Abstract
AIM
To evaluate and describe the efficacy of fecal microbiota transplantation (FMT) for Clostridium difficile infection (CDI) in a national Israeli cohort.
METHODS
All patients who received FMT for recurrent (recurrence within 8 wk of the previous treatment) or refractory CDI from 2013 through 2017 in all the five medical centers in Israel currently performing FMT were included. Stool donors were screened according to the Israeli Ministry of Health guidelines. Clinical and laboratory data of patients were collected from patients’ medical files, and they included indications for FMT, risk factors for CDI and disease severity. Primary outcome was FMT success (at least 2 mo free of CDI-related diarrhea post-FMT). Secondary outcomes included initial response to FMT (cessation of diarrhea within 7 d) and recurrence at 6 mo.
RESULTS
There were 111 FMTs for CDI, with a median age of 70 years [interquartile range (IQR): 53-82], and 42% (47) males. Fifty patients (45%) were treated via the lower gastrointestinal (LGI, represented only by colonoscopy) route, 37 (33%) via capsules, and 24 (22%) via the upper gastrointestinal (UGI) route. The overall success rate was 87.4% (97 patients), with no significant difference between routes of administration (P = 0.338). In the univariant analysis, FMT success correlated with milder disease (P = 0.01), ambulatory setting (P < 0.05) and lower Charlson comorbidity score (P < 0.05). In the multivariant analysis, only severe CDI [odd ratio (OR) = 0.14, P < 0.05] and inpatient FMT (OR = 0.19, P < 0.05) were each independently inversely related to FMT success. There were 35 (32%) patients younger than 60 years of age, and 14 (40%) of them had a background of inflammatory bowel disease.
CONCLUSION
FMT is a safe and effective treatment for CDI, with capsules emerging as a successful and well-tolerated route. Severe CDI is less likely to respond to FMT.
Keywords: Clostridium difficile infection, Capsules, Israel, Fecal microbiota transplantation
Core tip: Fecal microbiota transplantation (FMT) emerged as a promising treatment for Clostridium difficile infection (CDI). Our aim was to summarize the national Israeli experience in FMT. One-hundred and eleven patients with CDI underwent FMT, 37 (35%) of which via oral capsules and 50 (45%) via colonoscopy. The overall success rate was 87.4%, with no difference between administration routes. Success was independently related to mild disease and an ambulatory setting. One-third of the patients were younger than 60 years. 14 of which (40%) also suffered from inflammatory bowel disease. FMT is an effective treatment for recurrent CDI. FMT via capsules was shown to be a successful alternative to endoscopy.
INTRODUCTION
Background
The incidence of Clostridium difficile infection (CDI) is rising in parallel to the increased use of broad-spectrum antibiotics, with more than 500000 cases and 29000 related deaths annually in the United States alone[1]. The resistance to current treatment with metronidazole and vancomycin is also increasing, with recurrence rates of up to 20% after the first episode and 40%-65% after the second[2-4]. Fecal microbiota transplantation (FMT) has cure rates ranging between 85%-95%, and it has emerged as being a safe and promising treatment option that is widely accepted for recurrences and refractory or severe cases of CDI[5-10]. FMT is considered the most effective treatment for recurrent CDI[11,12] in both young and elderly patient populations[13]. FMT it is also a promising potential treatment for conditions other than CDI, such as inflammatory bowel diseases (IBD), irritable bowel syndrome, neuropsychiatric conditions, obesity, insulin resistance, and autoimmune diseases[14,15].
Current techniques for FMT administration vary considerably between institutions and can be performed via a nasogastric/nasojejunal tube, gastroscopy, oral capsules, enema, sigmoidoscopy or colonoscopy[6,15-18]. The procedure is considered safe and is mostly free of severe adverse events, although peri-procedural transient gastrointestinal (GI) symptoms may develop in some patients. The mortality cited in previous studies was attributed to the patients’ pre-morbid conditions, and generally occurred in elderly and critically ill patients[5,15-17,19].
Objective
We had earlier described the initial Israeli experience of 22 patients that were treated with FMT for CDI and experienced an overall cure rate above 89%[20]. However, during the past 5 years, the procedure has been performed in additional Israeli centers that processed feces from a wide range of donors in a significantly larger number of patients with different disease conditions, and using all acceptable transplantation routes. Therefore, our aim was to examine whether FMT continued to demonstrate efficacy despite this wider range of donors and patients, to investigate FMT-dependent variables, and to examine the efficacy of individual FMT routes.
MATERIALS AND METHODS
Study design and settings
This multi-center retrospective study included all the patients who were treated with FMT for CDI in Israel between January 2013 and October 2017. The participating medical centers were the Tel Aviv Medical Center (TLVMC), Tel Aviv; Shaare Zedek Medical Center (SZMC) and Hadassah Medical Center (HMC), Jerusalem; Assaf Harofe Medical Center (AHMC), Zerifin; and Kaplan Medical Center (KMC), Rehovot. All the patients or their legal surrogates provided written informed consent. The study was approved by the ethics committees of each medical center. Patients were followed routinely at 48 h, 7 d, 2 mo, and 6 mo after the procedure for the assessment of side effects and treatment outcome.
Following the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) practice guidelines[21], CDI was defined as diarrhea (≥ 3 unformed stools per day) and a stool test that was positive for Clostridium difficile antigen and its toxins by either an ELISA (immunocheck™) or by PCR if the glutamate dehydrogenase antigen was positive and the toxin assessment was negative[22]. Recurrence was defined as another episode occurring within the 8 wk following the previous treatment. Refractory CDI was defined as disease that did not respond to medical therapy. Severe disease was defined by leukocytosis ≥ 15000 cells/μL and/or a serum creatinine level ≥ 1.5 times the premorbid level[21]. Initial response to FMT was defined as fewer than 3 liquid stools per day within the 7 d following FMT, and cure was defined as at least 2 mo free of CDI-related diarrhea post-FMT.
Donor stool preparation
The donor stool was delivered to the institution within a few hours of evacuation in a clean closed plastic container. It was immediately diluted with sterile saline (NaCl 0.9%) and blended into a homogenous liquid. The liquid was then filtered to remove particulate matter. Stool donations were processed and kept frozen at -80 °C until use at TLVMC or AHMC, while a fresh donor stool was delivered to SZMC or HMC on the day of FMT for processing and transplantation. KMC used both frozen and fresh donor stools. The preparation of capsules was the same as that described in our previous reports[6,23].
Participants
All patients aged 10 to 92 years who were treated with FMT for refractory, recurrent, or severe CDI in all five centers were included. Donor selection, FMT procedure and patient follow-up were the same as those described in our earlier report[20], and they were carried out according to practice guidelines[24]. Capsule FMT was administrated following practice guidelines as reported elsewhere[6,7].
Variables
The primary outcome was FMT success (at least 2 mo free of CDI-related diarrhea post-FMT). Secondary outcomes included initial response to FMT (see above) and recurrence at 6 mo. The key variables were age, Charlson comorbidity score, and the risk factors for CDI in the 3 mo preceding the infection (hospitalization, exposure to antibiotics, IBD and chemotherapy).
Data sources
Clinical data were obtained from medical records, and they included epidemiologic information, risk factors for CDI, the Charlson comorbidity score[25], and follow-up information up to 6 months post-FMT. Patients were excluded if FMT was indicated for an etiology other than CDI or if follow-up was incomplete.
Statistical analysis
All continuous variables were displayed as mean [standard deviation (SD)] or median [interquartile range (IQR)], while categorical variables were displayed as number (percent) of patients within each group. Continuous variables were analyzed by the Student t test for normally distributed variables and by the Mann-Whitney U test and the Kruskal-Wallis test for non-normally distributed variables. We used the χ2 test to assess associations among categorical variables. Statistical significance is expressed as P ≤ 0.05 or P ≤ 0.01. Multivariate logistic regression analysis was used to test the association between patient and FMT characteristics and successful FMT while controlling for potential confounders. The SPSS 22.0 statistical package was used to perform all statistical analyses (SSPS Inc., Chicago, IL, United States).
RESULTS
Participants
A total of 113 CDI patients were treated with FMT in five Israeli medical centers. Two patients were excluded due to insufficient follow-up. The median age of the 111 participating patients was 70 years (IQR: 53-82), 47 (42%) of them were males, and the median 1-year Charlson comorbidity score for the cohort was 6 (IQR: 3-7 points; expected one-year survival of 79% ± 9%).
Descriptive data
The risk factors for CDI in the 3 mo preceding the infection included hospitalization (80 patients, 72%), exposure to antibiotics (65 patients, 59%), IBD (20 patients, 18%) and chemotherapy (19 patients, 17%). The patients’ demographics, epidemiological data, and risk factors for CDI are summarized in Table 1.
Table 1.
Study population characteristics according to fecal microbiota implantation route
|
Overall |
Upper GI1 |
Lower GI |
Capsules |
P value | |||||
| n (valid) | % (IQR) | n | % (IQR) | n | % (IQR) | n | % (IQR) | ||
| Patients | 111 | 100 | 24 | 222 | 50 | 45 | 37 | 33 | |
| Male gender | 47 (111) | 42 | 16 | 673 | 19 | 38 | 12 | 32 | |
| Age, median (IQR) | 70 (111) | (53-82) | 68 | (51-86) | 78 | (61-83) | 68 | (45-75) | 0.114 |
| Charlson comorbidity score, median | 6 (81) | 3-7 | 5.5 | (3-7.25) | 6 | (1.75-7.5) | 5 | (2-7) | 0.734 |
| CDI risk factors | |||||||||
| Prior hospitalization | 80 (111) | 72 | 18 | 753 | 26 | 52 | 36 | 97 | < 0.01 |
| Prior antibiotics use | 66 (108) | 59 | 15 | 633 | 15 | 30 | 36 | 97 | 0.012 |
| PPI usage | 37 (110) | 33 | 8 | 333 | 14 | 28 | 15 | 41 | 0.470 |
| Chemotherapy | 19 (111) | 17 | 4 | 173 | 3 | 6 | 12 | 32 | 0.005 |
| IBD | 20 (111) | 18 | 5 | 213 | 7 | 14 | 8 | 22 | 0.606 |
| Previous CDI episode, median | 2 (107) | (2-3) | 2 | (1-3) | 2 | (1-3) | 3 | (2-4) | 0.275 |
| Prior therapy | |||||||||
| Metronidazole | 89 (110) | 80 | 17 | 713 | 41 | 82 | 31 | 84 | 0.365 |
| Vancomycin | 109 (110) | 98 | 24 | 1003 | 48 | 96 | 37 | 100 | 0.534 |
| Combination | 31 (110) | 28 | 10 | 423 | 15 | 30 | 6 | 16 | 0.099 |
| Severe CDI | 20 (111) | 18 | 8 | 33.33 | 10 | 20 | 2 | 5.40 | 0.019 |
| Indication | |||||||||
| Refractory | 29 (111) | 26 | 6 | 253 | 19 | 38 | 4 | 11 | 0.017 |
| Recurrent | 82 (111) | 74 | 18 | 753 | 31 | 62 | 33 | 89 | |
| Outpatient | 78 (111) | 70 | 12 | 503 | 36 | 72 | 30 | 81 | 0.032 |
Upper GI: Gastroscopy, nasogastric tube or through percutaneous endoscopic gastrostomy;
Percentages refer to the total number of subjects in the cohort;
Percentages refer to the number of subjects in the specific group. CDI: Clostridium difficile infection; IBD: Inflammatory bowel diseases; PPI: Proton pump inhibitor; GI: Gastrointestinal; FMT: Fecal microbiota implantation; IQR: Interquartile range; Prior hospitalization: Hospitalization in the 3 mo prior to the FMT; Prior antibiotics: Antibiotics use in the 3 mo prior to the FMT.
Seventy-eight (70%) of the FMT procedures were performed in an ambulatory setting. FMT was performed through the lower GI route (LGI; only by colonoscopy) in 50 patients (45%), followed by capsules in 37 patients (33%), and through the upper GI route (UGI; gastroscopy, nasogastric tube or through their percutaneous endoscopic gastrostomy) in 24 patients (22%). The median age of the patients in the LGI group was 78 years (IQR: 61-83), which was significantly older than the median age of 68 years for the capsules group (IQR: 45-75) (P < 0.05). Twenty (18%) patients had severe CDI infection: they included 8 of the 24 patients (33.3%) who received FMT via the UGI route compared with 10 of the 50 patients [20% who received FMT via the LGI route (P = 0.21) and 5.4% (2/37) who received FMT via capsules (P = 0.004)].
Outcome data and main results
Successful FMT is more likely in ambulatory patients with milder CDI: A total of 97 (87.4%) patients achieved clinical remission (79% UGI, 88% LGI, and 92% capsules, P = 0.338). Ninety-nine patients were followed-up 6 mo after FMT initiation (11 patients died, and one had not reached this endpoint by study closure). Sixteen of them (16.2%) had a recurrence of CDI: 3 in the UGI group (15.8% of the UGI group), 5 in the LGI group (11.4%), and 8 (22.2%) in the capsule group (P = 0.141) (Figure 1 and Table 2). Four patients required multiple FMT infusions due to recurrence: 3 underwent a second FMT infusion (of which 2 were via colonoscopy and 1 was via capsules) and one patient had 3 FMTs (all via colonoscopy). Only 2 had a successful FMT (1 via capsules and 1 via colonoscopy).
Figure 1.
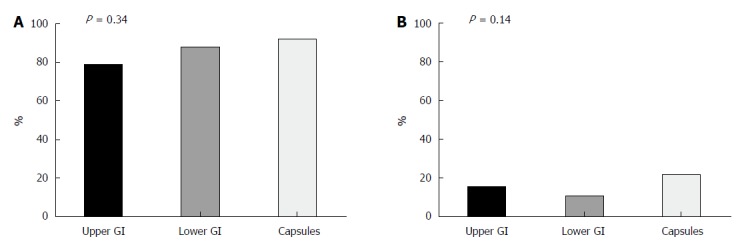
Comparison of success and recurrence rates of Clostridium difficile infection fecal microbiota transplantation treatment according route of administration. Fecal microbiota transplantation (FMT) was performed in recurrent Clostridium difficile infection (CDI) patients through upper gastrointestinal (GI) (n = 24), lower GI (n = 50) or capsules (n = 37). Success rates at 2 mo (A) and recurrence rates (B) at 6 mo post-FMT were similar between the groups. GI: Gastrointestinal.
Table 2.
Fecal microbiota implantation outcome and patients’ follow-up
|
Overall |
Upper GI |
Lower GI |
Capsules |
P value | |||||
| n (valid) | %/IQR | n | %/IQR | n | %/IQR | n | %/IQR | ||
| Patients | 111 (111) | 24 | 222 | 50 | 45 | 37 | 33 | ||
| Response within 7 d | 94 (111) | 84.72 | 17 | 713 | 42 | 84 | 34 | 92 | 0.198 |
| Days to response, median | 2 (111) | 1-2 | 2 | 1-3 | 1 | 0-2 | 2 | 2-3 | 0.069 |
| No. of patients in follow-up at 6 mo5 | 99 (99) | 89.22 | 19 | 793 | 44 | 88 | 36 | 97 | |
| Recurrence at 6 mo | 16 (99) | 16.22 | 3 | 15.83 | 5 | 11.40 | 8 | 22.20 | 0.141 |
| Total death | 11 (111) | 9.92 | 5 | 213 | 6 | 12 | 0 | 0 | 0.023 |
| Death - unrelated | 6 (11) | 5.42 | 4 | 803 | 2 | 33 | 0 | 0 | |
| Death - CDI-related | 3 (11) | 2.72 | 1 | 203 | 4 | 66 | 0 | 0 | |
| No. of adverse events | 19 (107) | 17.12 | 1 | 43 | 14 | 28 | 4 | 11 | 0.006 |
| Success4 | 97 (111) | 87.42 | 19 | 793 | 44 | 88 | 34 | 92 | 0.338 |
1Upper GI: Gastroscopy, nasogastric tube or through percutaneous endoscopic gastrostomy;
Percentages refer to the total number of subjects in the cohort;
Percentages refer to the number of subjects in the specific group;
Success: diarrhea-free two months post-fecal microbiota implantation;
Eleven patients died before this endpoint, and one had not yet reached it at study closure. Patients who died before this endpoint from a reason other than Clostridium difficile infection (CDI) and were diarrhea-free were regarded as success, while patients who died before this endpoint from their CDI were considered as failures. CDI: Clostridium difficile infection; IQR: Interquartile range; GI: Gastrointestinal.
We further divided the cohort based on FMT success and achievement/non-achievement of clinical remission (Table 3). The 97 patients who experienced clinical remission were more likely to have undergone an ambulatory FMT (92.3% compared with 73.8% hospitalized patients, P < 0.05), were less likely to have a severe form of disease (14% compared with 44% of the treatment failure group, P = 0.01), and had a lower Charlson comorbidity score (average 4.82 ± 3.2 compared with 6.6 ± 2.3, mean difference 1.78, 95%CI: 0.02-3.55, P < 0.05). In addition, patients who had a successful FMT tended to be younger (mean age 63.2 ± 22.4 years compared with 73.7 ± 12.1 years; 95%CI: -1.61 to 22.6, P = 0.09). Finally, more patients in the FMT failure group died during the study period (37.5% vs 6.2% for the treatment success groups, P < 0.001) (Figure 2 and Table 3). Frozen stool was used in 91 of all the patients in the cohort, with slightly higher success rates than those obtained by fresh stool (89% vs 80%, P = 0.272).
Table 3.
Fecal microbiota implantation success and failure
| Valid | No. |
Success |
Failure |
P value | |||
| Number | % | Number | % | ||||
| Patients | 111 | 111 | 97 | 87.42 | 14 | 12.6 | |
| Male gender | 111 | 47 | 40 | 41.23 | 7 | 50.0 | 0.535 |
| Ambulatory | 111 | 78 | 72 | 92.32 | 6 | 7.7 | 0.016 |
| Hospitalized | 111 | 33 | 25 | 75.82 | 8 | 24.2 | |
| CDI risk factors | |||||||
| Prior hospitalization | 111 | 80 | 68 | 70.13 | 12 | 85.7 | 0.224 |
| Antibiotics use | 108 | 89 | 77 | 81.13 | 12 | 92.3 | 0.318 |
| PPI usage | 110 | 37 | 32 | 33.33 | 5 | 35.7 | 0.86 |
| Chemotherapy | 111 | 19 | 17 | 17.53 | 2 | 14.3 | 0.764 |
| IBD | 111 | 20 | 18 | 18.63 | 2 | 14.3 | 0.698 |
| Steroid usage | 111 | 17 | 13 | 13.43 | 4 | 28.6 | 0.141 |
| Fever | 76 | 21 | 19 | 27.93 | 2 | 25.0 | 0.86 |
| Severe disease | 111 | 20 | 14 | 14.43 | 6 | 42.9 | 0.01 |
| Death | 111 | 11 | 6 | 6.23 | 5 | 37.5 | 0.001 |
| Adverse events | 107 | 19 | 15 | 16.03 | 4 | 30.8 | 0.19 |
| Route: Lower GI FMT | 111 | 50 | 45 | 88.02 | 5 | 12.0 | 0.338 |
| Upper GI FMT1 | 111 | 24 | 19 | 79.22 | 5 | 20.8 | |
| Capsules FMT | 111 | 37 | 34 | 91.92 | 3 | 8.1 | |
| Frozen stool | 111 | 91 | 81 | 89.02 | 10 | 11.0 | 0.272 |
| Fresh stool | 111 | 20 | 16 | 80.02 | 4 | 20.0 | |
| 0-1 Previous CDI | 107 | 24 | 23 | 24.23 | 1 | 8.3 | 0.214 |
| 2+ previous CDI | 107 | 83 | 72 | 74.23 | 11 | 78.6 | |
| Indication | 0.824 | ||||||
| Refractory | 111 | 29 | 25 | 86.22 | 4 | 13.8 | |
| Recurrent | 111 | 82 | 72 | 87.82 | 10 | 12.2 | |
| Prior therapy | |||||||
| Metronidazole | 110 | 89 | 80 | 83.33 | 9 | 64.3 | 0.09 |
| Vancomycin | 110 | 109 | 95 | 99.03 | 14 | 100.0 | 0.701 |
| Combination | 109 | 31 | 27 | 28.13 | 4 | 30.8 | 0.843 |
| Valid | Mean | SD | Mean | SD | Sigma | ||
| Age (yr) | 111 | 63.2 | 22.4 | 73.7 | 12.1 | 0.089 | |
| Charlson comorbidity score | 81 | 4.82 | 3.18 | 6.6 | 2.32 | 0.048 | |
| Previous CDI episodes (n) | 107 | 2.47 | 1.7 | 2.38 | 0.9 | 0.864 | |
| Time from 1st CDI (d) | 89 | 140.4 | 200.8 | 108.56 | 120.3 | 0.643 | |
| Creatinine (mgl/dL) | 76 | 1.37 | 1.2 | 2.19 | 2.6 | 0.098 | |
| Albumin (mg/L) | 54 | 3.32 | 0.8 | 2.76 | 0.5 | 0.053 | |
| WBC (103/dL) | 76 | 13.6 | 18.7 | 15.6 | 10.4 | 0.756 | |
Upper GI: Gastroscopy, nasogastric tube or through percutaneous endoscopic gastrostomy;
Percentages referral is the total number of subjects in the cohort;
Percentages referral is the number of subjects in the specific group. CDI: Clostridium difficile infection; IBD: Inflammatory bowel diseases; PPI: Proton pump inhibitor; GI: Gastrointestinal; FMT: Fecal microbiota implantation; IQR: Interquartile range; WBC: White blood cells; Prior hospitalization: Hospitalization during the 3 mo prior to the FMT; Prior hospitalization: Antibiotics use in the 3 mo prior to the FMT.
Figure 2.
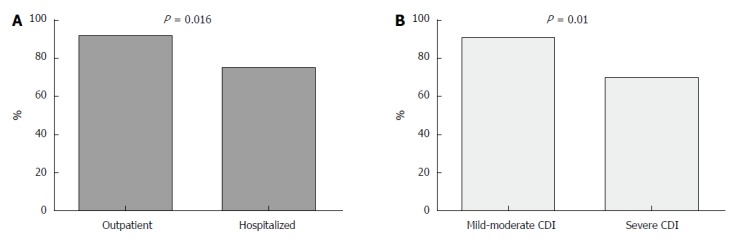
Comparison of fecal microbiota transplantation success rates among hospitalized and ambulatory patients. Seventy-eight patients who underwent ambulatory fecal microbiota transplantation had a success rate of 92.3% compared with 75.8% for hospitalized patients, P = 0.016 (A). Success rates were much lower among patients with severe Clostridium difficile infection compared with mild-moderate disease (B). CDI: Clostridium difficile infection.
The multivariance analysis revealed severe disease and inpatient status as being independently inversely related to FMT success, with odd ratios (ORs) of 0.14 (P < 0.05) and 0.19 (P < 0.05), respectively (Table 4). The Charlson score did not affect FMT success or failure.
Table 4.
Multivariate analysis of the association between patient characteristics and a successful fecal microbiota implantation procedure
| Patient characteristics | OR (95%CI) | P value |
| Hospitalized patient | 0.19 (0.04-0.86) | 0.032 |
| Severe disease | 0.14 (0.02-0.76) | 0.023 |
| Charlson score categories | 0.82 (0.38-1.76) | 0.622 |
Adjusted to previous hospitalization, metronidazole treatment prior to fecal microbiota implantation (FMT), previous Clostridium difficile infection (CDI), recurrent CDI, inflammatory bowel diseases, route of administration, stool preparation method (fresh/frozen) and FMT dosage.
Other analysis
IBD is prevalent among young FMT patients: We next divided the cohort into one group of those below 60 years of age (mean 37.2 ± 14.7 years) and another group above 60 years of age (mean 77.1 ± 8.9 years, mean difference 39.8, 95%CI: 35.3-44.3, P < 0.001) (Table 5). The success rates were slightly higher in the former group, but this difference did not reach a level of significance. Forty percent (14/35) of the patients younger than 60 years of age had IBD compared to only 7.9% (6/76) of the older group (P < 0.001). FMT was performed significantly earlier after diagnosis among the older patients compared to the younger ones (within 102 ± 112 d compared to 198 ± 279 d, respectively, P = 0.024). The elderly were less likely to be treated as outpatients (63% vs 86%, P < 0.05) and less likely to receive corticosteroids (10.5% vs 26%, P < 0.05). No patient in the younger group died during the follow-up period (Table 5).
Table 5.
Comparison of patients’ characteristics by age
| Valid | No. |
Below 60 yr |
Above 60 yr |
P value | |||
| Number | % | Number | % | ||||
| Patients | 111 | 111 | 35 | 31.52 | 76 | 68.5 | |
| Male gender | 111 | 47 | 15 | 42.93 | 32 | 42.1 | 0.941 |
| Ambulatory | 111 | 78 | 30 | 85.73 | 48 | 63.2 | 0.011 |
| CDI risk factors | |||||||
| Prior hospitalization | 111 | 80 | 22 | 62.93 | 58 | 76.3 | 0.142 |
| Antibiotics use | 108 | 89 | 28 | 80.03 | 61 | 83.6 | 0.649 |
| PPI usage | 110 | 37 | 9 | 25.73 | 28 | 37.3 | 0.230 |
| Chemotherapy | 111 | 19 | 7 | 20.03 | 12 | 15.8 | 0.584 |
| IBD | 111 | 20 | 14 | 40.03 | 6 | 7.9 | 0.000 |
| Steroid usage | 111 | 17 | 9 | 25.73 | 8 | 10.5 | 0.039 |
| Fever | 76 | 21 | 7 | 35.03 | 14 | 25.0 | 0.391 |
| Severe | 111 | 20 | 3 | 8.63 | 17 | 22.4 | 0.079 |
| Route | |||||||
| Lower GI | 111 | 50 | 12 | 34.33 | 38 | 50.0 | 0.254 |
| Upper GI1 | 111 | 24 | 8 | 22.93 | 16 | 21.1 | |
| Capsules | 111 | 37 | 15 | 42.93 | 22 | 28.9 | |
| 0-1 Previous CDI | 107 | 24 | 8 | 22.93 | 16 | 22.2 | 0.941 |
| 2+ previous CDI | 107 | 83 | 27 | 77.13 | 56 | 77.8 | |
| Indication | |||||||
| Refractory | 111 | 29 | 8 | 22.93 | 21 | 27.6 | |
| Recurrent | 111 | 82 | 27 | 77.13 | 55 | 72.4 | |
| Prior therapy | |||||||
| Metronidazole | 110 | 98 | 29 | 82.93 | 69 | 80.0 | 0.722 |
| Vancomycin | 109 | 109 | 35 | 100.03 | 74 | 98.7 | 0.493 |
| Combination | 109 | 31 | 12 | 34.33 | 19 | 25.7 | 0.352 |
| Outcomes | |||||||
| Success | 111 | 97 | 33 | 94.33 | 64 | 84.2 | 0.137 |
| Recurrence by 6 m | 99 | 16 | 7 | 203 | 9 | 14.1 | 0.443 |
| Death | 111 | 11 | 0 | 03 | 11 | 100.0 | 0.018 |
| Adverse events | 107 | 19 | 5 | 14.33 | 14 | 19.4 | 0.512 |
| Mean | SD | Mean | SD | Sigma | |||
| Age (yr) | 111 | 37.26 | 14.72 | 77.07 | 8.9 | 0.000 | |
| Charlson comorbidity score | 81 | 1.29 | 1.95 | 6.35 | 2.3 | 0.000 | |
| Previous CDI episodes (n) | 107 | 2.63 | 1.9 | 2.38 | 1.51 | 0.455 | |
| Time from 1st CDI (d) | 89 | 198.75 | 279.04 | 102.68 | 112.19 | 0.024 | |
| Creatinine (mg/dL) | 76 | 0.91 | 0.51 | 1.67 | 1.63 | 0.051 | |
| Albumin (mg/L) | 54 | 3.51 | 0.99 | 3.17 | 0.69 | 0.153 | |
| WBC (103/dL) | 76 | 11.33 | 90.36 | 14.78 | 19.86 | 0.468 | |
Upper GI: Gastroscopy, nasogastric tube or through percutaneous endoscopic gastrostomy;
Percentages refer to the total number of subjects in the cohort;
Percentages refer to the number of subjects in the specific group. CDI: Clostridium difficile infection; IBD: Inflammatory bowel diseases; PPI: Proton pump inhibitor; GI: Gastrointestinal; FMT: Fecal microbiota implantation; IQR: Inter quartile range; WBC: White blood cells; Prior hospitalization: Hospitalization in the 3 mo prior to the FMT; Prior hospitalization: Antibiotics use in the 3 mo prior to the FMT.
Adverse effects: Peri-procedural side effects were recorded in 19 patients. They were mild and self-limiting in 17 of them, and included abdominal discomfort, constipation, nausea/vomiting, flatulence and decreased appetite. Most of these effects occurred in patients who underwent colonoscopy [14 (28%) compared with 4 (11%) for capsules, and 1 (4%) for UGI routes, P < 0.01]. There were 2 cases of post-endoscopy aspirations, one involving a patient with severe CDI who had an aspiration after FMT via colonoscopy and died from sepsis 12 d post-FMT, and the other involving a severely ill patient who was hospitalized in the intensive care unit (ICU) due to fulminant CDI, with distended colon and increased intra-abdominal pressure.
Mortality: The characteristics of the 11 patients in our cohort who did not survive are compared to the rest of the cohort in Table 6. They were much older (84.0 ± 5.8 years compared with 62.4 ± 21.6 years, mean difference 21.6 years, 95%CI: 8.6-34.7, P < 0.001), had higher Charlson comorbidity index scores (8.0 ± 2.4 compared with 4.57 ± 2.98, mean difference 3.42, 95%CI: 1.54-5.31, P = 0.001), and had fewer episodes of CDI (average of 1.45 ± 1.4 compared with 2.57 ± 1.6, mean difference 1.1, 95%CI: 0.1-2.1, P < 0.05). None of the deceased had undergone FMT via capsules, and only one was performed on an ambulatory basis. All 11 deceased patients were hospitalized at least once within the 3 months prior to undergoing the FMT procedure. The CDI of 6 of the deceased patients had improved significantly, and they died from causes unrelated to either CDI or to the FMT procedure itself. The other 5 showed no clinical response to FMT and they died shortly after undergoing it, with continuing deterioration of their general clinical condition.
Table 6.
Mortality
| Valid | No. |
Alive |
Dead |
P value | |||
| Number | % | Number | % | ||||
| Patients | 100 | 90.12 | 11 | 9.9 | |||
| Male gender | 111 | 47 | 38 | 38.03 | 9 | 81.8 | 0.005 |
| Ambulatory | 104 | 75 | 74 | 77.93 | 1 | 10.0 | 0.000 |
| CDI risk factors | |||||||
| Prior hospitalization | 111 | 80 | 69 | 69.03 | 11 | 100.0 | 0.030 |
| Antibiotics use | 108 | 89 | 81 | 82.73 | 8 | 80.0 | 0.834 |
| PPI usage | 110 | 37 | 31 | 31.33 | 6 | 54.5 | 0.122 |
| Chemotherapy | 111 | 19 | 19 | 19.03 | 0 | 0.0 | 0.112 |
| IBD | 111 | 20 | 20 | 20.03 | 0 | 0.0 | 0.101 |
| Steroid usage | 111 | 17 | 16 | 16.03 | 1 | 9.1 | 0.546 |
| Fever | 76 | 21 | 15 | 23.13 | 6 | 54.5 | 0.031 |
| Severe | 110 | 20 | 13 | 13.03 | 7 | 63.6 | 0.000 |
| Adverse events | 107 | 19 | 15 | 15.53 | 4 | 40.0 | 0.053 |
| Route | |||||||
| Lower GI | 111 | 50 | 44 | 44.03 | 6 | 54.5 | 0.023 |
| Upper GI1 | 24 | 19 | 19.03 | 5 | 45.5 | ||
| Capsules | 37 | 37 | 37.03 | 0 | 0.0 | ||
| 0-1 previous CDI | 107 | 24 | 19 | 19.83 | 5 | 45.5 | 0.053 |
| 2+ previous CDI | 83 | 77 | 6 | ||||
| Indication | 111 | 0.306 | |||||
| Refractory | 23 | 19 | 19.03 | 4 | 36.4 | ||
| Recurrent | 82 | 76 | 76.03 | 6 | 54.5 | ||
| Prior therapy | |||||||
| Metronidazole | 110 | 89 | 80 | 80.83 | 9 | 81.8 | 0.936 |
| Vancomycin | 110 | 109 | 98 | 99.03 | 11 | 100.0 | 0.738 |
| Combination | 109 | 31 | 25 | 25.53 | 6 | 54.5 | 0.043 |
| Outcomes | |||||||
| Adverse events | 107 | 19 | 15 | 15.53 | 4 | 40.0 | 0.053 |
| Response to FMT | 111 | 94 | 88 | 88.03 | 6 | 54.5 | 0.003 |
| Mean | SD | Mean | SD | Sigma | |||
| Age (yr) | 111 | 62.37 | 21.656 | 84 | 5.797 | 0.000 | |
| Charlson comorbidity score | 81 | 4.57 | 2.98 | 8 | 2.4 | 0.001 | |
| Previous CDI episodes (n) | 107 | 2.57 | 1.633 | 1.45 | 1.368 | 0.025 | |
| Time from 1st CDI (d) | 89 | 138.47 | 197.991 | 110.75 | 63.036 | 0.493 | |
| Creatinine (mg/dL) | 76 | 1.44 | 1.507 | 1.74 | 1.193 | 0.482 | |
| Albumin (mg/L) | 54 | 3.34 | 0.782 | 2.4 | 0.408 | 0.010 | |
| WBC (103/dL) | 76 | 13.65 | 18.87 | 15.64 | 78.62 | 0.564 | |
Upper GI: Gastroscopy, nasogastric tube or through percutaneous endoscopic gastrostomy;
Percentages refer to the total number of subjects in the cohort;
Percentages refer to the number of subjects in the specific group. CDI: Clostridium difficile infection; IBD: Inflammatory bowel diseases; PPI: Proton pump inhibitor; GI: Gastrointestinal; FMT: Fecal microbiota implantation; IQR: Inter quartile range; WBC: White blood cells; Prior hospitalization: Hospitalization within the 3 mo prior to the FMT; Prior hospitalization: Antibiotics use in the 3 mo prior to the FMT.
DISCUSSION
Key results and interpretation
In this multi-center cohort study, we described the real-world experience of FMT procedures for CDI in a heterogeneous national Israeli population during the 5 years since the procedure was approved. We examined the distribution of the various techniques, routes and success rates in 111 FMT procedures.
There was an 85% response to treatment within the first 7 d post-FMT, and the success rates rose to 88% at 2 mo. These rates are compatible with reports and reviews of others[5,7-9,12,15-19]. Comparison of the 3 usual methods of FMT administration, UGI, LGI and capsules, revealed a higher success rates for the capsules and LGI routes over the UGI route although not to a level of statistical significance. Nevertheless, capsules-treated patients had the highest recurrence rates (22% at 6 mo), again not reaching a level of statistical significance. These results can be interpreted in several ways. First, there were higher rates of severe CDI within the UGI group compared with the 2 other groups (Figure 1 and Table 1). Second, previous reports showed lower success rates for the UGI route compared with LGI: one systematic review of 325 cases of FMT for CDI suggested a lower success rate for upper gut administration (76%) compared with colonoscopy (89%) and enema (95%) administration[17], while Kassam et al[19] reported a trend for higher resolution rates through the LGI route compared with the UGI route. Cure from CDI was associated with a milder disease and undergoing treatment in an outpatient setup. Other factors, such as age, Charlson score, albumin levels, and creatinine levels were not significantly different between the groups, since the study was probably underpowered to detect significant differences in these variables. Multiple infusions were seldom and relatively unsuccessful in our cohort (4 patients, 50% success rate), but the numbers are too low to arrive at any conclusions and the results cannot be compared with those of recent meta analyses which showed increased success rates with multiple FMTs[26,27].
Multivariant analysis revealed that severe CDI (OR = 0.14, P < 0.05) and inpatient FMT (OR = 0.19, P < 0.05) were each independently inversely related to FMT success, while patients’ background illnesses as reflected by the Charlson comorbidity score were not associated with either success or failure of FMT. Similar results were reported by Ianiro et al[28] in their single-center cohort study that showing that severe CDI and inadequate bowel preparation were independent predictors of FMT failure, and by Fischer et al[29] in their multi-center study, in which predictors for FMT failure included severe or severe-complicated CDI, inpatient status during FMT and previous CDI-related hospitalization. Taken together, this data implies that the severe form of CDI is less likely to be successfully treated with FMT, and that future studies are warranted in order to find the optimal treatment. Other factors, including the patients’ comorbidities as determined by the Charlson comorbidity score, did not seem to affect the FMT outcome.
To maintain continuity with a previous report[13], we compared the outcomes of the patients below and above 60 years of age and found that almost one-third (35/111) of the patients who underwent FMT for treatment of CDI in Israel were below 60 years of age (mean age 37.2 years). These patients had much lower Charlson comorbidity scores, underwent FMT on an ambulatory basis, and tended to have lower rates of severe CDI. Interestingly, there were no differences between the groups regarding hospitalizations in the 3 mo prior to the FMT. In a population-based study from Olmested county, Minnesota, United States, community-acquired CDI accounted for 41% of CDI cases and was characterized by a younger population with less severe disease, which is in line with our findings[30]. We observed a significantly higher percentage (40%) of IBD patients among this group compared to the older group (8%). Interestingly, the waiting period between the first CDI episode to undergoing FMT was longer among the younger patients compared to the older ones, possibly due to a delay in diagnosis or to a lower compliance rate to undergo the procedure, as well as a lower index of suspicion among physicians caring for younger patients with diarrhea compared to older ones leading to a delay in diagnosis. The higher rates of IBD among younger patients might also contribute to the delay in diagnosis, since diarrhea can be attributed to illnesses, such as IBD and other GI disorders other than CDI. Indeed, time to FMT from first CDI in IBD patients tended to be longer compared to non-IBD patients (207.2 and 116.9 d, respectively, P = 0.066). Interestingly, the IBD patients in our study experienced higher success rates than reported in the literature (90% compared to 74.4% reported by Khoruts et al[31]), although the group of IBD patients group in our study is much smaller (n = 20) compared with theirs (n = 272), and that might explain the difference in results.
Finally, severe CDI may be a fatal disease, especially in elderly and frail populations. The patients in our study group who died were much older, had significantly higher Charlson comorbidity scores, and much higher rates of severe CDI than the survivors. The CDI-associated diarrhea had improved significantly in 6 of them, while the condition of the others continued to deteriorate despite broad-spectrum antibiotic coverage treatment and ICU support, including mechanical ventilation and vasopressors. None of the deaths were attributed to the FMT procedure in any of them, which correlates with previous reports[13,19]. Although an approximately 10% mortality rate is quite high for FMT, it represents the natural history of weakened senior patients with multiple comorbidities in a large cohort rather than the FMT itself, as reported earlier[19,32]. Similar numbers can be found in other long follow-up studies, such as the one by Brandt et al[12] which reported the demise of 7 of the 77 patients in their long-term study (mean follow-up 17 mo).
Most of the adverse events were mild and self-resolving, and they included abdominal discomfort, nausea, flatulence and constipation, which can be attributed to the procedure itself (i.e., most of these complications occurred in the LGI group). In addition, they are generally self-limiting and rather common post-colonoscopy events, occurring after up to 33% of colonoscopies[33]. Severe complications were recorded for 2 patients (< 2% of the cohort) who were severely ill in an ICU setting and each suffered post-endoscopy aspirations.
The strength of this study is its ability to capture real-life data from the 5 medical centers throughout Israel that perform FMT through various routes and use different donors with negligible differences in preparing donors’ fecal filtrate. These are important for creating balanced data regarding the efficacy and safety of FMT.
Limitations
There were several limitations in the present study. Firstly, it is retrospective in design, warranting a prospective double blind randomized placebo-controlled study. Secondly, some of the data were collected a posteriori and information on laboratory findings, class of antibiotic used prior to CDI and Charlson scores of some of the patients (especially the ambulatory ones) were not available. Other limitations were the power of the study and the fact that the study population consisted only of Israeli patients.
Generalizability
The results of this study correlate with previous works regarding overall success rates[5,7,8,12,15-19], different routes of FMT administration[17,19] and predictors of failure[28,29] (see above), and reflect a multi-center data of heterogenous population from several districts in Israel and from different stool donors, making its results generalizable worldwide.
The corresponding author can provide complete data upon request.
In conclusion, FMT is a safe and effective treatment for CDI, which has been occurring in growing numbers in both older and younger populations. While both LGI and capsule administration of FMT seem to be more efficient than the UGI endoscopic route, FMT via capsules has emerged as a successful and well-tolerated alternative. Severe CDI and inpatient status were related to FMT failure. Prospective and well-powered studies are needed to conclusively determine the best route of administration, regarding patient safety, ease of administration, side effects and costs.
ARTICLE HIGHLIGHTS
Research background
The incidence of Clostridium difficile infection (CDI) is rising, and the increase is associated with significant morbidity and mortality rates. Fecal microbiota transplantation (FMT) is a promising and safe treatment. FMT has been performed in 5 medical centers in Israel since 2013, but its efficacy and safety had not yet been assessed.
Research motivation
To summarize all the FMT procedures performed in Israel for CDI between 2013-2017 and provide a detailed report on its current status, success rates, safety, and modes of administration.
Research objectives
The main objectives were to assess FMT success and failure rates as well as predictors of success with respect to mode of administration.
Research methods
This multi-center retrospective study included all the patients who were treated with FMT for CDI in Israel between January 2013 and October 2017. Clinical data were obtained from medical records, and they included epidemiologic information, risk factors for CDI, Charlson co-morbidity scores, and follow-up information up to 6 mo post-FMT. The Student t test was used for normally distributed variables and the Mann-Whitney U test was applied for non-normally distributed variables. We also used the χ2 test to assess associations among categorical variables. Multivariate logistic regression analysis tested the association between patient and FMT characteristics and successful FMT, controlling for potential confounders.
Research results
A total of 111 CDI patients were included. The median age of the 111 participating patients was 70 years [interquartile range (IQR): 53-82], 47 (42%) of the patients were males, and the median 1-year Charlson comorbidity score for the entire cohort was 6 (IQR: 3-7 points; expected one-year survival of 79% ± 9%). Seventy-eight (70%) of the FMT procedures were performed in an ambulatory setting. FMT was performed through the lower gastrointestinal route (LGI) in 50 patients (45%), followed by capsules in 37 patients (33%), and through the upper GI route (UGI) in 24 patients (22%). A total of 97 (87.4%) patients achieved clinical remission (79% UGI, 88% LGI, and 92% capsules, P = 0.338). Multiple FMT infusions were rare and unsuccessful. The multi-variance analysis revealed that severe disease and inpatient status were independently inversely related to FMT success, with an OR of 0.14 and 0.19, respectively. Patients younger than 60 years (n = 35, 32%) had higher percentages of background inflammatory bowel disease (IBD; 14/35, 40%) compared patients older than 60 years (6/76, 8%, P < 0.01), and waited much longer to undergo the FMT procedure (time between diagnosis of CDI to FMT of 102 d for the older group and 198 d for the younger group, P < 0.05). Eleven patients who died during the study period (no death was attributed to the FMT procedure) were much older (84 years compared with 62 years, P < 0.01), and had higher Charlson comorbidity index scores (8 compared with 4.5, P < 0.01).
Research conclusions
This is the first comprehensive description of the FMT experience in Israel since the procedure was introduced in 2013. This study shows FMT success rates which are similar to other reports worldwide, and provides the information about the efficacy and safety of the procedure, with respect to different administration routes. Predictors of FMT failure were CDI-related (severe disease and inpatient status) but not patient-related (as reflected by the Charlson comorbidity score). Concomitant IBD did not affect clinical outcomes. FMT through capsules was an efficient mode of FMT administration, and emerged as being a safe and well-tolerated alternative to endoscopy.
Research perspectives
FMT is a safe and effective treatment for CDI. The association between severe CDI with higher FMT failure rates raises the need for the future investigation of other approaches. FMT through capsules seems to be at least as good as FMT by means of endoscopies and its potential as a primary route should be investigated.
ACKNOWLEDGMENTS
The authors thank Esther Eshkol for editorial assistance.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by the institutional review board of Tel Aviv Sourasky Medical Center.
Informed consent statement: Informed consent was obtained from the patients.
Conflict-of-interest statement: The authors have declared no conflicts of interest.
Data sharing statement: No additional data are available.
STROBE statement: The authors have checked the manuscript according to STROBE checklist.
Peer-review started: August 10, 2018
First decision: October 24, 2018
Article in press: December 6, 2018
P- Reviewer: Ianiro G, Konig J, Tahan V S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
Contributor Information
Sharon A Greenberg, Department of Gastroenterology and Liver Diseases, Tel Aviv Sourasky Medical Center, affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6423906, Israel.
Ilan Youngster, Assaf Harofe Medical Center, Zerifin 70300, Israel.
Nathaniel A Cohen, Department of Gastroenterology and Liver Diseases, Tel Aviv Sourasky Medical Center, affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6423906, Israel.
Dan M Livovsky, Digestive Diseases Institute, Shaare Zedek Medical Center, Jerusalem 91031, Israel.
Jacob Strahilevitz, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University, Jerusalem 91120, Israel.
Eran Israeli, Department of Gastroenterology and Liver Diseases, Hadassah-Hebrew University, Jerusalem 91120, Israel.
Ehud Melzer, Gastrointestinal and Liver Diseases Institute, Kaplan Medical Center, Rehovot 76100, Israel.
Kalman Paz, Digestive Diseases Institute, Shaare Zedek Medical Center, Jerusalem 91031, Israel.
Naomi Fliss-Isakov, Department of Gastroenterology and Liver Diseases, Tel Aviv Sourasky Medical Center, affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6423906, Israel.
Nitsan Maharshak, Department of Gastroenterology and Liver Diseases, Tel Aviv Sourasky Medical Center, affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6423906, Israel; Bacteriotherapy Clinic, Tel Aviv Sourasky Medical Center, affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6423906, Israel. nitsanm@tlvmc.gov.il.
References
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Petrella LA, Sambol SP, Cheknis A, Nagaro K, Kean Y, Sears PS, Babakhani F, Johnson S, Gerding DN. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis. 2012;55:351–357. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;8:1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 6.Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, Kaplan JL, Hohmann EL. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. doi: 10.1186/s12916-016-0680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98; quiz 499. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 10.Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, Moore DJ, Colville A, Bhala N, Iqbal TH, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67:1920–1941. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 11.Mattila E, Uusitalo-Seppälä R, Wuorela M, Lehtola L, Nurmi H, Ristikankare M, Moilanen V, Salminen K, Seppälä M, Mattila PS, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–496. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 13.Friedman-Korn T, Livovsky DM, Maharshak N, Aviv Cohen N, Paz K, Bar-Gil Shitrit A, Goldin E, Koslowsky B. Fecal Transplantation for Treatment of Clostridium Difficile Infection in Elderly and Debilitated Patients. Dig Dis Sci. 2018;63:198–203. doi: 10.1007/s10620-017-4833-2. [DOI] [PubMed] [Google Scholar]
- 14.Cohen NA, Maharshak N. Novel Indications for Fecal Microbial Transplantation: Update and Review of the Literature. Dig Dis Sci. 2017;62:1131–1145. doi: 10.1007/s10620-017-4535-9. [DOI] [PubMed] [Google Scholar]
- 15.Brandt LJ, Aroniadis OC. An overview of fecal microbiota transplantation: techniques, indications, and outcomes. Gastrointest Endosc. 2013;78:240–249. doi: 10.1016/j.gie.2013.03.1329. [DOI] [PubMed] [Google Scholar]
- 16.Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013;15:337. doi: 10.1007/s11894-013-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 18.König J, Siebenhaar A, Högenauer C, Arkkila P, Nieuwdorp M, Norén T, Ponsioen CY, Rosien U, Rossen NG, Satokari R, et al. Consensus report: faecal microbiota transfer - clinical applications and procedures. Aliment Pharmacol Ther. 2017;45:222–239. doi: 10.1111/apt.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 20.Cohen NA, Livovsky DM, Yaakobovitch S, Ben Yehoyada M, Ben Ami R, Adler A, Guzner-Gur H, Goldin E, Santo ME, Halpern Z, et al. A Retrospective Comparison of Fecal Microbial Transplantation Methods for Recurrent Clostridium Difficile Infection. Isr Med Assoc J. 2016;18:594–599. [PubMed] [Google Scholar]
- 21.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 22.Ota KV, McGowan KL. Clostridium difficile testing algorithms using glutamate dehydrogenase antigen and C. difficile toxin enzyme immunoassays with C. difficile nucleic acid amplification testing increase diagnostic yield in a tertiary pediatric population. J Clin Microbiol. 2012;50:1185–1188. doi: 10.1128/JCM.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 24.Woodworth MH, Neish EM, Miller NS, Dhere T, Burd EM, Carpentieri C, Sitchenko KL, Kraft CS. Laboratory Testing of Donors and Stool Samples for Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. J Clin Microbiol. 2017;55:1002–1010. doi: 10.1128/JCM.02327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 26.Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, Iqbal TH. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46:479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 27.Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, Gasbarrini A, Cammarota G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficil infection: A systematic review and meta-analysis. United European Gastroenterol J. 2018;6:1232–1244. doi: 10.1177/2050640618780762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ianiro G, Valerio L, Masucci L, Pecere S, Bibbò S, Quaranta G, Posteraro B, Currò D, Sanguinetti M, Gasbarrini A, et al. Predictors of failure after single faecal microbiota transplantation in patients with recurrent Clostridium difficile infection: results from a 3-year, single-centre cohort study. Clin Microbiol Infect. 2017;23:337.e1–337.e3. doi: 10.1016/j.cmi.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Fischer M, Kao D, Mehta SR, Martin T, Dimitry J, Keshteli AH, Cook GK, Phelps E, Sipe BW, Xu H, et al. Predictors of Early Failure After Fecal Microbiota Transplantation for the Therapy of Clostridium Difficile Infection: A Multicenter Study. Am J Gastroenterol. 2016;111:1024–1031. doi: 10.1038/ajg.2016.180. [DOI] [PubMed] [Google Scholar]
- 30.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoruts A, Rank KM, Newman KM, Viskocil K, Vaughn BP, Hamilton MJ, Sadowsky MJ. Inflammatory Bowel Disease Affects the Outcome of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2016;14:1433–1438. doi: 10.1016/j.cgh.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuya-Kanamori L, Doi SA, Paterson DL, Helms SK, Yakob L, McKenzie SJ, Garborg K, Emanuelsson F, Stollman N, Kronman MP, et al. Upper Versus Lower Gastrointestinal Delivery for Transplantation of Fecal Microbiota in Recurrent or Refractory Clostridium difficile Infection: A Collaborative Analysis of Individual Patient Data From 14 Studies. J Clin Gastroenterol. 2017;51:145–150. doi: 10.1097/MCG.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 33.Ko CW, Dominitz JA. Complications of colonoscopy: magnitude and management. Gastrointest Endosc Clin N Am. 2010;20:659–671. doi: 10.1016/j.giec.2010.07.005. [DOI] [PubMed] [Google Scholar]


