Abstract
Cervical cancer screening is a critical preventive healthcare service for all women. Sexual minority women (SMW) in the United States experience multiple health disparities including decreased access to and use of cervical cancer screening. The mechanisms driving these disparities are not clear and SMW with multiple marginalized identities may be more likely to miss recommended cervical cancer screening. This study aimed to identify subgroups of SMW that are more and less likely to be screened for cervical cancer according to American Cancer Society guidelines. We used cross-sectional data from the latest (2010–2012) wave of the Chicago Health and Life Experiences of Women (CHLEW) Study (N = 691). Informed by intersectionality theory, we performed classification and regression tree (CART) modeling to construct a data-driven, predictive model of subgroups of SMW who were more and less likely to receive guideline-recommended screening. Notably, the CART model did not include commonly tested variables such as race/ethnicity or level of income or education. The model did identify subgroups with low likelihood of receiving screening and several novel variables that may be important in understanding SMW's use of cervical cancer screening; lifetime number of sexual partners, age at drinking onset, childhood physical abuse, and internalized homonegativity. Our results point to the importance of early life experiences and identity development processes in shaping patterns of preventive healthcare use among adult SMW. Our analysis also demonstrated the potential value of CART modeling techniques for evaluating how multiple variables interact in complex ways to predict cervical cancer screening.
Highlights
-
•
Subgroups of SMW experience differential risk for missing cervical cancer screening.
-
•
Early life experiences explain some variation in Pap testing among SMW.
-
•
Findings corroborate that age and sexual history influence Pap testing among SMW.
-
•
CART methods can guide resources to subgroups at highest risk for missing screening.
1. Introduction
Guidelines published by the American Cancer Society (ACS) (2015) specify that anyone with a cervix should be screened regularly for cervical cancer via Papanicoloau (Pap) testing regardless of sexual history, orientation, or identity. However, previous studies have demonstrated that lesbian and bisexual women are less likely to be screened for cervical cancer than their heterosexual counterparts (Agénor et al., 2014a; Charlton et al., 2011, Charlton et al., 2014; National Academies of Medicine, 2011). In the United States (US), sexual minority women (SMW), or those who identify as lesbian, gay, bisexual, or queer, experience multiple health disparities including lower rates of preventive healthcare service use (Agénor et al., 2014a; Charlton et al., 2011, Charlton et al., 2014; National Academies of Medicine, 2011; Agénor et al., 2014b; Matthews et al., 2004). Sexual orientation-related health disparities are explained by the stigma associated with minority sexual identities. This includes social stigma, which increases health risks, and healthcare-specific stigma, which creates barriers to high-quality care (Meyer, 1995, Meyer, 2003). However, SMW are not a homogenous group and wide variability in preventive healthcare use suggests that SMW with multiple marginalized identities (e.g., SMW who have a minority racial or ethnic identity or low socioeconomic status) face unique barriers to care (Agénor et al., 2015, Agénor et al., 2016; Calabrese et al., 2014, Calabrese et al., 2015; Szymanski and Meyer, 2008; Bowleg et al., 2003; Wilson et al., 2011).
The mechanisms driving disparities in Pap testing are not clear but misinformation among providers and the public about SMW's sexual health risks may contribute. Most SMW, including lesbian-identified women, are at risk for human papillomavirus (HPV), the most common cause of cervical cancer. Most SMW have some history of sexual contact with men (Mustanski et al., 2013; Diamant et al., 1999) and HPV can be transmitted through female-to-female contact (Anderson et al., 2014; Moszynski, 2009). Other research suggests that “cues to screening” that are common among heterosexual women, such as receiving other sexual and reproductive healthcare services, may be less common among SMW (Charlton et al., 2014; Agénor et al., 2014b, Agénor et al., 2015; Greene et al., 2018; Reiter and McRee, 2015; Eaton et al., 2008; Johnson et al., 2016a; Tracy et al., 2010). Healthcare provider- and system-level factors such as provider recommendation of Pap testing (Reiter and McRee, 2015; Johnson et al., 2016a; Marrazzo et al., 2001; Tracy et al., 2013), good communication with providers (Agénor et al., 2015; Johnson et al., 2016a, Johnson et al., 2016b; Clark et al., 2003), and disclosing one's sexual minority identity to providers (Reiter and McRee, 2015; Tracy et al., 2013; Clark et al., 2003; Diamant et al., 2000) have been associated with higher rates of screening among SMW. However, the impact of these potential “cues to screening” may vary among groups of SMW with different specific sexual histories, racial or ethnic identities, level of education, and socioeconomic resources (Agénor et al., 2015; Calabrese et al., 2014; Bowleg et al., 2003; Miles-Richardson et al., 2017).
Existing research suggests that trends in some demographic and structural barriers to Pap testing among SMW are similar to those among heterosexual women (Plourde et al., 2016; Doescher and Jackson, 2009; Coughlin et al., 2008; Centers for Disease Control and Prevention, 2012). Nuanced analyses of race and ethnicity and other aspects of identity have been limited among SMW because representative samples of sexual minority populations are difficult to define (National Academies of Medicine, 2011). Additionally, most published studies of SMW include samples that are largely white, well educated, and predominantly lesbian-identified (National Academies of Medicine, 2011). Fewer studies of the sexual and reproductive health of SMW have included bisexual women and racial and ethnic minority SMW (Bostwick et al., 2014), and very few studies have evaluated how individual and system-level factors intersect to drive cervical cancer screening.
Thus, this study aimed to identify subgroups of SMW that are more and less likely to be screened for cervical cancer according to ACS guidelines, based on the intersections of demographic characteristics, sexual identity, sexual history, and other known risk factors for poor health outcomes among SMW.
1.1. Theoretical foundation
This study was informed by intersectionality theory, developed from Black feminist scholarship and introduced by Kimberlé Crenshaw. Intersectionality theory proposes that multiple aspects of identity and experience intersect to create unique forms of discrimination (Crenshaw, 1991). The theory explains how individual components of identity intersect in unique ways among individuals with multiple marginalized identities (Crenshaw, 1991). Intersectionality theory also stresses that individual characteristics or identities such as gender, race, class, and sexual orientation are intricately linked with institutional structures (Bradford and van Wagenen, 2012; Bowleg, 2012). Previous researchers have used intersectionality specifically to study Black SMW, as they constitute a population with multiple marginalized identities and who may experience various forms of institutionalized sexism, racism, and homophobia (Bradford and van Wagenen, 2012).
Major gaps in the literature persist in understanding how multiple marginalizations and barriers to care converge to drive lower rates of screening among SMW. Classification and regression tree (CART) modeling is a recursive partitioning method that is uniquely suitable for evaluating how multiple factors intersect to predict cervical cancer screening. We used concepts from intersectionality theory to select variables that have potential to predict cervical cancer screening including race/ethnicity, income and employment status, and experiences of discrimination.
2. Study design and methods
We used existing cross-sectional data from the most recent wave (Wave 3, 2010–12) of the Chicago Health and Life Experiences of Women (CHLEW) study.
2.1. Sample
The CHLEW study is an ongoing longitudinal cohort study of SMW's health. The CHLEW sample includes a large, diverse, community-based sample that was recruited in the Chicago metropolitan area (Brown and Tracy, 2008; Waterman and Voss, 2015). Recruitment for the study began in 2000 and involved a broad range of strategies including print and online advertisements, networking at social events and community-based organizations, and individual social networks (snowball sampling). Concerted efforts were made to reach subgroups of SMW typically underrepresented in research, such as older (>50 years) and younger (<25 years) women, racial and ethnic minorities, and those with lower educational attainment.
Recruitment for the first wave of CHLEW targeted women who identified as exclusively or mostly lesbian, though some of these participants indicated other sexual orientations at subsequent interviews. At the third wave of data collection, 354 of the original 447 participants were re-interviewed (response rate = 79%), and an additional sample of 373 women was recruited using components of respondent-driven sampling (Heckathorn, 1997; Heckathorn, 2002). Recruitment of the new sample focused on African American and Latina, bisexual, and young (18–25 years) women. Data were collected using computer assisted personal interviewing methods. In the current study we included all women interviewed in Wave 3 who were 21 years old or older and had complete Pap testing data (N = 691).
2.2. Ethical considerations and data management
The Institutional Review Board at the University of Illinois at Chicago approved the parent study and the Institutional Review Board at the University of Pennsylvania approved the current study. All CHLEW data were de-identified before sharing and were password-protected and stored on the research network at the University of Pennsylvania. CHLEW interviewers received 20 h of training in general field-interviewing techniques and study-specific, potentially sensitive topics including discrimination, substance use, and sexual history. Interviewers obtained informed consent during their face-to-face meeting with participants after a review of the purpose and procedures of the study. Participants privately completed sections of the interview that addressed potentially sensitive subjects. During interviews, a distress protocol was in place (though never employed) and every CHLEW participant received a referral list of local and national crisis response agencies and hotlines.
2.3. Measures
2.3.1. Outcomes
The primary outcome was self-report of cervical cancer screening via Pap test within the year prior to interview. Although current ACS guidelines recommend cervical cancer screening every 3–5 years beginning at age 21, at the time of the Wave 3 interviews, consensus guidelines endorsed annual Pap testing for most women (ACS, 2015).
2.3.2. Potential predictors
We included 25 potential covariates (see Table 1). These variables included demographic characteristics (sexual orientation, age, race/ethnicity, income, and education). We created binary income and education variables indicating whether participants' annual household income and highest level of education were above or below the sample median. Potential covariates also included healthcare-related factors (e.g., insurance status, past-year experiences of discrimination in a healthcare setting) and factors related to sexual minority identity and sexual history (e.g., internalized homonegativity, number of sex partners). The Internalized Homonegativity scale measures the extent to which an individual has internalized negative social messages or stereotypes about sexual minority people and incorporated them into their own self-image (Herek et al., 1997). Internalized Homonegativity scores range from 1 to 5, with higher scores indicating higher levels of internalized homonegativity. This scale has previously been found to have an internal consistency of 0.71 among SMW (Herek et al., 1997). Self-described masculinity and femininity were based on questions from the Sex Role Identity Scale (Storms, 1979). We also included variables that are risk factors for multiple negative health outcomes among SMW, including experiences of childhood abuse, sexual victimization, and age at drinking onset. We generated the list of variables by reviewing the existing literature and CHLEW survey items.
Table 1.
Characteristics of participants who did and did not report a past-year Pap test, including all 25 variables inputted into CART analysis software (N = 691); frequency(percent) or mean ± standard deviation (Chicago, 2010–2012).
| Did not report past-year Pap N = 299 (42.3) |
Reported past-year Pap N = 392 (56.7) |
p Value | |
|---|---|---|---|
| Demographics | |||
| Agea | 43.4 ± 14.5 | 39.2 ± 12.6 | <0.0001⁎⁎ |
| Sexual orientation | 0.01⁎ | ||
| Lesbian | 227 (32.9) | 258 (37.3) | |
| Bisexual | 53 (7.7) | 104 (15.1) | |
| Other | 19 (2.8) | 30 (4.3) | |
| Race/ethnicity | 0.007⁎⁎ | ||
| White | 135 (19.5) | 127 (18.4) | |
| Black/African American | 93 (13.5) | 153 (22.1) | |
| Hispanic/Latina | 59 (8.5) | 97 (14.0) | |
| Other | 12 (1.7) | 15 (2.2) | |
| Education level | 0.38 | ||
| High school diploma or less | 139 (20.1) | 196 (28.4) | |
| Bachelor's degree or higher | 159 (23.0) | 196 (28.4) | |
| Income | 0.94 | ||
| <$40,000/year | 141 (21.2) | 188 (28.3) | |
| >$40,000/year | 145 (21.8) | 191 (28.7) | |
| Income “not enough to meet basic needs” | 101 (14.7) | 166 (24.2) | 0.09 |
| Unemployment | 33 (4.8) | 68 (9.8) | 0.02⁎ |
| Healthcare related variables | |||
| Has health insurancea | 205 (29.7) | 291 (42.2) | 0.12 |
| Any recent discrimination in healthcare | 29 (4.2) | 35 (5.1) | 0.73 |
| Out to all healthcare providers | 204 (29.5) | 267 (38.6) | 0.97 |
| Any previous pregnancy | 119 (17.2) | 188 (27.2) | 0.03 |
| Sexual identity and history | |||
| Masculinity score | 11.3 ± 4.7 | 11.5 ± 4.6 | 0.65 |
| Femininity score | 12.3 ± 5.0 | 13.1 ± 4.9 | 0.03⁎ |
| Internalized homonegativity scorea | 1.36 ± 0.5 | 1.48 ± 0.6 | 0.004⁎⁎ |
| Age of coming out | 20.1 ± 8.7 | 19.3 ± 8.0 | 0.20 |
| In a committed relationship | 181 (26.4) | 245 (35.7) | 0.55 |
| Age at sexual debut | 17.5 ± 4.5 | 17.0 ± 4.4 | 0.15 |
| Lifetime sexual partners (quartiles) | 0.33 | ||
| 0–6 | 91 (13.2) | 96 (13.9) | |
| 7–11 | 70 (10.1) | 97 (14.0) | |
| 12–20 | 67 (9.7) | 103 (14.9) | |
| >20 | 71 (10.3) | 96 (13.9) | |
| Lifetime sexual partners (cont.)a | 15.9 ± 17.7 | 18.3 ± 22.9 | 0.14 |
| >1 Male sexual partners | 202 (29.3) | 292 (42.3) | 0.045⁎ |
| Lifetime male partners (cont.) | 7.0 ± 13.9 | 8.4 ± 16.4 | 0.24 |
| Risk factors | |||
| Age at drinking onseta | 16.7 ± 4.1 | 17.0 ± 3.8 | 0.45 |
| Childhood sexual abuse | 114 (20.3) | 152 (27.1) | 0.51 |
| Childhood physical abusea | 58 (8.4) | 103 (15.0) | 0.03 |
| Adult sexual victimization | 139 (20.1) | 198 (28.7) | 0.29 |
Note. STI: sexually transmitted infection.
p-Value < 0.05 based on Chi square or t-test.
p-Value < 0.01 based on Chi square or t-test.
Variable appeared in CART model.
2.4. Data analysis
Descriptive statistics were generated for all variables (SAS version 9.4, SAS Institute Inc., Cary, NC). We calculated means and standard deviations for all continuous variables and frequencies and percentages for all categorical variables. We tested differences between participants who reported a past-year Pap test and those who did not using chi-square tests for categorical variables and two-sample t-tests for continuous variables.
To identify subgroups of SMW in the sample more and less likely to report past-year cervical cancer screening, we used CART analysis (RPART package in R version 3.3.1) (R Core Team, 2013). CART analysis software recursively splits data by one predictor variable at a time to generate a series of splits that best predict the outcome variable. The location of splits in continuous or count variables occur where the split best homogenizes the outcome variable. This process results in small subgroups of the larger study population (depicted in “terminal nodes”) that are more homogenous in the outcome than the entire sample (depicted in the “root node”). The final CART model reveals how individual predictor variables intersect to predict an outcome (Neville, 1999), making it an appropriate method for a study framed by intersectionality.
The CART modeling software chooses optimum splits in the data from a large number of possible splits by minimizing the tree's misclassification rate (R Core Team, 2013). The cost-complexity parameter controls the size of the decision tree and reflects the tradeoff between the cost of adding a variable to the tree and the overall fit of the data. Our tree was generated using a complexity parameter of 0.011 according to the one minus standard error rule. CART analysis generates nonparametric, predictive models, therefore traditional statistical power analyses are not applicable.
3. Results
3.1. Distributions of key variables
Table 1 presents the distributions of all potential predictor variables by the outcome variable of past-year Pap test. A total of 392 participants (56.7%) reported a past-year Pap test. According to chi square and two-sample t-tests, participants who reported a past-year Pap test were younger (39.2 years vs. 43.4 years, p < 0.0001), more likely to be unemployed (9.8% vs. 4.8%, p = 0.02), higher in self-reported femininity (13.1 vs. 12.3, p = 0.03) and internalized homonegativity (1.48 vs. 1.36, p = 0.004) scores, and more likely to report more than one male sexual partner (42.3% vs. 29.3%, p = 0.045) than those who did not report a past-year Pap test. Sexual orientation (p = 0.01) and race/ethnicity (p = 0.007) distributions also differed significantly between the two groups.
3.2. Overall decision tree model performance
As shown in Table 2, the CART model had a 64.8% accuracy predicting the Pap test outcome (CI 61.1%, 68.4%), which was a significant improvement over the predictive error in the root node, or the entire sample (43.3%). The model's sensitivity was 89.8% (CI 86.4%, 92.6%), reflecting the model's ability to identify those who did receive Pap testing. The model's specificity was 32.1% (CI 26.8%, 37.7%), indicating that the model less accurately identified those who did not report past year Pap testing. The positive predictive value of the model was 63.4% (CI 61.4%, 65.4%), while the negative predictive value of the model was 70.6% (CI 63.2, 77.1%).
Table 2.
Performance statistics for the CART model predicting past-year Pap test (Chicago, 2010–2012).
| Statistic | Value |
|---|---|
| Root node error | 0.433 |
| Accuracy | 0.648 |
| 95% CI | (0.611, 0.684) |
| p-Value [Acc > NIR] | 8.5e−06 |
| Sensitivity | 0.898 |
| 95% CI | (0.864, 0.926) |
| Specificity | 0.321 |
| 95% CI | (0.268, 0.377) |
| Positive predictive value | 0.634 |
| 95% CI | (0.614, 0.654) |
| Negative predictive value | 0.706 |
| 95% CI | (0.632, 0.771) |
Note. In this model, Sensitivity represents the proportion of participants that were correctly identified in the model as having received Pap testing. Specificity represents the proportion of participants that did not receive a Pap test in the previous year and were correctly identified in the model. Positive predictive value is the proportion of participants who actually received Pap testing out of all those identified as having received Pap testing in the model. Negative predictive value of the model is the proportion of participants who actually did not receive Pap testing out of all those identified as not having received Pap testing in the model. The p-value represents the probability that the model accuracy is higher than the no information rate.
3.3. Individual participant profiles
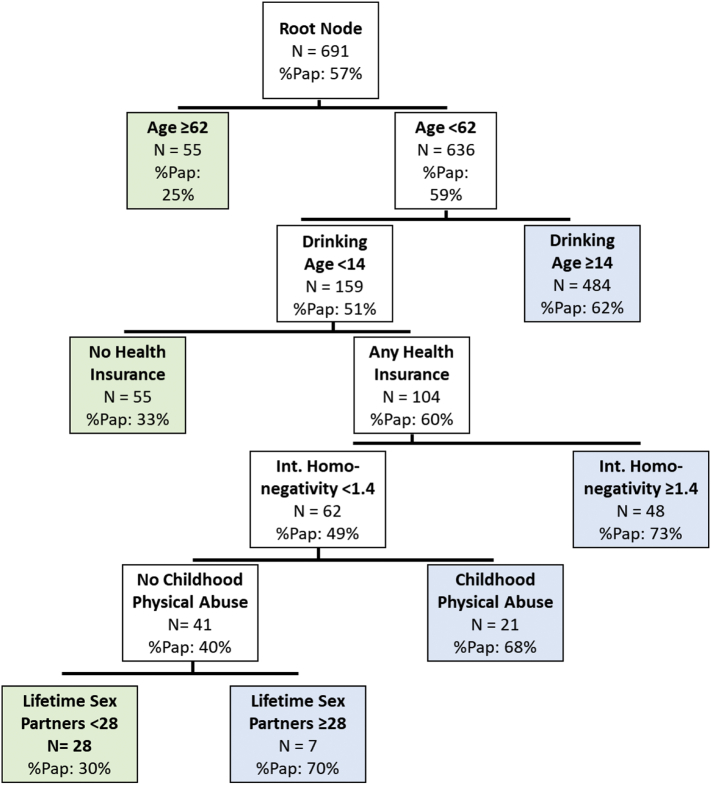
Fig. 1 displays the CART model predicting past year Pap test use. The model yielded a total of six predictor variables: participant age, age at drinking onset, health insurance status, internalized homonegativity score, childhood physical abuse, and number of lifetime sexual partners. Thus, among women in this sample, these six of the 25 inputted potential variables were most important in predicting the Pap test outcome.
Fig. 1.
CART model predicting past-year Pap testing with complexity parameter set to 0.011 (Chicago, 2010–2012).
Note: Decision tree models are interpreted based on both their overall performance in predicting the outcome accurately as well as individual terminal nodes that predict the outcome for specific subgroups of data. The “root node” of the decision tree displays the distribution of the outcome variable in the entire data set. Each subsequent “node” displays the next splitting variable, the number of participants represented by that node, and the percent of those participants with the outcome of interest. “Terminal nodes” display the outcome distribution (in this case, past-year Pap test) in final subgroups for which further splits would not improve prediction.
There were three terminal nodes that predicted low probability of screening (25% to 33%) and four terminal nodes that predicted high probability of screening (62% to 73%). Relatively good accuracy in individual terminal nodes allowed us to identify specific subgroups of SMW that may be more or less likely to receive recommended Pap testing. Participants who were age 62 or older were not likely to receive a Pap test in the previous year (25%). Other subgroups with high accuracy among women younger than 62 years were also identified. Participants younger than 62, who began drinking before age 14, were insured, and reported higher internalized homonegativity scores were more likely to have received a past-year Pap test (73%). In contrast, women younger than 62, who began drinking before age 14, were insured, reported lower internalized homonegativity, did not report any childhood abuse, and had fewer than 28 lifetime sexual partners were less likely to have had a past-year Pap test (30%). However, in this group, women who had 28 or more sexual partners were more likely to have had a past-year Pap test (70%).
4. Discussion
Although it made a statistically significant improvement over the error in the full sample, or the root node error (p = 8.5e−06), the CART model predicting Pap test use had moderate accuracy. This suggests that the set of potential covariates included in our analysis did not completely explain patterns of cervical cancer screening in this diverse sample of SMW. Future studies should include other potential correlates of cervical cancer screening among SMW. These may include additional measures of experiences of discrimination in healthcare and elsewhere, low perception of risk for cervical cancer, or elevated discomfort with the Pap test procedure. Since public health and clinical interventions designed to increase screening will be concerned about women most likely to miss recommended screening, the relatively low specificity of the model, or higher rate of “false negatives” may be an acceptable feature of the model. Our findings confirm that many factors intersect in complex ways to predict cervical cancer screening among SMW.
Two variables were consistent with previous research on preventive healthcare seeking among SMW: age and health insurance status. First, only 25% of women over age 62 in the sample had been screened. This likely includes participants who had multiple previous normal Pap tests and were not recommended for further annual testing (ACS, 2015). However, research on aging within sexual minority communities has demonstrated unique risks and needs among sexual minorities as they age, including limited access to safe and affirming healthcare (Fredriksen-Goldsen and Muraco, 2010). The relative invisibility of aging SMW may contribute to low rates of preventive screening in this population. Health insurance status also distinguished between groups with low and high rates of past-year Pap testing in our sample; those with no health insurance were part of a subgroup with a low rate of screening. Despite federal funding that supports free or low-cost sexual and reproductive health services, insurance status continues to be a barrier to seeking preventive healthcare services in the general public and among SMW in particular (U. S. Department of Health and Human Services, 2017; Centers for Disease Control and Prevention Division of Cancer Prevention and Control, 2017).
Several specific variables emerged from this analysis that contradict or add to existing research. Specifically, race/ethnicity and sexual identity, which have been previously identified as predictors of screening, did not appear in our CART model. While race/ethnicity did not appear in our model, racial and ethnic identity are deeply intertwined with multiple socioeconomic indicators and access to healthcare services in the US (Jackson et al., 2016; Williams and Purdie-Vaughns, 2016; Williams et al., 2016). Race/ethnicity tends to act as a proxy for a confluence of factors including economic status and opportunity, experiences of bias and discrimination, and social mobility (Roberts, 2011). Therefore it is likely that it is related to SMW's use of preventive care both independently and through other variables in our model (e.g., health insurance status).
Our analysis also revealed other variables that have not been tested in more traditional studies of screening, including number of sexual partners, age at drinking onset, childhood physical abuse, and internalized homonegativity. First, our findings suggest that having >28 lifetime sexual partners may predict Pap testing among SMW. This may reflect an accurate understanding of the increased risk of HPV infection from multiple sexual partners, or the misunderstanding that SMW—especially those with primarily or exclusively female partners—have low or no risk. In reality, SMW with fewer than 28 sexual partners (regardless of partner gender) are still at risk for HPV infection and cervical cancer and should be screened according to guidelines.
Other novel variables point to the importance of early life and developmental experiences in SMW's lives. The predictive value of these variables suggests a need to examine patterns of sexual identity development among SMW to understand variation in perceived need for and actual use of preventive health services such as Pap testing. Previous research has established that experiencing childhood trauma including physical abuse is a predictor of multiple negative health outcomes in adulthood (Gilbert et al., 2015; Felitti et al., 1998), and significant evidence exists to suggest that sexual minority youth may be at increased risk for sexual, physical, and emotional abuse (Roberts et al., 2010). Early age of drinking onset is associated with alcohol dependence in adulthood (Hingson et al., 2006), and a large body of research has demonstrated disproportionate rates of problematic alcohol use among SMW, including early age of drinking onset (Hughes, 2003; Hughes et al., 2010; Talley et al., 2014; Wilson et al., 2016). Among some SMW, drinking problems may be a response to minority stress or discrimination (Hughes, 2003; Everett et al., 2016). Avoidance of or lack of access to preventive healthcare services including Pap testing may be a measurable consequence of this combination of childhood abuse, early drinking, and subsequent problematic drinking.
Although internalized homonegativity may also be considered a consequence of early adverse experiences related to sexual identity, higher levels of internalized homonegativity were associated with a higher likelihood of receiving Pap testing. This may suggest that the more proximate consequences of internalized homonegativity, such as more sexual partners, riskier sexual encounters, or earlier and more problematic drinking (Berg et al., 2016) may actually promote regular Pap testing.
4.1. Study limitations
This study had several notable limitations. First, CART methods do not identify causal relationships. However, these methods facilitate the generation of new hypotheses. Second, we conducted a secondary data analysis and while the CHLEW study includes many variables likely to be important in predicting Pap testing, our analysis was limited to measures included in the parent study. Additionally, the CHLEW sample is not a random sample and therefore we cannot generalize our findings to all SMW. Specifically, the CHLEW sample is comprised of women who are “out” as sexual minorities and resided in or near Chicago, IL in 2000. Patterns of preventive sexual healthcare usage may be different among SMW living in more rural and other geographic areas and who have not disclosed their minority sexual identity.
Our CART model predicting Pap test use has limited clinical or practical utility. However, findings imply that investigators should continue to examine other factors that may better predict regular cervical cancer screening among SMW. Additionally, our measure of cervical cancer screening does not reflect newer screening guidelines, which extend the length of time between recommended Pap tests, or regular use of Pap testing over time. Recent guideline changes may significantly affect rates of screening among SMW, who may already be less likely to seek regular healthcare. However, using guidelines that were current at the time of interview best reflects guideline-adherent screening. We were also unable to measure patterns of cervical cancer screening over time. The Pap testing outcome in this study reflects screening at one time in participants' lives. While one incidence of Pap testing can act as a proxy for regular screening, regular and repeated screening is crucial for effectively preventing cervical cancer. Notwithstanding these limitations, our study included age- and race-diverse SMW and a novel analytical method allows for generating nuanced hypotheses about on how early experiences including sexual identity development influence future health and healthcare outcomes can be generated from these findings.
4.2. Recommendations for future study
Future studies should examine Pap test use among SMW longitudinally, and in the context of other life events and health behaviors. This study provides further evidence that early experiences such as childhood abuse, early drinking, and the formation of internalized homonegativity are important in predicting future use of Pap testing. Longitudinal studies can also contribute to understanding causal relationships between life experiences, sexual identity, and healthcare service use among SMW.
Future studies should also gather system- and provider-level data on where and how SMW seek cervical cancer screening. Recent studies of the general population of women (Plourde et al., 2016) as well as SMW (Reiter and McRee, 2015; Plourde et al., 2016) have shown that providers' specific recommendation for Pap testing is highly correlated with receiving a Pap test. SMW may seek other kinds of sexual and reproductive healthcare during which Pap testing is discussed less frequently (Agénor et al., 2014a, Agénor et al., 2014b; Charlton et al., 2014), and experiences of discrimination and discomfort in healthcare settings can influence SMW's decisions about seeking preventive care (Li et al., 2015; Macapagal et al., 2016). Importantly, future investigators should recruit samples diverse in race/ethnicity, socioeconomic status, age, and sexual identity to further illuminate how multiple minority identities intersect in SMW's lives to impact their utilization of preventive healthcare.
5. Conclusions
Our findings demonstrate that intersecting components of individual and structural factors impact cervical cancer screening among SMW. Health insurance status was the variable most explicitly tied to structural inequality in our model, but experiences such as childhood physical abuse, early drinking, and internalized homonegativity also reflect structural influences on individuals' experiences. Our findings also highlight the significance of early life experiences in shaping patterns of health and preventive healthcare utilization in adulthood.
This study demonstrated the potential value of CART analysis in identifying population subgroups that may be at higher or lower risk of preventive care outcomes. Because CART models and other recursive partitioning strategies are data-driven and do not rely on theoretical explanations of healthcare use, they may help uncover novel predictors of screening outcomes. Ultimately, findings from this study and other studies can help guide the distribution of public health and research resources to population subgroups that are at highest risk of missing cervical cancer screening and other critical preventive health services.
Acknowledgments
Acknowledgements
The authors would like to acknowledge Kelly Martin at the University of Illinois at Chicago and Dr. Cindy Veldhuis at Columbia University for their expertise and assistance with the CHLEW data set and relevant variables.
Funding
This work was supported by the National Institutes of Health under two Ruth L. Kirschstein National Research Service Awards, through the National Institute of Nursing Research (T32 NR007100, PI: Medoff-Cooper, B.) and the National Institute of Child Health and Human Development (T32HD049302, PI: Ehrenthal, D.).
Conflicts of interest
The authors have no conflicts of interest to report.
Contributor Information
Madelyne Z. Greene, Email: mgreene8@wisc.edu.
Tonda L. Hughes, Email: th2696@cumc.columbia.edu.
Alexandra Hanlon, Email: alhanlon@upenn.edu.
Liming Huang, Email: limingh@upenn.edu.
Marilyn S. Sommers, Email: ssommer@upenn.edu.
Salimah H. Meghani, Email: meghanis@upenn.edu.
References
- ACS . The American Cancer Society Medical and Editorial Content Team. 2015. History of ACS recommendations for the early detection of cancer in people without symptoms. [Google Scholar]
- Agénor M., Krieger N., Austin S.B., Haneuse S., Gottlieb B.R. At the intersection of sexual orientation, race/ethnicity, and cervical cancer screening: assessing Pap test use disparities by sex of sexual partners among black, Latina, and white US women. Soc. Sci. Med. 2014;116:110–118. doi: 10.1016/j.socscimed.2014.06.039. [DOI] [PubMed] [Google Scholar]
- Agénor M., Krieger N., Austin S.B., Haneuse S., Gottlieb B.R. Sexual orientation disparities in Papanicolaou test use among US women: the role of sexual and reproductive health services. Am. J. Public Health. 2014;104(2):e68–e73. doi: 10.2105/AJPH.2013.301548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agénor M., Bailey Z., Krieger N., Austin S.B., Gottlieb B.R. Exploring the cervical cancer screening experiences of black lesbian, bisexual, and queer women: the role of patient-provider communication. Women Health. 2015;55(6):717–736. doi: 10.1080/03630242.2015.1039182. [DOI] [PubMed] [Google Scholar]
- Agénor M., Austin S.B., Kort D., Austin E.L., Muzny C.A. Sexual orientation and sexual and reproductive health among African American sexual minority women in the U.S. South. Womens Health Issues. 2016;26(6):612–621. doi: 10.1016/j.whi.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T.A., Schick V., Herbenick D., Dodge B., Fortenberry J.D. A study of human papillomavirus on vaginally inserted sex toys, before and after cleaning, among women who have sex with women and men. Sex. Transm. Infect. 2014;90(7):529–531. doi: 10.1136/sextrans-2014-051558. [DOI] [PubMed] [Google Scholar]
- Berg R.C., Munthe-Kaas H.M., Ross M.W. Internalized homonegativity: a systematic mapping review of empirical research. J. Homosex. 2016;63(4):541–558. doi: 10.1080/00918369.2015.1083788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick W.B., Meyer I., Aranda F. Mental health and suicidality among racially/ethnically diverse sexual minority youth. Am. J. Public Health. 2014;104(6):1129–1136. doi: 10.2105/AJPH.2013.301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L. The problem with the phrase women and minorities: intersectionality—an important theoretical framework for public health. Am. J. Public Health. 2012;102(7):1267–1273. doi: 10.2105/AJPH.2012.300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L., Huang J., Brooks K., Black A., Burkholder G. Triple jeopardy and beyond: multiple minority stress and resilience among black lesbians. J. Lesbian Stud. 2003;7(4):87–108. doi: 10.1300/J155v07n04_06. [DOI] [PubMed] [Google Scholar]
- Bradford J., van Wagenen A. Research on the health of sexual minority women. In: Goldman M.B., Troisi R., Rexrode K.M., editors. Women and Health. Academic Press; 2012. pp. 77–91. [Google Scholar]
- Brown J.P., Tracy J.K. Lesbians and cancer: an overlooked health disparity. Cancer Causes Control. 2008;19(10):1009–1020. doi: 10.1007/s10552-008-9176-z. [DOI] [PubMed] [Google Scholar]
- Calabrese S.K., Earnshaw V.A., Underhill K., Hansen N.B., Dovidio J.F. The impact of patient race on clinical decisions related to prescribing HIV pre-exposure prophylaxis (PrEP): assumptions about sexual risk compensation and implications for access. AIDS Behav. 2014;18(2):226–240. doi: 10.1007/s10461-013-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese S.K., Meyer I.H., Overstreet N.M., Haile R., Hansen N.B. Exploring discrimination and mental health disparities faced by black sexual minority women using a minority stress framework. Psychol. Women Q. 2015;39(3):287–304. doi: 10.1177/0361684314560730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cancer screening - United States, 2010. Morb. Mortal. Wkly Rep. 2012;61(3):41–45. [PubMed] [Google Scholar]
- Charlton B.M., Corliss H.L., Missmer S.A. Reproductive health screening disparities and sexual orientation in a cohort study of U.S. adolescent and young adult females. J. Adolesc. Health. 2011;49(5):505–510. doi: 10.1016/j.jadohealth.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton B.M., Corliss H.L., Missmer S.A. Influence of hormonal contraceptive use and health beliefs on sexual orientation disparities in Papanicolaou test use. Am. J. Public Health. 2014;104(2):319–325. doi: 10.2105/AJPH.2012.301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.A., Bonacore L., Wright S.J., Armstrong G., Rakowski W. The cancer screening project for women: experiences of women who partner with women and women who partner with men. Women Health. 2003;38(2):19–33. doi: 10.1300/J013v38n02_02. [DOI] [PubMed] [Google Scholar]
- Coughlin S.S., Leadbetter S., Richards T., Sabatino S.A. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc. Sci. Med. 2008;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Crenshaw K. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev. 1991;43(6):1241–1299. [Google Scholar]
- Diamant A.L., Schuster M.A., McGuigan K., Lever J. Lesbians' sexual history with men: implications for taking a sexual history. Arch. Intern. Med. 1999;159(22):2730–2736. doi: 10.1001/archinte.159.22.2730. [DOI] [PubMed] [Google Scholar]
- Diamant A.L., Schuster M.A., Lever J. Receipt of preventive health care services by lesbians. Am. J. Prev. Med. 2000;19(3):141–148. doi: 10.1016/s0749-3797(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Division of Cancer Prevention and Control . US Department of Health and Human Services; 2017. National Breast and Cervical Cancer Early Detection Program (NBCCCEDP)https://www.cdc.gov/cancer/nbccedp/about.htm [Google Scholar]
- Doescher M.P., Jackson J.E. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J. Public Health Manag. Pract. 2009;15(3):200–209. doi: 10.1097/PHH.0b013e3181a117da. [DOI] [PubMed] [Google Scholar]
- Eaton L., Kalichman S., Cain D. Perceived prevalence and risks for human papillomavirus (HPV) infection among women who have sex with women. J. Women's Health. 2008;17(1):75–83. doi: 10.1089/jwh.2006.0256. [DOI] [PubMed] [Google Scholar]
- Everett B.G., McCabe K.F., Hughes T.L. Unintended pregnancy, depression, and hazardous drinking in a community-based sample of sexual minority women. J. Women's Health. 2016;25(9):904–911. doi: 10.1089/jwh.2015.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am. J. Prev. Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fredriksen-Goldsen K., Muraco A. Aging and sexual orientation: a 25-year review of the literature. Res. Aging. 2010;32(3):372–413. doi: 10.1177/0164027509360355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L.K., Breiding M.J., Merrick M.T. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am. J. Prev. Med. 2015;48(3):345–349. doi: 10.1016/j.amepre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Greene M.Z., Hughes T.L., Sommers M.S., Hanlon A., Meghani S.H. Association of pregnancy history and cervical cancer screening in a community sample of sexual minority women. J. Women's Health (Larchmt) 2018 doi: 10.1089/jwh.2018.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn D.D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc. Probl. 1997;44(2):174–199. [Google Scholar]
- Heckathorn D.D. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc. Probl. 2002;49(1):11–34. [Google Scholar]
- Herek G.M., Cogan J.C., Gillis J.R., Glunt E.K. Correlates of internalized homophobia in a community sample of lesbians and gay men. J. Gay Lesbian Med. Assoc. 1997;2:17–25. [Google Scholar]
- Hingson R.W., Heeren T., Winter M.R. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch. Pediatr. Adolesc. Med. 2006;160(7):739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hughes T.L. Lesbians' drinking patterns: beyond the data. Subst. Use Misuse. 2003;38(11–13):1739–1758. doi: 10.1081/ja-120024239. [DOI] [PubMed] [Google Scholar]
- Hughes T., McCabe S.E., Wilsnack S.C., West B.T., Boyd C.J. Victimization and substance use disorders in a national sample of heterosexual and sexual minority women and men. Addiction. 2010;105(12):2130–2140. doi: 10.1111/j.1360-0443.2010.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J.W., Williams D.R., VanderWeele T.J. Disparities at the intersection of marginalized groups. Soc. Psychiatry Psychiatr. Epidemiol. 2016;51(10):1349–1359. doi: 10.1007/s00127-016-1276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.J., Mueller M., Eliason M.J., Stuart G., Nemeth L.S. Quantitative and mixed analyses to identify factors that affect cervical cancer screening uptake among lesbian and bisexual women and transgender men. J. Clin. Nurs. 2016;25(23-24):3628–3642. doi: 10.1111/jocn.13414. [DOI] [PubMed] [Google Scholar]
- Johnson M.J., Nemeth L.S., Mueller M., Eliason M.J., Stuart G.W. Qualitative study of cervical cancer screening among lesbian and bisexual women and transgender men. Cancer Nurs. 2016;39(6):455–463. doi: 10.1097/NCC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- Li C.C., Matthews A.K., Aranda F., Patel C., Patel M. Predictors and consequences of negative patient-provider interactions among a sample of African American sexual minority women. LGBT Health. 2015;2(2):140–146. doi: 10.1089/lgbt.2014.0127. [DOI] [PubMed] [Google Scholar]
- Macapagal K., Bhatia R., Greene G.J. Differences in healthcare access, use, and experiences within a community sample of racially diverse lesbian, gay, bisexual, transgender, and questioning emerging adults. LGBT Health. 2016;3(6):434–442. doi: 10.1089/lgbt.2015.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrazzo J.M., Koutsky L.A., Kiviat N.B., Kuypers J.M., Stine K. Papanicolaou test screening and prevalence of genital human papillomavirus among women who have sex with women. Am. J. Public Health. 2001;91(6):947–952. doi: 10.2105/ajph.91.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A.K., Brandenburg D.L., Johnson T.P., Hughes T.L. Correlates of underutilization of gynecological cancer screening among lesbian and heterosexual women. Prev. Med. 2004;38(1):105–113. doi: 10.1016/j.ypmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Meyer I.H. Minority stress and mental health in gay men. J. Health Soc. Behav. 1995;36(1):38–56. [PubMed] [Google Scholar]
- Meyer I.H. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol. Bull. 2003;129(5):674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles-Richardson S., Allen S., Claridy M.D., Booker E.A., Gerbi G. Factors associated with self-reported cervical cancer screening among women aged 18 years and older in the United States. J. Community Health. 2017;42(1):72–77. doi: 10.1007/s10900-016-0231-5. [DOI] [PubMed] [Google Scholar]
- Moszynski P. Cervical cancer virus can be transmitted through same sex relationships, report warns. BMJ. 2009:339. doi: 10.1136/bmj.b5667. [DOI] [PubMed] [Google Scholar]
- Mustanski B., Birkett M., Greene G.J., Rosario M., Bostwick W., Everett B.G. The association between sexual orientation identity and behavior across race/ethnicity, sex, and age in a probability sample of high school students. Am. J. Public Health. 2013;104(2):237–244. doi: 10.2105/AJPH.2013.301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Medicine . The National Academies Press; 2011. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. [PubMed] [Google Scholar]
- Neville P.G. SAS Institute, Inc.; 1999. Decision Trees for Predictive Modeling. [Google Scholar]
- Plourde N., Brown H.K., Vigod S., Cobigo V. Contextual factors associated with uptake of breast and cervical cancer screening: a systematic review of the literature. Women Health. 2016;56(8):906–925. doi: 10.1080/03630242.2016.1145169. [DOI] [PubMed] [Google Scholar]
- Reiter P.L., McRee A.L. Cervical cancer screening (Pap testing) behaviours and acceptability of human papillomavirus self-testing among lesbian and bisexual women aged 21–26 years in the USA. J. Fam. Plann. Reprod. Health Care. 2015;41(4):259–264. doi: 10.1136/jfprhc-2014-101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. The New Press; New York, NY: 2011. Fatal Invention: How Science, Politics, and Big Business Re-create Race in the Twenty-first Century. [Google Scholar]
- Roberts A.L., Austin S.B., Corliss H.L., Vandermorris A.K., Koenen K.C. Pervasive trauma exposure among US sexual orientation minority adults and risk of posttraumatic stress disorder. Am. J. Public Health. 2010;100(12):2433–2441. doi: 10.2105/AJPH.2009.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms M.D. Sex role identity and its relationships to sex role attributes and sex role stereotypes. J. Pers. Soc. Psychol. 1979;30(10):1779–1789. [Google Scholar]
- Szymanski D.M., Meyer D. Racism and heterosexism as correlates of psychological distress in African American sexual minority women. J. LGBT Issues Couns. 2008;2(2):94–108. [Google Scholar]
- Talley A.E., Hughes T.L., Aranda F., Birkett M., Marshal M.P. Exploring alcohol-use behaviors among heterosexual and sexual minority adolescents: intersections with sex, age, and race/ethnicity. Am. J. Public Health. 2014;104(2):295–303. doi: 10.2105/AJPH.2013.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 3.3.1 ed. R Foundation for Statistical Computing; Vienna, Austria: 2013. A Language and Environment for Statistical Computing. [Google Scholar]
- Tracy J.K., Lydecker A.D., Ireland L. Barriers to cervical cancer screening among lesbians. J. Women's Health (Larchmt) 2010;19(2):229–237. doi: 10.1089/jwh.2009.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy J.K., Schluterman N.H., Greenberg D.R. Understanding cervical cancer screening among lesbians: a national survey. BMC Public Health. 2013;13:442. doi: 10.1186/1471-2458-13-442. (2458-2413-2442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services . vol. 2017. U.S. Department of Health and Human Services, Office of Population Affairs; 2017. About Title X Grants. [Google Scholar]
- Waterman L., Voss J. HPV, cervical cancer risks, and barriers to care for lesbian women. Nurse Pract. 2015;40(1):46–53. doi: 10.1097/01.NPR.0000457431.20036.5c. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Purdie-Vaughns V. Needed interventions to reduce racial/ethnic disparities in health. J. Health Polit. Policy Law. 2016;41(4):627–651. doi: 10.1215/03616878-3620857. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Priest N., Anderson N.B. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407–411. doi: 10.1037/hea0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.D.M., Okwu C., Mills S.A. Brief report: the relationship between multiple forms of oppression and subjective health among black lesbian and bisexual women. J. Lesbian Stud. 2011;15(1):15–24. doi: 10.1080/10894160.2010.508393. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Gilmore A.K., Rhew I.C., Hodge K.A., Kaysen D.L. Minority stress is longitudinally associated with alcohol-related problems among sexual minority women. Addict. Behav. 2016;61:80–83. doi: 10.1016/j.addbeh.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]