Abstract
The purpose of this study was to evaluate the bioequivalence of 5% ceftiofur hydrochloride sterile suspension in two formulations, a test formulation (Saifukang 5% CEF, Hvsen) and a reference formulation (Excenel®RTU 5% CEF, Pfizer). Twenty-four healthy pigs were assigned to a two-period, two-treatment crossover parallel trial, and both formulations were administered at a single intramuscular dose of 5 mg/kg weight, with a 7-day washout period. Blood samples were collected consecutively for up to 144 hr after administration. The concentrations of ceftiofur- and desfuroylceftiofur-related metabolites in the plasma were determined by high-performance liquid chromatography. In addition, the major pharmacokinetic parameters (Cmax, AUC0-t and AUC0-∞) were computed and compared via analysis of variance, with 90% confidence intervals. Bioequivalence evaluation of Tmax was statistically analyzed with the nonparametric test. The comparison values between test and reference formulation for AUC0-t, AUC0-∞, Cmax, and Tmax were 376.7 ± 75.3 µg·hr/ml, 390.5 ± 78.6 µg·hr/ml, 385.9 ± 79.2 µg·hr/ml, 402.7 ± 80.4 µg·hr/ml, 34.6 ± 5.5 µg/ml, 36.1 ± 6.2 µg/ml, 1.27 ± 0.18 hr, and 1.26 ± 0.21 hr, respectively, and we observed no significant differences between the two formulations. The 90% CI values were within the recommended range of 80–125% (P>0.05), and the relative bioavailability of the test product was 96.47 ± 10.92% according to AUC0-t values. Based on our results, the two formulations exhibit comparable pharmacokinetic profiles, and the test product is bioequivalent to the reference formulation.
Keywords: bioequivalence, ceftiofur, confidence interval, pharmacokinetic, pig
Ceftiofur (CEF) is a third-generation cephalosporin and widely used abroad in the treatment of respiratory diseases in pigs, cattle, sheep, dogs, and poultry [1, 4, 20, 36]. It exhibits good antibacterial activity against gram-positive and gram-negative bacteria as well as some anaerobic bacteria, including β-lactamase-producing strains, both in vitro and in vivo [3, 39]. Similar to other cephalosporins, its antibacterial activity is based on the inhibition of cell wall synthesis [19]. The clinically approved dosage of treatment CEF equivalents for pigs ranges from 3 to 5 mg/kg body weight in the U.S. and in European countries, administered intramuscularly once daily for 3–5 consecutive days. In recent years, the hydrochloride salt of CEF was successfully prepared as a sterile suspension with a more stable form and has been approved for treating respiratory diseases in many animals [16, 17]. It has a rapid absorption rate and maintains high drug concentrations in plasma and tissue, which presented a longer half-life and prolonged therapeutic concentrations of CEF and defuroylceftiofur (DFC)-related metabolites, requiring less frequent injections and thereby minimizing handling and stress.
Some researchers have reported the metabolism of ceftiofur in rats [21], dairy cattle [22] and pigs [14]. They show similar metabolism in all animal species studied to date and is characterized by rapid cleavage of the thioester bond to active metabolite DFC [38]. CEF hydrochloride, irrespective of the route of administration, is rapidly metabolized to DFC and furoic acid in the body [22]. DFC is further metabolized to disulfides and reversibly bound to macromolecules in plasma and tissues [2, 38]. CEF is undetectable in plasma, bound DFC conjugated with glutathione, cysteine, and protein can be detected in plasma [33]. Free DFC is the primary metabolite and the active moiety of CEF.
In recent years, the evaluation of bioequivalence has gradually attracted the attention of the veterinary drug departments of the world; it can reduce the drugs registration period, costs of medication, time-to-market and increasing the selection range in veterinary clinical treatment. In particular, the European Medicine Agency (EPA) and the US Food and Drug Administration (FDA) guidelines noted that drugs produced from two different pharmaceutical companies contain the same active ingredients or that the same drugs of different formulations show similar bioavailability and therapeutic efficacy; therefore, the reference product can be replaced by the test product [6, 31]. At present, in the evaluation of bioequivalence, the pharmacokinetic method is generally selected as the classical method. Descriptive pharmacokinetic parameters, including maximum plasma concentration (Cmax), the time to reach maximum concentration (Tmax), the area under the plasma concentration-time curve from 0 to the last point (AUC0-t), and the area under the plasma concentration-time curve from 0 to infinity (AUC0-∞), are used for statistical analysis [24]. When bioequivalence has been demonstrated between two formulations, it is generally considered that two drug products are pharmaceutically equivalent [10, 11, 25].
The purpose of this study was to investigate the pharmacokinetic profiles of two 5% CEF hydrochloride sterile suspensions in pigs under standard conditions. This study will provide availability data of the two formulations for bioequivalence evaluation.
MATERIALS AND METHODS
Drugs and reagents
The desfuroylceftiofur reference standard (98.0% purity) was purchased from Sigma (St. Louis, MO, U.S.A.). Two kinds of commercial products of CEF hydrochloride sterile suspension containing 5% CEF, namely Saifukang (lot number: 20110510) as a test formulation (Hvsen, Wuhan, China) and Excenel®RTU (lot number: 1A5YW) as a reference formulation (Pfizer, Madison, NJ, U.S.A.), were used for the bioequivalence study. Solid phase extraction (SPE) cartridges (Dikma, Beijing, China) were used in the analytical method. Dithioerythritol (purity >99%) was obtained from Acros Organics (Geel, Antwerp, Belgium). Trifluoroacetic acid (TFA), chromatographic grade, was purchased from Tedia (Fairfield, OH, U.S.A.). All other reagents and solvents used for this study were of analytical grade.
Animals
All animal experiments were conducted according to the guidelines of the committee and approved by the Laboratory of Animal Use and Care Committee, Hubei Science and Technology Agency, China (permit number SYXK2013-0044). Humane methods were used to reduce the pain of the experimental animals.
The study was performed in 24 healthy landrace pigs (50% males, and 50% females), the animals were 6–7 weeks old, with a body weight of 30 ± 5 kg. All animals were allowed to acclimatize for 1 week prior to the study. Throughout the experimental period, they were fed antibiotic-free food twice daily and had free access to water. The animals were kept in a building with a temperature of 25 ± 2°C and a relative humidity of 45–65%.
Bioequivalence study design and sample collection
The bioequivalence study used a similar two-period, two-treatment, randomized crossover design. Pigs were randomly allocated to one of two groups (50% males and 50% females per group) receiving either the test or a reference formulation, respectively, in two periods.
In the first period, 12 pigs were given a single dose of the reference product (Excenel®RTU 5% CEF, Pfizer), while the other 12 pigs received a single dose of the test product (Saifukang 5% CEF, Hvsen). After a washout period of 7 days, the study was repeated in the same way to achieve the crossover design. Both products were administered intramuscularly at a dosage of 5 mg/kg body weight. Blood samples of 5 ml each were collected in heparinized tubes from the jugular vein before drug administration and at 0.13, 0.5, 1, 2, 4, 5, 8, 12, 24, 48, 72, 96, 120, and 144 hr after administration of the 5 mg/kg dose. The plasma samples were separated by centrifugation at 4,000 rpm for 15 min and stored at −20°C until analysis.
Analysis of plasma CEF and DFC-related metabolites concentrations
Plasma concentrations of ceftiofur and DFC-related metabolites were analyzed by using an Agilent 1100 series (Santa Clara, CA, U.S.A.) high-performance liquid chromatography (HPLC). An Agilent ZORBAX SB C18 column (250 × 4.6 mm, 5 µm i,d: Agilent) was used for separation. Detection and quantification were conducted at a wavelength of 266 nm. The mobile phase conditions consisted of 0.1% TFA (phase A) and acetonitrile (phase B) (86/14; V/V). The flow rate and injection volumes were 1 ml/min and 50 µl, respectively.
After administration of CEF hydrochloride, it is rapidly metabolized to active the ingredient, DFC. Therefore, plasma DFC concentrations were determined for the pharmacokinetics of CEF. CEF is extracted from plasma samples using the extraction solution to fracture thioester bond method that converts CEF and all metabolites to DFC. After thawing at room temperature, 7 ml of extraction solution (0.4% dithioerythritol dissolved in 0.05 mol/l borate buffer solution) were added to 500 µl of plasma. The samples were thoroughly mixed and incubated in a water bath (50°C) for 15 min; every 3 min, the tubes were shaken for 30 sec. Subsequently, the samples were cooled to 25°C and centrifuged (4,000 rpm) for 10 min. The supernatant was pipetted into a tube and prepared for extraction, and the mixture was then cleaned up on a ProElut PLS (60 mg/3 ml Dikma ProElutTM, Dikma), preconditioned with 3 ml of methanol and equilibrated with 3 ml of water. Cartridges were washed with 3 ml of water (5% methanol) and the derivative was eluted with 6 ml methanol. The samples were then evaporated to dryness with a stream of nitrogen at 35°C, followed by reconstitution in 0.5 ml of the mobile phase and centrifugation at 4,000 rpm for 10 min; subsequently, the samples were filtered through 0.22-µm organic membranes into autosampler vials.
Pharmacokinetic and bioequivalence analysis
All pharmacokinetic parameters were calculated using linear model software (WinNonlin version 5.2.1., Pharsight, Mountain View, CA, U.S.A.). Values for maximum plasma concentration (Cmax) and the time to reach the maximum concentration (Tmax) were determined directly from the data. The linear trapezoidal rule was used to calculate the area under the plasma concentration-time curve from 0 to the last point (AUC0-t) and the area under the plasma concentration-time curve from 0 to infinity (AUC0-∞) All pharmacokinetic parameters, except for Tmax, were logarithmically transformed prior to data analysis, according to bioequivalence technical guidelines for the veterinary drug in China [15]. Values for Tmax were not normally distributed, even after logarithmic transformation. Therefore, a non-parametric test was used to compare mean values for Tmax [32]. Pharmacokinetic parameters were compared between the test product and the reference product with 2-way ANOVA, using statistical software [12]. The criteria for accepting bioequivalence was that the 90% confidence interval of the difference between the test formulation and the reference formulation for the variables AUC0-t and AUC0-∞ ranged within 80–125% [5]. The acceptable range of Cmax was wider than that of AUC, with an FDA recommended range of 70–143% [26]. All statistical analysis were performed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IN, U.S.A.), version 17.0.
Statistical analysis
Values were considered statistically significant and highly significant at P≤0.05 and P≤0.01, respectively (*P<0.05 and **P<0.01).
RESULTS
All animals remained in a healthy state, and no adverse reactions were observed in this study. Data were presented as the mean and standard deviation (mean ± S.D.).
DFC HPLC analysis in plasma
The detection limit (DFC) of the analytical method was 0.05 µg/ml. The calibration curves were in good linearity over the range of 0.1–40 µg/ml, with a correlation coefficient of 0.9996. The lowest limit of quantification was 0.1 µg/ml in plasma. Plasma samples could be stored at −20°C in the dark for at least 15 days and were stable. The inter-day and intra-day coefficients of variation at three different concentrations (0.25, 5, 20) were all below 8% in plasma. Moreover, mean recoveries were in the range of 85.2 ± 4.45% to 87.8 ± 6.53% (Table 1), meeting the requirements of the Guidance for Industry, Bioanalytical Method Validation [13].
Table 1. Precision and accuracy of determination of desfuroylceftiofur in blank plasma (Mean ± S.D., n=15).
| Concentration (µg/ml) |
Mean recovery (%) |
Intra-day CV (%) |
Inter-day CV (%) |
Accuracy (RE%) |
|---|---|---|---|---|
| 0.25 | 85.2 ± 4.42 | 4.43 ± 1.36 | 6.75 ± 2.25 | 14.80 |
| 5 | 87.8 ± 6.53 | 4.35 ± 1.89 | 7.18 ± 2.47 | 12.20 |
| 20 | 86.2 ± 7.26 | 3.26 ± 1.02 | 7.96 ± 3.14 | 13.80 |
CV, the coefficient of variability; RE, the accuracy.
Pharmacokinetic analysis
A comparison of the mean ± S.D. plasma concentration-time curves of DFC-related metabolites after intramuscular administration of the two formulations, as shown in Fig. 1. The main pharmacokinetic parameters of free DFC, calculated from plasma data, are listed in Table 2. Pharmacokinetic parameters of CEF hydrochloride did not differ significantly between male and female pigs.
Fig. 1.
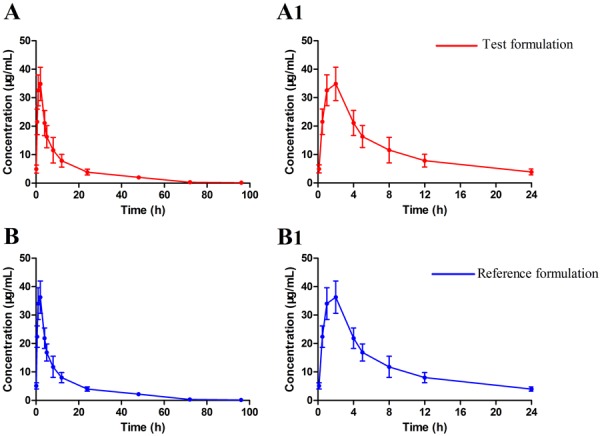
The mean plasma concentration-time curves of ceftiofur and desfuroylceftiofur-related metabolites in pigs (n=24 for each group) after a single intramuscular injection of 5 mg/kg body weight of either test formulation and reference formulation. (A) the test one in 96 hr, (A1) Amplification of A at 0–24 hr, (B) the reference one in 96 hr, (B1) Amplification of B at 0–24 hr.
Table 2. Pharmacokinetics parameters of ceftiofur and desfuroylceftiofur-related metabolites in pigs after a single intramuscular administration (5 mg/kg body weight) of test formulation and reference formulation, and relative bioavailability (Mean ± S.D., n=24).
| Parameters | Unit | Test | Reference | F (%) |
|---|---|---|---|---|
| AUC0-t | µg·hr/ml | 376.7 ± 75.3 | 390.5 ± 78.6 | 96.47 ± 10.92 |
| AUC0-∞ | µg·hr/ml | 385.9 ± 79.2 | 402.7 ± 80.4 | |
| Cmax | µg/ml | 34.6 ± 5.5 | 36.1 ± 6.2 | |
| Tmax | hr | 1.27 ± 0.18 | 1.26 ± 0.21 |
AUC0-t, Area under the plasma concentration-time curve from 0 to the last point; AUC0-∞, Area under the plasma concentration-time curve from 0 to infinity; Cmax, Maximum plasma concentration; Tmax, Time to reach maximum concentration; F, the relative bioavailability.
Bioequivalence analysis
To assess the bioequivalence of the two formulations, AUC and Cmax were considered as the primary parameters. Bioequivalence was evaluated by analysis of variance (ANOVA) for crossover design and by determining 90% intervals (CI) of the ratio of test/reference formulation, using log-transformed data. According to the AUC0-t values, the relative bioavailability of the test formulation was 96.47 ± 10.92% (Table 2). The values of Cmax, AUC0-t, and AUC0-∞ showed no statistically significant differences between the products, periods, and individuals (Table 3).
Table 3. ANOVA results of pharmacokinetic parameters in 24 pigs after a single intramuscular dose of test formulation and reference formulation.
| Parameters | F value | ||
|---|---|---|---|
| Between-formulations | Between-periods | Between-individuals | |
| lnAUC0-t (µg·hr/ml) | 0.86 | 2.15 | 1.42 |
| lnAUC0-∞ (µg·hr/ml) | 0.64 | 1.53 | 1.38 |
| lnCmax (µg/ml) | 0.016 | 0.08 | 0.08 |
| lnTmax (hr) | 0.009 | 0.05 | 0.09 |
F0.05 (1,22)=4.30; F0.05 (23,22)=2.07. *P<0.05.
Based on the results of two one-sided T-tests and 90% CI analysis, the Cmax, AUC0-t, and AUC0-∞ (after log-transformation of data) showed no statistically significant difference between the test and reference products (t1 and t2), which were all above 1.717 and had significant differences (P<0.05) with each other (Table 4). The 90% CI values of the test formulation in AUC0-t and AUC0-∞ were 92.3–117.3% and 92.1–117.9%, respectively, which is within the bioequivalence range (80–125%) of the reference formulation. The 90% CI values in Cmax ranged between 93.1 and 116.3%, which also was within the bioequivalence range (70–143%) of the reference formulation (Table 4).
Table 4. Two one-sided T-test and 90% confidence interval (CI) results of the parameters after a single intramuscular administration of test formulation and reference formulation.
| Parameters | t1 | t2 | 90%CI (%) |
Ratio (T/R) (%) |
Acceptable Range (%) |
|---|---|---|---|---|---|
| AUC0-t | 3.62a) | 2.48a) | 92.3–117.3 | 99.3 | 80–125 |
| AUC0-∞ | 3.48a) | 2.41a) | 92.1–117.9 | 99.3 | 80–125 |
| Cmax | 5.67a) | 4.59a) | 93.1–116.3 | 98.6 | 70–143 |
T(1–0.05)(22)=1.717. a) P<0.05.
DISCUSSION
Cephalosporins represent an important class of antibacterial agents. CEF, which belongs to this group, is effective against bacteria and widely used in domestic animal species (cattle, pigs, horse, dogs) [7, 18, 29, 30]; studies have also reported pharmacokinetic (PK) parameters in Asian elephants, water buffalo, sea lions, and other exotic animals [8, 28, 32]. Several studies have reported the PK and bioequivalence of CEF sodium after intramuscular or subcutaneous administration to cattle, sheep, and chickens, while only a few works have focused on CEF hydrochloride in pigs [5, 7, 9]. The present work was designed to determine the basic pharmacokinetic parameters of CEF hydrochloride sterile suspensions and to compare a test product with a reference formulation in terms of bioequivalence.
The Cmax values of the test and the reference formulation were 34.6 ± 5.5 and 36.1 ± 6.2 µg/ml, respectively, which were considerably higher than those previously found for pigs (29.7 ± 6.72 µg/ml) or cattle (11.0 ± 1.69 µg/ml) [3, 5]. In the preliminary reports, different dosage forms of CEF exhibit the different Cmax, the Cmax of CEF sodium was 28.3 ± 4.45 and 15.22 ± 0.57 µg/ml for long-acting CEF hydrochloride suspension in pigs [36], when administered intramuscularly at 5 mg/kg. It took the same time to reach maximum plasma concentration (Tmax), approximately 1.3 hr after administration, indicating high absorption rates of the drugs. According to its characteristics of PK/PD (pharmacodynamics), CEF belongs to time-dependent drugs, its antibacterial activity was closely related to time above minimum inhibitory concentration (MIC) rather than maximum plasma or tissue concentrations. Therefore, the concentrations of CEF should be maintained above the MIC for as long as possible during the treatment of respiratory diseases in pigs. Concentrations of CEF and its DFC-related metabolites 72 hr after the 5 mg/kg injection were 0.32 ± 0.07 µg/ml after the test administration and 0.33 ± 0.06 µg/ml after the reference formulation administration [3], which was more than five times the MIC (0.06 µg/ml) for CEF against major pathogens including Pasteurella multocida, Actinobacillus (Hemophilus) pleuropneumoniae, and Streptococcus suis [35, 39], both formulations displayed a long effective plasma concentration and slow elimination, it was of greater benefit for a therapeutic effect and decrease frequent injections. The area under the concentration-time curve was an important parameter to express the extent of absorption. The values of AUC0-t and AUC0-∞ were 376.7 ± 75.3 and 385.9 ± 79.2 µg·hr/ml, respectively, after the test formulation administration and 390.5 ± 78.6 and 402.7 ± 80.4 µg·hr/ml, respectively, after administration of the reference formulation; these values are similar to previously reported values in pigs [3]. The main PK parameters between male and female pigs had no statistical differences, indicating the metabolism of CEF hydrochloride was not relevant to sex. According to the AUC0-t values, the mean relative bioavailability of the test product to the reference product was 96.47 ± 10.92%, which was within the range recommended by the FDA. The values of Cmax, AUC0-t, and AUC0-∞ were similar for the two formulations, indicating similar therapeutic effects as well as absorption rates and extents. We also found no statistical differences in PK parameters between two formulations.
To assess the bioequivalence of two formulations, several PK indicators have been suggested in the past, including a direct comparison for determining concentration-time curves between the test product and the reference product [23, 34]. In addition, the ratios of Cmax/AUC and Cmax/Tmax, as well as the y-intercept of ln (C/t) versus t plot, have also been used for bioequivalence evaluation [27, 37]. The parameter Cmax does not specifically describe the rate of absorption, but it is affected by the extent of absorption; other indicators were also controversial and had numerous restrictions in practical application. Many regulatory authorities worldwide have issued specific criteria and approaches, and the standard PK method has been used for bioequivalence assessment.
Conventional bioequivalence studies select the main pharmacokinetic parameters (AUC0-t, AUC0-∞, Cmax and Tmax) to make a statistical comparison. In our study, ANOVA and two one-sided tests for Cmax, AUC0-t, and AUC0-∞ (after log-transformation of data) showed no statistically significant differences, while the 90% CI also demonstrated that these parameters of the two formulations lie within the recommended range of 80–125% (70–143% for Cmax). Therefore, the two products were considered to be bioequivalent. The factor Tmax was used in the non-parametric analysis and presented no significant difference between the two formulations. Our study therefore verifies that the test formulation (Saifukang 5% CEF, Hvsen) is bioequivalent to the reference formulation (Excenel®RTU 5% CEF, Pfizer).
Acknowledgments
The work was supported by National Natural Science Foundation of China (grant No. 31572572, 31601922) and the Natural Science Foundation of Hubei Province, China (grant No. 2017CFB446).
REFERENCES
- 1.Beconi-Barker M. G., Hornish R. E., Vidmar T. J., Dame K. J., Brown S. A.1996. Ceftiofur hydrochloride: plasma and tissue distribution in swine following intramuscular administration at various doses. J. Vet. Pharmacol. Ther. 19: 192–199. doi: 10.1111/j.1365-2885.1996.tb00038.x [DOI] [PubMed] [Google Scholar]
- 2.Beconi-Barker M. G., Roof R. D., Millerioux L., Kausche F. M., Vidmar T. H., Smith E. B., Callahan J. K., Hubbard V. L., Smith G. A., Gilbertson T. J.1995. Determination of ceftiofur and its desfuroylceftiofur-related metabolites in swine tissues by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 673: 231–244. doi: 10.1016/0378-4347(95)00258-1 [DOI] [PubMed] [Google Scholar]
- 3.Brown S. A., Hanson B. J., Mignot A., Millérioux L., Hamlow P. J., Hubbard V. L., Callahan J. K., Kausche F. M.1999. Comparison of plasma pharmacokinetics and bioavailability of ceftiofur sodium and ceftiofur hydrochloride in pigs after a single intramuscular injection. J. Vet. Pharmacol. Ther. 22: 35–40. doi: 10.1046/j.1365-2885.1999.00182.x [DOI] [PubMed] [Google Scholar]
- 4.Brown S. A., Chester S. T., Robb E. J.1996. Effects of age on the pharmacokinetics of single dose ceftiofur sodium administered intramuscularly or intravenously to cattle. J. Vet. Pharmacol. Ther. 19: 32–38. doi: 10.1111/j.1365-2885.1996.tb00005.x [DOI] [PubMed] [Google Scholar]
- 5.Brown S. A., Chester S. T., Speedy A. K., Hubbard V. L., Callahan J. K., Hamlow P. J., Hibbard B., Robb E. J.2000. Comparison of plasma pharmacokinetics and bioequivalence of ceftiofur sodium in cattle after a single intramuscular or subcutaneous injection. J. Vet. Pharmacol. Ther. 23: 273–280. doi: 10.1046/j.1365-2885.2000.00271.x [DOI] [PubMed] [Google Scholar]
- 6.Chow S. C., Liu J. P.2000. Design and Analysis of Bioavailability and Bioequivalence Studies, 2nd ed., Chapman and Hall, London. [Google Scholar]
- 7.Craigmill A. L., Brown S. A., Wetzlich S. E., Gustafson C. R., Arndt T. S.1997. Pharmacokinetics of ceftiofur and metabolites after single intravenous and intramuscular administration and multiple intramuscular administrations of ceftiofur sodium to sheep. J. Vet. Pharmacol. Ther. 20: 139–144. doi: 10.1046/j.1365-2885.1997.00820.x [DOI] [PubMed] [Google Scholar]
- 8.Dumonceaux G., Isaza R., Koch D. E., Hunter R. P.2005. Pharmacokinetics and i.m. bioavailability of ceftiofur in Asian elephants (Elephas maximus). J. Vet. Pharmacol. Ther. 28: 441–446. doi: 10.1111/j.1365-2885.2005.00686.x [DOI] [PubMed] [Google Scholar]
- 9.El-Sayed M. G., El-Komy E. H., Elbarawy E. A. M., Ibrahim D. M.2015. Pharmacokinetics and tissue residues of ceftiofur in normal and Escherichia coli Infected Chickens. J. Physiol. Pharmacol. Adv 5: 574–582. doi: 10.5455/jppa.20141203095345 [DOI] [Google Scholar]
- 10.Endrenyi L., Tothfalusi L.2012. Metrics for the evaluation of bioequivalence of modified-release formulations. AAPS J. 14: 813–819. doi: 10.1208/s12248-012-9396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food U. S., Administration D.2003. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations, Rockvile. [Google Scholar]
- 12.Gao Y., He B., Qin G., Yang X., Zheng W.2010. Identification and drug sensitivity test of pathogenic bacterium of endometritis in dairy buffalo. Zhongguo Nainiu 6: 38–40. [Google Scholar]
- 13.United States Food and Drug Administration (US-FDA). 2001. Guidance for Industry, Bioanalytical Method Validation. Rockvile: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER).
- 14.Gilbertson T. J., Roof R. D., Nappier J. L., Zaya M. J., Robins R. H., Stuart D. J., Krzeminski L. F., Jaglan P. S.1995. Disposition of ceftiofur sodium in swine following intramuscular treatment. J. Agric. Food Chem. 43: 229–234. doi: 10.1021/jf00049a041 [DOI] [Google Scholar]
- 15.Government of Canada H, C., Health Products, and Food Branch V. D. D. 2014. VICH guideline 52: Bioequivalence-Blood Level Bioequivalence Study (step 4)−Health Canada Consultation Notice.
- 16.Halbur P., Thanawongnuwech R., Brown G., Kinyon J., Roth J., Thacker E., Thacker B.2000. Efficacy of antimicrobial treatments and vaccination regimens for control of porcine reproductive and respiratory syndrome virus and Streptococcus suis coinfection of nursery pigs. J. Clin. Microbiol. 38: 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halloy D. J., Cambier C., Gustin P. G.2006. Efficacy of ceftiofur and flunixin in the early treatment of bronchopneumonia in weaners. Vet. Rec. 158: 291–296. doi: 10.1136/vr.158.9.291 [DOI] [PubMed] [Google Scholar]
- 18.Halstead S. L., Walker R. D., Baker J. C., Holland R. E., Stein G. E., Hauptman J. G.1992. Pharmacokinetic evaluation of ceftiofur in serum, tissue chamber fluid and bronchial secretions from healthy beef-bred calves. Can. J. Vet. Res. 56: 269–274. [PMC free article] [PubMed] [Google Scholar]
- 19.Hornish R. E., Kotarski S. F.2002. Cephalosporins in veterinary medicine - ceftiofur use in food animals. Curr. Top. Med. Chem. 2: 717–731. doi: 10.2174/1568026023393679 [DOI] [PubMed] [Google Scholar]
- 20.Jaglan P. S., Cox B. L., Arnold T. S., Kubicek M. F., Stuart D. J., Gilbertson T. J.1990. Liquid chromatographic determination of desfuroylceftiofur metabolite of ceftiofur as residue in cattle plasma. J. Assoc. Off. Anal. Chem. 73: 26–30. [PubMed] [Google Scholar]
- 21.Jaglan P. S., Kubicek M. F., Arnold T. S., Cox B. L., Robins R. H., Johnson D. B., Gilbertson T. J.1989. Metabolism of ceftiofur. Nature of urinary and plasma metabolites in rats and cattle. J. Agric. Food Chem. 37: 1112–1118. doi: 10.1021/jf00088a066 [DOI] [Google Scholar]
- 22.Jaglan P. S., Yein F. S., Hornish R. E., Cox B. L., Arnold T. S., Roof R. D., Gilbertson T. J.1992. Depletion of intramuscularly injected ceftiofur from the milk of dairy cattle. J. Dairy Sci. 75: 1870–1876. doi: 10.3168/jds.S0022-0302(92)77946-6 [DOI] [PubMed] [Google Scholar]
- 23.Karalis V., Macheras P.2003. Pharmacodynamic considerations in bioequivalence assessment: comparison of novel and existing metrics. Eur. J. Pharm. Sci. 19: 45–56. doi: 10.1016/S0928-0987(03)00064-2 [DOI] [PubMed] [Google Scholar]
- 24.Kaushal N., Singh S. K., Gulati M., Vaidya Y., Kaushik M.2016. Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: Impact on generic drug substitution. J. Appl. Pharm. Sci. 6: 206–222. doi: 10.7324/JAPS.2016.60430 [DOI] [Google Scholar]
- 25.Lei Z., Liu Q., Yang B., Ahmed S., Xiong J., Song T., Chen P., Cao J., He Q.2017. Evaluation of bioequivalence of two long-acting 20% oxytetracycline formulations in pigs. Front. Vet. Sci. 4: 61–64. doi: 10.3389/fvets.2017.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L., Qi M., Wang P., Li H.2004. High-performance liquid chromatographic method for the bioequivalence evaluation of desloratadine fumarate tablets in dogs. J. Pharm. Biomed. Anal. 34: 1013–1019. doi: 10.1016/j.jpba.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 27.Macheras P., Symillides M., Reppas C.1996. An improved intercept method for the assessment of absorption rate in bioequivalence studies. Pharm. Res. 13: 1755–1758. doi: 10.1023/A:1016421630290 [DOI] [PubMed] [Google Scholar]
- 28.Meegan J., Collard W. T., Grover G. S., Pussini N., Van Bonn W. G., Gulland F. M. D.2013. Pharmacokinetics of ceftiofur crystalline-free acid (EXCEDE sterile suspension) administered via intramuscular injection in wild California sea lions (Zalophus californianus). J. Zoo Wildl. Med. 44: 714–720. doi: 10.1638/2013-0001R.1 [DOI] [PubMed] [Google Scholar]
- 29.Meyer J. C., Brown M. P., Gronwall R. R., Merritt K.1992. Pharmacokinetics of ceftiofur sodium in neonatal foals after intramuscular injection. Equine Vet. J. 24: 485–486. doi: 10.1111/j.2042-3306.1992.tb02883.x [DOI] [PubMed] [Google Scholar]
- 30.Meyer S., Giguère S., Rodriguez R., Zielinski R. J., Grover G. S., Brown S. A.2009. Pharmacokinetics of intravenous ceftiofur sodium and concentration in body fluids of foals. J. Vet. Pharmacol. Ther. 32: 309–316. doi: 10.1111/j.1365-2885.2008.01041.x [DOI] [PubMed] [Google Scholar]
- 31.Morais J. A., Lobato M. R.2010. The new European medicines agency guideline on the investigation of bioequivalence. Basic Clin. Pharmacol. Toxicol. 106: 221–225. doi: 10.1111/j.1742-7843.2009.00518.x [DOI] [PubMed] [Google Scholar]
- 32.Nie H., Feng X., Peng J., Liang L., Lu C., Tiwari R. V., Tang S., He J.2016. Comparative pharmacokinetics of ceftiofur hydrochloride and ceftiofur sodium after administration to water buffalo (Bubalus bubalis). Am. J. Vet. Res. 77: 646–652. doi: 10.2460/ajvr.77.6.646 [DOI] [PubMed] [Google Scholar]
- 33.Olson S. C., Beconi-Barker M. G., Smith E. B., Martin R. A., Vidmar T. J., Adams L. D.1998. In vitro metabolism of ceftiofur in bovine tissues. J. Vet. Pharmacol. Ther. 21: 112–120. doi: 10.1046/j.1365-2885.1998.00118.x [DOI] [PubMed] [Google Scholar]
- 34.Polli J. E., McLean A. M.2001. Novel direct curve comparison metrics for bioequivalence. Pharm. Res. 18: 734–741. doi: 10.1023/A:1011067908500 [DOI] [PubMed] [Google Scholar]
- 35.Salmon S. A., Watts J. L., Yancey R. J., Jr1996. In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance. J. Vet. Diagn. Invest. 8: 332–336. doi: 10.1177/104063879600800309 [DOI] [PubMed] [Google Scholar]
- 36.Tang S., Xiao J., Guo G., He J., Hao Z., Xiao X.2010. Preparation of a newly formulated long-acting ceftiofur hydrochloride suspension and evaluation of its pharmacokinetics in pigs. J. Vet. Pharmacol. Ther. 33: 238–245. doi: 10.1111/j.1365-2885.2009.01126.x [DOI] [PubMed] [Google Scholar]
- 37.Tothfalusi L., Endrenyi L.1995. Without extrapolation, Cmax/AUC is an effective metric in investigations of bioequivalence. Pharm. Res. 12: 937–942. doi: 10.1023/A:1016237826520 [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Peng H., Kong J., Zhao T., Zhang S., Cao X.2018. Pharmacokinetic profile of ceftiofur hydrochloride injection in lactating holstein dairy cows. J. Vet. Pharmacol. Ther. 41: 301–306. doi: 10.1111/jvp.12469 [DOI] [PubMed] [Google Scholar]
- 39.Yancey R. J., Jr, Kinney M. L., Roberts B. J., Goodenough K. R., Hamel J. C., Ford C. W.1987. Ceftiofur sodium, a broad-spectrum cephalosporin: evaluation in vitro and in vivo in mice. Am. J. Vet. Res. 48: 1050–1053. [PubMed] [Google Scholar]


