Abstract
Telomeres are repetitive non-coding DNA sequences located at the ends of chromosomes in eukaryotic cells. Their most important function is to protect chromosome ends from being recognized as DNA damage. They are also implicated in meiosis and synapse formation. The length of telomeres inevitably shortens at the end of each round of DNA replication and, also, as a consequence of the exposure to oxidative stress and/or genotoxic agents. The enzyme telomerase contributes to telomere lengthening. It has been reported that telomerase is exclusively expressed in germ cells, granulosa cells, early embryos, stem cells, and various types of cancerous cells. Granulosa cells undergo many mitotic divisions and either granulosa cells or oocytes are exposed to a variety of genotoxic agents throughout folliculogenesis; thus, telomerase plays an important role in the maintenance of telomere length. In this review article, we have comprehensively evaluated the studies focusing on the regulation of telomerase expression and activity, as well as telomere length, during folliculogenesis from primordial to antral follicles, in several mammalian species including mice, bovines, and humans. Also, the possible relationships between female infertility caused by follicular development defects and alterations in the telomeres and/or telomerase activity are discussed.
Keywords: Female infertility, Granulosa cell, Oocyte, Telomerase, Telomere
Introduction
Telomeres are specialized nucleoprotein structures located at the ends of all eukaryotic chromosomes and are composed of repetitive DNA sequences and associated proteins [1]. They were first identified in Drosophila melanogaster by Herman Muller [2]. He named them “telomeres” from the Greek words telos, meaning ‘end’, and meros, meaning ‘part’ [2]. Telomeric repetitive DNA sequences exhibit differences among organisms, e.g., TTTTGGGG in Oxytricha [3], TTGGGG in Tetrahymena [4], and TTAGGG in mammals [5]. Moreover, the length of telomeres differs in mammals, ranging from 50 to 150 kb in mice [6], 12–23 kb in bovines [7], and 10–15 kb in humans [8].
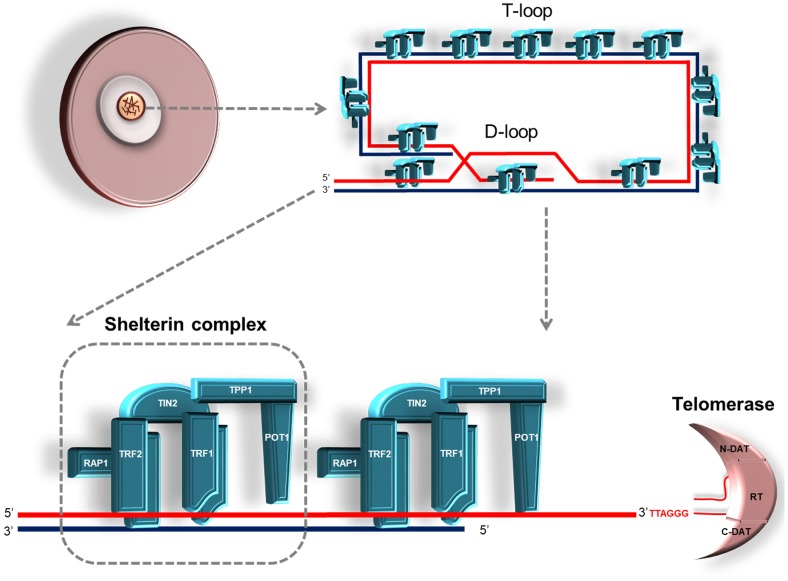
Telomeres contain two main DNA sequences: the subtelomere and the main telomere [9]. The subtelomere (1–200 kb in length), adjacent to the main telomere, includes either a low copy number of telomeric DNA repeats or duplicated DNA strands [10]. It has been shown that the subtelomeric region plays an important role in maintaining telomeric integrity, as well as in telomere lengthening, by a mechanism known as alternative lengthening of telomeres (ALT) [11, 12]. The main telomere region consists of single and double DNA strands, mostly comprising specific DNA repetitive TTAGGG sequences, in mammals. At the 3' end of the telomere region, within the single-stranded part, there is a guanine-rich sequence, 50 to 500 nucleotides in length, called G overhang or G-rich region [13]. The G overhang region invades the double-stranded telomeric DNA to protect the single-stranded DNA from being recognized as a DNA break, forming both D-loop and T-loop structures at the ends of all chromosomes [14] (Fig. 1).
Fig. 1.
Schematic diagram showing telomere structure, telomere-associated proteins (TRF1, TRF2, POT1, TIN2, TPP1, and RAP1; forming the shelterin complex), and telomerase in a mammalian oocyte. The 3' overhang of telomere end enters the doubled-stranded DNA to create a displacement (D-loop) and a telomere (T-loop) loop. The shortened telomeres are elongated by the telomerase enzyme, composed of a TERT and a TERC subunits, as well as by other components.
Telomeres were previously considered as transcriptionally silent regions; however, later studies have shown that telomeres are transcribed into telomere repeat-containing RNAs (TERRAs) [15,16,17]. TERRA molecules have been characterized in rodents and humans [15, 18]. They contain UUAGGG repeats and range from 100 bp to 9 kb in length [15]. Interestingly, TERRAs possess multiple promoter regions at the subtelomeric site [19]. Since they reside at telomeres during the S phase of the cell cycle, the main function of TERRAs is thought to be the maintenance of telomeric stability [20, 21]. Moreover, they participate in the re-extension of telomeres by organizing the recruitment of telomerase to the shortened telomeres [20]. Consistently, TERRA molecules interact with telomerase reverse transcriptase (TERT), telomeric repeat factor 1 (TRF1), and telomeric repeat factor 2 (TRF2) [16, 17]. TERRAs are found to be expressed in the human fetal oocytes throughout the germinal vesicle stage [17] and in the human spermatogenic cells [22].
Telomere-associated Proteins
In addition to DNA, telomeres include telomere-associated proteins including TRF1, TRF2, protection of telomeres 1 (POT1), TRF1-interacting nuclear protein-2 (TIN2), TIN2 interacting protein (TPP1), repressor-activator protein 1 (RAP1), as well as TRF-interacting proteins (Fig. 1). The primary role of these proteins is the maintenance of chromosomal end integrity and the regulation of the telomere lengthening process [23]. A complex of six telomere-associated proteins (TRF1, TRF2, POT1, TIN2, TPP1, and RAP1) are known as shelterin or telosome [24] (Fig. 1).
The most extensively studied telomere-associated proteins, TRF1 and TRF2, specifically bind to the double-stranded telomeric DNA [25] (Fig. 1). TRF1 forms homodimers that bind to the telomeric DNA and thereby control telomerase activity, preventing further telomere elongation [26, 27]. Moreover, TRF1 also interacts with TRF2 in cooperation with TIN2, contributing to the shelterin complex stability [28]. Notably, the absence of TRF1 causes replication fork-dependent defects, telomeric fusion, and altered genome packaging [29]. TRF2 also associates with double-stranded DNA upon formation of TRF2-TRF2 homodimers [25]. TRF2 is structurally similar to TRF1, except that its N-terminal end contains more basic amino acid residues [30]. RAP1 interacts with TRF2, and both play a critical role in regulating the expression of specific genes [31]. As TRF2 is implicated in the protection of the G-overhang stability, its lack leads to loss of the G-overhang structure [32].
Another member of the shelterin complex, TPP1, interacts with either TIN2 or POT1 and this event facilitates the interaction between telomere-binding proteins and the G-overhang which, in turn, prevents the activation of the DNA damage response [33]. In addition, both POT1 and TPP1 are involved in the regulation of telomere length [34]. Notably, although TPP1 does not directly bind to telomeric DNA, it increases the binding affinity of POT1 to telomeric DNA by 5–10 fold [35]. Moreover, TPP1 promotes telomerase activity [34]; however, POT1 masks the 3' telomeric end and prevents telomerase access to the telomeric area [36]. Consistently, POT1 knockdown by RNA interference causes loss of G-overhangs, chromosomal instability, senescence, and induction of apoptosis [37]. In summary, the telomere-binding proteins preserve telomeric integrity and ensure the formation and maintenance of D-loop and T-loop structures. Interestingly, these proteins may combine with each other forming various homo- or heterodimers, reflecting their multifaceted regulation of telomeric functions. Examples of these complexes are TRF1-TRF1, TRF2-TRF2, TRF1-TIN2-TPP1/POT1, TRF2-RAP1-TIN2-TPP1/POT1, and TRF2-RAP1 [38].
Telomerase is Mainly Responsible for Telomere Lengthening
Telomere lengthening is carried out by two different mechanisms: the telomerase-based pathway and the ALT process. The telomerase enzyme was first discovered in the ciliate “Tetrahymena thermophila” [4] and it is evolutionarily conserved in mammals. Telomerase consists of two main subunits: TERT and the telomerase RNA component (TERC) [39] (Fig. 1). Moreover, mass spectrometric analysis of affinity-purified telomerase from HeLa cells revealed that the telomerase complex comprises accessory proteins such as dyskerin, NHP2, NOP10, pontin/reptin, and TCAB1 [40, 41]; nevertheless, these proteins are not required for telomerase activity [42].
The TERT subunit of telomerase contains three main structural elements, i.e., a long N-terminal (N-DAT) domain including conserved DNA- and RNA-binding regions, a central catalytic reverse transcriptase domain (RT domain), and a C-terminal C-DAT domain [43] (Fig. 1). The N-DAT region of TERT involves two subdomains: the telomerase essential N-terminal (TEN) domain and the telomerase RNA-binding domain (TRBD), both of which are responsible for binding to nucleic acids [44]. The RT domain of TERT consists of two subdomains, called ‘fingers’ and ‘palm’ [45, 46]. These subdomains are the functional regions of the enzyme facilitating the direct interaction with the RNA-DNA hybrids [47]. It is important to note that the C-DAT domain exhibits poor sequence conservation, unlike other regions of TERT [35]. The TERC subunit of telomerase has three conserved regions: the pseudoknot/template core, the CR4/CR5, and the box H/ACA domains [48]. While the core domain is only required for telomerase activity [49], the box H/ACA domain is important for the stability and the nuclear localization of the TERC subunit [49, 50]. The exact function of the CR4/CR5 domain remains elusive.
In addition to telomerase-driven elongation, telomeres can be lengthened by an alternative mechanism known as ALT, especially in the absence of telomerase activity [51]. Although the ALT mechanism has not been fully elucidated, it is mainly based on homologous recombination (HR) between telomeres of sister chromatids [52]. The Werner syndrome helicase (WRN) and the Bloom syndrome helicase (BLM) are necessary for the proper functioning of the ALT process [53]. Further molecular and functional studies are required to characterize the mechanisms underlying ALT activation in eukaryotic cells.
Telomerase Activity and Telomere Length During Folliculogenesis in Mammals
Female primordial germ cells (PGCs) originate from the extra-embryonic yolk sac endoderm and migrate to the genital ridge [54], after which they undergo additional mitotic divisions and become oogonia [55]. Once they have entered meiosis, the oogonia in the genital ridges are defined as oocytes. Before birth, primordial follicles are formed. These are composed of oocytes arrested at the diplotene stage of prophase I of the first meiotic division, as well as of squamous follicular cells [56]. Folliculogenesis may be defined as the development of the primordial follicles into antral follicles by consecutive gonadotropin-independent and -dependent mechanisms [57, 58]. First, primordial follicles, with squamous follicular cells surrounding the oocyte, mature into primary follicles, covered by one layer of cuboid follicular cells [57]. Thereafter, the cuboidal follicular cells undergo multiple mitotic divisions, resulting in the formation of secondary follicles containing a few layers of granulosa cells [57]. The secondary follicles develop into early antral follicles with small antral spaces among the granulosa cells [57]. Small antral spaces eventually join to form single large antrum at the antral follicular stage. In the antral follicles, oocytes remaining at the prophase I stage of the first meiosis undergo luteinize hormone-dependent nuclear and cytoplasmic maturation [58].
It is known that granulosa cells undergo many mitotic divisions during folliculogenesis, which result in telomeric shortening. Additionally, granulosa cells are exposed to many agents, including hormones and other factors, accumulating in the follicular fluid. This microenvironment most likely affects the length of telomere in these cells. The granulosa cells also possess telomerase activity capable of restoring the shortened telomeres [59, 60]. To date, a few studies have addressed the mechanisms controlling telomere length, as well as the expression and activity of telomerase, in the granulosa cells and the oocytes during folliculogenesis in rodents, bovines, pigs, and humans. It is important to note that telomerase activity is largely controlled through the expression levels of TERT protein and mRNA [61]. However, the TERC subunit of telomerase also affects telomerase activity since the lack of TERC (TERC–/–) results in telomeric loss at late stages of mouse embryogenesis [62]. Thus, although telomerase activity is largely dependent on TERT expression, the TERC subunit seems to be a limiting factor for sustained telomerase activity.
Telomerase activity as a function of follicular development was initially evaluated in rats by Eisenhauer et al. [63]. Enzyme activity was found to be higher in small and healthy follicles than in large and atretic follicles [63]. High telomerase activity was detected in oocytes from both preantral and preovulatory follicles, whereas it was relatively low in ovulated oocytes [63]. A key role of estrogen in the regulation of telomerase activity in the granulosa cells, was suggested by the observation that cell exposure to estrogen causes increased telomerase activity, most likely as a result of enhanced TERT expression [64]. Consistently, a recent in vitro study showed that high concentrations of estradiol-17b (E2) facilitate telomere elongation in granulosa cells obtained from early antral follicles [65]. As it is known, luteinizing hormone surges resume meiotic activity, resulting in mature metaphase II (MII) ovulating oocytes from antral follicles. In mice, telomerase activity is lower in MII oocytes in comparison with germinal vesicle (GV) oocytes [66]. Telomere length was also measured in different mouse strains such as hybrid B6C3F1 and outbred CD1 by quantitative fluorescent in situ hybridization (Q-FISH) [66]. The length of telomeres was found to be 18.03 ± 0.47 kb and 16.09 ± 0.78 kb in the MII oocytes of hybrid B6C3F1 and CD1 mice, respectively [66]. The difference between these values suggests the presence of distinct telomere lengthening mechanisms in the MII oocytes of the two strains. However, the potential impact of telomere length on the lifespan of these mice still remains elusive.
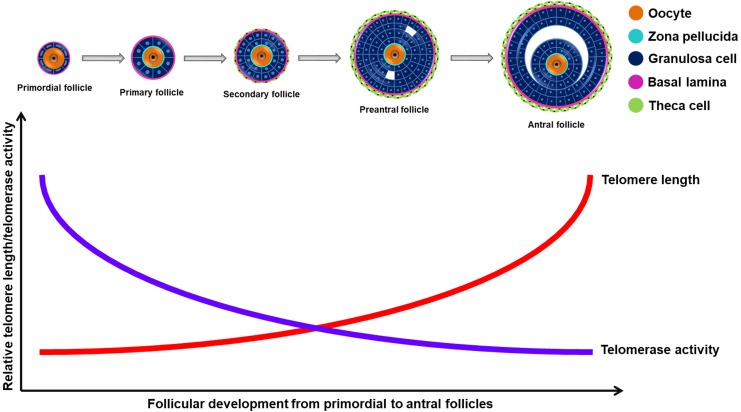
In bovines, similar to that observed in rats, high telomerase activity was found in the smallest pre-antral follicles (60–100 μm in diameter), which gradually declined during the transition from small- (1 mm diameter) to medium-sized (3 mm diameter) follicles [67]. In the same study, the TERC subunit of telomerase was found to localize to the granulosa cells of growing follicles, but it was not detected in the primordial follicles [67]. In pigs, TERT protein expression was detected by immunohistochemistry in the primary, preantral, small antral, and medium/large antral ovarian follicles [68]. Notably, the subcellular distribution of TERT exhibited maturation stage-dependent differences: the protein was localized in the cytoplasm of oocytes in the antral follicles, whereas it was in the nuclei of oocytes in either primary or pre-antral follicles [68] (Fig. 2). In the MII oocytes, TERT was abundant in the subcortical cytoplasm, while no TERT expression was found in the polar bodies [68]. When Russo et al. (2006) analyzed the telomere length in porcine granulosa cells by using fluorescence in situ hybridization (FISH), longer telomeres were found in the preantral and antral follicles with respect to early-stage follicles [68] (Fig. 2). However, oocytes in the preantral follicle had shorter telomeres compared to the oocytes of the primary, small-antral, and medium/large antral follicles [68]. Thus, regulation of telomere length was somehow divergent in granulosa cells and oocytes. This may result from a different activation timing of telomere lengthening processes during developmental maturation. Newly designed studies, analyzing telomerase activity and telomere length during folliculogenesis in either the oocytes or granulosa cells are required to directly address this issue.
Fig. 2.
Telomerase activity and telomere length during folliculogenesis. The studies performed in pigs and bovines suggest that the telomerase activity gradually decreases and the length of telomeres progressively increases from primordial follicles to antral follicles. The blue line represents telomerase activity, whereas the red line represents telomere length.
Telomerase activity was also evaluated during oocyte maturation in bovines by using the TRAP method [69]. The activity was high in the immature oocytes, gradually decreased during oocyte maturation, and reached the lowest level in the mature oocytes [69] (Fig. 2). When the expression of TRF1, TRF2, and TERC was examined in the bovine GV and MII oocytes, as well as in the early embryos, no significant differences were detected [70]. Notably, measurement of the relative telomere length by quantitative real time PCR (qPCR) revealed the presence of longer telomeres in GV oocytes compared to MII oocytes, in bovines [70] (Fig. 2). On the other hand, Meerdo et al. (2005) observed no differences in telomere length between immature and mature oocytes in bovines [71]. This discrepancy may derive from the use of different techniques for telomere measurement or the different samples employed.
Ovarian Function and Telomeres/Telomerase Activity in Humans
A few studies have explored telomerase expression and telomere length in human oocytes. Generally, the surplus human oocytes donated by couples undergoing treatment with assisted reproductive technologies (ARTs) are used in this kind of investigations. Relative telomerase activity was measured in the GV and MII oocytes, as well as in the early embryos, using the TRAP assay [72]. Telomerase activity is significantly higher in the GV oocytes compared to that in the MII oocytes [72], as also found in mouse [66] and bovine oocytes [69]. The length of telomeres in human GV oocytes obtained from patients undergoing in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), was found to be 11.12 kb, as assessed by Q-FISH [73]. In another study conducted by the same group, telomere lengths were measured in human oocytes, spermatozoa, male and female pronuclei [74]. The average telomere length of the human spermatozoa and mature oocytes was 6.32 ± 2.00 kb and 8.79 ± 0.86 kb, respectively, whereas it was 6.16 kb and 8.63 kb in the male and female pronuclei, respectively [74]. It is important to note that telomere length in the mature oocytes (8.79 ± 0.86) was significantly shorter than in the immature oocytes (11.41 ± 0.81) [74]. Consistently, there is no telomerase activity in the unfertilized mature human oocytes [75]. Most likely, in the absence of telomerase activity, progressive telomere shortening during oogenesis results in shorter telomeres in the mature oocytes. On the other hand, genetically induced telomeric shortening in mice triggers cytoplasmic fragmentation and disrupts meiosis and chiasm formation [76], resulting in defective oocyte fertilization and abnormal embryo cleavage [77]. Telomeric shortening has also been demonstrated to induce apoptosis in human preimplantation embryos [78]. Interestingly, successful conception was associated with the presence of longer telomeres in the oocytes [79]. Consistently, Keefe et al. (2003) found that IVF patients with relatively long telomeres in their oocyte polar bodies (7.5 ± 1.17 kb) had higher chances of becoming pregnant as compared to patients with shorter telomeres (6.2 ± 1.69 kb) [80]. Importantly, pregnancy seems to be precluded to patients with telomere lengths below 6.32 kb [80]. In the granulosa cells, telomerase activity is a more suitable parameter, as compared to telomere length, for predicting the pregnancy outcome following IVF treatment [81]. Consistently, the length of telomeres in the mature oocytes and early embryos seems to be crucial for successful pregnancy outcome. Ovarian granulosa cells surround the oocytes and support their development. It has been suggested that IVF treatments carried out in the presence of high levels of telomerase activity in granulosa cells may result in increased chances of pregnancy [82]. However, in another study, no differences were found in the telomere length of granulosa cells between the different IVF patient groups, albeit telomerase activity was higher in the transferred blastocysts that actually resulted in pregnancy [81]. This study suggested that the level of telomerase activity in granulosa cells is a more effective indicator of pregnancy success, as compared to other parameters including telomere length, age, FSH, and estrogen levels. On the other hand, estrogen deficiency leads to the inhibition of TERT gene expression and telomerase activity and results in telomere shortening in mouse ovarian granulosa cells [64]. Estrogen is produced by granulosa cells in cooperation with theca cells and luteinizing cells in the corpus luteum [83] and its level during folliculogenesis seems to be important for the development of high-quality oocytes and for successful pregnancy.
As telomeres play important roles in many cellular events including meiosis, nuclear organization, and genomic integrity throughout the lifespan of eukaryotic cells, their dysfunctions associate with various types of diseases including Werner syndrome [84], dyskeratosis congenita [85], Bloom syndrome [86], Fanconi anemia [87], and cancer [88]. Furthermore, there is a close relationship between telomeres/telomerase activity and female infertility caused by ovarian aging [89], polycystic ovary syndrome (PCOS) [90], and premature ovarian failure (POF) [23].
Telomere length and telomerase activity have been investigated in the granulosa cells of 54 women aged 37 years or less, suffering from ovarian insufficiency and under treatment with ARTs [89]. This study revealed that the granulosa cells from the patient group lacked telomerase activity and displayed shorter telomeres when compared to those in their control counterparts [89]. Therefore, telomerase activity and telomere length may be used as molecular markers for ovarian insufficiency, a common condition in infertile women [89].
PCOS is one of the commonly encountered reproductive disorders exhibiting menstrual irregularity, hyperandrogenism, and polycystic ovaries as prominent clinical symptoms. Moreover, 74% of the PCOS patients experience infertility [6, 91]. The relative telomere length in peripheral blood samples from PCOS patients [0.764 ± 0.016 T/S (telomere/single copy gene)] was found be significantly shorter than that of the control group (0.876 ± 0.023 T/S) [92]. Hormonal alterations and increased oxidative stress in the PCOS patients may negatively affect the structural integrity of telomeres, leading to telomere attrition in the blood cells. It is important to note that, in order to determine the precise impact of the histopathological/hormonal changes on telomere length, telomeres should be measured in the granulosa cells of PCOS patients. In recent studies, telomere lengths were measured by qPCR in the leukocytes obtained from either PCOS patients or control subjects and no remarkable differences were detected [93,94,95]. However, the length of telomeres in the granulosa cells was found to be significantly longer in the PCOS group (1.57 ± 0.67) than in the controls (1.00 ± 0.37) [93] (Table 1). Most likely, this was due to a decreased number of mitotic divisions in granulosa cells and also to altered hormonal levels in the PCOS patients. Wang et al. (2017) also analyzed telomere length and TERRA levels in peripheral blood leukocytes of patients with PCOS and control subjects [96] and found that the telomeres were significantly longer in the patient-derived leukocytes [96]. Of note, the level of TERRAs, contributing to the regulation of telomere length, was found to be lower in the PCOS patients [96]. Previous studies have shown that, after aromatization, androgens may regulate telomerase expression and activity, acting through ERα [97, 98]. Consistently, the increased testosterone concentration observable in patients with PCOS might lead to enhanced telomerase activity, resulting in extended telomeres. In contrast, Li et al. (2017) reported that telomeres in the granulosa cells were shorter in the PCOS patients (0.971) than in the controls (1.118) (Table 1), whereas they could not detect any difference in telomerase activity [90]. Further studies with larger numbers of samples will be necessary to comprehensively evaluate the mechanisms underlying the altered regulation of telomeric length characterizing patients with PCOS.
Table 1. The studies in humans, focusing on the relationship between alteration of telomeres/telomerase activity and polycystic ovary syndrome (PCOS)/premature ovarian failure (POF) development, are summarized. qPCR, quantitative polymerase chain reaction; TRAP, telomere repeat amplification protocol.
| Ovarian disease | Analyzed parameter | The finding | Sample | Method | Species | Reference |
|---|---|---|---|---|---|---|
| PCOS | Telomere length | Increased telomere length | Follicles | qPCR | Human | Wei D et al., 2017 |
| Telomere length | Decreased telomere length | Follicles | qPCR | Human | Li Y et al., 2017 | |
| POF | Telomere length | Decreased telomere length | Follicles | qPCR | Human | Butts S et al., 2009 |
| Telomerase activity | High in the patients with dysfunctional follicles Low in the patients with follicular depletion | Follicles | TRAP | Human | Kinugawa C et al., 2000 | |
POF is an irreversible pathologic loss of the ovarian function occurring before age 40 and leading to early female infertility [99]. The predominant clinical symptoms include premature menopause, primary ovarian failure, hypergonadotropic hypogonadism, and gonadal dysgenesis [37, 93]. In the ovary of POF patients, two main defects are observed: follicle dysfunction and follicle depletion [99]. Thus, reduced ovarian function and infertility result from a declined follicle reserve and/or poor follicular response to the gonadotropins [99]. To date, only a few studies have addressed the question of whether alterations in telomere length and/or telomerase activity play a role in POF development. Kinugawa et al. (2000) first compared telomerase activity in POF patients and normal subjects by using the TRAP method [100]. Whereas the patients with follicular dysfunction displayed high ovarian telomerase activity, those with follicle depletion possessed low telomerase activity [100] (Table 1). The interpretation of these findings was that decreased telomerase activity in the ovary may arise from age-related primordial follicle depletion and that telomerase activity may represent a marker of ovarian function [100]. It has been found that women lacking telomerase activity in the granulosa cells have an 11-fold risk of experiencing POF as compared to those with normal telomerase activity [89]. Consistently, the length of telomeres was remarkably shorter in the granulosa cells of POF patients (1.88) in comparison to the controls (3.15) [89] (Table 1).
In conclusion, telomeres are conserved structures located at the ends of eukaryotic chromosomes and play a critical role in protecting genomic integrity during the cellular lifespan. Telomeres can be extended by two main pathways: the telomerase-dependent pathway and the ALT process. In addition to telomere-associated proteins, recently identified TERRA molecules have been discovered to contribute to telomere reorganization. A limited number of studies have addressed the expression of telomerase, telomere-associated proteins, and TERRA molecules, as well as telomere length, during folliculogenesis in mammals. We think that additional studies are required to evaluate the relationship between the altered expression of telomerase and telomere-associated proteins, on the one hand, and the development of female infertility, on the other. Finally, the cellular mechanisms regulating telomerase activity and telomere structure in oocytes and granulosa cells during folliculogenesis should be the focus of extensive research for the elucidation of the events underlying female infertility.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Mol Cell Biol 1990; 10: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller HJ. The remaking of chromosomes. Collect Net1938: 181-95. [Google Scholar]
- 3.Klobutcher LA, Swanton MT, Donini P, Prescott DM. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3′ terminus. Proc Natl Acad Sci USA 1981; 78: 3015–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 1978; 120: 33–53. [DOI] [PubMed] [Google Scholar]
- 5.Brown WR. Molecular cloning of human telomeres in yeast. Nature 1989; 338: 774–776. [DOI] [PubMed] [Google Scholar]
- 6.Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol 2013; 50: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal K, Kues WA, Baulain U, Garrels W, Herrmann D, Niemann H. Species-specific telomere length differences between blastocyst cell compartments and ectopic telomere extension in early bovine embryos by human telomerase reverse transcriptase. Biol Reprod 2011; 84: 723–733. [DOI] [PubMed] [Google Scholar]
- 8.Gomes NM, Shay JW, Wright WE. Telomere biology in Metazoa. FEBS Lett 2010; 584: 3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingner J, Cech TR. Telomerase and chromosome end maintenance. Curr Opin Genet Dev 1998; 8: 226–232. [DOI] [PubMed] [Google Scholar]
- 10.Riethman H, Ambrosini A, Paul S. Human subtelomere structure and variation. Chromosome Res 2005; 13: 505–515. [DOI] [PubMed] [Google Scholar]
- 11.Azzalin CM, Nergadze SG, Giulotto E. Human intrachromosomal telomeric-like repeats: sequence organization and mechanisms of origin. Chromosoma 2001; 110: 75–82. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Herrera A, García F, Azzalin C, Giulotto E, Egozcue J, Ponsà M, Garcia M. Distribution of intrachromosomal telomeric sequences (ITS) on Macaca fascicularis (Primates) chromosomes and their implication for chromosome evolution. Hum Genet 2002; 110: 578–586. [DOI] [PubMed] [Google Scholar]
- 13.Wellinger RJ, Sen D. The DNA structures at the ends of eukaryotic chromosomes. Eur J Cancer 1997; 33: 735–749. [DOI] [PubMed] [Google Scholar]
- 14.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell 1999; 97: 503–514. [DOI] [PubMed] [Google Scholar]
- 15.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007; 318: 798–801. [DOI] [PubMed] [Google Scholar]
- 16.Reig-Viader R, Vila-Cejudo M, Vitelli V, Buscà R, Sabaté M, Giulotto E, Caldés MG, Ruiz-Herrera A. Telomeric repeat-containing RNA (TERRA) and telomerase are components of telomeres during mammalian gametogenesis. Biol Reprod 2014; 90: 103. [DOI] [PubMed] [Google Scholar]
- 17.Reig-Viader R, Brieño-Enríquez MA, Khoriauli L, Toran N, Cabero L, Giulotto E, Garcia-Caldés M, Ruiz-Herrera A. Telomeric repeat-containing RNA and telomerase in human fetal oocytes. Hum Reprod 2013; 28: 414–422. [DOI] [PubMed] [Google Scholar]
- 18.Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol 2008; 10: 228–236. [DOI] [PubMed] [Google Scholar]
- 19.Nergadze SG, Farnung BO, Wischnewski H, Khoriauli L, Vitelli V, Chawla R, Giulotto E, Azzalin CM. CpG-island promoters drive transcription of human telomeres. RNA 2009; 15: 2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cusanelli E, Romero CA, Chartrand P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol Cell 2013; 51: 780–791. [DOI] [PubMed] [Google Scholar]
- 21.Reig-Viader R, Garcia-Caldés M, Ruiz-Herrera A. Telomere homeostasis in mammalian germ cells: a review. Chromosoma 2016; 125: 337–351. [DOI] [PubMed] [Google Scholar]
- 22.Reig-Viader R, Capilla L, Vila-Cejudo M, Garcia F, Anguita B, Garcia-Caldés M, Ruiz-Herrera A. Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertil Steril 2014; 102: 728–738.e1. [DOI] [PubMed] [Google Scholar]
- 23.De Boeck G, Forsyth RG, Praet M, Hogendoorn PC. Telomere-associated proteins: cross-talk between telomere maintenance and telomere-lengthening mechanisms. J Pathol 2009; 217: 327–344. [DOI] [PubMed] [Google Scholar]
- 24.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19: 2100–2110. [DOI] [PubMed] [Google Scholar]
- 25.Patel TN, Vasan R, Gupta D, Patel J, Trivedi M. Shelterin proteins and cancer. Asian Pac J Cancer Prev 2015; 16: 3085–3090. [DOI] [PubMed] [Google Scholar]
- 26.van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature 1997; 385: 740–743. [DOI] [PubMed] [Google Scholar]
- 27.Meng L, Hsu JK, Zhu Q, Lin T, Tsai RY. Nucleostemin inhibits TRF1 dimerization and shortens its dynamic association with the telomere. J Cell Sci 2011; 124: 3706–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome Biol 2008; 9: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009; 138: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet 2008; 42: 301–334. [DOI] [PubMed] [Google Scholar]
- 31.Yang D, Xiong Y, Kim H, He Q, Li Y, Chen R, Songyang Z. Human telomeric proteins occupy selective interstitial sites. Cell Res 2011; 21: 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu P, van Overbeek M, Rooney S, de Lange T. Apollo contributes to G overhang maintenance and protects leading-end telomeres. Mol Cell 2010; 39: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 2007; 448: 1068–1071. [DOI] [PubMed] [Google Scholar]
- 34.Baumann P, Price C. Pot1 and telomere maintenance. FEBS Lett 2010; 584: 3779–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 2007; 445: 506–510. [DOI] [PubMed] [Google Scholar]
- 36.Chang S. Cancer chromosomes going to POT1. Nat Genet 2013; 45: 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Q, Zheng YL, Harris CC. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol 2005; 25: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus 2011; 2: 119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science 2007; 315: 1850–1853. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 1999; 402: 551–555. [DOI] [PubMed] [Google Scholar]
- 41.Fu D, Collins K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol Cell 2007; 28: 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardano L, Holland L, Oulton R, Le Bihan T, Harrington L. Native gel electrophoresis of human telomerase distinguishes active complexes with or without dyskerin. Nucleic Acids Res 2012; 40: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autexier C, Lue NF. The structure and function of telomerase reverse transcriptase. Annu Rev Biochem 2006; 75: 493–517. [DOI] [PubMed] [Google Scholar]
- 44.Rouda S, Skordalakes E. Structure of the RNA-binding domain of telomerase: implications for RNA recognition and binding. Structure 2007; 15: 1403–1412. [DOI] [PubMed] [Google Scholar]
- 45.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 1997; 276: 561–567. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997; 277: 955–959. [DOI] [PubMed] [Google Scholar]
- 47.Drosopoulos WC, Prasad VR. The active site residue Valine 867 in human telomerase reverse transcriptase influences nucleotide incorporation and fidelity. Nucleic Acids Res 2007; 35: 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell 2000; 100: 503–514. [DOI] [PubMed] [Google Scholar]
- 49.Theimer CA, Feigon J. Structure and function of telomerase RNA. Curr Opin Struct Biol 2006; 16: 307–318. [DOI] [PubMed] [Google Scholar]
- 50.Theimer CA, Jády BE, Chim N, Richard P, Breece KE, Kiss T, Feigon J. Structural and functional characterization of human telomerase RNA processing and cajal body localization signals. Mol Cell 2007; 27: 869–881. [DOI] [PubMed] [Google Scholar]
- 51.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J 1995; 14: 4240–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet 2000; 26: 447–450. [DOI] [PubMed] [Google Scholar]
- 53.Cohen H, Sinclair DA. Recombination-mediated lengthening of terminal telomeric repeats requires the Sgs1 DNA helicase. Proc Natl Acad Sci USA 2001; 98: 3174–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motta PM, Makabe S, Nottola SA. The ultrastructure of human reproduction. I. The natural history of the female germ cell: origin, migration and differentiation inside the developing ovary. Hum Reprod Update 1997; 3: 281–295. [DOI] [PubMed] [Google Scholar]
- 55.McLaren A, Monk M. X-chromosome activity in the germ cells of sex-reversed mouse embryos. J Reprod Fertil 1981; 63: 533–537. [DOI] [PubMed] [Google Scholar]
- 56.Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res 1961; 24: 495–507. [DOI] [PubMed] [Google Scholar]
- 57.Sánchez F, Smitz J. Molecular control of oogenesis. Biochim Biophys Acta 2012; 1822: 1896–1912. [DOI] [PubMed] [Google Scholar]
- 58.Sato E. Intraovarian control of selective follicular growth and induction of oocyte maturation in mammals. Proc Jpn Acad B 2015; 91: 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo V, Berardinelli P, Capacchietti G, Scapolo PA. Localization of the telomerase catalytic subunit (TERT) in pig ovarian follicles. Vet Res Commun 2003; 27(Suppl 1): 623–626. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, Zhu GJ. Expression of telomerase in human ovarian luteinized granulosa cells and its relationship to ovarian function. Zhonghua Fu Chan Ke Za Zhi 2003; 38: 402–404 (in Chinese). [PubMed] [Google Scholar]
- 61.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res 1998; 58: 4168–4172. [PubMed] [Google Scholar]
- 62.Schaetzlein S, Lucas-Hahn A, Lemme E, Kues WA, Dorsch M, Manns MP, Niemann H, Rudolph KL. Telomere length is reset during early mammalian embryogenesis. Proc Natl Acad Sci USA 2004; 101: 8034–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eisenhauer KM, Gerstein RM, Chiu CP, Conti M, Hsueh AJ. Telomerase activity in female and male rat germ cells undergoing meiosis and in early embryos. Biol Reprod 1997; 56: 1120–1125. [DOI] [PubMed] [Google Scholar]
- 64.Bayne S, Li H, Jones ME, Pinto AR, van Sinderen M, Drummond A, Simpson ER, Liu JP. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell 2011; 2: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Endo M, Kimura K, Kuwayama T, Monji Y, Iwata H. Effect of estradiol during culture of bovine oocyte-granulosa cell complexes on the mitochondrial DNA copies of oocytes and telomere length of granulosa cells. Zygote 2014; 22: 431–439. [DOI] [PubMed] [Google Scholar]
- 66.Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, Wu C, Czerwiec E, Sandler L, Seyfang A, Blasco MA, Keefe DL. Telomere lengthening early in development. Nat Cell Biol 2007; 9: 1436–1441. [DOI] [PubMed] [Google Scholar]
- 67.Lavranos TC, Mathis JM, Latham SE, Kalionis B, Shay JW, Rodgers RJ. Evidence for ovarian granulosa stem cells: telomerase activity and localization of the telomerase ribonucleic acid component in bovine ovarian follicles. Biol Reprod 1999; 61: 358–366. [DOI] [PubMed] [Google Scholar]
- 68.Russo V, Berardinelli P, Martelli A, Di Giacinto O, Nardinocchi D, Fantasia D, Barboni B. Expression of telomerase reverse transcriptase subunit (TERT) and telomere sizing in pig ovarian follicles. J Histochem Cytochem 2006; 54: 443–455. [DOI] [PubMed] [Google Scholar]
- 69.Betts DH, King WA. Telomerase activity and telomere detection during early bovine development. Dev Genet 1999; 25: 397–403. [DOI] [PubMed] [Google Scholar]
- 70.Gilchrist GC, Kurjanowicz P, Mereilles FV, King WA, LaMarre J. Telomere length and telomerase activity in bovine pre-implantation embryos in vitro. Reprod Domest Anim 2015; 50: 58–67. [DOI] [PubMed] [Google Scholar]
- 71.Meerdo LN, Reed WA, White KL. Telomere-to-centromere ratio of bovine clones, embryos, gametes, fetal cells, and adult cells. Cloning Stem Cells 2005; 7: 62–73. [DOI] [PubMed] [Google Scholar]
- 72.Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod 2001; 7: 947–955. [DOI] [PubMed] [Google Scholar]
- 73.Turner S, Wong HP, Rai J, Hartshorne GM. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol Hum Reprod 2010; 16: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner S, Hartshorne GM. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol Hum Reprod 2013; 19: 510–518. [DOI] [PubMed] [Google Scholar]
- 75.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 1996; 18: 173–179. [DOI] [PubMed] [Google Scholar]
- 76.Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci USA 2004; 101: 6496–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu L, Blasco M, Trimarchi J, Keefe D. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev Biol 2002; 249: 74–84. [DOI] [PubMed] [Google Scholar]
- 78.Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, Agarwal S, Blasco MA. Telomere length predicts embryo fragmentation after in vitro fertilization in women— toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol 2005; 192: 1256–1260, discussion :1260–1261. [DOI] [PubMed] [Google Scholar]
- 79.Keefe DL. Telomeres and meiosis in health and disease. Cell Mol Life Sci 2007; 64: 115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keefe DL, Franco S, Liu L, et al. Short telomeres in eggs are associated with decreased outcomes following IVF – toward a telomere theory of reproductive aging in women. Am Soc Reprod Med Gen Prize Paper Session 10.2003.
- 81.Wang W, Chen H, Li R, Ouyang N, Chen J, Huang L, Mai M, Zhang N, Zhang Q, Yang D. Telomerase activity is more significant for predicting the outcome of IVF treatment than telomere length in granulosa cells. Reproduction 2014; 147: 649–657. [DOI] [PubMed] [Google Scholar]
- 82.Chen H, Wang W, Mo Y, Ma Y, Ouyang N, Li R, Mai M, He Y, Bodombossou-Djobo MM, Yang D. Women with high telomerase activity in luteinised granulosa cells have a higher pregnancy rate during in vitro fertilisation treatment. J Assist Reprod Genet 2011; 28: 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu JP, Li H. Telomerase in the ovary. Reproduction 2010; 140: 215–222. [DOI] [PubMed] [Google Scholar]
- 84.Si X, Shao C, Li J, Jia S, Tang W, Zhang J, Yang J, Wu X, Luo Y. Loss of p21 promoted tumorigenesis in the background of telomere dysfunctions induced by TRF2 and Wrn deficiency. Int J Biol Sci 2018; 14: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garofola C, Gross GP. Dyskeratosis Congenita. StatPearls. Treasure Island (FL); 2018. [PubMed] [Google Scholar]
- 86.Root H, Larsen A, Komosa M, Al-Azri F, Li R, Bazett-Jones DP, Stephen Meyn M. FANCD2 limits BLM-dependent telomere instability in the alternative lengthening of telomeres pathway. Hum Mol Genet 2016; 25: 3255–3268. [DOI] [PubMed] [Google Scholar]
- 87.Sarkar J, Liu Y. Fanconi anemia proteins in telomere maintenance. DNA Repair (Amst) 2016; 43: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pilsworth JA, Cochrane DR, Xia Z, Aubert G, Färkkilä AEM, Horlings HM, Yanagida S, Yang W, Lim JLP, Wang YK, Bashashati A, Keul J, Wong A, Norris K, Brucker SY, Taran FA, Krämer B, Staebler A, van Meurs H, Oliva E, Shah SP, Kommoss S, Kommoss F, Gilks CB, Baird DM, Huntsman DG. TERT promoter mutation in adult granulosa cell tumor of the ovary. Mod Pathol 2018; 31: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 89.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab 2009; 94: 4835–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Deng B, Ouyang N, Yuan P, Zheng L, Wang W. Telomere length is short in PCOS and oral contraceptive does not affect the telomerase activity in granulosa cells of patients with PCOS. J Assist Reprod Genet 2017; 34: 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vitek W, Hoeger K, Legro RS. Treatment strategies for infertile women with polycystic ovary syndrome. Minerva Ginecol 2016; 68: 450–457. [PubMed] [Google Scholar]
- 92.Li Q, Du J, Feng R, Xu Y, Wang H, Sang Q, Xing Q, Zhao X, Jin L, He L, Wang L. A possible new mechanism in the pathophysiology of polycystic ovary syndrome (PCOS): the discovery that leukocyte telomere length is strongly associated with PCOS. J Clin Endocrinol Metab 2014; 99: E234–E240. [DOI] [PubMed] [Google Scholar]
- 93.Wei D, Xie J, Yin B, Hao H, Song X, Liu Q, Zhang C, Sun Y. Significantly lengthened telomere in granulosa cells from women with polycystic ovarian syndrome (PCOS). J Assist Reprod Genet 2017; 34: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pedroso DC, Miranda-Furtado CL, Kogure GS, Meola J, Okuka M, Silva C, Calado RT, Ferriani RA, Keefe DL, dos Reis RM. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome. Fertil Steril 2015; 103: 542–7.e2. [DOI] [PubMed] [Google Scholar]
- 95.Kalyan S, Patel MS, Kingwell E, Côté HCF, Liu D, Prior JC. Competing factors link to bone health in polycystic ovary syndrome: chronic low-grade inflammation takes a toll. Sci Rep 2017; 7: 3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang C, Shen F, Zhu Y, Fang Y, Lu S. Telomeric repeat-containing RNA (TERRA) related to polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 2017; 86: 552–559. [DOI] [PubMed] [Google Scholar]
- 97.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 2009; 114: 2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou C, Steplowski TA, Dickens HK, Malloy KM, Gehrig PA, Boggess JF, Bae-Jump VL. Estrogen induction of telomerase activity through regulation of the mitogen-activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS One 2013; 8: e55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jankowska K. Premature ovarian failure. Przegl Menopauz 2017; 16: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinugawa C, Murakami T, Okamura K, Yajima A. Telomerase activity in normal ovaries and premature ovarian failure. Tohoku J Exp Med 2000; 190: 231–238. [DOI] [PubMed] [Google Scholar]