Abstract
The control of inflammation, which arises from complex biological responses to harmful stimuli, is an important determinant of both clinical outcomes and patient comfort. However, the side effects of many current therapies such as non-steroidal anti-inflammatory drugs mean that new safe treatments are required. We previously reported that 12.5 µg/ml hydroxytyrosol (HT) suppressed gene expression of the inducible nitric oxide (NO) synthase (iNOS) isoform and NO production, in mouse peritoneal macrophages treated with lipopolysaccharide (LPS), where nuclear factor-κB (NF-κB) gene expression was not altered. The present study evaluated the anti-inflammatory effects of various concentrations of HT in LPS-induced RAW264.7 mouse macrophages. HT suppressed NF-κB signaling and downregulated LPS-mediated expression of iNOS, cyclooxygenase-2, tumor necrosis factor alpha, and interleukin-1β at 12.5 µg/ml, resulting in reduced production of NO and prostaglandin E2. At lower concentrations, HT seemed to act via another signaling pathway to regulate the inflammatory response. In contrast, HT did not suppress LPS-induced expression of phosphorylated p44/42 mitogen-activated protein kinase. This study showed that HT had anti-inflammatory effects on LPS-stimulated RAW264.7 cells. HT is already available as a nutritional supplement and no toxic effects have been reported. Hence, HT represents a potential novel anti-inflammatory agent.
Keywords: hydroxytyrosol, inflammation, lipopolysaccharide, macrophage, nitric oxide
Inflammation can result from complex biological responses of body tissues to harmful stimuli such as pathogens, damaged cells, or irritants; these protective responses involve immune cells, blood vessels, and molecular mediators. Inflammation eliminates the initial cause of cell injury, clears out necrotic cells and tissues damaged by the original insult and the inflammatory process, and initiates tissue repair. However, excessive inflammation causes chronic inflammatory conditions including arthritis, asthma, multiple sclerosis, and atherosclerosis [13]. The activation of pro-inflammatory cells, mainly macrophages, plays a critical role in inflammatory response. Lipopolysaccharide (LPS), which is a component of the cell wall of gram-negative bacteria, is known to activate cytokine networks by inducing the release of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and inflammatory mediators including nitric oxide (NO) and prostaglandin E2 (PGE2) [1, 27]. NO can be synthesized from l-arginine by a family of NO synthases (NOS). An inducible isoform of NOS (iNOS) is only expressed after exposure to pro-inflammatory conditions. Once expressed, iNOS generates large amounts of NO, which plays an important role in acute and chronic inflammation [10, 26]. Although the suppression of excessive inflammation is important to control chronic inflammatory diseases, current therapies using non-steroidal anti-inflammatory drugs sometimes cause harmful side effects [17, 20]. Hence, novel anti-inflammatory agents with reduced side effects are required.
Hydroxytyrosol (HT), which is a small phenolic molecule found in olive oil, exerts strong antioxidant activity and acts as an anti-inflammatory, antithrombotic, antitumor, and antimicrobial agent [22]. In a previous study, we reported that HT suppressed iNOS gene expression and NO production in mouse peritoneal macrophages treated with LPS. However, no suppression of the nuclear factor-κB (NF-κB) signaling pathway was observed. These results indicate that HT suppresses LPS-induced NO production in an NF-κB pathway-independent manner in mouse peritoneal macrophages [21]. On the other hand, Zhang et al. [28] reported that HT suppressed the translocation of NF-κB to the nucleus in the presence of LPS in human monocytic THP-1 cells. Although these two studies employed similar HT concentrations of 80 µM (12.5 µg/ml) and 100 µM, different cells were studied. These results suggest that low HT concentrations may not always be able to suppress the NF-κB-mediated inflammatory response. Alternatively, different macrophage lineage cells cultured with HT could show different responses to LPS stimulation. In addition to its effects on NF-κB signaling, LPS has been reported to induce other signaling pathways. In particular, p44/42 mitogen-activated protein kinase (ERK1/2) was strongly induced by LPS in monocytes and macrophages [8, 23], and HT may exert an anti-inflammatory effect by suppressing this signaling pathway.
In this study, we aimed to evaluate the anti-inflammatory effects of various concentrations of HT, and to clarify its mechanism of action in an LPS-induced RAW264.7 mouse macrophage cell line.
MATERIALS AND METHODS
HT
HT (>98% purity; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was dissolved in distilled water (Otsuka Pharmaceutical Factory, Inc., Tokyo, Japan) to 100 µg/ml and was stored at −80°C.
In vitro culture of RAW264.7 cells and cytotoxicity testing
A mouse RAW264.7 macrophage cell line (DS Pharma Biomedical Co., Ltd., Osaka, Japan) was cultured in Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Industries Ltd., Osaka, Japan) containing 10% fetal bovine serum at 37°C in a humidified 5% CO2 incubator.
The cells were added to 24-well plates (1 × 106 cells per well). Two hours later, the medium was replaced with fresh medium to remove non-adherent cells. Fresh medium containing serially diluted HT (0−50 µg/ml) was added to the wells, and the cells were cultured at 37°C for 24 hr. The cytotoxicity of HT was determined by measuring the amount of lactate dehydrogenase (LDH) released from the cells using an automatic biochemical analysis system (LABOSPECT 006; Hitachi High-Technologies Co., Ltd., Tokyo, Japan).
Effect of HT on RAW264.7 cells stimulated with LPS
RAW264.7 cells were added to 12-well plates (2 × 106 per well), and non-adherent cells were removed 2 hr later. Adherent cells were cultured in medium containing HT (1.56−12.5 µg/ml) with LPS (0.25 µg/ml) at 37°C for 3 or 24 hr. The cells cultured in the absence of LPS and HT served as controls. The cultured cells were harvested, and real-time reverse transcription polymerase chain reaction (RRT-PCR) was used to measure the levels of mRNA-encoding proteins involved in inflammation. The NO, PGE2, TNF-α, and IL-1β concentrations in cell culture media harvested following 24 hr of incubation were determined using a Nitric Oxide (total) detection kit (Enzo Life Sciences, New York, NY, U.S.A.), Prostaglandin E2 ELISA kit (Abcam, Cambridge, U.K.), Mouse TNF alpha SimpleStep ELISA® kit (Abcam), and IL-1 beta Mouse SimpleStep ELISA® Kit (Abcam), respectively. The cellular COX-2 concentrations were determined using a Mouse COX2 SimpleStep ELISA® kit (Abcam). For western blotting, the cells were harvested after 15 min to analyze the expression of phosphorylated NF-κB (pNF-κB) and ERK1/2 (pERK1/2), and after 6 hr to analyze the expression of iNOS.
RRT-PCR analysis of gene expression
For RRT-PCR analysis of gene expression, the cells were harvested after 3 and 24 hr. Total RNA was extracted from cells using the RNeasy® Mini Kit (QIAGEN, Venlo, Netherlands) and reverse-transcribed using the QuantiTectTM Reverse Transcription Kit (QIAGEN) as follows: 42°C for 15 min and 95°C for 3 min. RRT-PCR was performed using the cDNAs and QuantiFast SYBR® Green PCR (QIAGEN) with AriaMx (Agilent Technologies, Santa Clara, CA, U.S.A.). The primers (QuantiTectTM Primer Assays, QIAGEN) are listed in Table 1. The PCR reactions were conducted as follows: 95°C for 5 min, with 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The threshold cycle (Ct) was defined as the number of cycles required for the intensity of fluorescence to rise above the threshold value. The Ct values were normalized to those of glyceraldehyde 3-phosphate dehydrogenase, and expressed in relation to the signals observed in control (LPS-treated) cells. In samples with levels that were undetectable using RRT-PCR, the Ct value was defined as 45. The results were expressed as the relative differences in gene expression, in accordance with the MIQE guidelines [3].
Table 1. Prime sets (QuantiTect Primer Assay, QIAGEN) used in real-time RT-PCR.
| Gene name | Assay name |
|---|---|
| iNOS (Nitric oxide synthase 2, inducible) | Mm_LOC673161_1_SG |
| COX-2 (Prostaglandin-endoperoxide synthase 2) | Mm_Ptgs2_1_SG |
| TNF-α (Tumor necrosis factor alpha) | Mm_Tnf_1_SG |
| NF-κB (Nuclear factor of kappa light polypeptide gene enhancer in B-cells) | Mm_Nfkb1_1_SG |
| IL-1β (Interleukin 1 beta) | Mm_Il1b_2_SG |
| IL-6 (Interleukin 6) | Mm_Il6_1_SG |
| IL-10 (Interleukin 10) | Mm_Il10_1_SG |
| Gapdh (glyceraldehyde-3-phosphate dehydrogenase) | Mm_Gapdh_3_SG |
Western blotting analysis
Lysates of cells harvested after 15 min or 6 hr were electrophoresed using Mini-PROTEAN TGX Precast Gels (Bio-Rad, Hercules, CA, U.S.A.), and the proteins were transferred to a polyvinylidene difluoride membrane using a Trans-Blot Turbo Transfer Pack (Bio-Rad). The membranes were blocked with phosphate-buffered saline containing 0.3% skim milk and then incubated with the relevant primary antibodies: anti-NF-κB, anti-pNF-κB, anti-ERK1/2, anti-pERK1/2, or anti-iNOS (Cell Signaling Technology, Danvers, MA, U.S.A.). Antigen-antibody complexes bound to the membrane were detected using the SuperSignalTM West Pico (Thermo Scientific, Waltham, MA, U.S.A.), and analyzed with a LAS-3000 (Fujifilm, Tokyo, Japan). As an internal control, β-actin was detected using the anti-β-Actin HRP-DirecT kit (Medical & Biological Laboratories, Nagoya, Japan).
Statistical analysis
Results are presented as the mean ± standard error of the mean (SE). Statistical analysis was performed using the Williams’ multiple comparison test. Statistical values of P<0.05 were considered significantly different.
RESULTS
Low concentrations of HT are not cytotoxic to RAW264.7 cells
First, we evaluated the cytotoxicity of 0–50 µg/ml (0–320 µM) HT in RAW264.7 cells. No increase in the LDH concentration, as an indicator of cytotoxicity, was observed at any of these concentrations. However, the LDH concentration was slightly decreased in cells exposed to ≥25 µg/ml (160 µM); the reason for this was not known (Fig. 1). Thus, subsequent experiments were carried out at HT concentrations of 0–12.5 µg/ml.
Fig. 1.
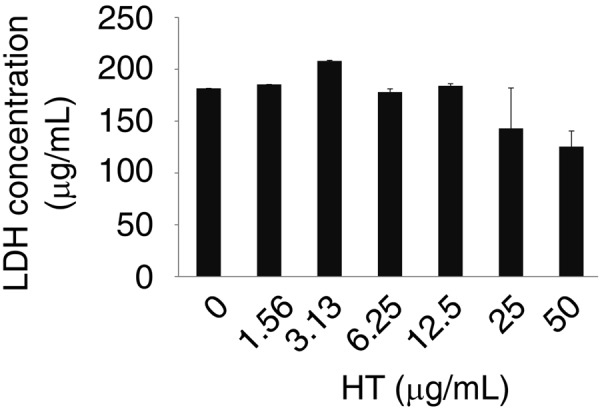
A low concentration of HT was not cytotoxic to RAW264.7 cells. RAW264.7 cells were cultured with HT (0–50 µg/ml) for 24 hr. LDH concentration in cell culture media was evaluated. Error bars indicate means ± SE (n=3).
HT suppresses the expression of LPS-induced inflammatory genes
We next evaluated the effects of HT in RAW264.7 cells stimulated with LPS (0.25 µg/ml). LPS-stimulated RAW264.7 cells were cultured in the presence of HT (0–12.5 µg/ml) for 3 and 24 hr.
At 3 hr, cells exposed to LPS alone showed significantly greater gene expression of iNOS, COX-2, TNF-α, IL-1β, and IL-6, as compared with control cells. Although NF-κB and IL-10 gene expression was also increased, the increase was not statistically significant. The addition of HT significantly suppressed these LPS-induced changes in gene expression. Cells exposed to HT (1.56–12.5 µg/ml) showed significantly lower TNF-α and IL-1β mRNA levels (Fig. 2).
Fig. 2.
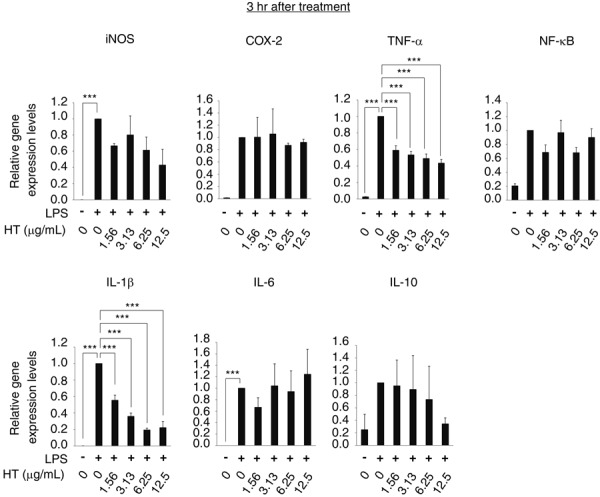
HT suppresses the early phase-expression of LPS-induced inflammatory genes in RAW264.7 cells. RAW264.7 cells were stimulated by LPS (0.25 µg/ml) and cultured with HT (0–12.5 µg/ml) for 3 hr. Expression of the iNOS, COX-2, TNF-α, NF-κB, IL-1β, IL-6 and IL-10 genes in RAW264.7 cells were analyzed by RRT-PCR. Error bars indicate means ± SE (n=4). ***P<0.001.
At 24 hr, cells treated with LPS alone showed significant increases in the gene expression of iNOS, COX-2, IL-1β, and IL-6, as compared with control cells. In contrast, NF-κB, TNF-α, and IL-10 gene expression was not altered by exposure to LPS. HT significantly reduced the mRNA levels of iNOS, COX-2, and IL-1β at concentrations of 12.5, 1.56–12.5, and 1.56–12.5 µg/ml, respectively (Fig. 3). These results indicate that HT suppresses LPS-induced upregulation of inflammatory gene expression.
Fig. 3.
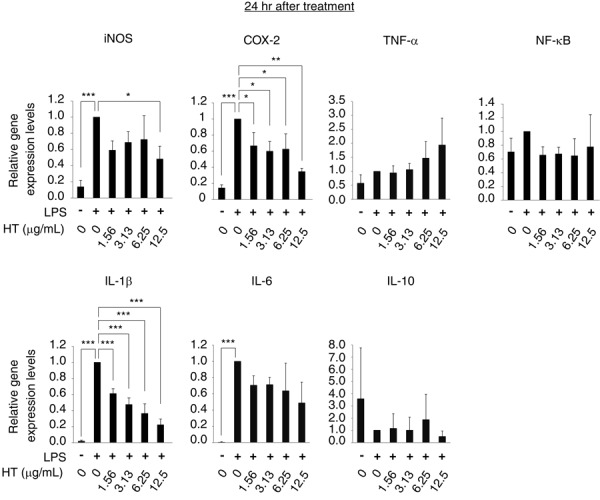
HT suppresses the late phase-expression of LPS-induced inflammatory genes in RAW264.7 cells. RAW264.7 cells were stimulated by LPS (0.25 µg/ml) and cultured with HT (0–12.5 µg/ml) for 24 hr. Expression of the iNOS, COX-2, TNF-α, NF-κB, IL-1β, IL-6 and IL-10 genes in RAW264.7 cells were analyzed by RRT-PCR. Error bars indicate means ± SE (n=4). *P<0.05, **P<0.01, ***P<0.001.
HT suppresses the production of LPS-induced inflammatory mediators
We also assessed the effects of HT on the production of LPS-induced inflammatory mediators. The concentrations of these molecules in culture media were assessed, and the COX-2 protein level in RAW264.7 cells was evaluated by ELISA. LPS significantly increased the levels of NO, PGE2, TNF-α, and IL-1β in conditioned media, and cellular COX-2 levels. The LPS-mediated induction of NO, PGE2, and IL-1β was significantly attenuated in cells exposed to 3.13–12.5, 1.56–12.5, and 1.56–12.5 µg/ml HT in a concentration-dependent manner, respectively. The cellular COX-2 level was slightly lower in cells exposed to HT, but this difference was not statistically significant. In contrast, the presence of HT did not reduce LPS-mediated TNF-α production (Fig. 4). These results indicate that HT suppresses the production of multiple LPS-induced inflammatory mediators.
Fig. 4.
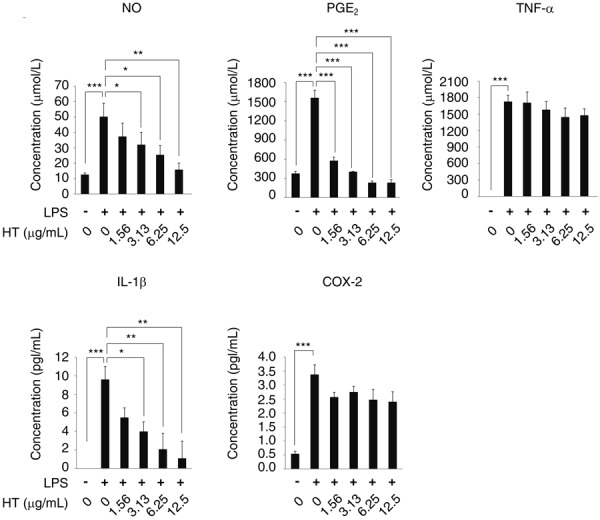
HT suppresses the production of LPS-induced inflammatory mediators in RAW264.7 cells. RAW264.7 cells were stimulated by LPS (0.25 µg/ml) and cultured with HT (0–12.5 µg/ml) for 24 hr. NO concentrations in cell culture media were evaluated using an NO detection kit. The concentrations of PGE2, TNF-α, IL-1β in cell culture media and of COX-2 in cell extracts were evaluated by ELISA. Error bars indicate means ± SE (n=3). *P<0.05, **P<0.01, ***P<0.001.
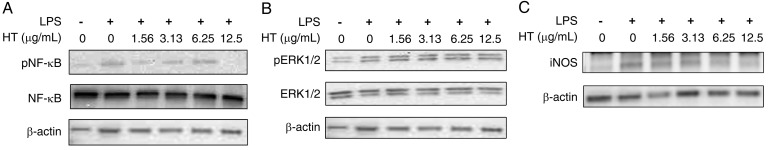
HT suppresses the LPS-induced phosphorylation of NF-κB, and iNOS expression
We next analyzed the phosphorylation of NF-κB and ERK1/2 to identify which signaling pathways were affected by HT. The iNOS expression level was also analyzed. Western blotting was used to determine the levels of pNF-κB and pERK1/2 in RAW264.7 cells cultured for 15 min, and of iNOS in cells cultured for 6 hr in the presence of LPS, with and without HT. Exposure of this cell line to LPS (0.25 µg/ml) increased pNF-κB, pERK1/2, and iNOS expression. The addition of 12.5 µg/ml HT suppressed this LPS-mediated induction of pNF-κB and iNOS expression. In contrast, the expression level of pERK1/2 was not affected by HT (Fig. 5).
Fig. 5.
HT suppresses the LPS-induced phosphorylation of NF-κB and iNOS in RAW264.7 cells. (A-C) RAW264.7 cells were stimulated by LPS (0.25 µg/ml) and cultured with HT (0–12.5 µg/ml) for 15 min (A, B) or 6 hr (C). The levels of NF-κB and pNF-κB (A), ERK1/2 and pERK1/2 (B), and iNOS (C) were analyzed by western blotting. β-Actin was an internal control. Representative results of duplicate experiments are shown.
These results indicate that HT (at ≤12.5 µg/ml) suppresses LPS-induced inflammatory cytokines in an ERK1/2 pathway-independent manner.
DISCUSSION
In the present study, we assessed the anti-inflammatory activities of HT in LPS-stimulated RAW264.7 mouse macrophages. HT suppressed LPS-induced NF-κB activation, TFN-α gene expression, IL-1β production, and activation of the COX-2-PGE2 and iNOS-NO axes. Toll-like receptor 4 is activated by LPS [14], triggering NF-κB signaling and inflammatory cytokine production, which activates the innate immune system [24]. IL-1β and TNF-α are rapidly translated and released at sites of tissue injury or infection [6, 11, 15]. iNOS and COX-2 are induced by IL-1β, TNF-α [7, 18, 19], and other cytokines, and are thus released later in the process. In the present study, HT suppressed the LPS-induced expression of TNF-α and IL-1β during the early phase (Fig. 2). Suppression of these inflammatory cytokines may therefore contribute to secondary inhibition of the iNOS-NO and COX-2-PGE2 axes.
Western blotting analysis showed that HT reduced the phosphorylation of NF-κB at 12.5 µg/ml (80 µM), but this activity was not observed at concentrations below 6.25 µg/ml (40 µM) (Fig. 5). Zhang et al. [28] showed that LPS-induced nuclear translocation of NF-κB in THP-1 cells was suppressed by 100 µM HT, but not by 50 µM. In the present study, even though the low HT concentration did not suppress NF-κB signaling, it did suppress TNF-α and IL-1β gene expression and the iNOS-NO axis. These results indicate that at a low concentration HT suppresses LPS-induced inflammatory responses in an NF-κB pathway-independent manner. pERK1/2 was strongly induced by LPS, as reported previously [8, 23]. However, HT did not suppress the expression of pERK1/2 at any of the tested concentrations. Chan et al. [5] showed that LPS-induced iNOS production in RAW264.7 cells was modulated by the c-Jun NH2-terminal kinase or stress-activated protein kinase pathway and the p38 MAPK pathway, in addition to the ERK pathway. Further studies are required to clarify the signaling pathway responsible for the mechanism of action of HT.
We previously showed that HT suppressed LPS-induced activation of the iNOS-NO axis in mouse peritoneal macrophages. However, in contrast to the present study, we did not observe suppression of NF-κB and COX-2 gene activation in mouse peritoneal macrophages [21]. Cardeno et al. [4] also reported that HT did not suppress COX-2 protein expression in LPS-activated mouse peritoneal macrophages. However, Maiuri et al. [16] and Zhang et al. [28] reported that HT suppressed LPS-mediated induction of both COX-2 gene expression and PGE2 production. These two studies used J774 murine macrophages and human monocytic THP-1 cells. These reports imply that HT responsiveness differs between macrophage types. LPS stimulation caused different molecular expression patterns in the RAW264.7 cell line, peritoneal macrophages, splenic macrophages, and bone marrow-derived macrophages [2, 25]. As many different types of macrophages are present in vivo, it is important to note that an experimental result acquired from a single cell type will not completely reflect the comprehensive in vivo phenomenon.
The side effects of non-steroidal anti-inflammatory drugs are known to include mucosal injuries to the stomach and small intestine [17], and renal disorder [20]. HT did not show any toxic effects in rodent studies [9], and is already widely commercially available in foods and nutritional supplements [12]. Although additional studies are required to fully understand the anti-inflammatory effects of HT, it would be worth testing whether HT effectively suppresses harmful inflammatory responses in an animal model.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Agarwal S., Piesco N. P., Johns L. P., Riccelli A. E.1995. Differential expression of IL-1 β, TNF-α, IL-6, and IL-8 in human monocytes in response to lipopolysaccharides from different microbes. J. Dent. Res. 74: 1057–1065. doi: 10.1177/00220345950740040501 [DOI] [PubMed] [Google Scholar]
- 2.Berghaus L. J., Moore J. N., Hurley D. J., Vandenplas M. L., Fortes B. P., Wolfert M. A., Boons G. J.2010. Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of Toll-like receptors 2, 3, and 4. Comp. Immunol. Microbiol. Infect. Dis. 33: 443–454. doi: 10.1016/j.cimid.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L., Vandesompele J., Wittwer C. T.2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55: 611–622. doi: 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 4.Cárdeno A., Sánchez-Hidalgo M., Aparicio-Soto M., Sánchez-Fidalgo S., Alarcón-de-la-Lastra C.2014. Extra virgin olive oil polyphenolic extracts downregulate inflammatory responses in LPS-activated murine peritoneal macrophages suppressing NFκB and MAPK signalling pathways. Food Funct. 5: 1270–1277. doi: 10.1039/C4FO00014E [DOI] [PubMed] [Google Scholar]
- 5.Chan E. D., Riches D. W. H.2001. IFN-γ + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38mapk in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 280: C441–C450. doi: 10.1152/ajpcell.2001.280.3.C441 [DOI] [PubMed] [Google Scholar]
- 6.Cho K. J., Yun C. H., Yoon D. Y., Cho Y. S., Rimbach G., Packer L., Chung A. S.2000. Effect of bioflavonoids extracted from the bark of Pinus maritima on proinflammatory cytokine IL-1 production in LPS-stimulated RAW 264.7. Toxicol. Appl. Pharmacol. 168: 64–71. doi: 10.1006/taap.2000.9001 [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury T. T., Bader D. L., Lee D. A.2003. Dynamic compression counteracts IL-1 β-induced release of nitric oxide and PGE2 by superficial zone chondrocytes cultured in agarose constructs. Osteoarthritis Cartilage 11: 688–696. doi: 10.1016/S1063-4584(03)00149-3 [DOI] [PubMed] [Google Scholar]
- 8.Chu A. J., Wang Z. G., Walton M. A., Seto A.2001. Involvement of MAPK activation in bacterial endotoxin-inducible tissue factor upregulation in human monocytic THP-1 cells. J. Surg. Res. 101: 85–90. doi: 10.1006/jsre.2001.6271 [DOI] [PubMed] [Google Scholar]
- 9.D’Angelo S., Manna C., Migliardi V., Mazzoni O., Morrica P., Capasso G., Pontoni G., Galletti P., Zappia V.2001. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 29: 1492–1498. [PubMed] [Google Scholar]
- 10.Denlinger L. C., Fisette P. L., Garis K. A., Kwon G., Vazquez-Torres A., Simon A. D., Nguyen B., Proctor R. A., Bertics P. J., Corbett J. A.1996. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J. Biol. Chem. 271: 337–342. doi: 10.1074/jbc.271.1.337 [DOI] [PubMed] [Google Scholar]
- 11.Dinarello C. A.1984. Interleukin-1 and the pathogenesis of the acute-phase response. N. Engl. J. Med. 311: 1413–1418. doi: 10.1056/NEJM198411293112205 [DOI] [PubMed] [Google Scholar]
- 12.Granados-Principal S., Quiles J. L., Ramirez-Tortosa C. L., Sanchez-Rovira P., Ramirez-Tortosa M. C.2010. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 68: 191–206. doi: 10.1111/j.1753-4887.2010.00278.x [DOI] [PubMed] [Google Scholar]
- 13.Hansson G. K., Hermansson A.2011. The immune system in atherosclerosis. Nat. Immunol. 12: 204–212. doi: 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 14.Kawai T., Akira S.2005. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17: 338–344. doi: 10.1016/j.coi.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Lawrence T.2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1: a001651. doi: 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiuri M. C., De Stefano D., Di Meglio P., Irace C., Savarese M., Sacchi R., Cinelli M. P., Carnuccio R.2005. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedebergs Arch. Pharmacol. 371: 457–465. doi: 10.1007/s00210-005-1078-y [DOI] [PubMed] [Google Scholar]
- 17.Matsui H., Shimokawa O., Kaneko T., Nagano Y., Rai K., Hyodo I.2011. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 48: 107–111. doi: 10.3164/jcbn.10-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyasaka N., Hirata Y.1997. Nitric oxide and inflammatory arthritides. Life Sci. 61: 2073–2081. doi: 10.1016/S0024-3205(97)00585-7 [DOI] [PubMed] [Google Scholar]
- 19.Newton R., Kuitert L. M., Bergmann M., Adcock I. M., Barnes P. J.1997. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1β. Biochem. Biophys. Res. Commun. 237: 28–32. doi: 10.1006/bbrc.1997.7064 [DOI] [PubMed] [Google Scholar]
- 20.Perneger T. V., Whelton P. K., Klag M. J.1994. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 331: 1675–1679. doi: 10.1056/NEJM199412223312502 [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y., Bui V. N., Iwasaki K., Kobayashi T., Ogawa H., Imai K.2014. Influence of olive-derived hydroxytyrosol on the toll-like receptor 4-dependent inflammatory response of mouse peritoneal macrophages. Biochem. Biophys. Res. Commun. 446: 1225–1230. doi: 10.1016/j.bbrc.2014.03.094 [DOI] [PubMed] [Google Scholar]
- 22.Tuck K. L., Hayball P. J.2002. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 13: 636–644. doi: 10.1016/S0955-2863(02)00229-2 [DOI] [PubMed] [Google Scholar]
- 23.van der Bruggen T., Nijenhuis S., van Raaij E., Verhoef J., van Asbeck B. S.1999. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect. Immun. 67: 3824–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaure C., Liu Y.2014. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 5: 316. doi: 10.3389/fimmu.2014.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C., Yu X., Cao Q., Wang Y., Zheng G., Tan T. K., Zhao H., Zhao Y., Wang Y., Harris D. C.2013. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol. 14: 6. doi: 10.1186/1471-2172-14-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisz A., Cicatiello L., Esumi H.1996. Regulation of the mouse inducible-type nitric oxide synthase gene promoter by interferon-gamma, bacterial lipopolysaccharide and NG-monomethyl-L-arginine. Biochem. J. 316: 209–215. doi: 10.1042/bj3160209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun K. J., Kim J. Y., Kim J. B., Lee K. W., Jeong S. Y., Park H. J., Jung H. J., Cho Y. W., Yun K., Lee K. T.2008. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-κ B inactivation in RAW 264.7 macrophages: possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 8: 431–441. doi: 10.1016/j.intimp.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Cao J., Jiang L., Zhong L.2009. Suppressive effects of hydroxytyrosol on oxidative stress and nuclear Factor-kappaB activation in THP-1 cells. Biol. Pharm. Bull. 32: 578–582. doi: 10.1248/bpb.32.578 [DOI] [PubMed] [Google Scholar]