ABSTRACT
Storage experiments were conducted to study the impacts of the environmental factors (temperature (T) (°C), relative humidity (RH) (%), and air flow velocity (VEL) (m/s)) on the hen egg quality indices and to develop kinetic model(s) for freshness prediction. VEL had negligible effect on relative weight loss (RWL). All factors had significant effect on Haugh unit (HU) but only T impacted S-ovalbumin content (SO). Fitted regression lines for the RWL and the HU had determination coefficient (R2) of 0.996 and 0.95, respectively. The HU equation reflected impacts of all factors, and the impact of temperature shift-up increases the HU decrease, where the impact decreases with RH and increases with flow velocity. Kinetic model for SO was developed using isothermal (5, 10, 20, 25, and 28.5°C) conditions and validated under dynamic (10 to 20 and 10 to 28.5°C) conditions. The accuracy and bias factor values were 1.091 and 0.917 at 10 to 20°C and 1.206 and 1.204 at 10 to 28.5°C, respectively, which indicates that the SO model performed well. The SO model can be used along with the HU model (as the HU model can reflect the combined effect of temperature, humidity, and air flow velocity) to predict hen egg freshness at 5 to 28.5°C storage condition.
Keywords: egg freshness, Haugh unit, air flow velocity, S-ovalbumin, kinetic model
INTRODUCTION
Shell eggs deteriorate in internal quality with time and this depends on the shell and internal content of the egg (Adeogun and Amole 2004). Poor storage conditions may cause deterioration of egg quality that can reduce eggs grade within a few days and consequently loss and waste of eggs may result. The principle degrading factors are high storage temperature and dehydration (Tabidi 2011). Research results by Jin et al. (2011) reported that egg weight loss, albumen pH, and Haugh unit (HU) are parameters that are greatly influenced by the storage temperature and time. Samli et al. (2005) has also reported that pH is a useful means for describing changes in albumen quality over time during storage and significant increases in yolk pH were observed with increasing storage time. Ovalbumin, the most abundant protein component in shell eggs, is gradually converted into S-ovalbumin during storage, which is an irreversible and extremely heat-stable form compared with ovalbumin, determined by a thermogram of the differential scanning calorimeter (Smith and Back 1965; Huntington et al. 1995). The formation of S-ovalbumin is affected by both pH and temperature and increases with time of storage at a rate which increased with pH (Smith and Back 1962). Huang et al. (2012b) also reported that S-ovalbumin formation positively correlated with the storage temperature, and high temperature could accelerate this formation. The S-ovalbumin content (SO) increases from 18% of the egg white to 86% after 6 mo of refrigerated storage (Smith and Nguyen 1984; Alleoni and Antunes 2004). Refrigeration and coating treatments reduce the rate of its formation (Alleoni and Antunes 2004).
An investigation by Whitelock et al. (1994) on peach quality during storage showed that storage conditions such as temperature, relative humidity, and storage air flow had significant (P < 0.05) effect on weight loss rate during storage. Huang et al. (2012a) reported that relative humidity did not affect the SO of eggs during storage. The storage conditions may vary during distribution (transportation and storage) than the specified value. To estimate the influence of these variations on egg quality during distribution, the different egg quality indices should be evaluated under different environmental conditions. Therefore, models predicting egg freshness quality change rate as influenced by environmental factors are helpful for estimating the shelf-life of shell eggs during storage and/or market display.
Studies including the impacts of significant environmental factors in storage on egg quality changes during expected storage life are needed. Several storage trials at different temperature conditions have been conducted during previous times to assess egg quality. There are some models that describe the effect of temperature on egg freshness quality indices. Studies carried out in order to set up techniques for prediction of the egg freshness parameters include the furosine analysis (Hidalgo et al. 2006), the biogenic amine concentrations analysis (Ramos et al. 2009), the dielectric properties analyses (Ragni et al. 2006), the use of an electronic nose (Dutta et al. 2003; Yongwei et al. 2009; Yimenu et al. 2017b), the use of spectroscopy (Kemps et al. 2007; Giunchi et al. 2008), the assessment of albumen viscosity (Kemps et al. 2010), the use of S-ovalbumin as an indicator (Huang et al. 2012a), and prediction at variable temperature conditions (Yimenu et al. 2017a). All these previous studies considered only the effect of temperature to estimate model parameters, which may not consider the significant influences of other environmental factors on the change rate of egg quality indices. Changes in egg freshness quality indices as influenced by other environmental factors such as relative humidity and air flow velocity were not investigated yet. There are no models to relate egg freshness change rates to combined effects of storage temperature, relative humidity, and air flow velocity. Therefore, experiments are needed to be conducted under different conditions by varying environmental factors in hen egg storage units.
The aim of the present study was to determine correlations between the freshness of hen eggs and the surrounding environmental conditions during storage. The specific objectives were (1) to study the impacts of the environmental factors (temperature (°C), relative humidity (%) and air flow velocity (m/s)) on the egg quality indices such as weight loss, Haugh unit, and S-ovalbumin content using regression analysis, (2) to develop kinetic model(s) for freshness prediction using constant temperature data and validate it under dynamic temperature conditions.
MATERIAL AND METHODS
Experimental Materials and Storage
Freshly laid special class (first or second grade eggs weighing between 60 and 68 g per egg) unfertilized and unwashed hen egg samples in their commercial packaging (cartons, each containing 10 egg specimens) were supplied directly from Ireefarm Egg Company, Seoul, and the storage experiments were conducted at Korea Food Research Institute. Storage experiments were performed in 2 parts at different times: part 1 for studying environmental impacts and part 2 for prediction modeling. The storage experiments in the respective parts of the study were conducted at the same time.
In part 1, samples were stored at 6 temperature controlled storage chambers (constant temperature rooms equipped with air blast fans) (REI Technology Co., Seoul, Korea). Three storage units were set at 15°C and the other 3 storages were set at 25°C. The storage conditions were shown in Table 1. The specific temperatures were chosen to include the common temperature during storage and the abusive temperature, which is well within the temperature range during egg distribution. Miniature, programmable, electronic data loggers were used to constantly monitor the temperature with ±0.3°C accuracy (HL-1D/TL-1D, Rotronic Measurement Solutions, Taiwan) and relative humidity with measurement range and accuracy of 0 to 99% RH (–20 to 70°C) and ±2.5% RH, respectively (TR-72i, T&D Corporation, Japan), in the storage chambers. Temperature data loggers were placed inside the storage chambers and plastic covers to monitor storage environment. Relative humidity data loggers were also placed in both storage chambers and plastic covers to monitor humidity during storage period. Air velocity inside the chambers and plastic covers was measured at intervals of 30 min for 24 h using a hand-held Testo 400 anemometer (Brandt Instruments, Inc., Prairieville, LA) with Vane and Pitot tube Measuring Range of 0 to 12,000 fpm (0 to 60 m/s) and 0 to 8,000/20,000 fpm (0 to 40/100 m/s), respectively, and the average value was considered.
Table 1.
Experimental conditions.
| Condition number | Relative humidity (%) | Temperature (°C) | Flow velocity (m/s) |
|---|---|---|---|
| 1 | 35.5 | 15 | 0.05 |
| 2 | 44.7 | 25 | 0.05 |
| 3 | 33.2 | 15 | 0.55 |
| 4 | 42.4 | 25 | 0.55 |
| 5 | 64.5 | 15 | 0.05 |
| 6 | 70.1 | 25 | 0.05 |
| 7 | 64.3 | 15 | 0.55 |
| 8 | 74.1 | 25 | 0.55 |
| 9 | 96.3 | 15 | 0.05 |
| 10 | 98.9 | 25 | 0.05 |
| 11 | 94.3 | 15 | 0.55 |
| 12 | 99 | 25 | 0.55 |
In part 2, similar storage chambers as in part 1 were used and set at 5, 10, 20, 25, and 28.5°C to create constant temperature conditions. In addition, 2 variable temperature conditions were established and specific time-temperature scenario for each variable profile was applied in temperature programmable storage chambers. The 2 variable temperature profiles used were set at 10 to 20°C (isothermal steps per repeated temperature cycle: 24 h at 10°C, 24 h at 20°C) and 10 to 28.5°C (isothermal steps per repeated temperature cycle: 24 h at 10°C, 24 h at 28.5°C). Similar temperature data loggers as the ones used in part 1 were placed inside the storage chambers as well as attached to egg surface and egg core, to monitor storage air, egg surface, and egg core temperature, respectively. The egg core temperature measurements were used during quality prediction model development, as the surrounding air temperature usually deviate from the egg core temperature.
Experimental Set-up
Part 1: 2 storage set-point temperatures (T) in °C (T: 15 and T: 25), 3 relative humidities (RH) in % (RH: low, RH: medium, and RH: high), and 2 storage air flow velocities (VEL) in m/s (VEL: high and VEL: low) were designed in the 6 temperature controlled storage chambers described in the above section. Egg samples were divided into 12 groups and moved to their corresponding treatments in 6 storages as shown in Table 1. Some egg cartons were stored on a shelf under a plastic cover having perforations (LDPE plastic) to allow gas exchange and positioning it in the chamber away from direct airflow so that negligible air velocity is attained; other egg cartons were stored on a separate shelf in the same chamber without covering and positioned to provide maximum airflow across the cartons to allow high air velocity. The high air flow velocities were attained by circulation using similar electric fans and the low air velocities were attained by free convection under plastic covers. The maximum and medium humidities were attained by using ultrasonic humidifier (DLE-US-06, 3Htech Co., Daejeon, Korea). The low humidity was attained under normal conditions without humidification.
The experiments were carried out for a total period of 29 and 10 d at 15 and 25°C, to observe the variation trends of weight loss rate, Haugh unit, yolk index, albumin index, yolk pH, albumin pH, and S-ovalbumin formation. Egg quality indices were measured per 3-d interval at 15°C and 2-d interval at 25°C.
Part 2: 7 storage experiments (5 at constant and 2 at variable temperatures) were designed as described in the previous section. Experiments were carried out for a total period of 10 d at 28.5°C, 10 d at 25°C, 21 d at 20°C, 26 d at 10 to 20°C, 13 d at 10 to 28.5°C, and 98 d at 5°C as well as 10°C storage temperatures. The egg quality indices were measured every 1, 2, 3, 2, 1, and 7 d for the 28.5, 25°C, 20, 10 to 20, 10 to 28.5, and 5°C as well as 10°C storage samples, respectively. The same egg quality indices were measured as in part 1. Data from fluctuating temperature conditions were used to validate the quality prediction models developed using data collected under isothermal conditions.
Egg sampling
Egg sampling was performed in a similar way during both part 1 and part 2 of the study. During each sampling time, 2 egg cartons (each containing 10 eggs) were randomly selected from each treatment group. One carton was used for albumen index, yolk index, and Haugh unit; another carton was used for pH (albumen, yolk) and S-ovalbumin content. For weight loss rate, on the initial day of the storage experiment, 3 packs (each containing 10 eggs) were randomly selected and individual eggs were labeled from 1 to 20. The eggs were identified and labeled by pasting a masking tape with appropriate identification tag around the sharp end of the egg.
Egg Quality Evaluation
Weight Loss
Weight (in grams) was established by individual weighting of each labeled egg, using an analytical balance (Carter 1975) during each experiment day. Egg weight was measured using a 200 g × 0.1 g capacity digital scale (Model CSG 201F, OHAUS Corporation). This is done by placing the egg on plastic holder on the flat surface of the scale ensuring that the scale is set to 0.0 g before measurement. Egg weight loss was determined as the difference between successive weights of eggs at different weighing days. Weight loss percentage was calculated in relation to day 0 egg weight (g). The difference between the day 0 egg weight and the weight after each time point was expressed as a percentage of the day 0 egg weight.
Yolk Index, Albumen Index, and Haugh Unit
Eggs were broken out on a flat transparent glass surface using a spatula to obtain various internal parameter measurements.
Yolk index was estimated from ratio of yolk height to yolk width. The height and diameter of the yolk were measured using a digimatic indicator (ID-C1050XB, Mitutoyo Co., Japan) and digimatic caliper (CD-15CPX, Mitutoyo Co., Japan), respectively. The measured values were expressed as the mean length and the mean breadth. The yolk index will be calculated from the resulting mean values using the following formula (Funk 1948):
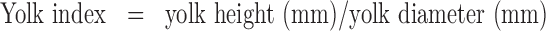 |
(1) |
Similarly, the height and diameter of the thick albumen were measured using the digimatic indicator and digimatic caliper, respectively. The long and short axes at 90o were measured and expressed as mean values. The albumen index was calculated from the resulting mean values using the following formula (Heiman and Carver 1936):
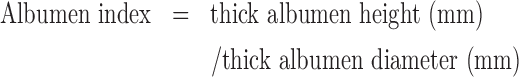 |
(2) |
Haugh unit was determined from egg weight and albumen height of a broken egg spread on a horizontal plate using the expression below (Haugh 1937):
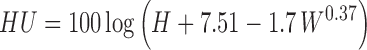 |
(3) |
where H = Albumen height (mm), W = the weight of egg when tested (g)
pH Measurement
After separating yolk and albumen, putting each into a beaker and homogenizing, pH values of the albumen and yolk were measured using a pH meter (TA-70, DKK-TOA Corporation, Japan). The pH meter was calibrated using buffer solutions at pH 4 and 7 before measurements. Measurements were performed on 3 replicates and average values were reported.
S-ovalbumin Content
The SO of the egg white was measured using the method described by the literature (Huang et al. 2012a).
Phosphate buffer: 13.7 g NaH2PO4·2H2O and 43.1 g Na2HPO4 was dissolved in 1 L of deionized water, 20-fold diluted, adjusted to pH 7.5.
Precipitant: 0.5 mol/L NaCl plus 0.1 mol/L CH3COONa, Values CH3COOH pH adjusted to 4.7.
Biuret solution: 1.8 g CuSO4·5H2O and 7.2 g C4O6H2KNa·4H2O was dissolved in 500 mL of deionized water, 36 g NaOH was dissolved in 360 mL deionized water, mixed 1 spare.
Five grams of egg whites was placed in a 100-mL beaker, and 25 mL of 0.5 mol/L phosphate buffer (pH 7.5) was added; the mixture was agitated for 5 min with a magnetic stirrer. Afterward, 5 mL of the suspension was placed into 2 test tubes; one of the tubes was heated at 75°C for 30 min. Upon cooling, 5 mL of the precipitating solution was added in each test tube, and the solution was transferred to centrifuge tubes with the addition of another 5 mL of precipitating solution. After resting for 10 min, the tubes were centrifuged at 10,733 × g for 5 min at 4°C and the supernatant was obtained and filtered. Two milliliters of the filtered supernatant was placed in a test tube with 4 mL of Biuret solution added. This supernatant aliquot was left to rest for 30 min, and the absorbance was determined at 540 nm using a Beckman DU-70 spectrophotometer (Fullerton, CA). The results were expressed as a percentage of S-ovalbumin in the total amount of ovalbumin.
Statistical Analysis
The obtained data were subjected to ANOVA analysis using the statistical analysis system (SAS 2008) whereby comparison of mean values were conducted with LSD test at P < 0.05 significance level. The R program for Windows was used for regression analysis of environmental factors and to fit the experimental data to model equations to obtain the coefficients of the equations (R Development Core Team 2011).
The comparison between the observed and predicted values of SO of the eggs stored under constant and variable temperature conditions was based on the bias and accuracy factors (Ross 1996). The accuracy and bias factors provide an indication of the average deviation between the model predictions and observed results (Ross 1996), and their closeness to a value of 1 is an effective and practical measure of predictive model validity. To evaluate the prediction error of each model, these statistical characteristic indices (bias factor (Bf) and accuracy factor (Af)) were employed:
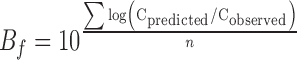 |
(4) |
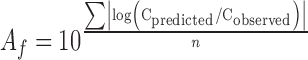 |
(5) |
where cobserved is the value of each data experimentally observed, cpredicted is the value predicted at the same time as the data were observed, and n is the number of observations.
RESULTS AND DISCUSSION
Egg Quality Changes During Storage
Table 1 shows the experimental condition considered in this study to investigate the impact of different environmental factors on the change of egg freshness indices. Storage time and temperature significantly affected almost all parameters of egg quality investigated in the present study. An analysis of variance showed that temperature and storage time had significant (P < 0.05) effect for all egg quality indices, although RH and airflow were not significant for SO content (Figure 3). Egg weight, Haugh unit, yolk index, and albumen index significantly (P < 0.001) decreased with increased storage time and temperature. Initially, albumen and yolk pH were shown to have an increasing trend and then shown a non-uniform trend by increased storage time and temperature, attaining higher values at the end of the experiments. S-ovalbumin content significantly (P < 0.001) increased by increased storage time and temperature.
Figure 3.
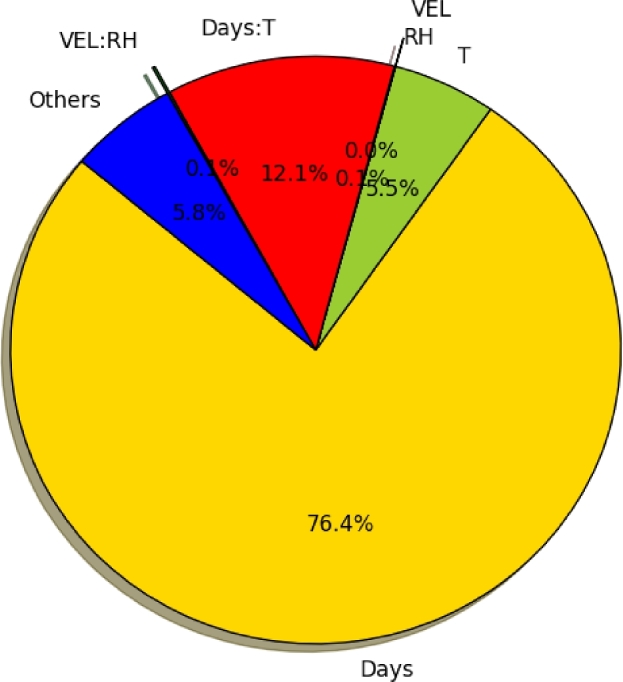
Relative impacts of environmental factors on S-ovalbumin change.
Increase in storage temperature accelerated egg weight loss and as the storage duration increased, egg weight loss was increased at considered temperatures (15 and 25o C). Similar results were reported by Okeudo et al. (2005), Samli et al. (2005), Akyurek and Okur (2009), Jin et al. (2011), Akter et al. (2014), Lee and Chung (2014), and Yimenu et al. (2017a). Normally eggs stored for longer periods of time loss from their initial weight, due to the evaporation of the water from their content and, to a much lesser extent, loss of CO2, ammonia, nitrogen, and hydrogen sulfide gas from the albumen (Haugh 1937; Obanu and Mpieri 1984; Scott and Silversides 2000).
Figure 1 shows the HUs change over time under different environmental conditions. The different colors compare the impact of RH. Blue, green, and red stand for low, middle, and high RH conditions. Circles are for low flow velocity conditions, whereas squares represent high flow velocity ones. Data with solid lines are taken from the temperature condition of 15°C, and those with dashed ones are for the condition of 25°C.
Figure 1.
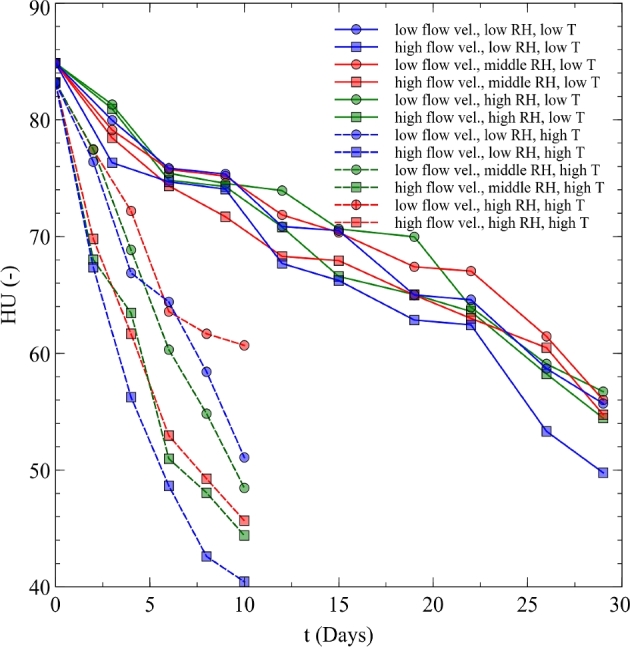
Haugh unit changes in time observed in the experiments.
Haugh unit values decreased with an increase in storage time at both storage temperatures (15 and 25o C), and a greater decrease in HU values were found at higher temperature (25oC) than at lower temperatures (15oC) (Figure 1). Haugh unit reduction happens due to the decrease in thick albumen height, because during egg storage albumen becomes thinner and loses CO2, allowing rupture of the electrostatic complex between lysozyme and ovomucin (Scott and Silversides 2000). These results are in agreement with those of Samli et al. (2005), Akyurek and Okur (2009), Jin et al. (2011), Tayeb (2012), Tebesi et al. (2012), Akter et al. (2014), Lee and Chung (2014), and Yimenu et al. (2017a), who reported a significant (P ≤ 0.05) decrease in HU due to storage time and temperature.
There was a confirmed rapid increase in SO after storage at 15 and 25°C, and the formation velocity at 25°C was significantly (P ≤ 0.05) higher than that at 15°C. The result indicated that high temperature could accelerate the S-ovalbumin formation, which supports the former reports (Alleoni and Antunes 2004; Huang et al. 2012a).
Modeling Environmental Impacts on Hen Egg Quality Indices
Changes in values of the relative weight loss, the Haugh units, and the S-ovalbumin content were analyzed statistically using regression analysis. The relative impact of each environmental factor (temperature, relative humidity, and air flow velocity) on the variation of egg quality indices analyzed by the open-source statistics program, R (R Development Core Team 2011) for windows.
Weight Loss
It was observed that the rate of weight change in time depends on RH (%) and temperature. The weight loss rate increases with temperature and decreases with RH as expected. Weight change was greatly affected by RH and the rate increased with lower RH. With decreasing RH, weight change increased for high and low airflow velocity. Although RH has the major influence on egg weight loss, air velocity is still a factor. The effects of the RH× airflow interaction were apparent because the weight change was higher for high airflow than low airflow.
The flow velocity was found not to affect the weight loss for the given range of change. The determination coefficient (R2) was estimated to be 0.996. The regression equation was
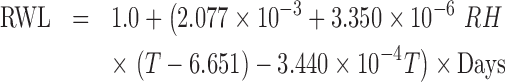 |
(6) |
where RH and T are the relative humidity and temperature, respectively.
The summation of the numbers in parenthesis in Eq. (6) was always negative for the conditions considered in this study. The magnitude of weight loss rate was the number in the parenthesis in Eq. (6), and found to increase with temperature and decrease with relative humidity. The interaction between RH and temperature in Eq. (6) indicated that the impact of RH increases with temperature, which implies RH becomes more impactive with the increase of storage temperature.
Figure 2 represents the relative impact of each environmental factor on the variation of the relative weight loss analyzed by the open-source statistics program, R. Time, relative humidity, and temperature were the significant factors, whereas that of flow velocity was negligible.
Figure 2.
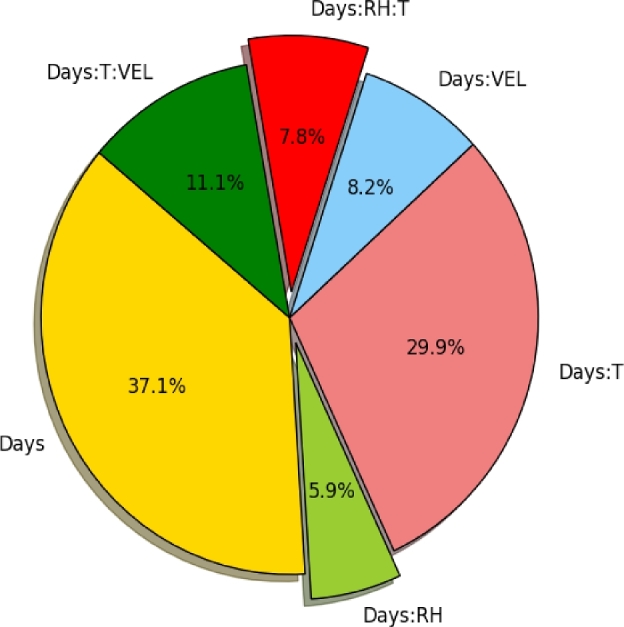
Relative impacts of environmental factors on Haugh unit change.
Haugh Units
Figure 1 shows the HU changes over time under different environmental conditions. The different colors compared the impact of RH. Blue, green, and red stand for low, middle, and high RH conditions. Circles were for low flow velocity conditions, whereas squares represented high flow velocity ones. Data with solid lines were taken from the temperature condition of 15°C, and those with dashed ones were from the condition of 25°C.
Both RH and air velocity had significant (P ≤ 0.05) effect on HU values (Figure 2). The difference between high airflow and negligible airflow treatment was significantly greater at low RHs and high temperature (Figure 1).
The variation of HU over time was analyzed using the regression analysis. The determination coefficient (R2) was estimated to be 0.95. The equation was shown below
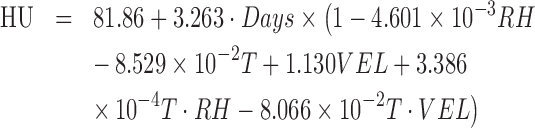 |
(7) |
where RH, T, and VEL are the relative humidity, temperature, and air flow velocity, respectively.
The decrease of HU under the combined effect of the environmental factors during storage time can be determined using Eq. (7). The impact of each environmental factor on the change of HU with time could be evaluated by taking the partial derivative of Eq. (7) with respect to each factor of interest.
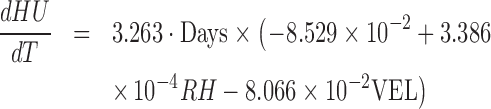 |
(8) |
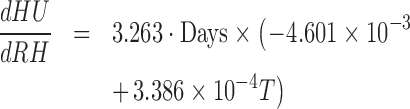 |
(9) |
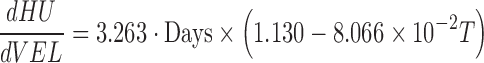 |
(10) |
The impact of temperature shift-up increased the negative slope in Eq. (8) to speed up the HU decrease, where the impact decreased with RH increased with flow velocity. Plugging in the temperature range considered, 15 to 25°C in Eq. (9), the value in the parenthesis turned to be positive indicating the high RH alleviating the decreasing rate of HU with time, and the impact was more effective under higher temperature. The flow velocity accelerated the decrease of HU, and the impact became more significant with temperature as shown in Eq. (10).
Ignoring the influence of RH and VEL,
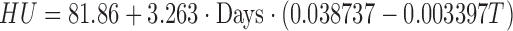 |
(11) |
S-ovalbumin Contents
It was statistically analyzed that S-ovalbumin change was independent of RH condition. Figure 3 clearly shows that the RH did not affect the change. The change was a strong function of time and temperature condition only. The difference between RH treatments as well as high airflow and low airflow treatments were not significant (P > 0.05) both at low temperature and high temperature conditions. The RH × airflow interaction was also not significant for SO change (Figure 3). Consequently, the change in SO during storage was expressed only as a function of time and temperature using a kinetic model described in the following section.
Kinetic Model Development For S-ovalbumin Variation
The optimum set of model parameters was determined from the iterative process to minimize the sum of the squared deviations of the indicators between the fittings and the experimental observations using the DEoptim package in R (Ardia and Mullen 2010). That was done by the process of minimizing the error between the experimental observations and the solutions of Eqs. (12) and (13) by assuming values of model parameters.
The time histories of SO showed convex upward shape. Equations (12) and (13) were used to mimic the behavior.
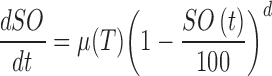 |
(12) |
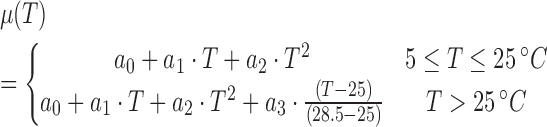 |
(13) |
where a0, a1, a2, a3, and d are model parameters, which are determined to be 4.347 × 10 − 1, 6.156 × 10 − 2, 7.264 × 10 − 3, and 7.640, respectively. t and T are the time and temperature, respectively.
Figure 4 shows the comparison of the observed S-ovalbumin histories with the model fitted ones under constant temperature conditions. They showed very good agreement with the accuracy and bias factor very close to 1 as listed in Table 2.
Figure 4.
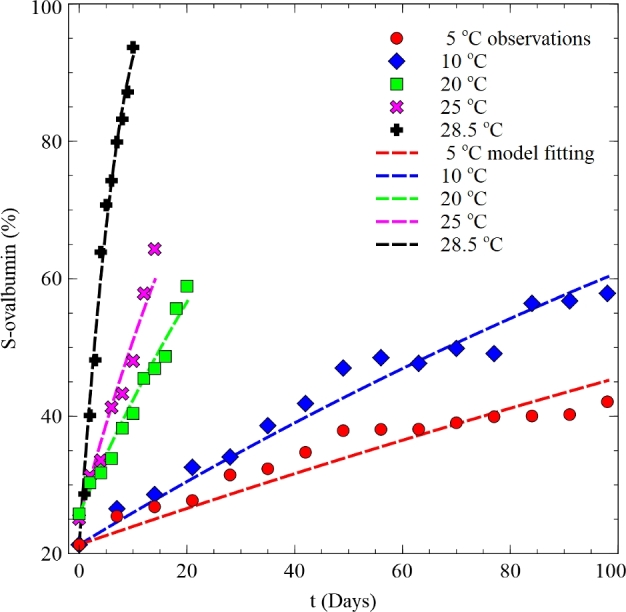
Comparison between observed S-ovalbumin histories with the model fitting data under constant temperature conditions.
Table 2.
The accuracy and bias factors of the model fitting the S-ovalbumin histories under constant temperature conditions.
| Temperature condition (°C) | Af (–) | Bf (–) |
|---|---|---|
| 5 | 1.059 | 0.972 |
| 10 | 1.040 | 0.982 |
| 20 | 1.033 | 1.013 |
| 25 | 1.043 | 1.009 |
| 28.5 | 1.039 | 1.015 |
Af = accuracy factor; Bf = bias factor.
Figure 5 compares the experimental observations with the model predictions under 2 fluctuating temperature conditions. The S-ovalbumin change in time was calculated by integrating Eqs. (12) and (13) with the measured temperature histories shown in Figure 5. The prediction underestimated the S-ovalbumin increase under 10 to 20°C condition, where it overestimated under 10 to 28.5°C. The accuracy and bias factors were 1.091 and 0.917 at 10 to 20°C and 1.206 and 1.204 at 10 to 28.5°C, respectively. According to Perez-Rodrı;guez and Valero (2013), the acceptable bias factor value for a predictive model could be 0.75 to 1.25, and the developed model was acceptable. However, the prediction performance was found to be inferior to the cases using other quality indices such as relative weight loss, Haugh unit, and yolk index, which had the Af and Bf values of 1.116 and 0.940, 1.028 and 1.001, and 1.038 and 0.966, respectively, at 10 to 30°C (see Yimenu et al. 2017a).
Figure 5.
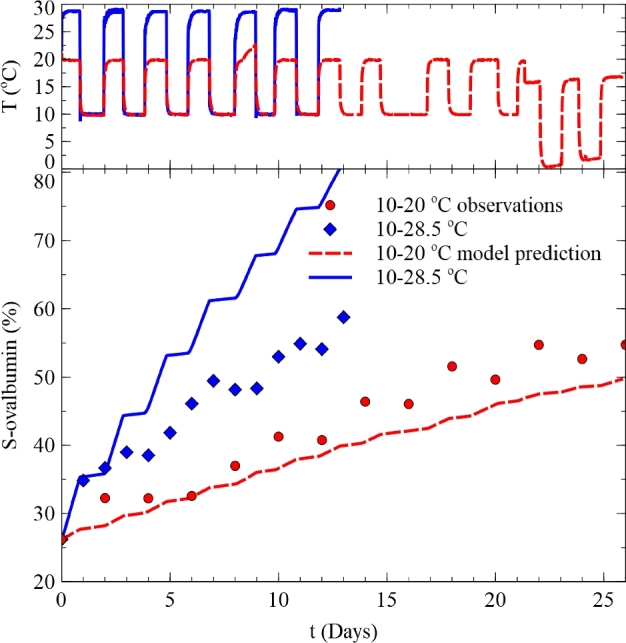
Comparison between observed S-ovalbumin histories with the model predictions under fluctuating temperature conditions.
CONCLUSIONS
The environmental impact analysis result in the current study showed that all storage factors had significant effect on the HU, whereas only temperature had significant effect on the (SO. This indicates that the HU regression model can be considered as the better model for prediction of egg freshness, as it reflect the combined effect of temperature, humidity, and air flow velocity. Therefore, it can be concluded that the SO kinetic model can be used to predict egg freshness along with the HU model at 5 to 28.5°C storage condition during hen egg distribution.
Acknowledgements
We thank u-Food Project, Korea Food Research Institute for funding and granting us access to its facilities.
REFERENCES
- Adeogun I. O., Amole F. O.. 2004. Some quality parameters of exotic chicken eggs under different storage conditions. Bull Anim. Health Prod. Afr. 52:43–47. [Google Scholar]
- Akter Y., Kasim A., Omar H., Sazili A. Q.. 2014. Effect of storage time and temperature on the quality characteristics of chicken eggs. J. Food Agric. Environ. 12:2. [Google Scholar]
- Akyurek H., Okur A. A.. 2009. Effect of storage time, temperature and hen age on egg quality in free-range layer hens. J. Anim. Vet. Adv. 8:1953–1958. [Google Scholar]
- Alleoni A. C. C., Antunes A. J.. 2004. Albumen foam stability and S-ovalbumin contents in eggs coated with whey protein concentrate. Rev. Bras. Cienc. Avic. 6:105–110. [Google Scholar]
- Ardia D., Mullen K.. 2010. DEoptim: Differential Evolution Optimization in R. R package version 2.0–4, http://CRAN.R-project.org/package=DEoptim. [Google Scholar]
- Carter J. C. 1975. The hen's egg: Estimation of shell superficial area and egg volume, using measurements of fresh egg weight and shell length and breadth alone or in combination. Br. Poult. Sci. 16:541–543. [Google Scholar]
- Dutta R., Hines E. L., Gardner J. W., Udrea D. D., Boilot P.. 2003. Non-destructive egg freshness determination: an electronic nose based approach. Meas. Sci. Technol. 14:190–198. [Google Scholar]
- Funk E. M. 1948. The relation of the yolk index determined in natural position to the yolk index as determined after separating the yolk from the albumen. Poult. Sci. 27:367–367. [Google Scholar]
- Giunchi A., Berardinelli A., Ragni L., Fabbri A., Silaghi F.. 2008. Non-destructive freshness assessment of shell eggs using FT-NIR spectroscopy. J. Food Eng. 89:142–148. [Google Scholar]
- Haugh R. R. 1937. The Haugh unit for measuring egg quality. US Poult. Mag. 43:552–573. [Google Scholar]
- Heiman V., Carver J. S.. 1936. The albumen index as a physical measurement of observed egg quality. Poult. Sci. 15:141–148. [Google Scholar]
- Hidalgo A., Rossi M., Pompei C.. 2006. Estimation of equivalent egg age through furosine analysis. Food Chem. 94:608–612. [Google Scholar]
- Huang Q., Ma M., Jin Y., Qiu N., Sun S., Geng F.. 2012a. Effect of storage condition on S-ovalbumin formation in albumen. Trans. Chin. Soc. Agric. Eng. 28:288–292. [Google Scholar]
- Huang Q., Qiu N., Ma M. H., Jin Y. G., Yang H., Geng F., Sun S. H.. 2012b. Estimation of egg freshness using S-ovalbumin as an indicator. Poult. Sci. 91:739–743. [DOI] [PubMed] [Google Scholar]
- Huntington J. A., Gettins P. G. W., Patston P. A.. 1995. S-ovalbumin, an ovalbumin conformer with properties analogous to those of loop-inserted serpins. Protein Sci. 4:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. H., Lee K. T., Lee W. I., Han Y. K.. 2011. Effects of storage temperature and time on the quality of eggs from laying hens at peak production. Asian-Aust. J. Anim. Sci. 24:279–284. [Google Scholar]
- Kemps B. J., Bamelis F. R., Mertens K., Decuypere E. M., DeBaerdemaeker J. G., DeKetelaere B.. 2010. The assessment of viscosity measurements on the albumen of consumption eggs as an indicator for freshness. Poult. Sci. 89:2699–2703. [DOI] [PubMed] [Google Scholar]
- Kemps B. J., DeKetelaere B., Bamelis F. R., Mertens K., Decuypere E. M., DeBaerdemaeker J. G., Schwagele F.. 2007. Albumen freshness assessment by combining visible near-infrared transmission and low-resolution proton nuclear magnetic resonance spectroscopy. Poult. Sci. 86:752–759. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Chung S. H.. 2014. Effect of hen age, storage duration and temperature on egg quality in laying hens. Int. J. Poult. Sci. 13:634–636. [Google Scholar]
- Obanu Z. A., Mpieri A. A.. 1984. Efficiency of dietary vegetable oils in preserving the quality of shell eggs under ambient tropical conditions. J. Sci. Food Agric. 35:1311–1317. [Google Scholar]
- Okeudo N. J., Ezetoha U., Akomas C., Akanno E. C.. 2005. Egg quality of gallus domesticus under domestic storage in Nigeria. Anim Res Int. 2:319–321. [Google Scholar]
- Perez-Rodriguez F., Valero A.. 2013. Predictive Microbiology in Foods. Springer, New York [Google Scholar]
- R Development Core Team 2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Ragni L., Gradari P., Berardinelli A., Giunchi A., Guarnieri A.. 2006. Predicting quality parameters of shell eggs using a simple technique based on the dielectric properties. Biosyst. Eng. 94:255–262. [Google Scholar]
- Ramos B., Pinho O., Ferreira I. M. P. L. V. O.. 2009. Changes of yolk biogenic amine concentrations during storage of shell hen eggs. Food Chem. 116:340–344. [Google Scholar]
- Ross T. 1996. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 81:501–508. [DOI] [PubMed] [Google Scholar]
- Samli H. E., Agna A., Senkoylu N.. 2005. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 14:548–553. [Google Scholar]
- SAS 2008. SAS/STAT User's Guide. Version 9.2. Cary, NC: SAS Institute Inc. [Google Scholar]
- Scott T. A., Silversides F. G.. 2000. The effect of storage and strain of hen on egg quality. Poult. Sci. 79:1725–1729. [DOI] [PubMed] [Google Scholar]
- Smith M. B., Back J. F. 1962. Modification of ovalbumin in stored eggs detected by heat denaturation. Nature 193:878–879. [DOI] [PubMed] [Google Scholar]
- Smith M. B., Back J. F.. 1965. Studies on ovalbumin II. The formation and properties of s-ovalbumin, a more stable form of ovalbumin. Aust. J. Biol. Sci. 18:365–377. [DOI] [PubMed] [Google Scholar]
- Smith M. B., Nguyen L.. 1984. Measuring the age of stored eggs. CSIRO Food Res. Q. 44:94–96. [Google Scholar]
- Tabidi M. H. 2011. Impact of storage period and quality on composition of table egg. Adv. Environ. Biol. 5:856–861. [Google Scholar]
- Tayeb I. T. 2012. Effects of storage temperature and length on egg quality parameters of laying hen. J. Anim. Scientist. 1:32–36. [Google Scholar]
- Tebesi T., Madibela O. R., Moreki J. C.. 2012. Effect of storage time on internal and external characteristics of guinea fowl (Numida meleagris) eggs. J. Anim. Sci. Adv. 2:534–542. [Google Scholar]
- Whitelock D. P., Brusewitz G. H., Smith M. W., Zhang X.. 1994. Humidity and airflow during storage affect peach quality. HortScience 29:798–801. [Google Scholar]
- Yimenu S. M., Kim J. Y., Koo J., Kim B. S.. 2017a. Predictive modeling for monitoring egg freshness during variable temperature storage conditions. Poult. Sci. 96:2811–2819. [DOI] [PubMed] [Google Scholar]
- Yimenu S. M., Kim J. Y., Kim B. S.. 2017b. Prediction of egg freshness during storage using electronic nose. Poult. Sci. 96:3733–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongwei W., Wang J., Zhou B., Lu Q.. 2009. Monitoring storage time and quality attribute of egg based on electronic nose. Anal. Chim. Acta. 650:183–188. [DOI] [PubMed] [Google Scholar]


