Abstract
Introduction
Normative cognitive data can help to distinguish pathological decline from normal aging. This study presents normative data from the Cambridge Neuropsychological Test Automated Battery, using linear regression and nonlinear quantile regression approaches.
Methods
Heinz Nixdorf Recall study participants completed Cambridge Neuropsychological Test Automated Battery tests: paired-associate learning, spatial working memory, and reaction time. Data were available for 1349-1529 healthy adults aged 57-84 years. Linear and nonlinear quantile regression analyses examined age-related changes, adjusting for sex and education. Quantile regression differentiated seven performance bands (percentiles: 97.7, 93.3, 84.1, 50, 15.9, 6.7, and 2.3).
Results
Normative data show age-related cognitive decline across all tests, but with quantile regression revealing heterogeneous trajectories of cognitive aging, particularly for the test of episodic memory function (paired-associate learning).
Discussion
This study presents normative data from Cambridge Neuropsychological Test Automated Battery in mid-to-late life. Quantile regression can model heterogeneity in age-related cognitive trajectories as seen in the paired-associate learning episodic memory measure.
Keywords: Normative data, Episodic memory, Cognition, Aging, Dementia, Mild cognitive impairment
Highlights
-
•
The study presents normative cognitive data from the Cambridge Neuropsychological Test Automated Battery in mid-to-late life.
-
•
Most tasks showed similar decline across performance bands with increasing age.
-
•
Quantile regression is sensitive for evaluating diverging trajectories with age.
-
•
Episodic memory showed accelerated decline in the average performance range.
1. Background
Normative data standardizes an individual's cognitive performance relative to peers. This helps to identify impairment and can reveal atypical declines [1]. However, in mid-to-late life, cognitive decline frequently occurs as a part of the normal aging process [2], affecting general cognitive functions, as well as a range of cognitive domains including fine motor skills, information processing, executive functions, visuospatial ability, and memory [3], [4], [5]. Understanding age-related cognitive change is important in the context of the rising worldwide incidence of dementia [6], since it is essential for early detection of atypical decline, which is important for early therapeutic intervention [7].
Normative data are often derived from a healthy reference population [8]. One method for deriving normative data is through stratifying test results for a defined age range, resulting in normative data grouped for specific age-ranges (often spanning multiple years [9]). However, changes in cognitive function have been reported year on year in healthy people aged over 60 years [10], and declines have been shown to accelerate with older age [4], [5]. Impairment thresholds for age-grouped norms are therefore likely to be biased toward the inclusion of older individuals, and normative data may be less suited to deliver the precision needed to differentiate between the normal aging process and clinically meaningful change.
Normative data derived from regression modeling can provide a more fine-grained view of age-related cognitive change. However this approach assumes equal rates of change across performance levels, which is problematic where different trajectories may be expected for those with high versus low performance [1]. As noted by Salthouse [11], it is unclear whether aging causes a cognitive shift in the entire distribution, or if differences between people increase with age, with only some individuals experiencing cognitive decline, and others remaining stable or improving. There is evidence to suggest that cognitive aging is heterogeneous, with different rates of change across individuals [12].
Nonlinear quantile regression modeling is another method which has been applied to provide normative and reference data for cognitive function and cognitive aging [1], [13], [14]. The method allows for different slopes to be fitted across performance ranges. This can identify discrete trajectories (e.g., high or low performers that may differ from the average), and is not biased by nonnormal distributions or nonconstant variances, which can undermine the validity of linear regression methods [1]. A detailed report of quantile regression modeling in comparison with linear regression in neuropsychological test scores is presented by Sherwood et al. [1].
The current report presents normative data for the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB) in a mid- to old-age German population sample using linear regression and nonlinear quantile regression modeling. Computerized cognitive assessments have advantages that may make them well suited to early detection of cognitive changes in the elderly. These include: minimization of floor and ceiling effects through flexibility of test difficulty levels, standardization of assessment, accuracy of response recordings, automated scoring, and reduced cost of administration [7], [15]. Here we present results from cognitive tests of episodic memory, executive functioning, and processing speed, which have previously shown sensitivity to mild cognitive impairment (MCI), dementia, and cognitive aging [16], [17], [18], [19], [20], [21], [22].
2. Methods
2.1. Participants
The Heinz Nixdorf Recall study is a large population-based sample recruited with the primary aim to examine risk factors for myocardial infarction and cardiovascular morbidity and mortality in mid-to-late life [16]. Ethical approval was provided by the ethics committee of the medical faculty, University Hospital Essen. Informed written consent was given by all participants.
The original sample included 4814 participants, recruited between 2000 and 2003 (baseline = T0), randomly selected from mandatory residential registries in the cities of Bochum, Essen, and Mülheim, in the urban Ruhr region in Germany. Exclusion criteria included medical or other conditions which precluded 5-year follow-up, severe psychiatric disorders, illegal substance abuse, and pregnancy [16]. People who were institutionalized (residing in nursing homes, institutions for the elderly, prisons), and people with severe illness or disability precluding their participation were also excluded [17]. Response rate was 55.5%, and a detailed analysis of nonresponse is presented in Stang et al. [17]. Although the sample was not selected based on ethnicity, all participants were Caucasian.
Participants were invited for follow-up examinations every 5 years (T1: n = 4157, 2006-2008; T2: n = 3087, 2011-2015). CANTAB was introduced at T2 in a subset of 2110 participants. CANTAB test results and complete clinical assessments were available in 1953 individuals at T2 (49.2% male, 50.8% female).
2.2. Clinical assessments
2.2.1. History of coronary disease and stroke
Hard coronary events were nonfatal acute myocardial infarction and coronary death defined as clinical symptoms, signs on ECG, increased enzymes (levels of creatinine kinase), and troponin T or I, as well, and necropsy changes [23], [24]. Hospital and nursing home records were collected. An external endpoint committee blinded for risk factor status reviewed all documents and classified hard coronary events at regular meetings twice a year. Stroke was defined as focal neurological deficits over a period of >24 hours of presumed cerebrovascular origin, classified by an expert panel consisting of four neurologists.
2.2.2. Dementia diagnosis
Dementia diagnosis was defined as physician's diagnosis of dementia, meeting the DSM-IV dementia criteria [25] or taking cholinesterase inhibitors (anatomic-therapeutic-chemical classification issued by the World Health Organization, code: N06DA) or other antidementia drugs (N06DX).
2.2.3. Mild cognitive impairment diagnosis
MCI was based on published criteria [26], [27]: A) objective cognitive impairment; B) subjective cognitive decline; C) no dementia diagnosis, and D) generally intact activities of daily living. Cognitive domains assessed included (1) attention—Trail Making Test A, color-word test card 1 and card 2; (2) executive function—Trail Making Test B, Labyrinth test, color-word test interference performance (card 3 minus card 2), verbal fluency “animals”; (3) verbal memory—eight word list immediate and delayed recall; (4) visuoconstruction—clock-drawing test. Criterion A was met where performance on at least one cognitive domain was one standard deviation below age- and education-adjusted test mean scores, or a score of ≥3 in the clock-drawing test. Individuals meeting criteria A-C but with additional impairment in activities of daily living were also present in the sample (MCI + impairment).
2.2.4. Depression
Elevated depression symptoms were identified using a cutoff score of ≥18 on the German 15-item Center for Epidemiologic Studies Depression Scale [28], [29].
2.2.5. Diabetes
Diabetes was defined present if participants reported a diagnosis of diabetes, used antidiabetic medication, or had a fasting glucose of >126 mg/dL [30].
2.2.6. Hypertension
Hypertension was defined as a systolic blood pressure of ≥140 mm Hg or a diastolic blood pressure of ≥90 mm Hg. Blood pressure was measured seated with an automated oscillometric blood pressure–measuring device (Omron, HEM-705CP), using the mean of the second and third value of three measurements [31].
2.3. CANTAB measures
Three nonverbal cognitive tests from the CANTAB computerized test battery were administered. Responses were logged via touch screen press. Average administration time was 23 minutes.
1. Paired-associate learning (PAL) is a test of episodic memory. Between 6 and 8 boxes are displayed and opened in a randomized order to reveal two or more patterns. Each pattern is then shown in the middle of the screen and the participant must select the box where the pattern was originally located. If an error is made, all pattern locations are revealed in sequence again. After four failed attempts within one stage, participants received an adjusted score (95% error, 5% correct by chance for remaining stages). Participants completed 2, 4, 6, and 8 pattern trials. Outcome measures included PAL total errors adjusted, with higher error scores denoting poorer performance; and PAL first attempt memory score, the frequency with which participants chose the correct box on their first attempt, with lower scores denoting poorer performance. Valid PAL scores were available for 1349 participants. A shorter version of the task was adopted part-way through the study because of time constraints, leading to data from the first 12% of participants (N = 186) being excluded. A further 42 participants did not complete the test.
2. Spatial working memory (SWM) examines retention and manipulation of visuospatial information. Participants find tokens in colored boxes presented on the screen and move them to a collection area. The key task instruction is that tokens will not be located in the same box twice in each trial. Outcome measures include SWM between errors: the number of times the participant incorrectly revisits a box, calculated across all assessed 4, 6, and 8 token trials; and SWM strategy: the number of unique boxes from which a participant starts a new search in the 6 and 8 box trials. More efficient searches are carried out by searching boxes in a fixed order [32]. For both outcome measures, higher scores are indicative of poorer performance. Valid scores were available for 1529 participants.
3. Reaction time (RTI) is a test of processing speed. Participants keep their finger on a button at the bottom of the screen until a target appears. The target appears inside one circle in the simple RTI variant, and inside one of five circles in the five-choice RTI variant. Outcome measures investigated included median simple RTI, and median five-choice RTI: the median duration to release the response button after the presentation of the target stimulus. Valid scores were available for 1501 participants for simple RTI and 1502 for five-choice RTI.
2.4. Statistical analysis
2.4.1. Linear regression modeling
Linear regression models were applied to assess the mean effects of age, sex (0 = male, 1 = female), and years in education (coded 0 for ≤13 years, and 1 for ≥14 years) on cognitive test performance. All variables were entered simultaneously into the regression model. Distributions of residuals were examined, and influential outliers (standardized residuals larger than ±3.29) were investigated and excluded if showing undue influence (19 data points for simple RTI, 9 from 5-choice RTI).
Impairment thresholds from linear regression can be calculated following methods described by van der Elst et al. [33], using data provided in Table 1. Each participant's predicted score was calculated using regression betas (predicted score = Intercept(0) + (Age*ßage) + (Sex*ßsex) + (Education*ßeducation). The residuals of each score were then calculated (ei = observed score − predicted score), and standardized (Zi = ei/SD[residual]). For test results where higher scores denote poorer performance, the sign of Zi was reversed (z-score = −Zi). A z-score ≤ −1.5 was considered indicative of impaired performance, and a z-score of ≤ −2 indicative of very impaired performance.
Table 1.
Performance on CANTAB tests and results from linear regression model
| Task | Outcome measures | A) Descriptives: Performance results |
B) Linear regression results |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants | Mean | Median | SD | Intercept (0) |
Age |
Sex |
Education |
Regression statistics |
|||||||
| β | SE | β (ßage) (unstd) | P Value | β (ßsex) (unstd) | P Value | β (ßeducation) (unstd) | P Value | SD of residuals (unstd) | Adjusted R2 | ||||||
| Paired associate learning | (1) Total errors adjusted | 1349 | 34.3 | 40 | 16.7 | −0.48 | 4.67 | 0.55 | <.001 | −1.20 | .21 | −4.73 | <.001 | 16.04 | 0.07 |
| (2) First attempt memory score | 1349 | 7.9 | 8 | 3.9 | 14.59 | 1.08 | −0.11 | <.001 | 0.35 | .11 | 1.41 | <.001 | 3.86 | 0.07 | |
| Spatial working memory | (3) Between errors | 1529 | 21.8 | 23 | 9.5 | −4.67 | 2.40 | 0.39 | <.001 | 1.73 | <.001 | −2.61 | <.001 | 8.96 | 0.12 |
| (4) Strategy | 1529 | 18.3 | 19 | 2.7 | 12.09 | 0.70 | 0.09 | <.001 | 0.87 | <.001 | −0.23 | .12 | 2.54 | 0.08 | |
| Processing speed | (5) Median simple reaction time | 1501 | 289.0 | 281.5 | 43.0 | 249.84 | 11.63 | 0.57 | .001 | 3.82 | .11 | −3.39 | .16 | 42.70 | 0.01 |
| (6) Median five-choice reaction time | 1502 | 327.7 | 322.5 | 43.6 | 263.50 | 11.65 | 0.92 | <.001 | 4.48 | .06 | −1.58 | .52 | 43.06 | 0.02 | |
Abbreviations: PAL, paired associate learning; SWM, spatial working memory; RTI, reaction time; SD, standard deviation; SE, standard error; unstd, unstandardized.
2.4.2. Nonlinear quantile regression modeling
Nonlinear quantile regression analysis was completed for PAL total errors adjusted, SWM between errors, and RTI measures. Quantile regression results from PAL first attempt memory score and SWM strategy (both count variables) are not presented because of more limited variance of these measures, precluding successful analysis.
Nonlinear quantile regression was completed in two steps. For each outcome measure an “age-effect equivalent” for sex (Ysex) was computed using the unstandardized ß coefficients (Table 1) from relevant linear regression models. These age-effect equivalents translate the performance differences because of sex into age adjustments, allowing men and women to be plotted together. These were calculated as follows: Ysex= (ßsex/ßage). Equally an “age-effect equivalent” for education (Yeducation) was computed as follows: Yeducation= (ßeducation/ßage).
Adjusted age for each participant in each outcome measure was computed in the following manner: Age (adjusted) = C + SYsex + EYeducation. Where S denotes sex (0 = male, 1 = female), E denotes educational level (coded as 0 for ≤13 years, and 1 for ≥14 years in education), and C denotes chronological age at testing (in years).
Quantile regression boundaries were established at 2.27%, 6.68%, 15.87%, 50%, 84.13%, 93.32%, and 97.73% categorizing performance into seven bands. Analyses were completed on adjusted ages, thereby controlling for sex and education. Statistical modeling was conducted in R software [26] using lm and the quantregGrowth package [34], specifying a cubic function, monotonicity restrictions, nonparametric bootstrapping, and a high level of smoothing. Impairment thresholds for quantile regression were extracted using the predict.gcrq command in the quantregGrowth package.
Normative curves plotted the following performance levels (centiles, z-scores): very impaired (2.27th centile, z = −2); impaired (6.68th centile, z = −1.5); low average (15.87th centile, z = −1); average (50th centile, z = 0); high average (84.13th centile, z = +1); superior (93.32nd centile, z = +1.5), and very superior (97.73rd centile, z = +2).
3. Results
3.1. Normative sample
Individuals with a history of stroke (n = 59), MCI (n = 254), MCI + impairment (n = 5), dementia (n = 11), and/or elevated depressive symptoms (n = 140) were excluded from the generation of normative data. Individuals at the age extremes were excluded to reduce variability where groups for each age were small (age 56 years, n = 7; age 85 years, n = 2; age 86 years, n = 1). A detailed review of exclusions is provided in Table 2.
Table 2.
Clinical conditions and exclusions frequency
| Participants | Frequency | % Of total sample | Frequency male/female |
|---|---|---|---|
| Clinical exclusions | |||
| Noncomorbid presentations | |||
| Dementia | 8 | 0.4 | 6/2 |
| MCI plus impairment | 1 | 0.1 | 0/1 |
| MCI | 208 | 10.7 | 107/101 |
| Depression | 96 | 4.9 | 29/67 |
| History of stroke | 37 | 1.9 | 20/17 |
| Comorbid presentations | |||
| Dementia and depression | 2 | 0.1 | 0/2 |
| Dementia and history of stroke | 1 | 0.1 | 1/0 |
| MCI and depression | 30 | 1.5 | 5/25 |
| MCI and stroke | 13 | 0.7 | 7/6 |
| MCI + impairment and depression | 4 | 0.2 | 1/3 |
| History of stroke and depression | 5 | 0.3 | 1/4 |
| MCI, stroke, and depression | 3 | 0.2 | 1/2 |
| Other exclusions | |||
| Age extremes (and ≤56 and ≥ 85 years) | 10 | 0.5 | 4/6 |
| Total excluded | 418 | 21.4 | 182/236 |
| Normative sample | 1535 | 78.6 | 779/756 |
| Totals | 1953 | 20.9 | 961/992 |
Abbreviation: MCI, mild cognitive impairment.
Exclusions resulted in a normative sample of 1535 participants (50.7% men, 49.3% women), with a mean age of 67.8 years (range 57-84). Men and women did not differ in age (means: men, 68.0 years (SD = 7.0); women, 67.7 years (SD = 6.7); t = 1.18, P = .32). However, most women were in formal education for ≤13 years (77.1%), whereas most men were in formal education for ≥14 years (56.6%; χ2 = 186.8 [df 2] P < .001). Diabetes was present in 14.7% (n = 227), a history of coronary heart disease in 9.3% (n = 143), and hypertension in 27.2% (n = 406).
3.2. Linear regression
Results from linear regression analyses are provided in Table 1. All outcome measures declined with age. With the exception of RTI tasks and SWM strategy, better performance was related to longer education. Overall, men performed better on SWM subtests, and no other sex differences were present.
3.3. Quantile regression
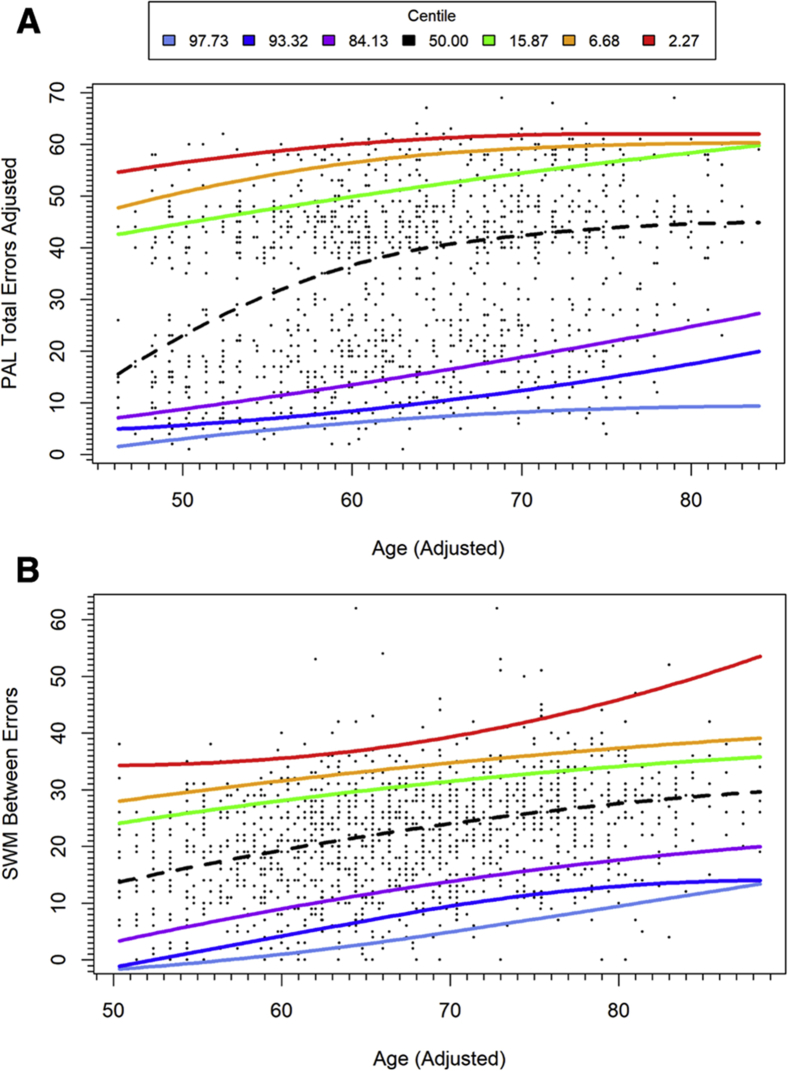
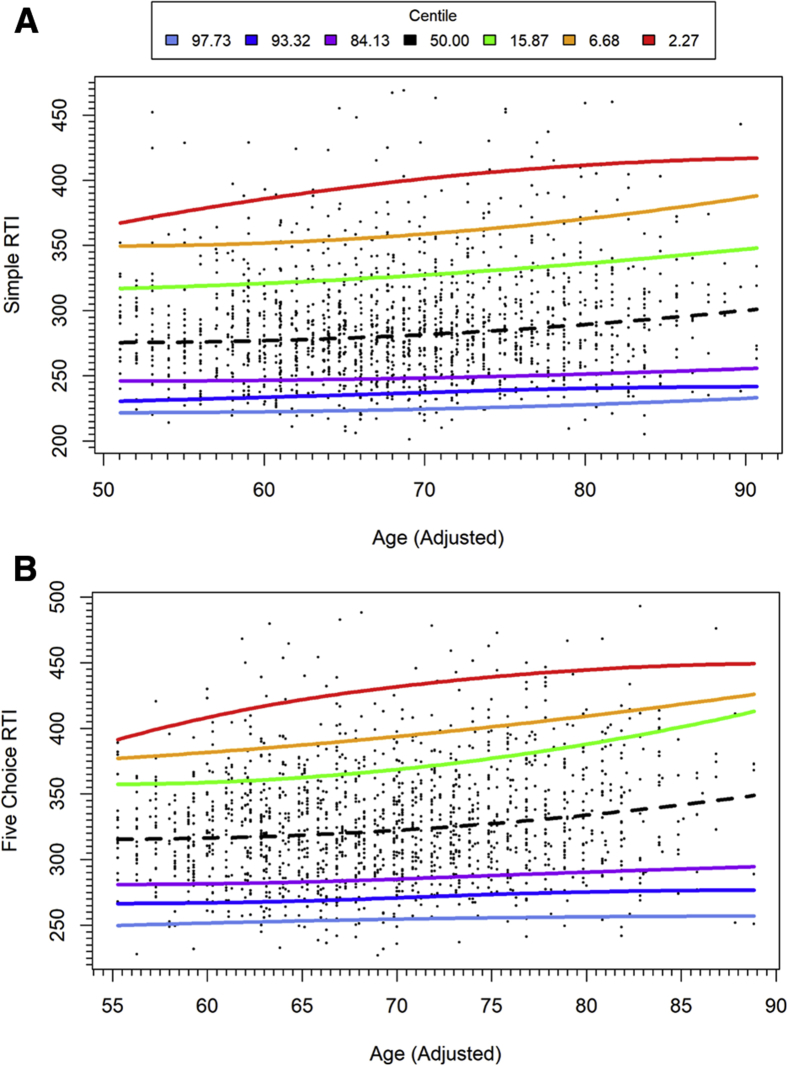
PAL total errors adjusted showed differential rates of age-related change between performance bands (Fig. 1A). From mid-life to 70 years, there was a marked increase in errors per year of age within individuals performing in the average range, but a slower rate of performance deterioration per year of age for participants in the higher performance bands and those in the low average, impaired, and very impaired performance bands. For SWM between errors, a similar trend was retained across performance bands, with roughly parallel slopes and consistent bandwidth (Fig. 1B). Broadly parallel slopes were seen for RTI, with an exception of the highest performance bands, which showed negligible decline with age (Fig. 2).
Fig. 1.
Normative data derived from quantile regression: (A) PAL total errors adjusted with adjusted age; (B) SWM between errors performance with adjusted age. Above average performance in blue and purple; below average performance in green, yellow and red. Adjusted age is calculated as described in the statistical analysis section. Abbreviations: PAL, paired-associate learning; SWM, spatial working memory.
Fig. 2.
Quantile regression plot for RTI measures with adjusted age: (A) RTI median simple reaction time; (B) RTI median five-choice reaction time. Above average performance in blue and purple; below average performance in green, yellow, and red. Adjusted age is calculated as described in the statistical analysis section. Abbreviation: RTI, reaction time.
3.4. Comparison of linear and quantile regression results: paired-associate learning
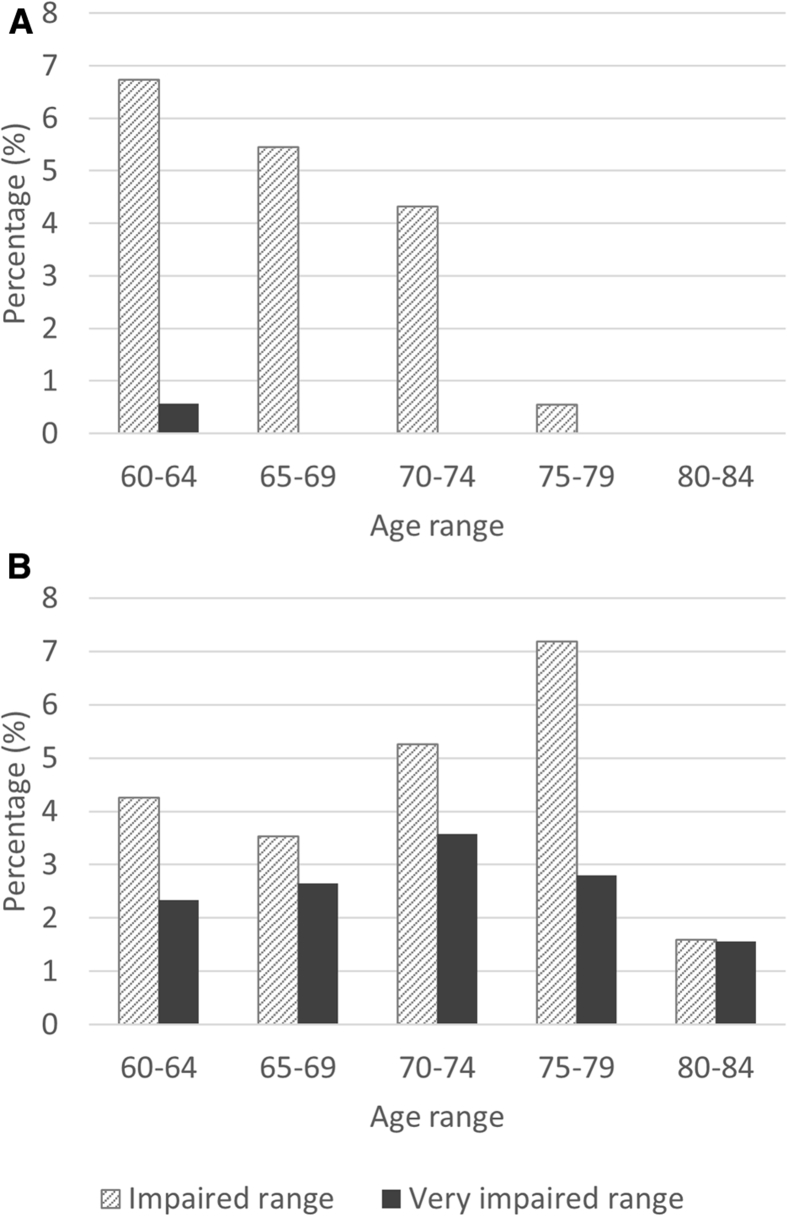
For measures with heterogeneity of age-related changes, such as in PAL, quantile regression modeling can help to refine normative thresholds of impairment. Using cutoffs for the impaired range and below (z ≤ −1.5), linear regression methods identified fewer individuals as impaired on PAL than nonlinear quantile regression methods (4.7% and 7%, respectively). Within the very impaired range (z ≤ −2) only three individuals (0.2%) were identified using cutoffs derived from linear regression, compared with 35 participants (2.7%) using limits from quantile regression.
Lower rates of impairment from linear regression are the result of lower sensitivity for detecting impairment in older participants (Fig. 3). No participants over the age of 62 years were found to perform in the very impaired range on PAL using limits derived from linear regression z scores.
Fig. 3.
Percentage of individuals in selected age ranges reaching thresholds for impairment using cutoffs from (A) linear regression, and (B) nonlinear quantile regression. Impaired range (between z = −1.5 and −2; very impaired range (z ≤ −2).
4. Discussion
The current report provides normative CANTAB data in mid-to-late life from a large population sample, screened in detail to exclude individuals with dementia, MCI, history of stroke, and elevated depressive symptoms. Our data show that CANTAB measures are sensitive to cognitive changes seen during healthy aging. As with previous research, we show age-related decline in cognitive performance measures [3], [5], including an increase in errors during tasks of working memory and episodic memory, as well as increased RTIs on measures of motor response speed.
Compared with previous publications in smaller samples of healthy participants where CANTAB performance is stratified across age ranges [35], [36], the present study provides a more fine-grained view of cognitive development in mid-to-late adulthood. This previous research shows large changes in CANTAB cognitive test performance with aging, including an approximately 30% increase in errors for visuospatial paired associates and around 20% increase in errors for spatial working memory between ages 60-64 and 65-69 years [36].
Quantile regression allows for differential curve estimation across performance bands. In the present study, a broadly parallel decline across performance quantiles was seen for SWM between errors with aging. For RTI tasks, a parallel decline was seen in all but the highest performing individuals, who showed no age-related change in RTIs. For episodic memory function, individuals performing in the average range showed a rapid age-related increase in PAL total errors adjusted. This rapid increase may be linked to the adjustment factor applied to PAL total errors adjusted, which provides an adjusted error score where participants fail a stage after four attempts and indicates that failure of PAL stages becomes more common for participants performing in the average range from mid to late life. Greater stability in test performance with aging was seen for the impaired and superior performance ranges.
For heterogeneous age-related changes across test performance bands, nonlinear quantile regression methods can help to improve sensitivity of normative thresholds for impairment. When derived from quantile regression methods, impairment thresholds for PAL total error adjusted provided more inclusive limits for older ages. Linear regression methods assume equal rates of change across performance levels, which may result in impracticably high thresholds for impairment in older adults.
Increased sensitivity of measures to assist in early detection of impairment and cognitive decline may help to improve the effectiveness of therapeutic interventions and pharmacological trials [37]. Dementia is a clinical syndrome characterized by cognitive and functional decline [38]. Current conceptions of chronic diseases such as dementia include extended premanifest disease stages [39], ushered in by the advent of prevention trials [40]. MCI was formulated as a transitional state between healthy cognitive functioning and dementia, and has evolved into a therapeutic target in prevention trials [41]. Amnestic MCI (characterized by impaired memory) is considered a possible prodromal stage of Alzheimer's disease (AD) [41].
PAL performance is sensitive to disease progression in AD, including amnestic MCI [17], [18], [19], [20], [42], [43]. Impairment or deterioration in PAL performance has been linked to biomarkers sensitive to disease progression in AD. These include higher CSF levels of tau and p-tau181, and reduced hippocampal volumes [19], presence of the apolipoprotein E (APOE) ε4 allele and above threshold amyloid β (Aβ) accumulation [44], [45]. Sensitive normative data from PAL may therefore be helpful for identifying impairment and accelerated cognitive decline in aging associated with disease progression in AD.
The current findings show a protective effect of education on cognitive function, in line with previous research [46], [47]. This may reflect a proxy influence of socioeconomic status or wealth and financial resources, also linked to lower dementia incidence [48]. In agreement with other studies, we find better performance by men on tests of spatial working memory [6], although sex differences for RTI and memory, reported previously [49], did not reach significance. Our findings and others [5], [47], highlight the importance of taking sex and educational level into consideration when examining cognitive function in aging and the importance of their inclusion in population norms [9].
Study limitations include (1) loss to follow-up: the subsample who completed CANTAB comprised a younger and more educated subgroup; (2) only a small proportion of men in the sample had been educated for <10 years, which may not reflect other population cohorts in this age range; and (3) the population was selected from a discrete geographic region, which may limit translation to other populations. These weaknesses highlight the need for replication in a separate population sample. However, this study also has specific strengths, including the large sample size, the epidemiological approach (reducing participant self-selection), the nonverbal nature of the tests used, and careful clinical characterization of participants. The current data set is cross-sectional, and longitudinal data are required to document cognitive trajectories across different performance ranges over time.
Research in Context.
-
1.
Systematic review: We examined the published literature on cognitive aging in mid-to-late life using traditional sources (e.g. PubMed). Research documents the effect of aging on a range of cognitive functions; however, normative data for the Cambridge Neuropsychological Test Automated Battery (CANTAB), a widely used computerized cognitive assessment, are not available.
-
2.
Interpretation: This article presents CANTAB results in a large mid-to-late life population sample. Linear regression and nonlinear quantile regression methodologies, adjusting for sex and education, provide detailed normative data of cognition in aging. In line with previous work, age-related cognitive decline affected all tests. Quantile regression identified discrete trajectories (high and low performers that differed from the average), which were most striking for episodic memory.
-
3.
Future directions: Normative data can assist in identifying cognitive impairment and decline. Replication in another sample would be desirable, and longitudinal data are now also required to examine trajectories across different performance ranges.
Acknowledgments
Advisory Board: T. Meinertz, Hamburg, Germany (Chair); C. Bode, Freiburg, Germany; P.J. de Feyter, Rotterdam, Netherlands; B. Güntert, Hall LT, Austria; F. Gutzwiller, Bern, Switzerland; H. Heinen, Bonn, Germany; O. Hess, Bern, Switzerland; B. Klein (†), Essen, Germany; H. Löwel, Neuherberg, Germany; M. Reiser, Munich, Germany; G. Schmidt (†), Essen, Germany; M. Schwaiger, Munich, Germany; C. Steinmüller, Bonn, Germany; T. Theorell, Stockholm, Sweden; S.N. Willich, Berlin, Germany.
Criteria and endpoint committee: Bode C, Freiburg (Chair); Berger K, Köpcke W & Ringelstein EB, Münster; Figulla HR, Jena; Hamm C, Bad Nauheim; Hanrath P, Aachen; Weimar C, Essen; Zeiher A, Frankfurt (all of Germany).
Funding: Heinz Nixdorf Foundation [Chairman: Martin Nixdorf; Past Chairman: Dr Jur Gerhard Schmidt (deceased)]; German Ministry of Education and Science (BMBF); German Aero-space Center; German Research Foundation (DFG project grant numbers: ER 155/6-2, SI 236/8-1, SI 236/9-1); Dr. Werner Jackstädt-Stiftung; Else Kröner-Fresenius-Stiftung (2015_A119); Johnson&Johnson and Cambridge Cognition.
Footnotes
Conflict of interest: M.J., A.W., N.P., and C.W. have nothing to disclose. R.A.A., C.S., P.J.N., P.R., and F.K.C. are employed by, or former employees of, Cambridge Cognition. M.T., L.v.N., J.S., and M.K. are employed or former employees of Janssen, Pharmaceutical companies of Johnson and Johnson. J.S. is currently employed by UCB. P.J.N. is an employee of Heptares Therapeutics.
References
- 1.Sherwood B., Zhou A.X.H., Weintraub S., Wang L. Using quantile regression to create baseline norms for neuropsychological tests. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2016;2:12–18. doi: 10.1016/j.dadm.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedden T., Gabrieli J.D.E. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 3.Hoogendam Y.Y., Hofman A., Van Der Geest J.N., Van Der Lugt A., Ikram M.A. Patterns of cognitive function in aging: The Rotterdam Study. Eur J Epidemiol. 2014;29:133–140. doi: 10.1007/s10654-014-9885-4. [DOI] [PubMed] [Google Scholar]
- 4.Verhaeghen P., Salthouse T.A. Meta-analyses of age–cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- 5.Zaninotto P., Batty G.D., Allerhand M., Deary I.J. 2018. Cognitive function trajectories and their determinants in older people : 8 years of follow-up in the English Longitudinal Study of Ageing; pp. 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Wild K., Howieson D., Webbe F., Seelye A., Kaye J. Status of computerized cognitive testing in aging: A systematic review. Alzheimer’s Dement. 2008;4:428–437. doi: 10.1016/j.jalz.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin P.K., Schroeder R.W., Baade L.E. A tale of two norms: the impact of normative sample selection criteria on standardized scores in older adults. Clin Neuropsychol. 2017;31:1204–1218. doi: 10.1080/13854046.2017.1349182. [DOI] [PubMed] [Google Scholar]
- 9.Busch R.M., Chapin J.S. Review of normative data for common screening measures used to evaluate cognitive functioning in elderly individuals. Clin Neuropsychol. 2008;22:620–650. doi: 10.1080/13854040701448793. [DOI] [PubMed] [Google Scholar]
- 10.Park H.L., Connell J.E.O., Thomson R.G. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry. 2003;18:1121–1134. doi: 10.1002/gps.1023. [DOI] [PubMed] [Google Scholar]
- 11.Salthouse T. Selective review of cognitive aging. J Int Neuropsychol. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabbitt P., Diggle P., Smith D., Holland F., Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia. 2001;39:532–543. doi: 10.1016/s0028-3932(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 13.Cheung Y.B., Xu Y., Feng L., Feng L., Nyunt M.S.Z., Chong M.S. Unconditional and conditional standards using cognitive function curves for the modified Mini-Mental State Exam: Cross-sectional and longitudinal analyses in older Chinese adults in Singapore. Am J Geriatr Psychiatry. 2015;23:915–924. doi: 10.1016/j.jagp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan R.M., Coen R.F., Kenny R.A., Lawlor B.A. Preservation of the semantic verbal fluency advantage in a large population-based sample: normative data from the TILDA study. J Int Neuropsychol Soc. 2016;22:570–576. doi: 10.1017/S1355617716000291. [DOI] [PubMed] [Google Scholar]
- 15.Schatz P., Browndyke J. Applications of computer-based neuropsychological assessment. J Head Trauma Rehabil. 2002;17:395–410. doi: 10.1097/00001199-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Sahakian B.J., Coull J.T. Tetrahydroaminoacridine (tha) in alzheimer’s disease: an assessment of attentional and mnemomic function using cantab. Acta Neurol Scand. 1993;88:29–35. doi: 10.1111/j.1600-0404.1993.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 17.Fowler K.S., Saling M.M., Conway E.L., Semple J.M., Louis W.J. Computerized neuropsychological tests in the early detection of dementia: prospective findings. J Int Neuropsychol Soc. 1997;3:139–146. [PubMed] [Google Scholar]
- 18.Fowler K.S., Saling M.M., Conway E.L., Semple J.M., Louis W.J. Paired associate performace in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- 19.Nathan P.J., Lim Y.Y., Abbott R., Galluzzi S., Marizzoni M., Babiloni C. Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI) Neurobiol Aging. 2017;53:1–10. doi: 10.1016/j.neurobiolaging.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Reijs B.L.R., Ramakers I.H.G.B., Köhler S., Teunissen C.E., Koel-Simmelink M., Nathan P.J. Memory Correlates of Alzheimer’s Disease Cerebrospinal Fluid Markers: A Longitudinal Cohort Study. J Alzheimer’s Dis. 2017;60:1119–1128. doi: 10.3233/JAD-160766. [DOI] [PubMed] [Google Scholar]
- 21.Robbins T.W., James M., Owen A.M., Sahakian B.J., McInnes L., Rabbitt P. Cambridge Neuropsychological Test Automated Battery ( CANTAB ): A Factor Analytic Study of a Large Sample of Normal Elderly Volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 22.Kuzmickienė J., Kaubrys G. Selective Ability of Some CANTAB Battery Test Measures to Detect Cognitive Response to a Single Dose of Donepezil in Alzheimer Disease. Med Sci Monit. 2015;21:2572–2582. doi: 10.12659/MSM.895381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmermund A., Möhlenkamp S., Berenbein S., Pump H., Moebus S., Roggenbuck U. Population-based assessment of subclinical coronary atherosclerosis using electron-beam computed tomography. Atherosclerosis. 2006;185:177–182. doi: 10.1016/j.atherosclerosis.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Erbel R., Mӧhlenkamp S., Moebus S., Schmermund A., Lehmann N., Stang A. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of Subclinical coronary atherosclerosis: The Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 26.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.-O. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 27.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff L.S. A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Hautzinger M., Bailer M. Belz Test. Weinheim; 1993. ADS - Allegemeine Depressionsskala. [Google Scholar]
- 30.Icks A., Kruse J., Dragano N., Broecker-Preuss M., Slomiany U., Mann K. Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabet Med. 2008;25:1330–1336. doi: 10.1111/j.1464-5491.2008.02585.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmermund A., Möhlenkamp S., Stang A., Grönemeyer D., Seibel R., Hirche H. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: Rationale and design of the Heinz Nixdorf RECALL study. Am Heart J. 2002;144:212–218. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 32.Rabbitt P., Lowe C. Patterns of cognitive ageing. Psychol Res. 2000;63:308–316. doi: 10.1007/s004269900009. [DOI] [PubMed] [Google Scholar]
- 33.van der Elst W., van Boxtel M.P.J., van Breukelen G.J.P., Jolles J. Rey’s verbal learning test: Normative data for 1855 healthy participants aged 24 – 81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11:290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 34.Muggeo V.M.R., Sciandra M., Tomasello A., Calvo S. Estimating growth charts via nonparametric quantile regression: A practical framework with application in ecology. Environ Ecol Stat. 2013;20:519–531. [Google Scholar]
- 35.De Luca C.R., Wood S.J., Anderson V., Buchanan J.-A., Proffitt T.M., Mahony K. Normative data from the CANTAB. I: development of executive function over the lifespan. J Clin Exp Neuropsychol. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- 36.Robbins T.W., James M., Owen A.M., Sahakian B.J., McInnes L., Rabbit P. Cambridge Neuropsychological Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 37.Cummings J.L., Doody R., Clark C. Disease-modifying therapies for Alzheimer disease. Neurology. 2007;69:1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- 38.Bouchard R.W. Diagnostic criteria of dementia. Can J Neurol Sci. 2007;34:S11–S18. doi: 10.1017/s0317167100005497. [DOI] [PubMed] [Google Scholar]
- 39.Cole J.H., Marioni R.E., Harris S.E., Deary I.J. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry. 2018:1–16. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malek-Ahmadi M. Reversion from Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Alzheimer Dis Assoc Disord. 2016;30:324–330. doi: 10.1097/WAD.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen K.F., Larsen J.P., Tysnes O.-B., Alves G. Prognosis of Mild Cognitive Impairment in Early Parkinson Disease. JAMA Neurol. 2013;70:580. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 42.De Jager C., Blackwell A.D., Budge M.M., Sahakian B.J. Predicting cognitive decline in healthy older adults. Am J Geriatr Psychiatry. 2005;13:735–740. doi: 10.1176/appi.ajgp.13.8.735. [DOI] [PubMed] [Google Scholar]
- 43.Egerhazi A., Berecz R., Bartok E., Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:746–751. doi: 10.1016/j.pnpbp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Abbott R.A., Nathan P.J., Lim Y.Y., Galluzzi S., Marizzoni M., Bagnoli C. Differential Effects of Apoe and CSF Amyloid on Memory Impairment in Individuals With Amnestic Mci Using the Cantab Cognitive Battery: Results From the European-Adni Study. Alzheimer’s Dement. 2016;12:P964–P965. [Google Scholar]
- 45.Nathan P.J., Abbott R.A., Lim Y.Y., Galluzzi S., Marizzoni M., Bagnoli C. Csf Beta-Amyloid- and Apoe Ɛ4-Related Decline in Episodic Memory Over 12 Months Measured Using the Cantab in Individuals With Amnestic Mci: Results From the European Adni Study. Alzheimer’s Dement. 2016;12:P751. [Google Scholar]
- 46.Meng X., D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Hooren S.A.H., Valentijn A.M., Bosma H., Ponds R.W.H.M., van Boxtel M.P.J., Jolles J. Cognitive Functioning in Healthy Older Adults Aged 64-81: A Cohorst Study into the Effects of Age, Sex, and Education.pdf. Aging Neuropsychol Cogn. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 48.Cadar D., Lassale C., Davies H., Llewellyn D.J., Batty G.D., Steptoe A. Individual and area-based socioeconomic factors associated with dementia incidence in England: Evidence from a 12-year follow-up in the English longitudinal study of ageing. JAMA Psychiatry. 2018;75:723–732. doi: 10.1001/jamapsychiatry.2018.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenehan M.E., Summers M.J., Saunders N.L., Summers J.J., Vickers J.C. Does the Cambridge Automated Neuropsychological Test Battery (CANTAB) Distinguish Between Cognitive Domains in Healthy Older Adults? Assessment. 2016;23:163–172. doi: 10.1177/1073191115581474. [DOI] [PubMed] [Google Scholar]