translation system. Luminescence readings were taken after incubation for 1 h at 37°C. Error bars indicate the standard error of the geometric mean of biological replicates, i.e., independently prepared cell-free extracts (n ≥ 3).
Our protocol for preparation of a B. subtilis transcription-translation system was based on that of Krinsky et al. (2016), which is specifically optimized to be rapid, cheap and relatively efficient – despite lacking a dedicated ATP regeneration system, it was able to generate 40–150 μg-protein/mL. The system is a binary mixture of cell-free extract and compound mix (Figure 1C). The cell-free extract contains a full set of cellular components carrying out protein synthesis, i.e., ribosomes, tRNAs, aminoacyl tRNA synthetases, methionyl-tRNA formyltransferase and translational factors. The compound mix contains (i) inorganic ions, importantly Mg2+, the key player in ribosomal function (Nierhaus, 2014) (ii) buffering (HEPES) and reducing (DTT) agents (iii) NTPs that serve both as the energy source and as the building blocks for mRNA synthesis (iv) template DNA in a form of pIVEX2.3MCs FFluc plasmid supplemented with recombinant T7 RNAP polymerase (v) amino acids and folinic acid that serve as building blocks for protein synthesis and, finally, (vi) stabilizing agents such as PEG-8000 and glucose.
To test the robustness of the effect of HPF loss on the efficiency transcription-translation system, we performed two titrations of the key components. As a first step, we have varied the ratio between the compound mix and cell-free extract (Figure 1D). To ensure the robustness of our results, we used at least three independently prepared cell-free extracts for each titration point. At a 1:1 ratio the activity is optimal, and the Δhpf lysate is robustly approximately twofold more active.
The second titration step was aimed at identifying the optimal concentration of magnesium ions (Zaher and Green, 2014). Magnesium is important both for optimizing transcriptional and translational efficiency in the coupled system. While using in vitro transcribed mRNA it is possible to discriminate between Mg2+ effects on transcription and translation, the specific effect of HPF elimination is unlikely to act on the transcriptional level.
Maintaining the ratio between the extract and compound mix at 1:1, we titrated the final concentration of Mg2+ from 7 to 22 mM (Figure 1E). While the activity of the Δhpf lysate peaks at 12 mM Mg2+, reaching an excess of 400,000 relative light units (RLU; the term “relative” is used since rather than providing absolute quantification of photons, the efficiency of detection is specific for a particular luminometer instrument), the Δhpf lysate is more active than the wild type at all magnesium concentrations tested. Therefore, we concluded that loss of HPF robustly improves the efficiency of B. subtilis lysates prepared from stationary phase cells.
Elimination of Stm1 Improves the Efficiency of S. cerevisiae Translation System
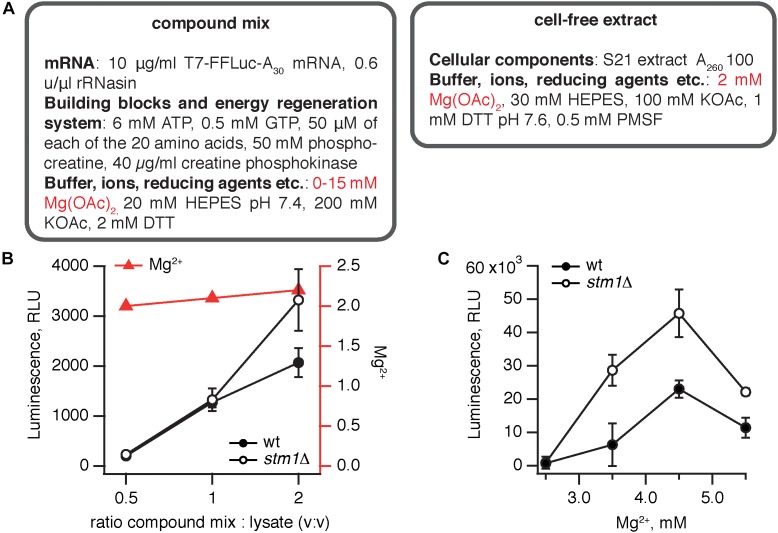
The S. cerevisiae stm1Δ strain was constructed by deleting the STM1 gene in the wild type MBS (Iizuka and Sarnow, 1997) strain. We have opted for a translation system supplemented with an in vitro transcribed, capped polyadenylated luciferase mRNA. Capping mRNA dramatically increases the efficiency of translation (Tarun and Sachs, 1995) but cannot be performed in situ in the lysate and must be added enzymatically to the mRNA after transcription. The translation protocol was based on that of Wu et al. (2007). Just as the bacterial coupled translation-transcription system, the yeast translation system is also a binary mixture of cell-free extract and a compound mix (Figure 2A). Since transcription is performed separately, the compound mix contains only the two NTP species necessary for translation, i.e., GTP and ATP, and mRNA is stabilized by RNase inhibitor (rRNasin). Creatine phosphokinase and phosphocreatine serve an energy regeneration system.
FIGURE 2.
Elimination of Stm1 improves the efficiency of S. cerevisiae translation system. (A) A cell-free translation system was assembled by combining the compound mix with the cell-free extract. The efficiency of translation was quantified by the activity of firefly luciferase using the Steady-Glo Luciferase Assay (Promega). Titrations of the compound mix to cell-free extract ratio (B) and magnesium ion concentration (C) in the cell-free translation system. Luminescence readings were taken after incubation for 1 h at 25°C. Error bars indicate the standard error of the geometric mean of biological replicates, i.e., independently prepared cell-free extracts (n ≥ 3).
Similarly to the B. subtilis system, we have performed two titrations, using at least three independently prepared cell-free extracts for each titration point: altering the ratio between the compound mix and cellular extract (Figure 2B) and the amount of Mg2+ in the extract (Figure 2C). The efficiency of the system gradually increases with the percentage of the compound mix in the reaction from 33 to 66% (Figure 2B). Notably, both at 33–50% compound mix there is no significant difference between the wild type and stm1Δ lysates; only in the presence of 66% compound mix does the stm1Δ translation reaction display higher activity. However, even in this case, the activity is two orders of magnitude lower than that seen in the B. subtilis system. A likely culprit is a sub-optimal concentration of Mg2+ ions; as the fraction of the compound mix increases, the Mg2+ concentration is moderately increased from 2 to 2.2 mM, following the concomitant increase in activity (Figure 2B). Therefore, we titrated Mg2+ from 2.5 to 5.5 mM (Figure 2C). The activity peaked at 4.5 mM Mg2+, reaching an excess of 40,000 RLU with the stm1Δ S. cerevisiae lysate robustly out-performing the wild type lysate.
Discussion
Here we demonstrated that genomic disruption of genes encoding ribosome inactivating factors – HPF in B. subtilis and Stm1 in S. cerevisiae – moderately but robustly (n ≥ 3 biological replicates – i.e., independently prepared cell-free extracts – were used for each data point) improves the activity of bacterial and yeast translation systems. Both of the proteins directly inactivate the ribosomes and are to cell-free translation lysates when added in trans (Ueta et al., 2010; Balagopal and Parker, 2011). Importantly, elimination of HPF leads to a complete loss of 100S ribosomes, thus despite only a moderate increase in the overall translational efficiency, the Δhpf lysate is particularly well-suited for disome-based approaches for preparation of stalled complexes for cryo-EM structural studies (Arenz et al., 2014; Crowe-McAuliffe et al., 2018).
The increase in efficiency of the delta Δhpf B. subtilis system is modest compared to the effects of reducing cellular proteolytic or RNase activities: a well-proven approach for genetic manipulation of strains for in vitro translation, as exemplified by the B. subtilis WB800N strain lacking eight protease-encoding genes (Wu et al., 2002) that is used for cell-free translation (Kelwick et al., 2016) and the E. coli MRE600 strain with low RNase I activity (Kurylo et al., 2016) that is used for preparation of active ribosomes (Rivera et al., 2015) and translation systems (Castro-Roa and Zenkin, 2015). In the case of B. subtilis WB800N, the cell-free translation system prepared from this strain is 72 times more active than that made from the wild type (Kelwick et al., 2016) – a significantly larger improvement compared to the twofold effect observed upon hpf deletion. A possible next step in developing strains for more efficient cell-free translation system is by combining genetic modifications targeting proteolysis, RNA degradation and ribosome hibernation. In the case of the S. cerevisiae system, optimization strategies used in recent years have been focused on cultivation conditions, preparation of lysates and approaches for replenishment of substrates (Zemella et al., 2015). Therefore, even the moderate improvement in efficiency yielded by Stm1 elimination has significance for biotechnological applications.
Materials and Methods
Preparation 6His-Tagged Recombinant T7 RNA Polymerase (T7 RNAP)
E. coli BL21 strain (NEB) lacking the λDE3 lysogen was transformed with pQE30-T7RNAP plasmid (Amp) (Antoun et al., 2004). An overnight culture in LB media supplemented with 50 μg/mL of ampicillin was used to inoculate a large-scale culture in the same media grown at 37°C with shaking. At OD600 of 0.5–0.6 expression of 6His-tagged T7 RNAP was induced by addition of IPTG to a final concentration of 1 mM. After an additional 2 h growth, cells were harvested by centrifugation, resuspended in buffer A (150 mM NaCl, 100 mM Tris:HCl pH 7.5, 2 mM MgCl2, 1 mM ß-mercaptoethanol) supplemented with 0.1 mM PMSF, 35 μg/mL lysozyme and 1 u/mL DNase I and lysed by one pass via Stansted Fluid Power SFPH-10 Stansted Pressure Cell/Homogenizer (1.5 bar). After removal of the cell debris by centrifugation (35,000 rpm for 40′), the clarified lysate was loaded onto a 1 mL HisTRAP HP column (GE Healthcare) equilibrated in buffer A. The column was washed with high salt buffer B (same as buffer A except for the addition of 2 M NaCl), and the protein was eluted by a gradient of buffer C (buffer A supplemented with 0.5 M imidazole), pure fractions were pooled, concentrated and buffer-exchanged into storage buffer (100 mM KCl, 20 mM Tris:HCl pH 7.5, 5 mM MgCl2, 1 mM DTT, 50% glycerol) using 50 MWCO centricons (Amicon). The purity of protein preparations was assessed by SDS PAGE and spectrophotometrically (A280/260 ratio of approximately 1.8). The protein was stored at −20°C.
Preparation of Firefly Luciferase mRNA for Use in Yeast Translational Lysates
Firefly luciferase mRNA containing a 30 nucleotide poly(A) tail was in vitro transcribed from the luciferase T7 control plasmid (Promega) linearized by Afel as a DNA template for the T7 RNA Polymerase (HiScribeTM T7 High Yield) RNA Synthesis Kit. A typical 20 μL reaction containing 1 pmole DNA was incubated for 2 h at 37°C prior to mRNA isolation with MEGAclearTM Kit (Ambion), followed by capping by the Vaccinia Capping System (NEB) and re-purification of mRNA with MEGAclearTM Kit (Ambion). The quality of the final product was confirmed by denaturing agarose electrophoresis.
B. subtilis and S. cerevisiae Strains
The wild type 168 (trpC2) B. subtilis strain was provided by Yuzuru Tozawa and the isogenic Δhpf knockout B. subtilis strain RIK2508 (trpC2 Δhpf)) was provided by Fujio Kawamura (Akanuma et al., 2016).
A S. cerevisiae strain deleted for STM1 (MJY1079, MATa ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 stm1::HIS3MX6 L-o M-o) was constructed by transforming the MBS strain (Iizuka and Sarnow, 1997) with a stm1::HIS3MX6 DNA fragment PCR amplified from pFA6a-HIS3MX6 (Wach et al., 1997). The oligonucleotides used were: 5′-AGTAGAAATAAACCAAGAAAGCATACACATTTTATTCTCACGGATCCCCGGGTTAATTAA-3′ and 5′TTATTGGATTCTTTCAGTTGGAATTATTCATATATAAGGCGAATTCGAGCTCGTTTAAAC-3′. The deletion was confirmed by PCR using primers that annealed outside of sequences present in the transformed DNA fragment.
Preparation of B. subtilis Bacterial Cell-Free Extract
The lysate preparation protocol is based on that of Krinsky et al. (2016) with minor modifications. Fifty milliliter LB cultures of B. subtilis 168 wild type and Δhpf strains were inoculated with single colonies from fresh LB plates and grown at 37°C with vigorous shaking overnight. To generate the biomass, two 2 L flasks containing 800 mL LB were inoculated to a starting OD600 of approximately 0.05 and bacteria were grown at 37°C with shaking. At the OD600 of 1.8–2.2 cells were collected by centrifugation at 10,000 g for 3 min (4°C, Beckman JLA-10.500 rotor), pellets were dissolved in 100 mL of ice-cold lysis buffer (10 mM Tris-acetate pH 8.0, 60 mM potassium glutamate, 14 mM magnesium acetate, 0.5 mM PMSF, 1mM DTT, pH 8.2), pelleted (3′ at 10,000 g, 4°C), taken up in 50 mL lysis buffer, and pelleted again in 50 mL Eppendorf centrifuge tubes (30′ at 3,000 g 4°C) yielding 4–5 g of biomass that was processed directly. To lyse the cells, lysis buffer was added to 4–5 g of cells to final volume of 12 mL, and cells were passed once though Stansted Fluid Power SFPH-10 Stansted Pressure Cell/Homogenizer at 2 bar. The lysate was clarified (10′ at 16,000 g, 4°C) and the supernatant was then desalted using Zeba Spin Desalting Columns 5 mL (ThermoFisher) equilibrated with lysis buffer. After adjusting A260 to 100 absorbance units with the lysis buffer, lysates were aliquoted in 50–100 μL fractions, snap frozen in liquid nitrogen and stored at −80°C.
Polysome Profile Analysis
B. subtilis 168 and Δhpf cell-free extracts were melted on ice and 6 AU260 units of each extract were loaded on 7–35% sucrose gradients made in HEPES:Polymix buffer (20 mM HEPES:KOH pH 7.5, 2 mM DTT, 5 mM MgOAc2, 95 mM KCl, 5 mM NH4Cl, 0.5 mM CaCl2, 8 mM putrescine, 1 mM spermidine; Antoun et al., 2004). Following centrifugation at 35,000 rpm for 2.5 h at 4°C (SW41 rotor, Beckman), the gradients were analyzed by measuring the continuous absorbance at 260 nm using a Piston Gradient Fractionator (Biocomp Instruments).
Preparation of S. cerevisiae Cell-Free Extract
The lysate preparation protocol is based on that of Wu et al. (2007) with minor modifications. The wild type MBS and MJY1079 strains were grown in YPD medium at 30°C for 24 h. To generate the biomass, two 2L flasks containing 800 mL YPD were inoculated to a starting OD600 of approximately 0.001 and yeast were grown overnight at 30°C until OD600 of 4.0–7.0. Cells were collected by centrifugation (10′ at 7,000 g, 4°C), washed (3′ at 10,000 g, 4°C) three times with 50 mL of ice-cold lysis buffer (30 mM HEPES, 100 mM potassium acetate, 2 mM magnesium acetate, 0.5 mM PMSF, 1 mM DTT, pH 7.6), and pelleted in 50 mL Falcon centrifuge tubes (30′ at 3,000 g, 4°C) yielding 10 g of cells. Cells were frozen as small pellets by mixing with lysis buffer and dropped into liquid nitrogen and stored at −80°C or processed directly. To lyse the cells, 10 g of frozen cells were combined with 1 mL of frozen lysis buffer and crushed with a mortar and pestle in liquid nitrogen for 20 min. Note that more robust lysis methods have been shown to reduce the ability to translate exogenous mRNA in a yeast in vitro translation system (Leibowitz et al., 1991). The resulting lysate was transferred to a 50 mL falcon tube and incubated on ice until melted. The lysate was clarified (30′ at 3,000 g followed by ultracentrifugation of the supernatant for 10′ at 21,000 g, all at 4°C). The supernatant was desalted using Zeba Spin Desalting Columns 5 mL (ThermoFisher) equilibrated with lysis buffer. After adjusting A260 to 100 absorbance units with the lysis buffer, lysates were aliquoted in 50–100 μL fractions, snap frozen in liquid nitrogen and stored at −80°C.
Preparation of B. subtilis Transcription-Translation System: Final Optimized Protocol
The protocol was based on that of Krinsky et al. (2016), with minor modifications. The translation system was assembled by mixing the B. subtilis lysate (see above) with the compound mix (10 mM Mg(OAc)2, 90 mM HEPES pH 8.2, 30 mM K2HPO4 pH 7.22, 135 mM potassium glutamate, 120 mM NH4OAc, 30 mg/mL PEG-8000, 1.8% (w/v) glucose, 3 mM DTT, 3 mM each of the 20 amino acid, 0.1 mM folinic acid, 3.5 mM ATP, 2.55 mM of GTP, CTP and UTP, 3 μg/mL plasmid pIVEX2.3MCs FFluc and 1.5 μM recombinant T7 RNAP) to a final volume of 30 μL per reaction point. The lysate and compound mix were combined at a 1:1 ratio. After gently mixing the binary system by pipetting, the reaction was incubated at 37°C for 1 h with shaking (500 rpm), and 10 μL of the reaction were added to 50 μL of Steady-Glo Luciferase Assay (Promega) (see below).
Preparation of S. cerevisiae Translation System: Final Optimized Protocol
The protocol was based on that of Wu et al. (2007) with minor modifications. The translation system was assembled by mixing the yeast lysate (see above) with the compound mix (7 mM Mg(OAc)2, 20 mM HEPES pH 7.4, 200 mM KOAc, 2 mM DTT pH 7.4, 50 μM each of the 20 amino acids, 6 mM ATP, 0.5 mM GTP, 50 mM phosphocreatine, 40 μg/mL creatine phosphokinase, 10 μg/mL capped firefly mRNA with a 30 nucleotide poly(A) tail and 0.6 units/μL rRNasin) to a final volume of 30 μL per reaction. Prior to use, the lysates were treated with micrococcal nuclease (NEB) (24 units/μL) activated by 0.5 mM CaCl2 (final concentration) at room temperature in order to degrade cellular mRNA species. After a 20′ treatment with the nuclease, the reaction was stopped by addition of EGTA (pH 7.5) to a final concentration of 2 mM. Capped luciferase mRNA was refolded (7′ at 70°C and kept on ice prior to use) and added last to the compound mix immediately prior to assembling the final reaction mixture. The lysate and compound mix were combined to a 1:1 ratio. After gently mixing the binary system by pipetting, the reaction was incubated at 25°C for 1 h on an Eppendorf Thermomixer with shaking (500 rpm), and 10 μL of the reaction were added to 50 μL of Steady-Glo Luciferase Assay (Promega) (see below).
Luciferase Assay
Steady-Glo Luciferase Assay (Promega) was used as per the manufacturer’s manual. The luciferase assay reagent was aliquoted in 50 μL fractions in 1.5 mL Eppendorf tubes kept in dark at room temperature. Readings were taken after addition of 10 μL reaction mixture to pre-aliquoted luciferase reagent using GloMax 20/20 Luminometer (Promega).
Author Contributions
VM and VH conceived and coordinated the study. TB, VM, and VH drafted the manuscript together with input from MJ, TN, and HT. VM, TB, and VH designed the experiments and analyzed the data. TB, VM, MJ, and HT performed the experiments. All authors have read and approved the manuscript as submitted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Yuzuru Tozawa for sharing wild type 168 (trpC2) B. subtilis strain and Fujio Kawamura for providing the isogenic Δhpf knockout (trpC) (Akanuma et al., 2016), Aivar Liiv for providing pQE30-T7RNAP plasmid (Antoun et al., 2004), Allan Jacobson for providing the MBS strain (Iizuka and Sarnow, 1997) and to Daniel N. Wilson for providing pIVEX2.3MCs FFluc plasmid (Starosta et al., 2010). The manuscript partially overlaps with a preprint at BioRxiv (Brodiazhenko et al., 2018).
Footnotes
Funding. This work was supported by the funds from European Regional Development Fund through the Centre of Excellence for Molecular Cell Technology (VH); the Molecular Infection Medicine Sweden (MIMS) (VH); Swedish Research council (Grant Nos. 2013–4680 to VH and Grant Nos. 2017-04663 to TN); Ragnar Söderberg foundation (VH); Magnus Bergvalls Foundation (2017-02098 to MJ), Åke Wibergs Foundation (M14-0207 to MJ).
References
- Agafonov D. E., Kolb V. A., Nazimov I. V., Spirin A. S. (1999). A protein residing at the subunit interface of the bacterial ribosome. Proc. Natl. Acad. Sci. U.S.A. 96 12345–12349. 10.1073/pnas.96.22.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanuma G., Kazo Y., Tagami K., Hiraoka H., Yano K., Suzuki S., et al. (2016). Ribosome dimerization is essential for the efficient regrowth of Bacillus subtilis. Microbiology 162 448–458. 10.1099/mic.0.000234 [DOI] [PubMed] [Google Scholar]
- Anger A. M., Armache J. P., Berninghausen O., Habeck M., Subklewe M., Wilson D. N., et al. (2013). Structures of the human and drosophila 80S ribosome. Nature 497 80–85. 10.1038/nature12104 [DOI] [PubMed] [Google Scholar]
- Antoun A., Pavlov M. Y., Tenson T., Ehrenberg M. M. (2004). Ribosome formation from subunits studied by stopped-flow and rayleigh light scattering. Biol. Proced. Online 6 35–54. 10.1251/bpo71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz S., Ramu H., Gupta P., Berninghausen O., Beckmann R., Vazquez-Laslop N., et al. (2014). Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide. Nat. Commun. 5:3501. 10.1038/ncomms4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal V., Parker R. (2011). Stm1 modulates translation after 80S formation in Saccharomyces cerevisiae. RNA 17 835–842. 10.1261/rna.2677311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Yap M. N. (2016). Ribosome hibernation factor promotes staphylococcal survival and differentially represses translation. Nucleic Acids Res. 44 4881–4893. 10.1093/nar/gkw180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckert B., Abdelshahid M., Schafer H., Steinchen W., Arenz S., Berninghausen O., et al. (2017). Structure of the Bacillus subtilis hibernating 100S ribosome reveals the basis for 70S dimerization. EMBO J. 36 2061–2072. 10.15252/embj.201696189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A., Garreau De Loubresse N., Melnikov S., Jenner L., Yusupova G., Yusupov M. (2011). The structure of the eukaryotic ribosome at 3.0 a resolution. Science 334 1524–1529. 10.1126/science.1212642 [DOI] [PubMed] [Google Scholar]
- Brodiazhenko T., Johansson M. J. O., Takada H., Nissan T., Hauryliuk V., Murina V. (2018). Elimination of ribosome inactivating factors improves the efficiency of Bacillus subtilis and Saccharomyces cerevisiae cell-free translational systems. bioRxiv [Preprint]. 10.1101/424044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Roa D., Zenkin N. (2015). Methodology for the analysis of transcription and translation in transcription-coupled-to-translation systems in vitro. Methods 86 51–59. 10.1016/j.ymeth.2015.05.029 [DOI] [PubMed] [Google Scholar]
- Crowe-McAuliffe C., Graf M., Huter P., Takada H., Abdelshahid M., Novacek J., et al. (2018). Structural basis for antibiotic resistance mediated by the Bacillus subtilis ABCF ATPase VmlR. Proc. Natl. Acad. Sci. U.S.A. 115 8978–8983. 10.1073/pnas.1808535115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Zhu M., Warren M., Balakrishnan R., Patsalo V., Okano H., et al. (2016). Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat. Microbiol. 2:16231. 10.1038/nmicrobiol.2016.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failmezger J., Rauter M., Nitschel R., Kraml M., Siemann-Herzberg M. (2017). Cell-free protein synthesis from non-growing, stressed Escherichia coli. Sci. Rep. 7:16524. 10.1038/s41598-017-16767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohara D. W., Yap M. F. (2018). Survival of the drowsiest: the hibernating 100S ribosome in bacterial stress management. Curr. Genet. 64 753–760. 10.1007/s00294-017-0796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka N., Sarnow P. (1997). Translation-competent extracts from Saccharomyces cerevisiae: effects of L-A RNA, 5′ cap, and 3′ poly(A) tail on translational efficiency of mRNAs. Methods 11 353–360. 10.1006/meth.1996.0433 [DOI] [PubMed] [Google Scholar]
- Kelwick R., Webb A. J., Macdonald J. T., Freemont P. S. (2016). Development of a Bacillus subtilis cell-free transcription-translation system for prototyping regulatory elements. Metab. Eng. 38 370–381. 10.1016/j.ymben.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Khusainov I., Vicens Q., Ayupov R., Usachev K., Myasnikov A., Simonetti A., et al. (2017). Structures and dynamics of hibernating ribosomes from Staphylococcus aureus mediated by intermolecular interactions of HPF. EMBO J. 36 2073–2087. 10.15252/embj.201696105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky N., Kaduri M., Shainsky-Roitman J., Goldfeder M., Ivanir E., Benhar I., et al. (2016). A simple and rapid method for preparing a cell-free bacterial lysate for protein synthesis. PLoS One 11:e0165137. 10.1371/journal.pone.0165137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurylo C. M., Alexander N., Dass R. A., Parks M. M., Altman R. A., Vincent C. T., et al. (2016). Genome sequence and analysis of Escherichia coli MRE600, a colicinogenic, nonmotile strain that lacks RNase I and the type I methyltransferase, EcoKI. Genome Biol. Evol. 8 742–752. 10.1093/gbe/evw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. J., Barbone F. P., Georgopoulos D. E. (1991). In vitro protein synthesis. Methods Enzymol. 194 536–545. 10.1016/0076-6879(91)94040-J [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. (2014). Mg2+, K+, and the ribosome. J. Bacteriol. 196 3817–3819. 10.1128/JB.02297-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A., Hellberg K., Enberg J., Karlsson B. G. (2011). Rational improvement of cell-free protein synthesis. N. Biotechnol. 28 218–224. 10.1016/j.nbt.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Rivera M. C., Maguire B., Lake J. A. (2015). Isolation of ribosomes and polysomes. Cold Spring Harb. Protoc. 2015 293–299. 10.1101/pdb.prot081331 [DOI] [PubMed] [Google Scholar]
- Rutkowska A., Beerbaum M., Rajagopalan N., Fiaux J., Schmieder P., Kramer G., et al. (2009). Large-scale purification of ribosome-nascent chain complexes for biochemical and structural studies. FEBS Lett. 583 2407–2413. 10.1016/j.febslet.2009.06.041 [DOI] [PubMed] [Google Scholar]
- Starosta A. L., Karpenko V. V., Shishkina A. V., Mikolajka A., Sumbatyan N. V., Schluenzen F., et al. (2010). Interplay between the ribosomal tunnel, nascent chain, and macrolides influences drug inhibition. Chem. Biol. 17 504–514. 10.1016/j.chembiol.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Tarun S. Z., Jr., Sachs A. B. (1995). A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev. 9 2997–3007. 10.1101/gad.9.23.2997 [DOI] [PubMed] [Google Scholar]
- Ueta M., Ohniwa R. L., Yoshida H., Maki Y., Wada C., Wada A. (2008). Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J. Biochem. 143 425–433. 10.1093/jb/mvm243 [DOI] [PubMed] [Google Scholar]
- Ueta M., Wada C., Wada A. (2010). Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 15 43–58. 10.1111/j.1365-2443.2009.01364.x [DOI] [PubMed] [Google Scholar]
- van den Elzen A. M., Schuller A., Green R., Seraphin B. (2014). Dom34-Hbs1 mediated dissociation of inactive 80S ribosomes promotes restart of translation after stress. EMBO J. 33 265–276. 10.1002/embj.201386123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Sanjurjo A., Schuwirth B. S., Hau C. W., Cate J. H. (2004). Structural basis for the control of translation initiation during stress. Nat. Struct. Mol. Biol. 11 1054–1059. 10.1038/nsmb850 [DOI] [PubMed] [Google Scholar]
- Wach A., Brachat A., Alberti-Segui C., Rebischung C., Philippsen P. (1997). Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast 13 1065–1075. [DOI] [PubMed] [Google Scholar]
- Wada A., Yamazaki Y., Fujita N., Ishihama A. (1990). Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc. Natl. Acad. Sci. U.S.A. 87 2657–2661. 10.1073/pnas.87.7.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Amrani N., Jacobson A., Sachs M. S. (2007). The use of fungal in vitro systems for studying translational regulation. Methods Enzymol. 429 203–225. 10.1016/S0076-6879(07)29010-X [DOI] [PubMed] [Google Scholar]
- Wu S. C., Yeung J. C., Duan Y., Ye R., Szarka S. J., Habibi H. R., et al. (2002). Functional production and characterization of a fibrin-specific single-chain antibody fragment from Bacillus subtilis: effects of molecular chaperones and a wall-bound protease on antibody fragment production. Appl. Environ. Microbiol. 68 3261–3269. 10.1128/AEM.68.7.3261-3269.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher H. S., Green R. (2014). In vitro synthesis of proteins in bacterial extracts. Methods Enzymol. 539 3–15. 10.1016/B978-0-12-420120-0.00001-3 [DOI] [PubMed] [Google Scholar]
- Zemella A., Thoring L., Hoffmeister C., Kubick S. (2015). Cell-Free protein synthesis: pros and cons of prokaryotic and eukaryotic systems. Chembiochem 16 2420–2431. 10.1002/cbic.201500340 [DOI] [PMC free article] [PubMed] [Google Scholar]