Abstract
Objective:
The efficacy of sedation during endoscopic injection sclerotherapy (EIS) for esophageal varices (EVs) in patients with liver cirrhosis remains unclear. The aim of this study is to compare the efficacy and safety between propofol- and midazolam-based sedation for EIS.
Methods:
Twenty-three patients with EVs were prospectively and randomly assigned to midazolam-based (Midazolam group) or propofol-based (Propofol group) sedation. All patients underwent a number connection test (NCT) to evaluate minimal hepatic encephalopathy (MHE) on the day before and at 2 and 24 hours following EIS. The primary endpoint was exacerbation of MHE after EIS, which was defined as deterioration of the NCT. The secondary endpoints were postoperative awareness, technical success rate, frequency of body movement, patient and operator satisfaction, cardiorespiratory dynamics during EIS, and adverse events.
Results:
Exacerbations of MHE at 2 hours after EIS compared with those before EIS were not significantly different between the two groups. In both groups, the deterioration of NCT scores before and 2 hours after EIS was observed (Propofol group: 60.0 vs. 70.0 s, P = 0.026; Midazolam group: 42.5 vs. 67.0 s, P = 0.002). There were no significant differences in awareness, technical success rate, or patient satisfaction. However, the frequency of body movement in the Propofol group was significantly lower than that in the Midazolam group (1 vs. 4, P = 0.045), and operator satisfaction in the Propofol group was significantly higher than that in the Midazolam group (P = 0.016). No adverse events were observed.
Conclusions:
Propofol-based sedation exacerbated MHE after EIS similarly to midazolam-based sedation in patients with liver cirrhosis. However, propofol-based sedation provided stable sedation with a lower frequency of body movements and high operator satisfaction.
Keywords: Endoscopy, esophageal varices, liver cirrhosis, sedation
Introduction
Endoscopic injection sclerotherapy (EIS) using ethanolamine oleate (EO) as an intra-variceal sclerosant is an established treatment for esophageal varices (EVs) in Japan1,2). EIS is a technically demanding and lengthy procedure involving injection into the variceal lumen and requires appropriate sedation. Midazolam is the main sedative agent used in endoscopic procedures3). However, it is difficult to achieve stable maintenance anesthesia through the intermittent intravenous administration of midazolam4). Recently, an increasing number of reports have been published on the use of propofol for various endoscopic procedures5-10). Propofol has a rapid onset and short duration of action11). Therefore, propofol can be continuously administered, enabling the constant maintenance of the depth of sedation. However, propofol has a narrow safety range that can result in the rapid depression of cardiovascular function12,13).
Many patients who undergo EIS suffer from liver cirrhosis. Patients with liver dysfunction are at increased risk of sedation-related adverse events, such as cardiorespiratory depression, particularly when they receive sedation using midazolam14,15). Propofol, however, has a favorable pharmacokinetic profile and does not require any dose adjustment in patients with liver cirrhosis16,17). In addition, the main problem with sedation in patients with liver cirrhosis is the potential influence on minimal hepatic encephalopathy (MHE). MHE is defined as a condition in which patients demonstrate quantifiable neuropsychological defects in psychometric tests, such as the number connection test (NCT), yet these individuals show a normal mental and neurological status upon clinical examination [18]. Sedation using midazolam has been reported to exacerbate MHE in patients with liver cirrhosis19-23). In contrast, sedation using propofol has not been reported to exacerbate MHE21-26). However, previous studies have only reported the use of sedation for esophagogastroduodenoscopy (EGD) or endoscopic variceal ligation (EVL). The efficacy and safety of sedation for EIS remain unclear. In addition, there have been no studies comparing propofol with midazolam as sedation for EIS.
Therefore, the aim of this prospective study is to compare the efficacy and safety between propofol-based and midazolam-based sedation for EIS.
Materials and Methods
Study Design and Patients
We conducted a prospective, randomized controlled parallel group trial at Fukushima Medical University Hospital between April 2015 and October 2016. Patients with EVs and liver cirrhosis who were scheduled for treatment with prophylactic EIS were randomly assigned to one of two groups: midazolam-based (Midazolam group) or propofol-based sedation (Propofol group) (simple randomization, allocation ratio 1: 1). Randomization was performed by sequentially opening numbered opaque envelopes with computer-generated group allocation cards in a random sequence. Although the operator and assistant were not blinded, the patients were blinded to their allocation. The randomization list was stored in the laboratory and completely concealed from the operator.
The following inclusion criteria were considered: 1) patients with liver cirrhosis who were scheduled for treatment with prophylactic EIS for EVs; 2) patients without a history of treatment or bleeding of EVs; 3) patients between 20 and 80 years of age; 4) patients with a performance status of 0; and 5) patients who provided consent to receive EIS and participate in this study. Indications for prophylactic EIS were moderate or large-sized varices (F2 or F3) and/or red color signs on varices diagnosed based on endoscopic findings according to the Japan Research Society for Portal Hypertension27). The diagnosis of liver cirrhosis was based on history, serologic testing, radiologic imaging, and liver histology when available. The following exclusion criteria were considered: 1) American Society of Anesthesiologists classification III, IV, or V; 2) severe hepatic disorder (Child-Pugh C classification and serum total bilirubin of 4 mg/dL or greater); 3) hepatocellular carcinoma with portal vein invasion; 4) alcohol intake during the past 2 weeks; 5) use of illicit drugs or drugs that act in the central nervous system, such as benzodiazepines; 6) clinically overt hepatic encephalopathy (HE); 7) dementia; 8) neurologic diseases, such as Alzheimer’s disease or Parkinson’s disease; 9) renal failure (serum creatinine > 2 mg/dL); 10) allergy to midazolam or propofol; and 11) patients who did not elect to undergo EIS. There was no modification of these criteria during this study period.
This study was conducted according to the Declaration of Helsinki, approved by the Ethics Committee of Fukushima Medical University (approval No. 2058) and registered in the University Hospital Medical Information Network (UMIN) as number UMIN 000017173. All the patients provided written consent to participate in the study.
EIS Procedures
EIS was performed using a GIF-Q240X endoscope (Olympus Medical Systems Corp., Tokyo, Japan) with an oral side balloon (CREATE MEDIC Co., Ltd., Yokohama, Japan) and a 23- or 25-gauge injection needle (TOP Corporation, Tokyo, Japan). The EIS procedure was performed by intra-variceal injection of 5% EO (Oldamin; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) under fluoroscopic guidance. EO was injected to fill the varices to the supplying vessels, such as the left gastric vein. The puncture was repeated for multiple varices until the injectable varices disappeared or the maximum amount of EO (0.4 mL/kg) was injected. All EIS procedures were performed by a single expert physician (K.W.) who was a qualified doctor accredited by the skill qualification system of the Japan Society for Portal Hypertension and had extensive experience in performing EIS over 100 times.
Monitoring and Sedation Protocols
During EIS, patients received oxygen via a nasal cannula at a rate of 2 L/min. Blood pressure (BP) was immediately measured after sedation, then every minute until the insertion of the endoscope and every 5 minutes thereafter. Heart rate (HR) and oxygen saturation (SpO2) were also continuously monitored. Sedation was administered by an independent physician with expertise in anesthesia and who was not involved in the endoscopic procedures.
In the Midazolam group, anesthesia was induced by a bolus intravenous (IV) infusion of 15 mg of pentazocine (Sosegon; Astellas Pharma, Tokyo, Japan) as an analgesic plus 2.5-5 mg of midazolam (Midazolam; Sandoz, Tokyo, Japan). In the event of body movement or signs of discomfort, an additional bolus IV infusion of 2.5 mg of midazolam was administered. Body movement was defined as movement disturbing the procedure and requiring control by a third person, such as a nurse. If cardiopulmonary suppression, such as hypotension with systolic BP < 80 mmHg or oxygen de-saturation < 90%, was observed, then the amount of drip infusion or flow volume of oxygen was increased. When needed, flumazenil (Flumazenil; Maruishi Pharmaceutical, Osaka, Japan) was administered as a reversal agent. After the completion of EIS, patients recovered from anesthesia with the administration of 0.5 mg of flumazenil.
In the Propofol group, anesthesia was induced by a bolus IV infusion of 15 mg of pentazocine plus 20 mg of propofol (1% Propofol; Maruishi Pharmaceutical, Osaka, Japan) followed by a continuous infusion of propofol using an infusion pump. The rate was maintained at 3-5 mg/kg/h during EIS. In the event of body movement or signs of discomfort, a bolus IV infusion of 20 mg of propofol was administered, and when needed, the continuous infusion rate was increased. If cardiopulmonary suppression was observed, the amount of drip infusion or flow volume of oxygen was increased, and the maintenance dose was reduced by 1 mg/kg/h. After the completion of EIS, the continuous propofol infusion was discontinued to allow the patient to recover from anesthesia. In both groups, 7.5 mg of pentazocine was administered every 30 min during EIS.
The sedation depth in both groups was adjusted to “deep sedation”, under which spontaneous breathing was maintained, according to the “Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists” published by the American Society of Anesthesiologists28).
Number Connection Test
The NCT consisted of documenting the time required to sequentially connect randomly placed circles labeled from 1 to 25. Different patterns were used to eliminate the learning effect of repetitive testing. The total time required was assessed using a stopwatch by a single examiner who was blinded to the randomization group and not involved in the endoscopic procedures. If the patient could not complete the NCT within 30 seconds, the result was defined as MHE29). The NCT was performed the day before and at 2 and 24 hours after EIS to evaluate exacerbation of MHE.
Outcomes
The primary endpoint was exacerbation of MHE after EIS, which was defined as deterioration of the NCT. The secondary endpoints were postoperative awareness, technical success rate, frequency of patient body movement during EIS, patient and operator satisfaction, cardiorespiratory dynamics during EIS, and adverse events. Postoperative awareness was evaluated at 2 and 24 hours after EIS according to the Observer’s Assessment of Awareness/Sedation Scale (OAAS: 1 point, awake; 2 points, somnolent/drowsy; 3 points, responsive to loud or repeated verbal stimuli; 4 points, responsive to physical/painful stimuli; and 5, no response to physical/painful stimuli)3). Technical success was defined as an injection of EO from the varices to the supplying vessels under fluoroscopic guidance. Patient and operator satisfaction were evaluated on a 10-point visual analog scale, where 1 point indicated the worst and 10 points indicated the best. This assessment was performed using a questionnaire after EIS. Adverse events related to sedation were defined as hypotension with a systolic BP (SBP) < 80 mmHg, SpO2 < 90%, or bradycardia with a HR < 45 beats/min. The characteristics of each patient were obtained before EIS, and procedural details were prospectively recorded in a database.
Sample Size Calculation and Statistical Analysis
Based on the results of previous studies, NCT scores were worse in greater than 70% of patients after sedation using midazolam19,20). However, NCT scores were not worse after sedation using propofol21-23,25). The required sample size was calculated as 10 patients for each group to detect a significant difference with a power of 0.8, a type I error of 0.05, and a type II error of 0.02 using Open-Epi version 3.0 (free and open source software available from http://www.openepi.com/Menu/OE_Menu.htm). Considering dropout cases, the inclusion number for this study was set at 23 patients for both groups.
Normally distributed data are reported as the means (± standard deviation [SD]), and data that were not normally distributed are reported as the median with a range. Changes in NCT scores before and after EIS were analyzed using the Wilcoxon’s signed-rank test. Continuous variables were analyzed using the Student’s t-test or the Mann-Whitney U test. Categorical variables were analyzed using the Chi-squared (χ2) test. Differences were considered statistically significant at P < 0.05. This analysis was performed using SPSS software (version 21 for windows; IBM Corp, Armonk, NY, USA).
Results
Patient Characteristics
A total of 23 consecutive patients with EVs were enrolled in this study. Twelve patients were assigned to the Midazolam group, and 11 patients were assigned to the Propofol group, as shown in Figure 1. The patient characteristics are summarized in Table 1. There was no significant difference in age, gender, cirrhosis etiology, Child-Pugh classification, and variceal size between groups.
Fig. 1.
Flowchart of patient enrollment.
Table 1.
Patient characteristics
| Midazolam group
(n = 12) |
Propofol group
(n = 11) |
P-value | |
| Age (years), mean ± SD | 62.7 ± 7.92 | 69.0 ± 9.56 | 0.097 |
| Gender (Male/Female) | 9/3 | 7/4 | 0.554 |
| BMI, mean ± SD | 25.7 ± 3.46 | 25.4 ± 6.36 | 0.895 |
| Etiology of liver cirrhosis, n (%) | 0.629 | ||
| HCV | 5 (41.7) | 4 (36.7) | |
| Alcohol | 2 (16.7) | 4 (36.7) | |
| NASH | 2 (16.7) | 2 (18.2) | |
| Others | 3 (25.0) | 1 (9.1) | |
| Child-Pugh classification, n (%) | 0.855 | ||
| A | 7 (58.3) | 6 (54.5) | |
| B | 5 (41.7) | 5 (45.5) | |
| C | 0 (0) | 0 (0) | |
| Serum albumin (g/dL), mean ± SD | 3.66 ± 0.63 | 3.55 ± 0.48 | 0.636 |
| Serum total bilirubin (mg/dL), mean ± SD | 1.20 ± 0.65 | 1.15 ± 0.62 | 0.839 |
| AST (IU/L), mean ± SD | 42.8 ± 21.8 | 44.5 ± 16.3 | 0.835 |
| ALT (IU/L), mean ± SD | 31.3 ± 14.0 | 27.3 ± 9.68 | 0.441 |
| Prothrombin time (%), mean ± SD | 73.4 ± 15.6 | 76.5 ± 11.1 | 0.591 |
| Platelet (×104/µL), mean ± SD | 8.7 ± 4.0 | 7.8 ± 2.9 | 0.529 |
| Ammonia (µg/dL), mean ± SD | 65.0 ± 37.2 | 72.8 ± 47.1 | 0.662 |
| Serum creatinine (mg/dL), mean ± SD | 0.976 ± 0.32 | 0.793 ± 0.22 | 0.129 |
| Variceal size, n (%) | 0.408 | ||
| Moderate (F2) | 8 (66.7) | 9 (81.8) | |
| Large (F3) | 4 (33.3) | 2 (18.2) |
SD: standard deviation, BMI: body mass index, HCV: hepatitis C virus, NASH: nonalcoholic steatohepatitis, AST: aspartate aminotransferase, ALT: alanine aminotransferase
Outcomes
All patients in the Propofol group had MHE, which was defined as being unable to complete the NCT within 30 seconds before EIS. Ten patients (83.3%: 10/12) in the Midazolam group had MHE before EIS. All patients in both groups had MHE 2 hours after EIS. NCT scores at 2 hours after EIS were compared to scores before EIS and expressed as the differences between the values before EIS and the values at 2 hours after EIS. These scores were not significantly different between groups (Table 2). In both groups, significant deterioration of NCT scores between before and 2 hours after EIS was observed (Propofol group: P = 0.026, Midazolam group: P = 0.002), which indicated propofol also exacerbated MHE similar to midazolam. However, no significant difference was observed in NCT scores before and at 24 hours after EIS in both groups (Figure 2a and b, Table 2). In both groups, there were no significant differences in NCT scores before and at 2 or 24 hours after EIS (Table 2). None of the patients developed overt hepatic encephalopathy after EIS.
Table 2.
Number connection test scores and postoperative awareness
| Midazolam group
(n = 12) |
Propofol group
(n = 11) |
P-value | |
| NCT scores before EIS (s), median (range) | 42.5 (26-79) | 60.0 (31-100) | 0.097 |
| NCT scores 2 hours after EIS (s), median (range) | 67.0 (26-79) | 70.0 (36-184) | 0.878 |
| NCT scores 24 hours after EIS (s), median (range) | 45.5 (25-71) | 61.1 (30-164) | 0.255 |
| Changes in NCT scores 2 hours after EIS compared with baseline | 17.0 (3-391) | 12.0 (-4-119) | 0.218 |
| (s), median (range) | |||
| Changes in NCT scores 24 hours after EIS compared with baseline | 2.5 (-12-18) | -1 (-23-64) | 1.000 |
| (s), median (range) | |||
| OAAS score 2 hours after EIS, median (range) | 2 (1-3) | 2 (1-2) | 0.212 |
| OAAS score 24 hours after EIS, median (range) | 1 (1) | 1 (1) | - |
NCT: number connection test, EIS: endoscopic injection sclerotherapy
OAAS: observer’s assessment of awareness/sedation scale
Fig. 2.
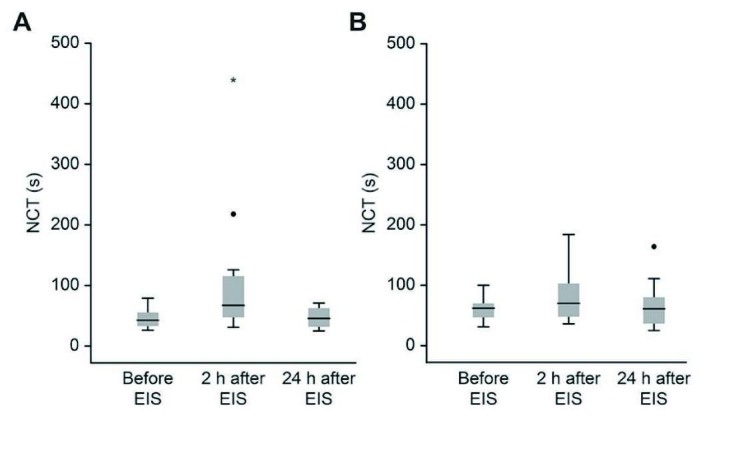
Changes in number connection test (NCT) scores.
A. Changes in NCT scores from baseline to 2 and 24 hours after EIS in the Midazolam group. A significant deterioration in NCT scores from baseline to 2 hours after EIS was observed. No significant difference was observed in NCT scores from baseline to 24 hours after EIS (Wilcoxon’s signed-rank test).
B. Changes in NCT scores from baseline to 2 and 24 hours after EIS in the Propofol group. A significant deterioration in NCT scores from baseline to 2 hours after EIS was observed. No significant difference was observed in NCT scores from baseline to 24 hours after EIS (Wilcoxon’s signed-rank test).
In terms of awareness, there was no significant difference between groups in OAAS at 2 hours after EIS (Table 2). The technical success rate, procedure time, and total amount of EO injected were not significantly different between groups (Table 3). The frequency of body movement during EIS was significantly lower in the Propofol group (1 count) than that in the Midazolam group (4 counts, P = 0.045, Table 3). There was no difference in patient satisfaction between groups; however, operator satisfaction in the Propofol group was significantly higher than that in the Midazolam group (10 vs. 7, P = 0.016, Table 3). Procedure discontinuation was not necessary in any case.
Table 3.
Treatment outcomes, cardiorespiratory dynamics and adverse events
| Midazolam group
(n = 12) |
Propofol group
(n = 11) |
P-value | |
| Dose of midazolam or propofol (mg), mean ± SD | 12.3 ± 4.32 | 214.2 ± 80.3 | - |
| Dose of pentazocine (mg), mean ± SD | 21.3 ± 2.92 | 21.8 ± 4.05 | 0.701 |
| Technical success, n (%) | 10 (83.3) | 10 (90.9) | 0.590 |
| Procedure time (min), mean ± SD | 43.6 ± 11.1 | 38.6 ± 10.7 | 0.290 |
| Total amount of injected EO (ml), mean ± SD | 14.3 ± 6.44 | 14.6 ± 5.78 | 0.895 |
| Frequency of body movement during EIS (times), median (range) | 4 (1-8) | 1 (0-7) | 0.045 |
| Patient satisfaction score, median (range) | 10 (7-10) | 10 (8-10) | 0.830 |
| Operator satisfaction score, median (range) | 7 (2-10) | 10 (5-10) | 0.016 |
| Initial SBP (mmHg), mean ± SD | 142.3 ± 27.5 | 144.1 ± 25.1 | 0.869 |
| Minimum SBP (mmHg), mean ± SD | 127.3 ± 25.1 | 112.6 ± 21.4 | 0.147 |
| Changes in minimum SBP compared with baseline (mmHg), mean ± SD | -14.9 ± 11.6 | -31.5 ± 15.6 | 0.009 |
| Initial SpO2 (%), mean ± SD | 98.7 ± 1.61 | 99.2 ± 1.08 | 0.383 |
| Minimum SpO2, (%), mean ± SD | 94.8 ± 2.77 | 94.3 ± 3.95 | 0.739 |
| Changes in minimum SpO2 compared with baseline, (%), mean ± SD | -3.92 ± 2.43 | -4.91 ± 3.08 | 0.399 |
| Initial HR (beats/min), mean ± SD | 66.8 ± 6.17 | 65.2 ± 9.25 | 0.635 |
| Minimum HR (beats/min), mean ± SD | 63.7 ± 5.12 | 58.1 ± 7.69 | 0.052 |
| Changes in minimum HR compared with baseline, (beats/min), mean ± SD | -3.08 ± 5.48 | -7.09 ± 6.96 | 0.138 |
| Adverse event, n (%) | 0 (0) | 0 (0) | - |
SD: standard deviation, EO: ethanolamine oleate, EIS: endoscopic injection sclerotherapy
SBP: systolic blood pressure, SpO2: oxygen saturation, HR: heart rate
In terms of cardiorespiratory dynamics, there were no differences in the minimum values of SBP, SpO2 and HR during EIS. Changes in the minimum SBP compared with baseline in the Propofol group were significantly higher than those in the Midazolam group (P = 0.009, Table 3). However, none of the patients experienced adverse events. EIS was safely performed in all cases when sedative administration was performed by physicians who were dedicated to sedation with an expertise in anesthesia.
Discussion
To the best of our knowledge, this study is the first trial to compare propofol-based sedation with midazolam-based sedation for EIS in patients with liver cirrhosis. This study showed that exacerbation of MHE at 2 hours after EIS appears in not only the Midazolam group but also in the Propofol group.
Liver dysfunction prolongs the elimination half-life of midazolam because midazolam is predominantly metabolized in the liver, and liver dysfunction impairs first-pass clearance15). Therefore, previous studies have reported that sedation using midazolam in patients with liver cirrhosis caused the deterioration of NCT scores21-23). Nevertheless, sedation using midazolam in patients without liver disease did not cause such deterioration19,20). However, propofol is quickly metabolized, and no adjustments in dosage are needed in patients with liver dysfunction16,17). In previous reports, sedation using propofol did not exacerbate MHE in patients with liver cirrhosis19-23), unlike sedation using midazolam21-26). However, in these reports, EGD or EVL, which have shorter procedural times (< 10 min), were performed16-25). Riphaus et al.21) conducted a randomized controlled study showing that the use of propofol sedation for EGD in patients with liver cirrhosis does not cause the deterioration of NCT scores at 2 hours after endoscopic procedures, unlike midazolam sedation. Riphaus showed that the mean procedure times were 9.5 versus 9.8 minutes21). Unlike these reports, EIS is a relatively long procedure that requires relatively large amounts of sedative agents. In addition, patients with EVs that required EIS were in a state of decompensated liver cirrhosis30). Based on the results of our study, in patients with decompensated liver cirrhosis, sedation using relatively large amounts of propofol exacerbates MHE. On the other hand, our study showed that NCT scores at 24 hours after EIS recovered to baseline scores in both groups; however, the NCT was performed only 1-2 hours after endoscopic procedures in previous reports19-25).
In terms of treatment outcomes, there was no significant difference in the technical success rate between both groups in this study. However, the frequency of body movement during EIS was significantly lower, and operator satisfaction was significantly higher in the Propofol group compared to the Midazolam group. Decreases in patient body movement due to the continuous infusion of propofol lead to a reduction in operator stress. In this study, all EIS procedures were performed by a single expert physician with extensive experience in EIS. If EIS will be performed by a non-expert physician with less experience, then propofol-based sedation may contribute to improved technical success due to the prevention of patient body movement.
This study has several limitations. First, in contrast to previous studies19-23), we used flumazenil as a reversal agent for the Midazolam group after the completion of EIS because we have used flumazenil routinely in all sedations with midazolam for EIS. Flumazenil has a half-life of 50 minutes, which is shorter than the half-life of midazolam28). However, flumazenil may contribute to the suppression of the duration of the effect of midazolam. Therefore, the use of flumazenil indeed affected the results of this research. Second, this study was conducted at a single institution. Third, we used the NCT to evaluate MHE because the NCT can be easily and repeatedly performed at the patient’s bedside with little burden. However, the mean OAAS score 2 hours after EIS was 2 in both groups, indicating that patients were somnolent/drowsy. The deterioration of the NCT score may be due to not only exacerbation of MHE but also delayed recovery from sedation. Finally, the operator and assistant were not blinded to the sedative agent. Thus, there was bias with regard to the treatment outcome and operator satisfaction.
In conclusion, propofol-based sedation caused the exacerbation of MHE after EIS like midazolam-based sedation in patients with liver cirrhosis. However, propofol-based sedation provided stable sedation with a lower frequency of body movement and higher operator satisfaction compared with midazolam-based sedation.
Conflicts of Interest
The authors declare that there are no conflicts of interest in this study.
Acknowledgments
We express our gratitude to all of the endoscopy medical staff in Fukushima Medical University Hospital for their collaboration and assistance with the endoscopic procedures.
References
- 1.Miyaaki H, Ichikawa T, Taura N, Miuma S, Isomoto H, Nakao K. Endoscopic management of esophagogastric varices in Japan. Ann Transl Med, 2: 42, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuki M, Kazumori H, Yamamoto S, Shizuku T, Kinoshita Y. Prognosis following endoscopic injection sclerotherapy for esophageal varices in adults: 20-year follow-up study. Scand J Gastroenterol, 43: 1269-1274, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology, 133: 675-701, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Diab FH, King PD, Barthel JS, Marshall JB. Efficacy and safety of combined meperidine and midazolam for EGD sedation compared with midazolam alone. Am J Gastroenterol, 91: 1120-1125, 1996. [PubMed] [Google Scholar]
- 5.McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest. Endosc, 67: 910-923, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Riphaus A, Gstettenbauer T, Frenz MB, et al. Quality of psychomotor recovery after propofol sedation for routine endoscopy: a randomized and controlled study. Endoscopy, 38: 677-683, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Tohda G, Higashi S, Wakahara S, Morikawa M, Sakumoto H, Kane T. Propofol sedation during endoscopic procedures: safe and effective administration by registered nurses supervised by endoscopists. Endoscopy, 38: 360-367, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Sipe BW, Rex DK, Latinovich D, et al. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest. Endosc, 55: 815-825, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Jung M, Hofmann C, Kiesslich R, Brackertz A. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy, 32: 233-238, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Kiriyama S, Gotoda T, Sano H, et al. Safe and effective sedation in endoscopic submucosal dissection for early gastric cancer: a randomized comparison between propofol continuous infusion and intermittent midazolam injection. J Gastroenterol, 45: 831-837, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Kanto J, Gepts E. Pharmacokinetic implications for the clinical use of propofol. Clin Pharmacokinet, 17: 308-326, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Faigel DO, Baron TH, Goldstein JL, et al. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest. Endosc, 56: 613-617, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Skues MA, Prys-Roberts C. The pharmacology of propofol. J Clin Anesth, 1: 387-400, 1989. [DOI] [PubMed] [Google Scholar]
- 14.McGuire BM. Safety of endoscopy in patients with end-stage liver disease. Gastrointest. Endosc. Clin N Am, 11: 111-130, 2001. [PubMed] [Google Scholar]
- 15.Allonen H, Ziegler G, Klotz U. Midazolam kinetics. Clin Pharmacol Ther, 30: 653-661, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Thuluvath PJ. Toward safer sedation in patients with cirrhosis: have we done enough ? Gastrointest. Endosc, 70: 269-271, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Riphaus A, Wehrmann T, Weber B, et al. [S3-guidelines—sedation in gastrointestinal endoscopy]. Z. Gastroenterol, 46: 1298-1330, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis, 19: 253-267, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Assy N, Rosser BG, Grahame GR, Minuk GY. Risk of sedation for upper GI endoscopy exacerbating subclinical hepatic encephalopathy in patients with cirrhosis. Gastrointest. Endosc, 49: 690-694, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Vasudevan AE, Goh KL, Bulgiba AM. Impairment of psychomotor responses after conscious sedation in cirrhotic patients undergoing therapeutic upper GI endoscopy. Am J Gastroenterol, 97: 1717-1721, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Riphaus A, Lechowicz I, Frenz MB, Wehrmann T. Propofol sedation for upper gastrointestinal endoscopy in patients with liver cirrhosis as an alternative to midazolam to avoid acute deterioration of minimal encephalopathy: a randomized, controlled study. Scand J Gastroenterol, 44: 1244-1251, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Khamaysi I, William N, Olga A, et al. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: a randomized controlled study. J Hepatol, 54: 72-77, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A, Sharma BC, Sharma P, Uppal R, Sarin SK. Randomized controlled trial for endoscopy with propofol versus midazolam on psychometric tests and critical flicker frequency in people with cirrhosis. J Gastroenterol Hepatol, 27: 1726-1732, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, Singh S, Sharma BC, et al. Propofol sedation during endoscopy in patients with cirrhosis, and utility of psychometric tests and critical flicker frequency in assessment of recovery from sedation. Endoscopy, 43: 400-405, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Amorós A, Aparicio JR, Garmendia M, Casellas JA, Martinez J, Jover R. Deep sedation with propofol does not precipitate hepatic encephalopathy during elective upper endoscopy. Gastrointest. Endosc, 70: 262-268, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Mao W, Wei XQ, Tao J, Zhen FP, Wen ZF, Wu B. The safety of combined sedation with propofol plus fentanyl for endoscopy screening and endoscopic variceal ligation in cirrhotic patients. J Dig Dis, 15: 124-130, 2014. [DOI] [PubMed] [Google Scholar]
- 27.The Japan Society for Portal Hypertension. The Rules for Study of Portal Hypertension. 3rd ed. Tokyo: Kanehara Co., 2013 [Google Scholar]
- 28.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology, 96: 1004-1017, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol, 34: 768-773, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet, 371: 838-851, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]