Abstract
Kisspeptin, identified as a natural ligand of GPR54 in 2001, is now considered as a master regulator of puberty and subsequent reproductive functions in mammals. Our previous studies using Kiss1 knockout (KO) rats clearly demonstrated the indispensable role of kisspeptin in gonadotropin-releasing hormone (GnRH)/gonadotropin secretion. In addition, behavioral analyses of Kiss1 KO rats revealed an organizational effect of kisspeptin on neural circuits controlling sexual behaviors. Our studies using transgenic mice carrying a region-specific Kiss1 enhancer-driven reporter gene provided a clue as to the mechanism by which estrogen regulates Kiss1 expression in hypothalamic kisspeptin neurons. Analyses of Kiss1 expression and gonadotropin secretion during the pubertal transition shed light on the mechanism triggering GnRH/gonadotropin secretion at the onset of puberty in rats. Here, we summarize data obtained from the aforementioned studies and revisit the physiological roles of kisspeptin in the mechanism underlying reproductive functions in mammals.
Keywords: Estrogen, Gonadotropin-releasing hormone (GnRH), Kiss1, Luteinizing hormone (LH), Puberty
Introduction
It is widely accepted that the hypothalamus plays a pinnacle role in the hierarchical mechanism regulating the gonadal axis through controlling the anterior pituitary gland in mammals. After the discovery of the gonadotropin-releasing hormone (GnRH) in the hypothalamus of domestic animals by two independent groups led by Schally and Guillemin in 1971 [1, 2], neuroendocrinologists have been keen to find out how GnRH release is controlled in mammals. The majority of GnRH neurons are scattered in the septum, preoptic and anterior hypothalamic regions of the brain in mammals [3-5], and most of them send axonal projections to the median eminence [6]. GnRH is secreted into the pituitary portal circulation at the median eminence, and activates the gonadotropin-secreting cells of the anterior pituitary gland through GnRH receptors [7, 8].
There are two modes of GnRH secretion in female mammals: one is pulsatile GnRH secretion [9, 10] and the other is the surge-mode of GnRH secretion [11, 12]. It is well known that pulsatile GnRH secretion controls tonic gonadotropin secretion [9, 10], which is found at most stages of the estrous cycle and is responsible for follicular development and steroidogenesis in the follicles and corpora lutea. Since the classical study performed by Moore and Price [13], it has been well accepted that pulsatile GnRH secretion is controlled by the negative feedback action of steroid hormones secreted from the follicles and corpora lutea [14]. On the other hand, the surge-mode of GnRH secretion induces a luteinizing hormone (LH) surge [11, 12], which is found in the preovulatory stage of the estrous cycle and evokes ovulation and corpus luteum formation. It is apparent that the surge-mode of GnRH secretion is induced by the positive feedback action of high levels of estrogen secreted by mature follicles [15]. During the last three decades of the 20th century, intensive studies were performed to address the involvement of various neurotransmitters and neuropeptides in the regulation of two modes of GnRH/gonadotropin secretion; however the mechanisms controlling GnRH/gonadotropin secretion were not fully elucidated until the discovery of kisspeptin at the beginning of the 21st century.
Kisspeptin, first named metastin, was originally found in human placenta as an endogenous ligand for GPR54, an orphan G-protein coupled receptor, in 2001 [16, 17]. The peptide was identical to the partial amino acid sequence of the KISS1 metastasis suppressor gene product reported elsewhere [18] and inhibited the chemotaxis, invasion, and metastasis of GPR54-expressing melanomas [16]. Kisspeptin was identified as a 52-amino-acid peptide in rats, and the amino acid sequences are highly conserved in mammals studied to date [16, 17, 19,20,21,22,23,24]. In particular, the C-terminal amidated 10-amino-acid sequences of kisspeptin, which are considered to be essential and sufficient for receptor interaction [16], are identical among mammals, except for a C-terminal tyrosine changed to phenylalanine in primates.
Two years after the discovery of kisspeptin [16, 17], a role for kisspeptin–GPR54 signaling as a key regulator for mammalian reproduction emerged. Two clinical studies independently revealed that loss-of-function mutations of the GPR54 gene caused hypogonadotropic hypogonadism, characterized by absence of sexual maturation, and low circulating gonadotropins and gonadal steroids [25, 26], suggesting that kisspeptin–GPR54 signaling plays a key role as a gatekeeper of reproduction in humans. To date, patients carrying loss-of-function mutations of the KISS1 gene recapitulated the phenotypes seen in patients carrying GPR54 mutations [27]. The incipient line of Gpr54 knockout (KO) mice also recapitulated human hypogonadal phenotypes [26]. Subsequently, several groups made efforts to generate Gpr54 or Kiss1 KO mice [26, 28,29,30,31] and further substantiated the essential role of kisspeptin–GPR54 signaling in puberty and fertility in mammals. However, detailed analyses of gonadotropin secretion in Gpr54 or Kiss1 KO mice were limited [32], because mice are simply too small to collect frequent blood samples for detailed analyses of the plasma profiles of gonadotropins. To overcome this disadvantage, a skillful frequent blood collection and a highly sensitive radioimmunoassay or enzyme-immunoassay are required for the successful detection of pulsatile and surge modes of gonadotropin secretion, as previously reported by ourselves and in other studies [32, 33].
The present article focuses on the roles of hypothalamic kisspeptin in the central mechanism regulating reproductive functions in mammals. First, we review the phenotypes of our Kiss1 KO rat model that have clearly demonstrated the indispensable role of kisspeptin in mammalian reproduction via controlling pulsatile and surge modes of GnRH/gonadotropin secretion. Second, we propose the molecular mechanisms by which estrogen regulates Kiss1 expression and consequently controls GnRH/gonadotropin secretion. Finally, we discuss a possible mechanism regulating pubertal changes in hypothalamic Kiss1 expression to trigger GnRH/gonadotropin secretion in rodents. A better understanding of the mechanism regulating reproductive functions in mammals would provide us with the basis for therapeutic approaches to solve infertility in livestock species.
Kiss1 KO Rats: An Animal Model to Demonstrate the Indispensable Role of Kisspeptin in Governing the Pulsatile and Surge Modes of GnRH/Gonadotropin Secretion
We generated Kiss1 KO rats by gene targeting in rat embryonic stem (ES) cells to demonstrate the indispensable role of kisspeptin in GnRH/gonadotropin secretion [34]. The gene-modified rat model has an advantage over the mouse model, as its large body size allows a detailed analysis of hormonal profiles in an individual. Briefly, a targeting vector carrying a tandem dimer Tomato (tdTomato) —a variant red fluorescent protein— reporter gene in the rat Kiss1 locus, was generated in order to disrupt the Kiss1 gene of rat ES cells by homologous recombination. Chimera rats were generated by microinjection of the Kiss1-targeted ES cells into the cavity of recipient rat blastocysts. Germline pups carrying the disrupted Kiss1 locus heterozygously were fertile and successfully intercrossed to produce Kiss1 KO rats.
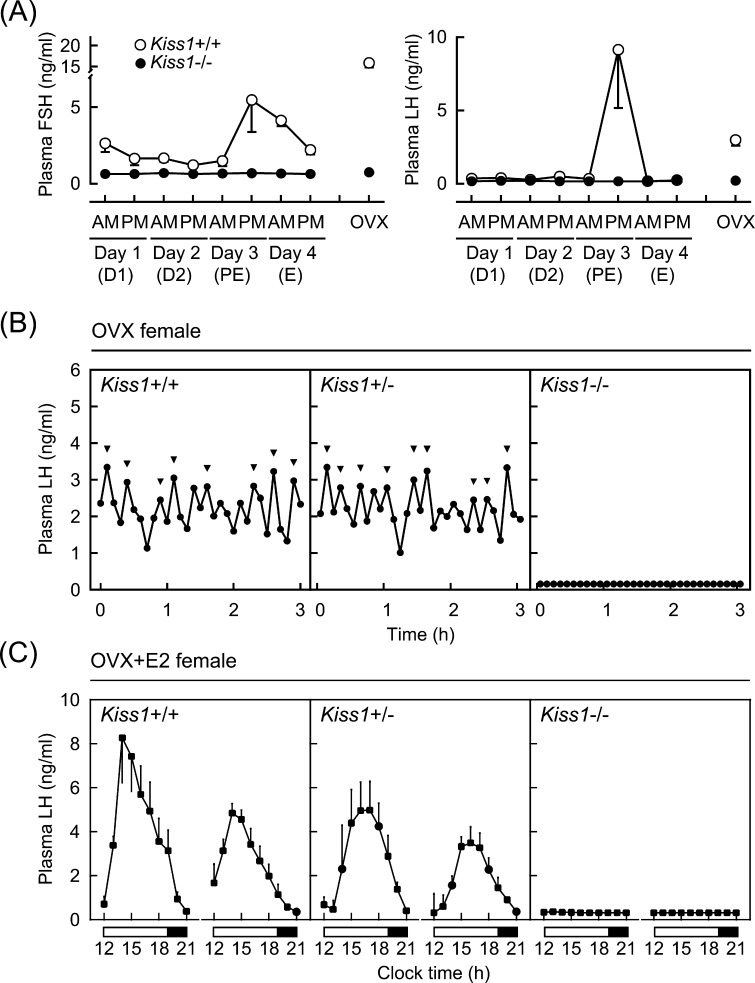
Kiss1 KO rats clearly reproduced the hypogonadal phenotypes of human and mouse models carrying KISS1/Kiss1 mutations [34]. Namely, Kiss1 KO rats showed pubertal failure and atrophic gonads in both sexes without growth retardation. Female Kiss1 KO rats showed a complete inhibition of both LH and follicle-stimulating hormone (FSH) secretion, whereas wild-type female littermates showed cyclic changes in plasma LH and FSH levels during their regular 4-day estrous cycle (Fig. 1A). Our more detailed analysis of plasma LH profiles with frequent blood collection, clearly showed that Kiss1 KO rats exhibited a complete suppression of pulsatile LH secretion even after ovariectomy (Fig. 1B). In addition, Kiss1 KO female rats exhibited no LH surge when animals received preovulatory levels of estradiol-17β (E2) (Fig. 1C). These findings clearly demonstrated that kisspeptin plays an indispensable role in controlling both the pulsatile and surge mode of secretion of GnRH/gonadotropin that regulate pubertal onset and subsequent normal reproductive functions in mammals. In addition, the administration of major stimulatory neurotransmitters, such as monosodium glutamate, N-methyl-D-aspartate (an agonist of a class of ionotropic glutamate receptor), or norepinephrine, failed to stimulate LH secretion in Kiss1 KO rats, though each stimulatory neurotransmitter stimulated LH secretion in wild-type littermates [34]. These findings suggest that kisspeptin neurons function as a hub, integrating major stimulatory neural inputs to GnRH neurons.
Fig. 1.
Lack of pulsatile and surge modes of gonadotropin secretion in Kiss1 knockout (Kiss1–/–) rats. (A) Plasma follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in wild-type (Kiss1+/+) and Kiss1–/– female rats. Plasma samples were collected from gonad-intact animals at 1000 h (AM) and 1700 h (PM) (1410 h light/dark cycle, light on 0500 h) for four consecutive days, and ovariectomized (OVX) rats at 1000 h. Stages of the estrous cycle were determined by vaginal smears in Kiss1+/+ rats. D1, diestrus 1; D2, diestrus 2; PE, proestrus; E, estrus. (B) Individual 3-h plasma LH profiles of representative OVX Kiss1+/+, heterozygous (Kiss1+/–), and Kiss1–/– rats. Arrowheads indicate LH pulses identified with the PULSAR computer program [70]. (C) Nine-hour plasma LH profiles of Kiss1+/+, Kiss1+/–, and Kiss1–/– female rats, which were OVX and received preovulatory levels of estradiol-17β (E2). Plasma samples were collected for two consecutive days. Open and closed horizontal bars indicate light and dark periods. Originally published in Uenoyama et al. (2015) [34].
In addition to the indispensable role of kisspeptin in GnRH/gonadotropin secretion, our behavioral analyses of Kiss1 KO rats revealed the involvement of kisspeptin in the defeminization/masculinization of neural circuits controlling sexual behaviors in male rats [35]. Namely, Kiss1 KO male rats exhibited no male-type sexual behaviors, such as mounting, intromission, and ejaculation, even though they received testosterone replacement in adulthood. The mounting and intromission in Kiss1 KO male rats were recovered by long-term testosterone replacement from the peripubertal period to adulthood, suggesting that kisspeptin is required for testosterone secretion from pubertal onset to adulthood to consequently induce masculinization of the neural circuits controlling male-type sexual behaviors. Notably, Kiss1 KO male rats exhibited the lordosis reflex, a female-type sexual behavior, as shown in wild-type females, when they received preovulatory levels of E2 in adulthood. The lordosis reflex in Kiss1 KO male rats was reduced to levels seen in wild-type males by kisspeptin replacement at the neonatal period, suggesting that kisspeptin is required for defeminization of the neural circuits controlling female-type sexual behaviors at the so-called “critical period” for sexual differentiation of the brain. Interestingly, Kiss1 KO female rats exhibited normal female-type sexual behaviors when they received preovulatory levels of E2 in adulthood. These findings clearly demonstrate the organizational effects of kisspeptin on the neural circuits controlling sexual behaviors in male rats.
Molecular Mechanism by which Estrogen Regulates Kiss1 Expression in Two Populations of Hypothalamic Kisspeptin Neurons
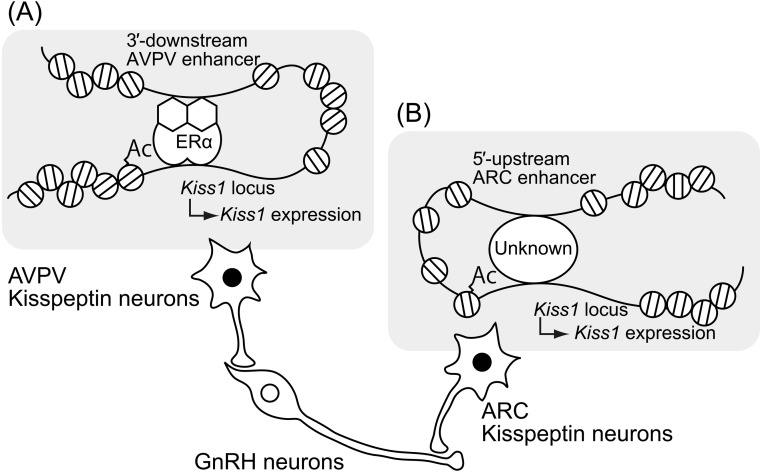
Distributions of kisspeptin neurons are largely similar in all mammals studied to date [20,21,22, 24, 36,37,38,39,40,41,42,43,44,45,46]. Our studies revealed the distribution of hypothalamic kisspeptin neurons in several mammals, such as rats [37, 41], Japanese monkeys [24], goats [20, 46], pigs [21, 45], and musk shrews (Suncus murinus) [22]. In rodents, kisspeptin neurons are mainly localized in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) [36, 37, 40, 41]. The distribution of kisspeptin neurons in rodents is largely based on studies in females, because males have few kisspeptin neurons in the AVPV [40, 41, 47, 48]. The sexual dimorphism of AVPV kisspeptin neurons provide a clue to their axonal projections; at least a portion of the AVPV kisspeptin neurons probably send axonal projections to GnRH cell bodies in the preoptic area (POA) [37, 40], because kisspeptin fibers adjusted to GnRH neuronal cell bodies were more apparent in female mice than in males [40]. On the other hand, ARC kisspeptin neurons probably send axonal projections to the median eminence, because our previous studies showed a close proximity of kisspeptin and GnRH axons in the median eminences of goats and rats [49, 50]. A schematic illustration of axonal projections of kisspeptin neurons is shown in Fig. 2.
Fig. 2.
Schematic illustrations of axonal projections of two populations of hypothalamic kisspeptin neurons and the molecular and epigenetic mechanism regulating Kiss1 expression in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC). (A) Estrogen-ERα complex bound on the Kiss1 promoter region induces histone acetylation in the Kiss1 promoter region and forms chromatin loops between the 3′-downstream region and promoter region of the Kiss1 locus to activate AVPV Kiss1 expression. (B) Histone acetylation of the Kiss1 promoter region and chromatin loops formed between the 5′-upstream region and promoter region of the Kiss1 locus via unknown transcriptional factor(s) appeared to be involved in ARC Kiss1 expression in the absence of estrogen. Ac, histone acetylation.
Estrogen robustly and differentially regulates Kiss1 expression in a region-specific manner: Kiss1 expression in the AVPV is up-regulated by estrogen treatment [36, 41], while ARC Kiss1 expression is down-regulated by this treatment [36, 37, 41]. The two-way regulation of Kiss1 expression by estrogen is suggested to be mediated by estrogen receptor-α (ERα), because estrogen had no effect on the Kiss1 expression in both the AVPV and ARC of ovariectomized (OVX) ERα KO mice [36, 51], in which it failed to exert its positive and negative feedback effects on gonadotropin secretion [52]. These studies suggest that AVPV kisspeptin neurons are responsible for the positive feedback action of estrogen to induce the surge mode of GnRH/LH secretion and that ARC kisspeptin neurons are involved in the negative feedback action of estrogen to modulate pulsatile GnRH/gonadotropin secretion in mammals. Given the potent stimulatory effect of kisspeptin on LH secretion and the successful blockade of spontaneous and E2-induced LH surges by the POA infusion of anti-kisspeptin antibody [37, 41, 53], AVPV kisspeptin neurons probably serve as a so-called “GnRH surge generator”. We recently reviewed the role of sexual dimorphic AVPV kisspeptin neurons in GnRH/LH surge generation [54]. Additionally, our previous studies in goats suggest that ARC kisspeptin neurons provably serve as a part of the GnRH pulse generating mechanism, because periodic bursts of multiple unit activity, corresponding to LH pulses, were successfully recorded from electrodes placed near the cluster of ARC kisspeptin neurons in goats [20, 55]. The current understanding of the molecular mechanism of GnRH pulse generation has been recently reviewed elsewhere [56, 57].
Our in vivo reporter assay using Kiss1-green fluorescent protein (GFP) reporter transgenic mice suggests the presence of region-specific enhancers of the Kiss1 locus and provides a clue as to the epigenetic and molecular mechanisms regulating Kiss1 expression in the AVPV and ARC. Briefly, transgenic mice carrying a so-called “full-length” Kiss1-GFP reporter construct (approximately 33 kb) successfully exhibited GFP signals in both populations of kisspeptin neurons localized in the AVPV and ARC [58]. On the other hand, transgenic mice carrying a 3′- downstream-truncated Kiss1-GFP reporter construct (approximately 28 kb) and transgenic mice carrying the 5′-upstream-truncated Kiss1-GFP reporter construct (approximately 13 kb) exhibited GFP signals only in the kisspeptin neurons localized in the ARC and AVPV, respectively [58, 59]. The results of this in vivo reporter assay suggest that the 3′-downstream region of the Kiss1 locus serves as an AVPV-specific enhancer for estrogen-induced increase in AVPV Kiss1 expression, whereas the 5′-upstream region of the Kiss1 locus serves as an ARC-specific enhancer for increase in ARC Kiss1 expression in the absence of estrogen. This scenario is supported by our previous gene conformation analyses, which indicated that the formation of chromatin loops between the Kiss1 promoter region and each putative region-specific enhancer is associated with the induction of Kiss1 expression in the AVPV and ARC [58, 59].
We have also demonstrated estrogen-dependent ERα recruitment and histone acetylation, an active histone modification, in the Kiss1 promoter region in the AVPV [58]. Interestingly, histone acetylation in the Kiss1 promoter region was also associated with an increase in ARC Kiss1 expression, because histones of the Kiss1 promoter region were highly acetylated in the absence of estrogen [59]. Further, the in vitro expression of Kiss1 was induced by a histone deacetylase inhibitor in the non-Kiss1-expressing immortalized neural cell line [58]. Thus, we estimate that AVPV Kiss1 expression is likely up-regulated by histone acetylation of the Kiss1 promoter region, and forms chromatin loops between the 3′-downstream region and promoter region of the Kiss1 locus under the control of an estrogen-ERα complex bound in the Kiss1 promoter region (Fig. 2A). Moreover, ARC Kiss1 expression is likely up-regulated by histone acetylation of the Kiss1 promoter region, and chromatin loops formed between the 5′-upstream region and promoter region of the Kiss1 locus in the absence of estrogen (Fig. 2B). The intracellular mechanism involved in the estrogen-dependent region-specific histone modification and consequent Kiss1 expression in AVPV and ARC kisspeptin neurons remains to be determined. Further studies are needed to address these issues.
Mechanism Regulating Pubertal Augmentation of ARC Kiss1 Expression to Trigger Pulsatile GnRH/Gonadotropin Secretion
As described above, kisspeptin–GPR54 signaling plays a key role as a gatekeeper of pubertal onset in mammals. Sexual maturation in the pubertal period seems to be timed by an increase in pulsatile GnRH/gonadotropin secretion in mammals [60,61,62,63,64], indicating a promising role of ARC kisspeptin neurons in the pubertal augmentation of GnRH/gonadotropin secretion in mammals. Classical studies highlighted the negative feedback action of estrogen in the mechanism controlling peripubertal GnRH/gonadotropin secretion in female rodents. Namely, Ramirez and McCann [65] and Eldridge et al. [66] proposed the ‘gonadostat hypothesis’, which states that a decrease in the sensitivity to the negative feedback action of estrogen would be associated with the pubertal augmentation of GnRH/gonadotropin secretion in rodents, because a lower amount of estrogen was required for the suppression of gonadotropin secretion in prepubertal animals compared to that in matured animals [65, 66]. After the discovery of kisspeptin, therefore, we hypothesized that pubertal changes in Kiss1 expression are robustly controlled by the negative feedback action of estrogen in female rats in a prepubertal period-specific manner [67]. Not surprisingly, ARC Kiss1 expression and pulsatile LH secretion increased immediately after ovariectomy in prepubertal rats. Estrogen replacement reverted female rats to the immature state. The strong suppression of ARC Kiss1 expression and pulsatile LH secretion was found only in the prepubertal period. Taken together, these findings suggest that the pulsatile GnRH/gonadotropin-secreting system seems to be already equipped before the onset of puberty, and the decrease in sensitivity to the negative feedback action of estrogen on ARC Kiss1 expression may trigger the pubertal augmentation of pulsatile GnRH/gonadotropin secretion in female rats.
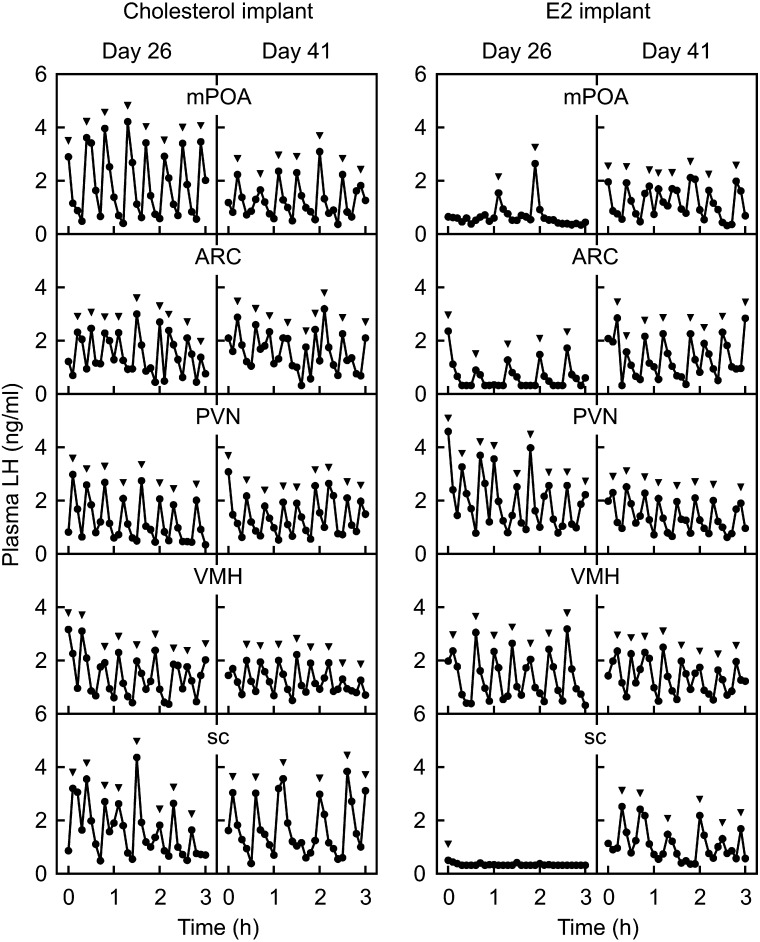
The negative feedback action of estrogen seems to directly inhibit Kiss1 expression via ERα expressed in ARC kisspeptin neurons, because kisspeptin neuron-specific ERα KO mice showed precocious ARC Kiss1 expression at birth, resulting in the precious pubertal onset [68]. Interestingly, our previous study [69] suggested another action site of estrogen negative feedback to suppress GnRH/LH secretion in the prepubertal period, because micro-implants of E2 in the POA, as well as the ARC, partly suppressed pulsatile LH secretion in prepubertal OVX rats (Fig. 3). It is unlikely that E2 implanted in the POA leaked into the ARC, because E2 implants in the nuclei close to the ARC, such as the paraventricular nucleus or ventromedial nucleus, showed no suppression of pulsatile LH secretion in prepubertal female rats (Fig. 3). Thus, we envision that POA estrogen-responsive neurons may mediate the negative feedback action of estrogen to insure the prepubertal suppression of ARC Kiss1 expression and subsequent pulsatile GnRH/gonadotropin secretion in female rats.
Fig. 3.
Estrogen-dependent prepubertal suppression of pulsatile LH secretion in female rats. Plasma LH profiles in cholesterol- (left panel) or E2-implanted (right panel) OVX rats at 26 (prepubertal period) and 41 (postpubertal period) days of age. Note that E2 implanted into the medial preoptic area (mPOA), ARC or subcutaneous (sc) space, but not into the paraventricular nucleus (PVN) or ventromedial nucleus (VMH), suppressed LH pulses in OVX rats in the prepubertal period. Arrowheads indicate LH pulses identified with the PULSAR computer program [70]. Originally published in Uenoyama et al. (2015) [69].
Taken together, the decrease in sensitivity to estrogen negative feedback action on ARC Kiss1 expression plays a critical role in triggering GnRH/gonadotropin secretion at the onset of puberty in female rats (Fig. 4). We speculate that a decrease in sensitivity to estrogen negative feedback action would occur in both POA estrogen-responsive neurons and ARC kisspeptin neurons during pubertal transition in rodents, because micro-implants of E2 in the POA and ARC failed to suppress pulsatile LH secretion in postpubertal OVX rats (Fig. 3). It is unlikely that the pubertal decrease in the sensitivity to estrogen is simply caused by a decrease in ERα expression, because ERα mRNA expression and the number of ERα-expressing cells in the POA and ARC of female rats were comparable between prepubertal and postpubertal periods [69]. Further studies are warranted to clarify the molecular mechanism decreasing the sensitivity of estrogen negative feedback action on ARC Kiss1 expression at the onset of puberty.
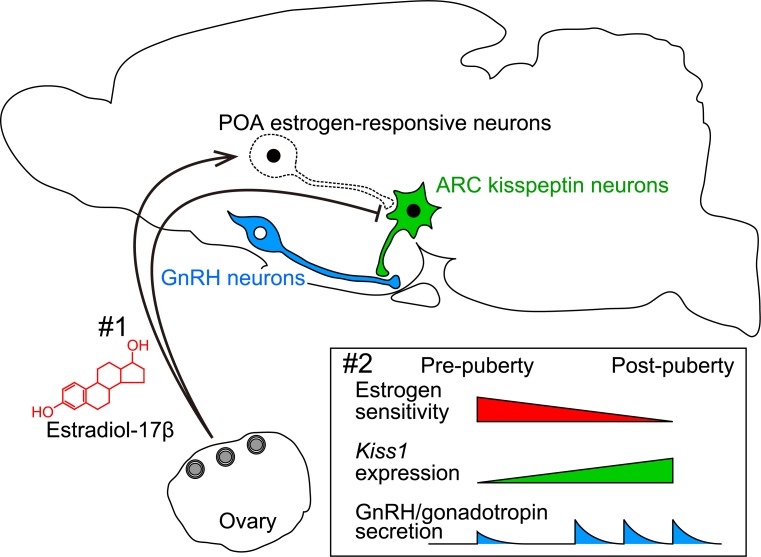
Fig. 4.
Schematic illustration showing a possible mechanism regulating the pubertal augmentation of ARC Kiss1 expression to trigger pulsatile GnRH/gonadotropin secretion. #1, Estrogen strongly suppresses ARC Kiss1 expression via direct and indirect pathways: estrogen-responsive neurons in the POA mediate estrogen negative feedback action to ensure the prepubertal suppression of ARC Kiss1 expression. #2, During the pubertal transition, the sensitivity to the estrogen negative feedback action on ARC Kiss1 expression somehow decreases, which results in an increase in Kiss1 expression, and the subsequent secretion of kisspeptin triggers GnRH/gonadotropin secretion at pubertal onset.
Conclusions and Perspective
Over the past 15 years, after the discovery of the key role of kisspeptin–GPR54 signaling in pubertal onset in humans, intensive studies on hypothalamic kisspeptin–GPR54 signaling elucidated the mechanism underlying reproductive functions in several mammalian species. As described in this article, kisspeptin plays indispensable roles in puberty and subsequent normal reproductive functions in mammals via controlling the pulse and surge modes of GnRH/gonadotropin secretion. Further analyses of Kiss1 expression and kisspeptin secretion at the cellular and molecular levels would provide a better understanding of the mechanism underlying reproductive functions in mammals. We envisage that these findings on the central mechanism of reproduction in mammals can be used as the basis for future therapeutic approaches to solve infertility of livestock species.
Acknowledgments
We would like to express our gratitude to the Society for Reproduction and Development (SRD) for awarding YU the SRD Outstanding Research Award in 2017. We would like to thank all the collaborators who contributed to this study, and Dr Nicola Skoulding for the editorial assistance. The present study was supported in part by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences and the Cooperative Study Program of the National Institute for Physiological Sciences (to KM), and by JSPS KAKENHI Grant Numbers 24380157 (to KM), 16K07987 (to NI), and 18H03973 (to HT).
References
- 1.Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, Nair RM, Debeljuk L, White WF. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 1971; 173: 1036–1038. [DOI] [PubMed] [Google Scholar]
- 2.Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun 1971; 44: 205–210. [DOI] [PubMed] [Google Scholar]
- 3.Silverman AJ, Antunes JL, Ferin M, Zimmerman EA. The distribution of luteinizing hormone-releasing hormone (LHRH) in the hypothalamus of the rhesus monkey. Light microscopic studies using immunoperoxidase technique. Endocrinology 1977; 101: 134–142. [DOI] [PubMed] [Google Scholar]
- 4.Witkin JW, Paden CM, Silverman AJ. The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology 1982; 35: 429–438. [DOI] [PubMed] [Google Scholar]
- 5.Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol 1986; 244: 19–35. [DOI] [PubMed] [Google Scholar]
- 6.Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci 1987; 7: 2312–2319. [PMC free article] [PubMed] [Google Scholar]
- 7.Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev 2004; 25: 235–275. [DOI] [PubMed] [Google Scholar]
- 8.Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci 2005; 88: 5–28. [DOI] [PubMed] [Google Scholar]
- 9.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 1982; 111: 1737–1739. [DOI] [PubMed] [Google Scholar]
- 10.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 1992; 130: 503–510. [DOI] [PubMed] [Google Scholar]
- 11.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990; 127: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 12.Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 1991; 129: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 13.Moore CR, Price D. Gonad hormone functions, and the reciprocal influence between gonads and hypophysis with its bearing on the problem of sex hormone antagonism. Am J Anat 1932; 50: 13–71. [Google Scholar]
- 14.Fink G. Feedback actions of target hormones on hypothalamus and pituitary with special reference to gonadal steroids. Annu Rev Physiol 1979; 41: 571–585. [DOI] [PubMed] [Google Scholar]
- 15.Clarke IJ. Generation of the GnRH surge and LH surge by the positive feedback effect of estrogen. In: Herbison AE, Plant TM (eds.), The GnRH Neuron and its Control. Wiley; 2018. [Google Scholar]
- 16.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411: 613–617. [DOI] [PubMed] [Google Scholar]
- 17.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276: 34631–34636. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996; 88: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 19.Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta 2004; 1678: 102–110. [DOI] [PubMed] [Google Scholar]
- 20.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 21.Tomikawa J, Homma T, Tajima S, Shibata T, Inamoto Y, Takase K, Inoue N, Ohkura S, Uenoyama Y, Maeda K, Tsukamura H. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol Reprod 2010; 82: 313–319. [DOI] [PubMed] [Google Scholar]
- 22.Inoue N, Sasagawa K, Ikai K, Sasaki Y, Tomikawa J, Oishi S, Fujii N, Uenoyama Y, Ohmori Y, Yamamoto N, Hondo E, Maeda K, Tsukamura H. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci USA 2011; 108: 17527–17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naniwa Y, Nakatsukasa K, Setsuda S, Oishi S, Fujii N, Matsuda F, Uenoyama Y, Tsukamura H, Maeda K, Ohkura S. Effects of full-length kisspeptin administration on follicular development in Japanese Black beef cows. J Reprod Dev 2013; 59: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, Inoue N, Maeda KI, Tsukamura H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol 2014; 26: 909–917. [DOI] [PubMed] [Google Scholar]
- 25.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 27.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 2012; 366: 629–635. [DOI] [PubMed] [Google Scholar]
- 28.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 2005; 102: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 2007; 104: 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 31.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol 2009; 21: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 2013; 154: 4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minabe S, Uenoyama Y, Tsukamura H, Maeda K. Analysis of pulsatile and surge-like luteinizing hormone secretion with frequent blood sampling in female mice. J Reprod Dev 2011; 57: 660–664. [DOI] [PubMed] [Google Scholar]
- 34.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinizing hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura S, Uenoyama Y, Ikegami K, Dai M, Watanabe Y, Takahashi C, Hirabayashi M, Tsukamura H, Maeda KI. Neonatal kisspeptin is steroid-independently required for defeminisation and peripubertal kisspeptin-induced testosterone is required for masculinisation of the brain: A behavioural study using Kiss1 knockout rats. J Neuroendocrinol 2016; 28. (in press). [DOI] [PubMed] [Google Scholar]
- 36.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. [DOI] [PubMed] [Google Scholar]
- 38.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 2005; 102: 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol 2006; 18: 806–809. [DOI] [PubMed] [Google Scholar]
- 40.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 42.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007; 148: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 43.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007; 92: 2744–2750. [DOI] [PubMed] [Google Scholar]
- 44.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008; 149: 4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ieda N, Uenoyama Y, Tajima Y, Nakata T, Kano M, Naniwa Y, Watanabe Y, Minabe S, Tomikawa J, Inoue N, Matsuda F, Ohkura S, Maeda K, Tsukamura H. KISS1 gene expression in the developing brain of female pigs in pre- and peripubertal periods. J Reprod Dev 2014; 60: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuda F, Nakatsukasa K, Suetomi Y, Naniwa Y, Ito D, Inoue N, Wakabayashi Y, Okamura H, Maeda KI, Uenoyama Y, Tsukamura H, Ohkura S. The luteinizing hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol 2015; 27: 57–65. [DOI] [PubMed] [Google Scholar]
- 47.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007; 148: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 48.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 49.Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, Maeda K, Ichikawa M, Okamura H. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology 2011; 94: 323–332. [DOI] [PubMed] [Google Scholar]
- 50.Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, Tsukamura H. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 2011; 23: 863–870. [DOI] [PubMed] [Google Scholar]
- 51.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 2009; 29: 9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 2007; 104: 8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pheng V, Uenoyama Y, Homma T, Inamoto Y, Takase K, Yoshizawa-Kumagaye K, Isaka S, Watanabe TX, Ohkura S, Tomikawa J, Maeda K, Tsukamura H. Potencies of centrally- or peripherally-injected full-length kisspeptin or its C-terminal decapeptide on LH release in intact male rats. J Reprod Dev 2009; 55: 378–382. [DOI] [PubMed] [Google Scholar]
- 54.Tsukamura H, Maeda KI, Uenoyama Y. Fetal/perinatal programming causing sexual dimorphism of the kisspeptin–GnRH neuronal network. In: Herbison AE, Plant TM (eds.), The GnRH Neuron and its Control. Wiley; 2018: 43-60. [Google Scholar]
- 55.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol 2013; 784: 297–323. [DOI] [PubMed] [Google Scholar]
- 57.Goodman RL, Ohkura S, Okamura H, Coolen LM, Lehman MN. KNDy hypothesis for generation of GnRH pulses: evidence from sheep and goats. In: Herbison AE, Plant TM (eds.), The GnRH Neuron and its Control. Wiley; 2018: 289-324. [Google Scholar]
- 58.Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, Abe H, Ieda N, Minabe S, Deura C, Inoue N, Sanbo M, Tomita K, Hirabayashi M, Tanaka S, Imamura T, Okamura H, Maeda K, Tsukamura H. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci USA 2012; 109: E1294–E1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goto T, Tomikawa J, Ikegami K, Minabe S, Abe H, Fukanuma T, Imamura T, Takase K, Sanbo M, Tomita K, Hirabayashi M, Maeda K, Tsukamura H, Uenoyama Y. Identification of hypothalamic arcuate nucleus-specific enhancer region of Kiss1 gene in mice. Mol Endocrinol 2015; 29: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews WW, Ojeda SR. A detailed analysis of the serum luteinizing hormone secretory profile in conscious, free-moving female rats during the time of puberty. Endocrinology 1981; 109: 2032–2039. [DOI] [PubMed] [Google Scholar]
- 61.Foster DL, Yellon SM, Olster DH. Internal and external determinants of the timing of puberty in the female. J Reprod Fertil 1985; 75: 327–344. [DOI] [PubMed] [Google Scholar]
- 62.Foster DL, Ebling FJP, Vannerson LA, Wood RI, Fenner DE. Regulation of puberty in the lamb: internal and external cues. In: Imura H, Shizume K, Yoshida S (eds.), Progress in Endocrinology. Amsterdam: Elsevier; 1988: 861-866. [Google Scholar]
- 63.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 1989; 125: 92–99. [DOI] [PubMed] [Google Scholar]
- 64.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 2001; 142: 2929–2936. [DOI] [PubMed] [Google Scholar]
- 65.Ramirez DV, McCANN SM. Comparison of the regulation of luteinizing hormone (LH) secretion in immature and adult rats. Endocrinology 1963; 72: 452–464. [DOI] [PubMed] [Google Scholar]
- 66.Eldridge JC, McPherson JC, 3rd, Mahesh VB. Maturation of the negative feedback control of gonadotropin secretion in the female rat. Endocrinology 1974; 94: 1536–1540. [DOI] [PubMed] [Google Scholar]
- 67.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 2009; 21: 527–537. [DOI] [PubMed] [Google Scholar]
- 68.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 2010; 107: 22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uenoyama Y, Tanaka A, Takase K, Yamada S, Pheng V, Inoue N, Maeda K, Tsukamura H. Central estrogen action sites involved in prepubertal restraint of pulsatile luteinizing hormone release in female rats. J Reprod Dev 2015; 61: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]