Abstract
The present study evaluated the effects of limestone particle size and Ca concentration on apparent ileal digestibility (AID) of P and Ca in the presence or absence of a 6-phytase derived from Buttiauxella sp., expressed in Trichoderma. Treatment diets were corn-soybean meal (SBM) based with no added inorganic Ca or P. The design consisted of a factorial arrangement of 2 particle sizes of limestone from the same source (mean particle size: pulverized, PUV < 75 μm; particulate, PAR = 402 μm); 3 Ca (0.6, 0.8, and 1.0%) and 2 phytase levels (0 and 1,000 (FTU)/kg) plus the corn-SBM basal diet with no added Ca (0.14% Ca), resulting a total of 13 treatments (Trts, n = 9, 3 birds/n). Starting at 27 d of age, broilers were fed the mash diets for 32 h, then sampled for gizzard pH and distal ileal digestibility. Gizzard pH was 1.94 in birds fed diet without added Ca and was similar to those fed diet with PAR limestone regardless of phytase. Gizzard pH was increased in birds fed PUV limestone diets irrespectively of phytase inclusion compared to birds fed 0.14% Ca (P < 0.05), except in birds fed 0.60% Ca diet. In the absence of phytase, AID P was reduced as a result of increases in limestone inclusion (P < 0.05), with a greater effect seen in PUV as compared to PAR limestone. The AID Ca in birds fed PUV limestone diets was lower than that of those fed PAR limestone diets (22.1% vs. 28.2%; P < 0.05). In the presence of phytase, the AID P was not affected by increasing Ca concentration when PAR limestone was used as the Ca source (average = 64.3%), whereas AID P decreased from 66.9% (0.6% Ca) to 51.0% (1.0% Ca) when PUV limestone was used as the Ca source (P < 0.05). In summary, impact of Ca concentration on AID Ca and P was dependent on limestone particle size and phytase inclusion.
Keywords: limestone particle size, calcium, phosphorus, phytase, broiler
INTRODUCTION
Practical plant seed-based poultry diets are normally deficient in Ca and require the addition of concentrated Ca sources to meet the Ca requirement for growth. Among all the Ca sources, limestone, a naturally occurring mineral primarily composed of CaCO3, is the predominant Ca supplement used in broiler production worldwide. However, considerable variations in limestone Ca solubility and Ca concentration have been reported even within the same country or region (Shih et al., 2000; Sa and Boyd, 2017) and preference of limestone particle size differs among countries/regions. In addition to its nutritional property, limestone is also widely used as flow agent (e.g., premixes and feed ingredients) and diluting material (e.g., ionophores, premixes, enzymes, and antibiotics) in the feed industry, of which the Ca is usually not accounted for in the feed formulation.
It is well documented that Ca has a detrimental impact on phytate P (PP) utilization in broilers, mainly through the formation of Ca-PP complexes (Tamim and Angel, 2003; Rutherfurd et al., 2012), especially when high Ca with severely deficient non-phytate P (nPP) diets are fed (Qian et al., 1996). Even though several studies have been published where the impact of Ca particle size on P utilization was evaluated, results have not been conclusive. This could be partially due to the confounding effect from either source differences in Ca (limestone, egg shell, etc.) and/or origin of the different limestones being tested (Anderson et al., 1984; Guinotte and Nys, 1991; Zhang and Coon, 1997a; Saunders-Blades et al., 2009) as well as particle size of the Ca used.
In broiler production, microbial phytase has been used widely as an effective approach to improve diet PP utilization. However, decreased phytase efficacy has been reported with increasing Ca concentration (Sebastian et al., 1996; Tamim et al., 2004; Plumstead et al., 2008; Walk et al., 2012; Amerah et al., 2014). Because different particle sizes of limestone are extensively used in feed manufacturing for its nutritional and non-nutritional properties, it is critical to better understand how Ca from limestone affects P digestibilities and whether and how much does particle size mediate these effects. In addition, because Ca needs to be solubilized to be reactive, and source and particle size of limestone has been shown to have profound effect on its solubility (Shih et al., 2000; Sa and Boyd, 2017), it is also important that the particle size effect is determined by using limestone from the same batch to avoid confounding effect of limestone source.
Therefore, the objective of current study was to determine the impact of limestone particle size and Ca concentration on apparent P and Ca digestibility in the presence and absence of phytase.
MATERIALS AND METHODS
Animal and Housing
All animal care procedures were approved by the University of Maryland Animal Care and Use Committee. Three hundred and fifty broiler hatchlings (Hubbard male 99 M x Cobb 500F, straight run) were purchased from a local hatchery (Allen Harim Farms, LLC. Seaford, DE). On arrival, birds were placed in temperature and light controlled floor pen rooms and allowed ad libitum access to feed and water. Birds were fed a commercial type corn-soybean meal (SBM)-based starter diet that met all NRC recommendations (National Research Council., 1994) as well as average nutrient usage concentrations in the USA for 2011 (AgriStats, 2012) from hatch to 26 d of age (1% Ca, 0.45% nPP, 22.5% crude protein, 1.20% digestible Lys, and 3,050 Kcal/kg MEn). On day 26, all birds were grouped (3 birds/pen), to minimize pen weight variation as well as within pen bird weight variations, and moved to battery pens (Petersime Incubator Co., Gettysburg, OH) previously assigned to treatments (Trt) for new environment acclimation and fed the same starter diet until day 27. On day 27, experimental diets were provided to the birds for ad libitum consumption for 32 h. Treatments were assigned such that each of 4 battery rooms was a block and each Trt was replicated at least twice in each room. The wire-floored battery pens (width × depth × height; 99.7 cm × 68.6 cm × 29.2 cm) were equipped with a water nipple system (2 nipples per pen) and 2 feed troughs (width × depth × height; 63.5 cm × 8.9 cm × 5.67 cm). Photoperiod was 24 light (L):0 dark (D) from hatch to 3 d, 14L:10D from 4 to 7 d, 16L:8D from 8 to 12 d, and 18L:6D from 13 to 28 d of age. Room temperature was kept at an average of 31°C from hatch to 3 d and was lowered by 1°C every 3 to 4 d up to 21°C and maintained until 28 d. Mortality was checked twice a day.
Preparation of different particle size limestone
A large batch of commercially used limestone (defined as particulate, PAR) was purchased from Irving Materials, Inc. (#20, IMI Cal Pro, IN). A subsample was taken from each 25 kg bag, well mixed, and subsampled for particle size determination. The PAR limestone, determined by ANSI/ASAE method S319.4 (2008), had a mean diameter (dgw) of 402 μm, with 255 μm geometric SD (Sgw) with distribution shown in Figure 1. An aliquot of 1 kg of the PAR limestone was carefully subsampled, and then ground (IKA®, Model 211 basic, Germany) to pass through a 75 μm sieve to prepare the pulverized (PUV) limestone sample.
Figure 1.
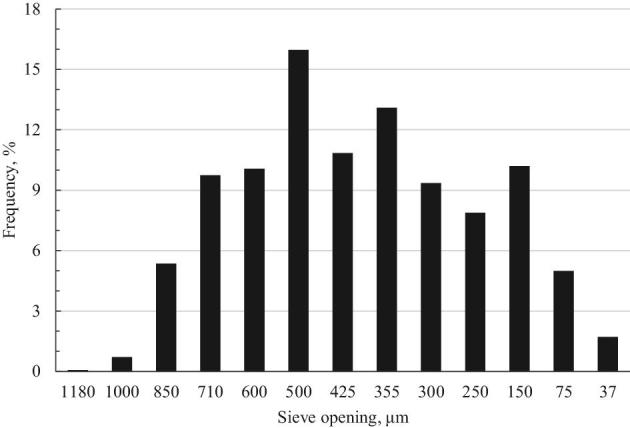
Particle size distribution of particulate limestone. Analyzed Ca concentration: 36.5%
Experimental Design and Diets
A corn and SBM mash basal diet (Table 1) was mixed in one batch and analyzed for dry matter, minerals, crude protein, ether extract, and amino acids. The basal was subdivided equally into 13 batches, such that it was represented equally in all final diets. Based on the analyzed Ca and P concentrations in the basal diet, pre-analyzed limestone, either PAR (dgw = 402 μm) or PUV (dgw < 75 μm), was added to the basal (94% basal inclusion in all Trt diets) to achieve the desired Ca concentrations in the final diets. Titanium dioxide (TiO2) was added as an inert marker at 0.3% and celite (Celite®, Celite Corp, CA) was included as a filler to achieve 100%. The final dietary arrangement consisted of 13 Trt, of which 12 Trt resulted from a 3 Ca concentration (0.60, 0.80, and 1.0%) × 2 limestone particle size × 2 phytase dose (0 and 1,000 (FTU)/kg) factorial arrangement, plus 1 low Ca diet containing 0.14% Ca with no added phytase. To prepare the final diets with phytase, 1 batch of the previously aliquoted basal was used to make each Ca concentration and limestone combination and divided into 2 equal lots to minimize potential variability. A 6-Phytase (Danisco Animal Nutrition, DuPont Industrial Biosciences, Marlborough, UK) (Axtra® PHY, Buttiauxella sp., expressed in Trichoderma reesei) was then added on top, at 0, and 1,000 FTU/kg, to 1 of the 2 lots and mixed so that the only difference among those 2 lots was the phytase concentration. Each Trt was replicated 9 times with each replicate having 3 birds.
Table 1.
Ingredient and chemical composition of the basal diet.
| Ingredient | Basal (%, as-fed basis) |
|---|---|
| Corn | 58.67 |
| Soybean meal (48% CP) | 34.58 |
| Soy oil | 5.25 |
| Salt | 0.52 |
| DL-Methionine, 99% | 0.29 |
| Biolys, 55% | 0.28 |
| L-Threonine, 98.5% | 0.08 |
| Choline chloride, 25% | 0.17 |
| Mineral Premix1 | 0.08 |
| Vitamin Premix2 | 0.08 |
| Total | 100.00 |
| Formulated (analyzed) concentrations (%) (mean ± SD)3 | |
| MEn, kcal/kg | 3217 |
| Crude protein | 21.82 (21.08) |
| Lysine | 1.31 (1.4) |
| Digestible lysine | 1.20 |
| Methionine + cysteine | 0.99 (0.97) |
| Calcium (Ca) | 0.14 (0.29 ± 0.01) |
| Total phosphorus (tP) | 0.42 (0.39 ± 0.00) |
| Phytate phosphorus (PP) | 0.30 (0.25 ± 0.01) |
| Non-phytate phosphorus (nPP)4 | 0.12 (0.14) |
1Supplied per kilogram of diet: zinc from zinc sulfate, 80 mg; manganese from manganese sulfate, 100 mg; iron from iron sulfate, 20 mg; copper from copper sulfate, 3 mg; iodine from calcium iodate, 3.9 mg; selenium from selenium sulfate, 0.3 mg.
2Supplied per kilogram of diet: vitamin A, 15,111 IU; vitamin D, 5,333 IU; vitamin E, 53.33 IU; vitamin B12, 26.66 mg; riboflavin, 17.78 mg; niacin, 71.11 mg; pantothenic acid, 24.89 mg; vitamin K3, 3.2 mg; folic acid, 2.13 mg; biotin, 0.142 mg; thiamine, 4.44 mg; pyridoxine, 6.22 mg.
3Crude protein, amino acids, and PP were analyzed in duplicate; Ca and tP were analyzed in triplicate.
4Concentration determined based on analyzed tP minus analyzed PP.
Sample Collection and Lab Analysis
After feeding the experimental diets for 32 h, all birds were euthanized by cervical dislocation and gizzard as well as distal half of the ileum, immediately (within 20 s of cervical dislocation) removed by experienced technicians to minimize potential digesta movements. A pH electrode (A57187, Beckman Coulter, Brea, CA) was introduced into 3 areas of the gizzard, and the average of the 3 measures was used as the mean pH per bird. The mean pH from the 3 birds per replicate was used as the replicate mean. The distal half of the ileum (last half of the segment encompassed between the Meckel's diverticulum and the ileocecal junction) was removed and the content was gently expressed by flushing with deionized distilled water. Distal ileal contents were pooled by pen, frozen at –20°C, and freeze dried. Dried ileal samples were ground using a mortar and pestle to pass through a 0.25 mm sieve and stored in airtight containers at 4°C until analyzed.
Diet samples were ground to pass through a 0.5 mm screen before analysis. Diet and ileal samples were analyzed in duplicate except where specified otherwise. Dry matter was determined by drying overnight in a 100°C oven (Shreve et al., 2006), Ca and P were determined, in triplicate, after acid digestion and analyzed using inductively coupled plasma atomic emission spectrometry (AOAC, 1999). Titanium (Ti) concentration was determined by a colorimetric method adopted from Short et al. (1996) where samples were first ashed at 580°C and then digested in 7.4 M H2SO4. Crude protein and ether extract in the basal diet were analyzed, according to AOAC methods 990.03 (2003) and 920.39 (2003), respectively. Amino acids, except Trp (AOAC method 994.12, 2003), and PP (Ellis et al., 1977) in the basal diet were also analyzed. Phytase activities in all Trt diets were determined according to the ISO 30024 (2009) procedure where 1 FTU is the amount of enzyme that releases 1 μmol of inorganic orthophosphate from a sodium phytate substrate per minute at pH 5.5 and 37°C.
In vitro Limestone Solubility
In vitro solubilities of PAR and PUV limestone were determined by a modified weight loss method according to Zhang and Coon (1997b). Briefly, 50 mL of 0.2 N HCl solution (pH = 0.7) was mixed with 0.5 g of limestone (PAR or PUV) in a 125 mL flask and incubated in a shaking water bath (42°C) for digestion. At 1, 3, 5, 7.5, and 10 min of incubation, 3 flasks from each limestone particle size were sampled, contents were vacuum filtered through a 2.5 μm filter paper (WhatmanTM, GE Healthcare Bio-Sciences, PA), dried at 100 °C for 24 h, and weighed to calculate the weight loss.
Calculations
The apparent ileal digestibility (AID) of nutrients was calculated based on the following formula using TiO2 as the inert marker (IM):
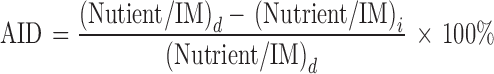 |
where (Nutritent/IM)d is the ratio of nutrient (Ca, P, AA, CP) to IM in the diet and (Nutrient/IM)i is the ratio of nutrient (Ca, P, AA, CP) to IM in the ileal contents.
Digestible P was calculated as follows:
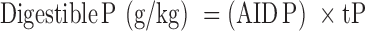 |
where tP is the analyzed total P concentration in the diet.
Net improvement of digestible P (DigP) as a result of phytase inclusion was calculated as follows:
 |
where DigPPhy and DigPnon-phy are the DigP in birds fed the phytase and non-phytase Trt, respectively, at the same Ca concentration.
Statistical Analysis
Data were analyzed using the MIXED procedure of SAS (SAS Institute Inc., 2008). Treatment was considered as a fixed effect and pen (flask) as a random effect. Dunnett's test (1955) was used to compare the Trt effect by assigning the Trt with no added Ca (0.14%) as the “control.” Contrast was used to compare the phytase effect between birds fed the same Ca and limestone source diet with and without phytase. Data from feeding Trt resulting from the factorial arrangement of 3 Ca (0.6, 0.8, and 1.0%) concentrations, 2 limestone particle sizes (PUV and PAR), and 2 phytase (0 and 1,000 FTU/kg) doses were tested for main effects and their interactions using 2 and 3-way ANOVA. Tukey's test (Tukey, 1949) was used to separate means when the model was significant. Significance was declared at P < 0.05.
RESULTS AND DISCUSSION
Analyzed Ca was higher than formulated in the basal diet (0.29 vs. 0.14%; Table 1), and analyzed concentrations of Trt Ca and phytase were all close to formulated values (Table 2). Phytase activities in non-phytase Trt were all below the detection limit, suggesting no cross-contamination among phytase and non-phytase Trt. Analyzed phytase activities in phytase Trt were within 12% of formulated values (Table 2).
Table 2.
Analyzed Ca and phytase activity (mean ± SD) in final diets (as is basis).1
| Ca, % | Phytase, FTU/kg | |||
|---|---|---|---|---|
| Limestone particle size | Fml2 | Ana2 | Fml2 | Ana2 |
| Basal | 0.14 | 0.27 ± 0.02 | 0 | <50 |
| Pulverized (dgw < 75 μm) | 0.60 | 0.60 ± 0.04 | 0 | <50 |
| 1,000 | 1090 ± 56 | |||
| 0.80 | 0.80 ± 0.06 | 0 | <50 | |
| 1,000 | 1110 ± 56 | |||
| 1.00 | 0.93 ± 0.02 | 0 | <50 | |
| 1,000 | 1145 ± 77 | |||
| Particulate (dgw = 402 μm) | 0.60 | 0.58 ± 0.03 | 0 | <50 |
| 1,000 | 1090 ± 28 | |||
| 0.80 | 0.78 ± 0.02 | 0 | <50 | |
| 1,000 | 1120 ± 26 | |||
| 1.00 | 0.91 ± 0.01 | 0 | <50 | |
| 1,000 | 1095 ± 7 | |||
1Diet Ca, P, and phytase concentrations for each treatment were analyzed in triplicate. All diets contained 0.13% non-phytate P.
2Fml, formulated concentrations; Ana, analyzed concentrations.
In vitro solubility of PUV limestone showed higher solubility than PAR limestone at all time points (P < 0.05, Table 3). More than 90% PUV limestone was solubilized within 1 min of incubation, whereas only 36.4% PAR limestone was solubilized (P < 0.05). Solubilized PAR limestone increased with time, and 89.4% PAR limestone was dissolved in the solution after 10 min of incubation. The 2 limestone particle sizes came from the same 1 kg of limestone that was quartered into 2 equal portions, and all of one portion ground to pass through a 75 μm sieve, they were exactly the same limestone with the only difference being particle size. Thus, the difference in solubility found in this trial has to be related only to particle size and not composition of the limestone or type of limestone source.
Table 3.
In vitro solubility of particulate and pulverized limestone (n = 3).
| Timepoint, min | Particulate1 | Pulverized1 | SEM | P-value |
|---|---|---|---|---|
| 1 | 36.4 | 94.6 | 1.80 | <0.001 |
| 3 | 61.6 | 95.5 | 0.68 | <0.001 |
| 5 | 74.3 | 96.9 | 0.71 | 0.002 |
| 7.5 | 82.3 | 97.6 | 1.75 | 0.025 |
| 10 | 89.4 | 99.1 | 0.17 | <0.001 |
1Particulate limestone: dgw = 402 μm, pulverized limestone: dgw < 75 μm.
In animal trials, due to 3-way interactions seen in AID Ca and P, to better illustrate the interaction between Ca and limestone particle size, all data were further analyzed using a 2-way ANOVA by separating the phytase effect.
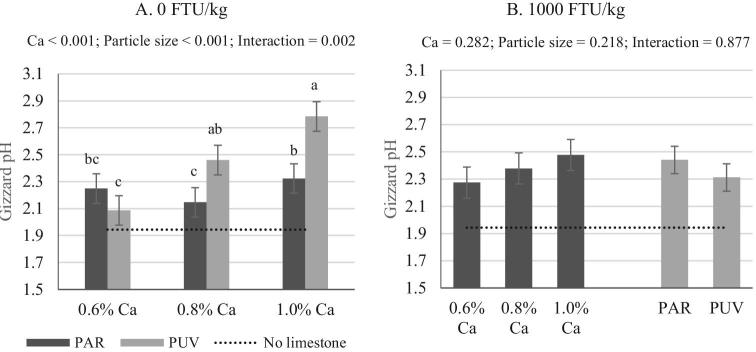
Gizzard pH ranged from 1.94 in birds fed diets containing no added limestone and 2.78 in those fed 1.0% Ca diet with PUV limestone (Table 4) but were all within the range reported in poultry (González-Alvarado et al., 2008). Impact of Ca concentration on gizzard pH varied and was dependent on limestone particle size and phytase (Figure 2 and Table 4). In broilers fed PAR limestone diets, there was no difference in gizzard pH irrespectively of Ca concentration in the final diet compared to those fed no added limestone diet regardless of phytase presence (average = 2.26; Table 4). However, when PUV limestone was used as the Ca source, increased gizzard pH was seen as Ca concentration increased from 0.14 (0.27% as analyzed) to 1.0%, in the presence or absence of phytase. Because the birds were fed diets containing the same Ca concentration, with only difference being limestone particle size, the pH increase seen in birds fed PUV limestone is an indication of greater amount of CaCO3 dissolved in the gizzard as shown previously from the in vitro solubility results (Table 3).
Table 4.
Effects of Ca and phytase concentrations on gizzard pH, apparent ileal Ca and P digestibilities (AID Ca and AID P) of broilers at 28 d of age.1
| Phytase2, FTU/kg | Ca2, % | Particle size3 | Gizzard pH | AID P4, % | AID Ca4, % |
|---|---|---|---|---|---|
| 0 | 0.14 | – | 1.94 | 54.1 | 40.2 |
| 0 | 0.60 | Pulverized | 2.09 | 23.55 | 38.1 |
| Particulate | 2.25 | 31.55 | 44.8 | ||
| 0.80 | Pulverized | 2.465 | 20.55 | 47.3 | |
| Particulate | 2.15 | 27.95 | 50.85 | ||
| 1.00 | Pulverized | 2.785 | 22.25 | 46.5 | |
| Particulate | 2.32 | 25.35 | 42.9 | ||
| 1000 | 0.60 | Pulverized | 2.30 | 66.85,6 | 53.45,6 |
| Particulate | 2.21 | 64.25,6 | 50.95 | ||
| 0.80 | Pulverized | 2.415 | 57.26 | 53.95,6 | |
| Particulate | 2.31 | 62.85,6 | 50.85 | ||
| 1.00 | Pulverized | 2.565 | 50.96 | 51.55,6 | |
| Particulate | 2.36 | 64.95,6 | 56.65,6 | ||
| SEM | 0.115 | 1.98 | 2.59 | ||
| P-values | <0.001 | <0.001 | <0.001 | ||
| Factorial analysis7 | |||||
| Ca | 0.001 | <0.001 | 0.079 | ||
| Particle size | 0.009 | <0.001 | 0.476 | ||
| Phytase | 0.815 | <0.001 | <0.001 | ||
| Ca × Particle size | 0.055 | 0.106 | 0.844 | ||
| Ca × Phytase | 0.445 | 0.374 | 0.078 | ||
| Phytase × Particle size | 0.554 | 0.821 | 0.394 | ||
| Ca × Particle size × Phytase | 0.186 | 0.001 | 0.024 | ||
1n = 9, 3 birds/n.
2Formulated concentrations and analyzed concentrations are shown in Table 2.
3Particulate limestone: dgw = 402 μm, pulverized limestone: dgw < 75 μm
4AID Ca or P was calculated as: AID % = [(Min/TiO2)d—(Min/TiO2)i]/(Min/TiO2)d × 100%. (Min/TiO2)d is the ratio of minerals (Ca or P) to TiO2 in the diet and (Min/TiO2)i is the ratio of minerals (Ca or P) to TiO2 in the ileal digesta.
5Denotes difference to no limestone added treatment with 0.14% Ca (P < 0.05, Dunnett's test).
6Denotes difference to non-phytase diets with same Ca and limestone particle size (contrast, t-test).
7Factorial analysis was conducted by excluding 0.14% Ca treatment.
Figure 2.
Impact of Ca concentration (formulated) and limestone particle size on gizzard pH with no phytase (A) or 1,000 FTU/kg (B) phytase inclusion (mean ± SEM). Comparisons were made across treatments (A) when interaction was significant (P < 0.05) or within a main effect when no interaction was seen (B). pH value from birds fed no added limestone diet (0.14% Ca, analyzed 0.27%) was provided as a reference. Analyzed Ca concentrations are shown in Table 2. PAR, particulate limestone (dgw = 402 μm); PUV, pulverized limestone (dgw < 75 μm).
Significant pH increase was seen in broilers fed the 0.8 and 1.0% Ca diets with PUV as compared to those fed the no added limestone diet (P < 0.05; Table 4). There were no differences in gizzard pH detected between birds fed the diets containing the PAR limestone regardless of amount versus birds fed the no added calcium diet. Solubility of Ca source is highly correlated with particle size, where increased solubility was reported to be associated with decreased particle size (Zhang and Coon, 1997a; Walk et al., 2012), which is also corroborated in this research (Table 4). The higher impact of Ca concentration on gizzard pH seen in birds fed diet containing PUV is therefore partly due to more rapid and higher solubilization of PUV as compared to PAR in vitro. There was no phytase effect on gizzard pH seen in this trial. In contrast, increased gizzard pH has been reported by Amerah et al. (2014), where gizzard pH was found to be 0.6 points higher (2.6 vs. 3.2) when 1,000 FTU Buttiauxella phytase/kg was added to corn-SBM-based diet with various Ca concentrations.
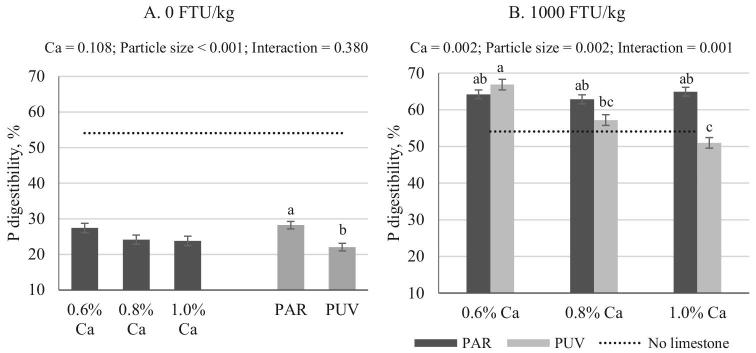
In the absence of phytase, the AID P was highest (54.1%; Table 4) when diets containing no additional limestone were fed as compared to birds fed diets with different particle sizes of limestone (P < 0.05). Similar but higher AID P (67.9%) was reported by Tamim et al. (2004) in 24-day-old broiler fed diet with no added limestone by using the same approach. Apart from the differences in animal age (22 vs. 28), and genetics (Ross 308 vs. Hubbard male 99 M x Cobb 500F), higher Ca concentration in the basal diet could also be contributing to the lower AID P seen in the current study. The corn-SBM basal diet in the Tamim et al. (2004) paper had an analyzed Ca concentration of 0.17% as compared to the basal analyzed Ca in the current trial of 0.29%.
In the absence of phytase, AID P was reduced by an average of 29 percentage points (range: 23 to 33 percentage points) when Ca from limestone was added into the diet, irrespective of Ca concentration or limestone particle size (Table 4; P < 0.05). However, the degree of AID P reduction due to increased dietary Ca was dependent on limestone particle size with the PUV limestone having a more profound negative effect on AID P (mean AID, P = 22%) as compared to PAR limestone, across all Ca concentrations (mean AID, P = 28%; Figure 3A; P < 0.05). The detrimental impact of Ca on AID P has been well established and reviewed extensively (Angel et al., 2002; Tamim et al., 2004; Selle and Ravindran, 2007; Walk et al., 2012; Li et al., 2014). For example, a 64% reduction in AID P was reported when 0.5% Ca from limestone was added to a diet as compared to that of birds fed the same diet without added limestone (Tamim et al., 2004). This is in agreement with the results from current study where a 23 and 31 percentage points reduction were seen even when only 0.33% Ca from limestone was added (0.6% minus 0.27% in the basal diet) from PAR and PUV limestone, respectively. However, despite the detrimental impact of Ca concentration on AID P (Sebastian et al., 1996; Plumstead et al., 2008; Walk et al., 2012; Amerah et al., 2014), increasing Ca from 0.6 to 1.0% did not decrease AID P regardless of limestone particle size (Figure 3A). This lack of increased Ca impact on AID P beyond 0.6%, in the absence of phytase, may be due to a saturated chelation between Ca and phytate having been reached between 0.6 and 0.8% (Ca: PP > 2.5). Because Ca interferes with AID P mainly through chelation with phytate and reduces the availability of PP (Tamim et al., 2004; Li et al., 2016), reactive Ca in the solution will interact with the phytic acid molecule. Therefore, pH in the gastric area that influences Ca solubility and phytic acid concentration that dictates the available substrate for chelation will affect the degree of Ca's impact on AID P. Increases in diet Ca to 1.0%, regardless of particle size, would therefore have no further negative impact on AID P if all available phytic acid has been chelated by Ca. Similarly, results reported by Plumstead et al. (2008) also suggest that Ca-phytate chelation was saturable, even though in their trials, the saturation point occurred at a higher Ca: PP ratio (>3.1). The results from Kim et al., (2013) are similar to those obtained in the current trial for PAR limestone, where maximum reduction on AID P was reached when Ca: PP ratio was greater than 2.9.
Figure 3.
Impact of Ca concentration (formulated) and limestone particle size on P digestibility with no phytase (A) or 1,000 FTU/kg (B) phytase inclusion (mean ± SEM). Comparisons were made across treatments (B) when interaction was significant (P < 0.05) or within a main effect when no interaction was seen (A). pH value from birds fed no added limestone diet (0.14% Ca, analyzed 0.27%) was provided as a reference. Analyzed Ca concentrations are shown in Table 2. PAR, particulate limestone (dgw = 402 μm); PUV, pulverized limestone (dgw < 75 μm).
It should be noted that the current trial adopted a short feeding period approach (32 h), which was also used by Tamim et al (2004), Kim et al. (2013), and Li et al. (2016), while the feeding length in other research was longer and usually from 7 to 21 d (Sebastian et al., 1996; Plumstead et al., 2008, Walk et al., 2012; Amerah et al., 2014). In a recent study, Li et al. (2014) compared the impact of Ca on AID P between broilers fed nPP-deficient diets for only 2 d (48 h, from 19 to 21 d of age) and 14 d (from 7 to 21 d of age), and found increased AID P in birds fed the test diets for 14 d vs. those fed the same test diet for only 2 d at the same age, irrespective of Ca. Their findings showed that when broilers were given prolonged exposure to P deficiency or Ca/P imbalance, they were capable of upregulating their capacity to digest and absorb P (Proszkowiec-Weglarz and Angel, 2013, Proszkowiec-Weglarz et al., 2013). Therefore, the detrimental impact of Ca could potentially be underestimated by feeding a P-deficient diet for periods greater than 48 h.
In the presence of 1,000 FTU phytase/kg, AID P were all significantly improved and were higher than those in bids fed diets containing no added limestone (Table 4; P < 0.05), except in birds fed diet containing 0.8 and 1.0% Ca from PUV limestone where the AID P (57.2%, 50.9%, respectively) was not different from that of birds fed 0.14% Ca diet (54.1%, P < 0.05). Unlike the lack of Ca effect or interaction seen in the absence of phytase (Figure 3A), there was an interaction between Ca and limestone particle size in the presence of phytase (Figure 3B). When PAR limestone was used as the Ca source, AID P was not affected by increased Ca concentration, with an average of 64% AID P across all Ca concentrations. However, when PUV limestone was used, AID P was reduced by 16 percentage points from 66.9 (0.6% Ca) to 50.9% (1.0% Ca; P < 0.05). Calculated phytase efficacy as net increase of digestible P was 0.16% (Figure 4) across all Ca concentrations when PAR limestone was used, whereas the increase in digestible P was 0.20, 0.17, and 0.14%, at 0.6, 0.8, and 1.0% Ca, respectively, when PUV limestone was used.
Figure 4.
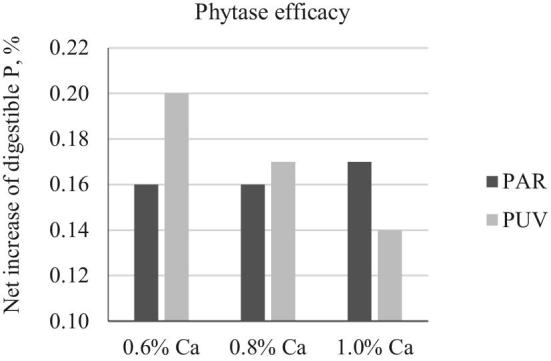
Impacts of Ca concentration and limestone particle size on phytase efficacy. Phytase efficacy was calculated as: net increase in digestible P (%) = (AID Pphy – AID Pnon-phy) × Pdiet.; AID Pphy, AID Pnon-phy, P digestibility in birds fed phytase and non-phytase diets, respectively; Pdiet, analyzed total P concentration in the diet. Values were calculated on treatment averages. PAR, particulate limestone (dgw = 402 μm); PUV, pulverized limestone (dgw < 75 μm).
Calcium forms complex with phytate regardless of pH, and complexes can remain highly soluble until pH < 3.5 (Angel et al., 2002; Tamim and Angel, 2003). In addition, chelation capacity of phytate decreases dramatically once 1 or 2 phosphate group(s) are released from the inositol ring (Persson et al., 1998; Yu et al., 2012). It is logical to conclude that: 1) the rapidity of the limestone solubility × Ca concentration in the diet will dictate the speed and concentration of Ca2+ available to be chelated by phytate in the digesta, and 2) the specific activity of phytase × dose will dictate the speed and number of phytate molecules where 2 phosphates groups that will be removed by phytase. We therefore hypothesize that in upper gastric-intestinal tract where phytate and the phytate-Ca complex remain highly soluble, if phytate chelates with Ca more quickly than the phytase can remove the first phosphate group from IP6 then the AID P and efficacy of phytase would be decreased.
The hypotheses proposed allow us to explain the discrepancies in the Ca effect between PAR and PUV limestone seen in the current study. With PUV limestone, a much higher solubility was seen as compared to that of the PAR limestone based in vitro solubility and on the gizzard pH and thus more reactive Ca would be in solution. At 0.6% Ca, even though most of the PUV Ca from limestone would be solubilized quickly in the gizzard, phytase was still able to access most of phytate molecules and remove P before chelation between Ca and IP6 occurred. In diets with PAR limestone, with slower limestone Ca solubility, phytase at the concentration used could access most the phytate molecules removing at least 1 or 2 phosphates thus decreasing the affinity of the phytate for divalent cations before enough Ca2+ would be available to fill the potential chelation sites in the phytate molecule. Therefore, PAR Ca2+ had no effect on AID P or phytase efficacy.
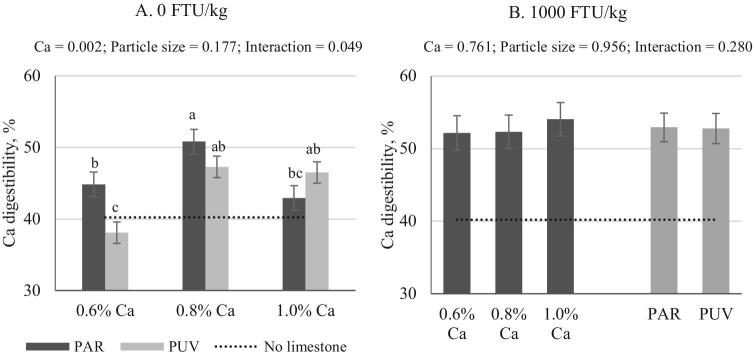
Unlike AID P, limestone per se had no effect on AID Ca in the absence of phytase (Table 4, Figure 5A). When phytase was used at 1,000 FTU/kg, no effect of Ca concentration or particle size was seen and no interaction was detected (Figure 5B). With phytase inclusion, AID Ca was significantly improved regardless of limestone particle size or Ca concentration as compared to that of birds fed diets with no added limestone (Table 4; P < 0.05). The effect of phytase varied when comparing it to birds fed the same diet without phytase, where the AID Ca was improved by 5 (1.0% Ca) to 15 (0.6% Ca) percentage points when PUV limestone was used as the Ca source (P < 0.05), while the increase due to phytase inclusion was only seen in birds fed 1.0% Ca with PAR limestone (P < 0.05).
Figure 5.
Impact of Ca concentration (formulated) and limestone particle size on Ca digestibility with no phytase (A) (mean ± SEM). Comparisons were made across treatments (A) when interaction was significant (P < 0.05) or within a main effect when no interaction was seen (B). pH value from birds fed no added limestone diet (0.14% Ca, analyzed 0.27%) was provided as a reference. Analyzed Ca concentrations are shown in Table 2. PAR, particulate limestone (dgw = 402 μm); PUV, pulverized limestone (dgw < 75 μm).
CONCLUSION
Limestone, the predominant Ca source in poultry feeds worldwide, can have particle sizes that are widely different which will impact the concentration of reactive (soluble) Ca. There is a well-documented detrimental impact of Ca on P digestibility, but the impact of limestone particle size which is highly correlated with its solubility and is a key determining factor on the degree of impact of Ca from limestone on P utilization has been less studied. In the current study, even though there was a detrimental effect of Ca on AID P in the absence of phytase, adding 1,000 FTU phytase/kg could alleviate the negative effect but only when PAR limestone was used. When limestone was provided in a PUV form, a greater impact of Ca on P digestibility was seen and reduction in P digestibility could only be partially recovered even with 1,000 FTU phytase/kg inclusion. Large variations exist between commercially used limestone sources, their particle sizes, and their solubility at low pHs. Because solubility of the limestone has such an impact on AID P, particle size of the Ca source must also be considered, in addition to total Ca concentration in the diet to properly formulate the diet.
Acknowledgements
The authors appreciate the supplying of Buttiauxella sp. phytase and partial financial support from Danisco Animal Nutrition, DuPont Industrial Biosciences.
REFERENCES
- AgriStats 2012. End of Year Summary: 2011. Agri stats, Inc, Fort Wayne, IN. [Google Scholar]
- Amerah A. M., Plumstead P. W., Barnard L. P., Kumar A.. 2014. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets1. Poult. Sci. 93:906–915. [DOI] [PubMed] [Google Scholar]
- American Society of Agricultural and Biological Engineers 2008. Method of Determining and Expressing Fineness of Feed Materials by Sieving (ANSI/ASAE S319.4). ASABE; St. Joseph, MI. [Google Scholar]
- Anderson J. O., Dobson D. C, Jack O. K.. 1984. Effect of particle size of the calcium source on performance of broiler chicksfed diets with different calcium and phosphorus levels. Poult. Sci. 63:311–316. [DOI] [PubMed] [Google Scholar]
- Angel R., Tamim N. M., Applegate T. J., Dhandu A. S., Ellestad L.E.. 2002. Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 11:471–480. [Google Scholar]
- Association of Official Analytical Chemists 1999. Macro and micro elements in plant tissues by ICP-AES. Pages 110–116 in Analytical Techniques for Inorganic Contaminants. Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Association of Official Analytical Chemists AOAC method 920.39 2003. Official Methods of Analysis. 17th ed, 2nd rev Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Association of Official Analytical Chemists AOAC method 990.03 2003. Official Methods of Analysis. 17th ed, 2nd rev Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Association of Official Analytical Chemists AOAC method 994.12 2003. Official Methods of Analysis. 17th ed, 2nd rev Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Dunnett C. W. 1955. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50:1096–1121. [Google Scholar]
- Ellis R., Morris E.R., Philpot C.. 1977. Quantitative determination of phytate in the presence of high inorganic phosphate. Anal. Biochem. 77:536–539. [DOI] [PubMed] [Google Scholar]
- González-Alvarado J. M., Jiménez-Moreno E., Valencia D. G., Lázaro R., Mateos G. G.. 2008. Effects of fiber source and heat processing of the cereal on the development of pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult. Sci. 87:1779–1795. [DOI] [PubMed] [Google Scholar]
- Guinotte F., Nys Y.. 1991. The effects of particle size and origin of calcium carbonate on performance and ossification characteristics in broiler chicks. Poult. Sci. 70:1908–1920. [DOI] [PubMed] [Google Scholar]
- ISO 30024 2009. Animal feeding stuffs – Determination of phytase activity.
- Kim S.-W., Angel R., Li W., Jiménez-Moreno E., Proszkowiec-Weglarz M., Plumstead P.W.. 2013. Impacts of dietary Ca on P digestibility in broilers fed diets with or without a 6-phytase from Buttiauxella sp. Poult. Sci. 92(E-Suppl. 1.):148. [Google Scholar]
- Li W., Angel R., Kim S.-W., Brady K., Yu S., Plumstead P. W.. 2016. Impacts of dietary calcium, phytate, and nonphytate phosphorus concentrations in the presence or absence of phytase on inositol hexakisphosphate (IP6) degradation in different segments of broilers digestive tract. Poult. Sci. 95:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Angel R., Kim S.-W., Jiménez-Moreno E., Proszkowiec-Weglarz M., Plumstead P. W.. 2014. Age and adaptation effects to Ca and P deficiencies: Effect on P digestibility. Poult. Sci. 93(E-Suppl. 1):84. [DOI] [PubMed] [Google Scholar]
- National Research Council , 1994. Nutrient Requirements of Poultry. 9th rev. ed National Academy of Science, Washington, DC. [Google Scholar]
- Persson H., Turk M., Nyman M., Sandberg A-S.. 1998. Binding of Cu2+, Zn2+, and Cd2+ to inositol tri-, tetra-, penta-, and hexaphosphates. J. Agric. Food Chem. 46:3194–3200. [Google Scholar]
- Plumstead P. W., Leytem A. B., Maguire R. O.. 2008. Interactionof calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult. Sci. 87:449–458. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Angel R., Jimenez-Moreno E., Kim S.-W., Miska K., Plumstead P. W.. 2013. Method development to determine digestible calcium and phosphorus in single ingredients for poultry 2: Impact of time and diet Ca and P deficiencies on their digestibility. Poult. Sci. 92(E-Suppl. 1):149. [Google Scholar]
- Proszkowiec-Weglarz M., Angel R.. 2013. Calcium and phosphorus metabolism in broilers: Effect of homeostatic mechanism on calcium and phosphorus digestibility1. J. Appl. Poul. Res. 22:609–627. [Google Scholar]
- Qian H., Kornegay E. T., Conner D. E. Jr. 1996. Adverse effects of wide calcium: phosphorus ratios on supplemental phytase efficacy for weanling pigs fed two dietary phosphorus levels. J. Anim. Sci. 74:1288–1297. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S. M., Chung T. K, Thomas D. V., Zou M. L., Moughan P. J.. 2012. Effect of a novel phytase on growth performance, apparent metabolizable energy, and the availability of minerals and amino acids in a low-phosphorus corn-soybean meal diet for broilers. Poult. Sci. 91:1118–1127. [DOI] [PubMed] [Google Scholar]
- Sa M. V. D. C. E., Boyd C. E.. 2017. Variability in the solubility of agricultural limestone from different sources and its pertinence for aquaculture. Aquacult. Res. 48:4292–4299. [Google Scholar]
- Shih S.-M., Lin J.-P., Shiau G-Y.. 2000. Dissolution rates of limestones of different sources. J. Hazard. Mater. 79:159–171. [DOI] [PubMed] [Google Scholar]
- SAS Institute 2008. Version 9.2 SAS Institute, Inc., Cary, NC, USA. [Google Scholar]
- Saunders-Blades J. L., MacIsaac J. L., Korver D. R., Anderson D. M.. 2009. The effect of calcium source and particle size on the production performance and bone quality of laying hens. Poult. Sci. 88:338–353. [DOI] [PubMed] [Google Scholar]
- Sebastian S., Touchburn S. P., Chavez E. R., Lagueë P. C.. 1996. Efficacy of supplemental microbial phytase at different dietary calcium levels on growth performance and mineral utilization of broiler chickens. Poult. Sci. 75:1516–1523. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Ravindran V.. 2007. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 135:1–41. [Google Scholar]
- Short F. J., Gorton P., Wiseman J., Boorman K. N.. 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 59:215–221. [Google Scholar]
- Shreve B., Thiex N., Wolf M.. 2006. National Forage Testing Association Reference Method: Dry Matter by Oven Drying for 3 Hours at 105°C. NFTA Reference Methods. National Forage Testing Association, Omaha, NB. [Google Scholar]
- Tamim N. M., Angel R.. 2003. Phytate phosphorus hydrolysis as influenced by dietary calcium and micro-mineral source in broiler diets. J. Agric. Food Chem. 5:4687–4693. [DOI] [PubMed] [Google Scholar]
- Tamim N. M., Angel R., Christman M.. 2004. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 83:1358–1367. [DOI] [PubMed] [Google Scholar]
- Tukey J. 1949. Comparing individual means in the analysis of variance. Biometrics. 5:99–114. [PubMed] [Google Scholar]
- Walk C. L., Bedford M. R., McElroy A. P.. 2012. Influence of diet, phytase, and incubation time on calcium and phosphorus solubility in the gastric and small intestinal phase of an in vitro digestion assay. J. Anim. Sci. 90:3120–3125. [DOI] [PubMed] [Google Scholar]
- Yu S., Cowieson A., Gilbert C., Plumstead P., Dalsgaard S.. 2012. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin1. J. Anim. Sci. 90:1824–1832. published online January 6, 2012. [DOI] [PubMed] [Google Scholar]
- Zhang B., Coon C.. 1997a. The relationship of calcium intake, source, size, solubility in vitro and in vivo, and gizzard limestone retention in laying hens. Poult. Sci. 76:1702–1706. [DOI] [PubMed] [Google Scholar]
- Zhang B., Coon C.. 1997b. Improved in vitro methods for determining limestone and oyster shell solubility. J. Appl. Poult. Res. 6:94–99. [Google Scholar]