Abstract
Interferon-tau (IFNT), a type I interferon (IFN), is known as pregnancy recognition signaling molecule secreted from the ruminant conceptus during the preimplantation period. Type I IFNs, such as IFN-alpha and IFN-beta, are known to activate cell-death pathways as well as induce apoptosis. In cows, induction of apoptosis with DNA fragmentation is induced by IFNT in cultured bovine endometrial epithelial cells. However, the status of cell-death pathways in the bovine endometrium during the preimplantation period still remains unclear. In the present study, we investigated the different cell-death pathways, including apoptosis, pyroptosis, and autophagy, in uterine tissue obtained from pregnant cows and in vitro cultured endometrial epithelial cells with IFNT stimulation. The expression of CASP7, 8, and FADD (apoptosis-related genes) was significantly higher in pregnant day 18 uterine tissue in comparison to non-pregnant day 18 tissue. The expression of CASP4, 11, and NLRP3 (pyroptosis-related genes) was significantly higher in the pregnant uterus in comparison to non-pregnant uterus. In contrast, autophagy-related genes were not affected by pregnancy. We also investigated the effect of IFNT on the expression of cell-death pathway-related genes, as well as DNA fragmentation in cultured endometrial epithelial cells. Similar to its effects in pregnant uterine tissue, IFNT affected the increase of apoptosis-related (CASP8) and pyroptosis-related genes (CASP11), but did not affect autophagy-related gene expression. IFNT also increased γH2AX-positive cells, which is a marker of DNA fragmentation. These results suggest that apoptosis- and pyroptosis-related genes are induced by IFNT in the pregnant bovine endometrial epithelial cells.
Keywords: Apoptosis, Autophagy, Interferon-tau, Pregnant bovine uterus, Pyroptosis
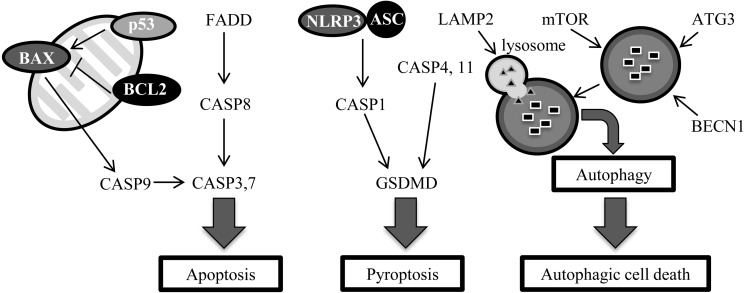
During the mammalian preimplantation period, endometrial tissue remodeling, including cell-death, occurs for preparing the uterus for implantation [1,2,3]. Apoptotic cell death in the preimplantation endometrium was observed in rats [1], pigs [2], mice, and monkeys [3]. However, the expression profile of cell-death related genes in the bovine pregnant uterus still remains unverified. Interferon-tau (IFNT) is a ruminant-specific interferon (IFN) secreted from the conceptus until day 25 of pregnancy in cattle [4], and the secretion peaks at day 18 [5]. IFNT is a type I IFN, whose characteristic features include their antiviral and proapoptotic activities [6]. Type I IFN-mediated apoptosis induction is initiated via diverse mechanisms and involves both the up-regulation of proapoptotic factors [7, 8] and the suppression of antiapoptotic responses [9]. Pyroptosis is another cell-death pathway that is induced by interferon regulatory transcription factor (IRF)-1, whose expression is mediated by IFNs [10, 11]. Furthermore, type I IFN has also been reported to induce autophagy in dendritic cells and B cells [12,13,14]. IFNT-mediated induction of cell death with increased DNA fragmentation was reported in cultured bovine uterine epithelial cells [7]. However, the detailed molecular mechanisms involved in the process of cell death in this tissue are not clearly understood (Fig. 1).
Fig. 1.
Cell-death signaling pathways for apoptosis, pyroptosis, and autophagy.
Recently, several forms of cell-death-related pathways were discovered [15]. Generally, cell death is classified as accidental cell death (ACD) or regulated cell death (RCD) [16]. ACD is caused by injury due to exposure to factors, such as acid, alkali, and heat shock. In contrast, RCD such as apoptosis involves genetic and molecular mechanisms encoded within cells [17].
A characteristic feature of apoptosis, pyknosis, involves the irreversible condensation of chromatin in the nucleus of cells, and blebbing of the cell membrane [17]. There are two major pathways to induce apoptosis. One pathway involves apoptosis via Caspase-9 (CASP9) and CASP8. CASP9 is activated by the release of cytochrome c from the mitochondria based on the expression of BAX, p53, BAK, BCL2, BH3-interacting domain death agonist (BID), and other molecules [18]. CASP8 is activated by binding with fas-associated death domain protein (FADD)-induced ligands, such as Tumor necrosis factor (TNF)–alpha [19]. Apoptotic pathways via CASP8 and 9 are not completely independent from each other [18,19,20]. When apoptosis is induced, cells release growth factors before dying [21]. Thus, apoptosis is not only related to the removal of cells, but is also involved in cell proliferation.
Pyroptosis is another form of RCD involving the rupture of the plasma membrane, efflux of the cytoplasm, and DNA fragmentation [20, 22, 23]. When membrane rupture occurs, diffusion of the cytoplasm passes the information (for example, bacterial infection) to nearby cells. Pyroptosis was determined to be a CASP1 dependent cell-death pathway [24]. After further study, a cell-death mechanisms that were similar to pyroptosis, but were dependent on CASP4 and 11, but not CASP1, was discovered [25, 26]. This CASP1-independent cell-death like pyroptotic pathway was recently classified as a form of pyroptosis [27]. In CASP1-dependent pyroptosis, CASP1 is activated by an inflammasome consisting of NLRP3 (nucleotide-binding domain leucine-rich repeat contain receptor family, pyrin domain-containing proteins), ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and pre-CASP1. While, the pathways depending on CASP4 and/or 11 are not necessary for the formation of an inflammasome. Destruction of the plasma membrane is induced by degradation of Gerdermin D (GSDMD) in both pathways [28,29,30].
Autophagy is a process for cellular survival, and is regulated by ATG, mTOR, LAMP2, and other proteins. Autophagic cell death was discovered using mouse embryonic fibroblast cells that were Bax and Bak double knockouts [31]. Autophagic cell death is related to differentiation; for example, metamorphosis in Drosophila [32, 33].
RCD (including apoptosis, pyroptosis, and autophagic cell death) is characterized by DNA fragmentation. Increase in DNA fragmentation has been observed in the preimplantation endometrium in some mammals [1,2,3]. Cell death relates to many phenomena, for example apoptosis relates to cell proliferation, pyrotosis relates to information diffusion, and autophagic cell death relates to differentiation. Thus, it is important to clarify the involvement of cell-death mechanisms in the bovine preimplantation uterus.
In the present study, we focused on several cell-death pathways (apoptosis, pyroptosis, and autophagic cell death) in the bovine preimplantation uterus. The purpose of the study was to clarify which cell death pathways in bovine endometrium is involved in implantation, and whether cell death is induced by IFNT in bovine uterus epithelial cells.
Materials and Methods
Collection of endometrial tissue samples
Uterine tissues were obtained from Japanese Black cows at the ranch of the NARO institute of Livestock and Grassland Science within 10-30 min of exsanguination, as previously described [34]. Briefly, the tissue samples were collected from cows on day 18 after artificial insemination (n = 3). The day of artificial insemination was designated as day 1. The uterine horn ipsilateral to the corpus luteum (CL) was obtained and immediately cut open to observe the endometrium. The presence or absence of fetal trophoblast was checked macroscopically to determine whether the cows were pregnant. The intercaruncular endometrial tissues (< 0.5 cm3) were collected and snap-frozen in liquid nitrogen, and then stored at –80°C until RNA extraction. All procedures for animal experiments were carried out in accordance with guidelines approved by the Animal Ethics Committee of the National Institute of Agrobiological Sciences, 2014 (#H18-036-3).
Recombinant bovine IFNT
Recombinant bovine IFNT (rbIFNT) was produced in Escherichia coli using cDNA (bTP-509A, gifted by Dr RM Roberts, University of Missouri, Columbia, MO, USA) and an expression vector [35]. Antiviral activity, determined by MDBK cells, was 8 × 106 IU/ml. The final IFNT concentration of 1,000 IU/ml was determined based on the antiviral activity of day 15 pregnant bovine uterine vein plasma sufficient to stimulate leukocytes locally in the uterine vicinity (500–1,000 U/ml) [36]. Recombinant bovine IFNT was added to 1 ml of culture medium, which was adjusted to approximately 500 IU/ml, according to the previous study [11].
Collection and culture of bovine endometrial epithelial cells
Non-pregnant bovine uteri were obtained from a local abattoir. Intercaruncular endometrial tissues were collected from the uterine horn and placed in sterile calcium- and magnesium-free Hank’s balanced salt solution (HBSS) (–); the tissues were them cut into small pieces (3 × 3 mm). These pieces were placed in 60 mm Petri dishes (IWAKI, Osaka, Japan). These pieces were cultured in 5% FBS (ICN Bio-Source International, Camarillo, CA, USA) in Dulbecco’s Modified Eagle’s medium (high glucose) (DMEM; Wako, Osaka, Japan) supplemented with 0.06 g/l penicillin G potassium (Nacalai Tesque, Kyoto, Japan) and 0.1 g/l streptomycin sulfate (Nacalai Tesque) at 38.5°C with 5% CO2 in air. After a week, the tissue pieces were removed and proliferating epithelial cells were cultured at 38.5°C with 5% CO2 in air. For the preprocessing removal of stromal cells, the culture dish was washed with calcium- and magnesium-free Phosphate buffered saline (PBS) (–). Subsequently, to separate the stromal cells, PBS (–) containing 0.05% trypsin and 0.53 mM EDTA was added to the dish and incubated for 2 min at 38.5°C in a CO2 incubator. After the incubation, the dish was washed with PBS (–) for removing stromal cells and TrypLETM Express (Thermo Fisher Scientific, Waltham, MA) was added to disperse epithelial cells at 38.5°C with 5% CO2 in air for 30 min. Next, 5% FBS in DMEM was added to inhibit trypsin activity in the TrypLETM Express; the cell suspension was centrifuged at 1,200 × g for 3 min. The pellet was washed with 5% FBS in DMEM and centrifuged at 1,200 × g for 3 min. Viable cells were plated at a dilution of 1.0 × 105 cells/ml onto 4-well culture plates (Thermo Fisher Scientific; for analysis of gene expression) or 8-well slides and chambers (Watson Bio Lab, Tokyo, Japan; for immunostaining) and cultured at 38.5°C with 5% CO2 in air. The medium was changed every 3 days. After cells became 70% confluent, they were cultured for 24 h with IFNT (500 IU/ml). Cycloheximide (Sigma-Andrich, St. Louis, USA), known as an apoptosis inducer, was used at 10 µM as a positive control.
RNA extraction and quantitative reverse-transcription polymerase chain reaction (RT-qPCR)
Bovine uterine tissues were homogenized using BioMasher® (Nippi, Tokyo, Japan) and centrifuged at 15,000 × g for 30 sec. Then, total RNA was extracted from the supernatant using ISOGEN II (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. To extract RNA from cultured uterine endometrial epithelial cells, ISOGEN II was dispensed in 4-well culture plates and RNA extraction was performed according to the manufacturer’s instructions. All RNA samples were stored in a freezer at –80°C until use. The RNA concentration was measured via spectrophotometry (NanoDrop ND-2000, Thermo Fisher Scientific). Complementary DNA was synthesized from 200 ng of total RNA by reverse transcription using the ReverTra Ace® qPCR RT Master Mix with gDNA remover (Toyobo Life Science, Osaka, Japan) according to the manufacturer’s instruction using a thermal cycler (ASTEC Program Temp Control System PC-815 or 816, ASTEC, Fukuoka, Japan). All cDNA samples were stored in a freezer at –30°C until use. Specific primers (Table 1) were designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The relative expression levels were assessed via qRT-PCR using a LightCycler® Nano (Roche Diagnostics, Basel, Switzerland) and THUNDERBIRDTM SYBR® qPCR Mix (Toyobo Life Science) at a final primer concentration of 0.5 µM for each primer. Thermal cycling conditions were as follows: 1 cycle at 95°C for 30 sec (denaturation), followed by 45 cycles at 95°C for 10 sec (denaturation), 55°C for 15 sec (primer annealing), and 72°C for 30 sec (extension). Relative mRNA abundance was calculated via the ΔΔCt method using the expression of H2AFZ as a reference gene.
Table 1. Primer sequences used for qRT-PCR.
| Accession No. | Gene | Forward (5'-3') | Reverse (5'-3') | |
|---|---|---|---|---|
| Apoptosis-related gene | ||||
| NM_173894 | BAX | GGCTGGACATTGGACTTCCTTC | TGGTCACTGTCTGCCATGTGG | |
| NM_001166486 | BCL2 | GGTGCCTATCTGGGCCATAAG | CAGCTTCACTTCAGTGGTGC | |
| NM_001007816 | FADD | AGGACCGAGACCTGCG | ACGTCAGATACTCCGAGGTG | |
| NM_001077840 | CASP3 | AGCGTCGTAGCTGAACGTAA | CCAGAGTCCATTGATTTGCTTC | |
| XM_604643 | CASP7 | TAACGACTGCTCTTGTGCCA | GCTGTCTTGCCATCTGTTCC | |
| NM_001045970 | CASP8 | AGCATAGCACGGAAGCAGG | GGTCTTATCCAAAGCGTCTGC | |
| NM_001205504 | CASP9 | CCGATCTGGCCTATGTCCTG | TCACAGTCGATGTTGGAGCC | |
| NM_174201 | p53 | AGTTGGAGCACATGACGGAG | GCGCGTAAATTCCCTTCCAC | |
| Pyroptosis-related gene | ||||
| NM_174730 | ASC1 | AGCAAGGGCCCTAGAAACGTG | GTGACCCGTGCGATGAGAG | |
| XM_019975839 | CASP1 | CTCAGGCATGCCAAATCTGC | TGTGAACCTGAAGTGAGCCC | |
| NM_176638 | CASP4 | CTGGCCTTTTGGATGACTTTGT | AGACTTGACCTGCCTCTTGG | |
| NM_001109796 | CASP11 | ACTCCACACCCAGGAGATTG | GAGATCGGGATCTGGCATAGG | |
| NM_001046160 | GSDMD | TTGTAGTGACCGAGGTGCTG | CCTTTGCCCTGTAAGCAGAAG | |
| NM_001102219 | NLRP3 | CTTTCTGGACTCTGACCGGG | TCCAGGTCCTCCAGGTAACG | |
| Autophagy-related gene | ||||
| NM_001075364 | ATG3 | AAGGGAAAGGCACTGGAAGT | GTGATCTCCAGCTGCCACAA | |
| NM_001033627 | BECN1 | AGTTGAGAAAGGCCAGACAC | GATGGAATAGGAACCACCAC | |
| NM_001034570 | LAMP2 | AAGAGCAGACCGTTTCCGTG | CGAACACTCTTGGGCAGTAG | |
| XM_015466779 | mTOR | ATGCTGTCCCTGGTCCTTATG | GGGTCAGAGAGTGGCCTTCAA | |
| Reference gene | ||||
| NM_174809 | H2AFZ | AGAGCCGGTTTGCAGTTCCCG | TACTCCAGGATGGCTGCGCTGT | |
Detection of γH2AX in cultured uterine epithelial cells
After 24 h of IFNT or Cycloheximide (CHX) treatment, cell samples were washed with PBS (–) and fixed in 4% paraformaldehyde diluted with PBS (–) for 15 min. After washing three times with PBS (–) for 5 min, the samples were permeabilized by PBS (–) containing 0.2% (v/v) Triton X-100 (PBS-T) for 10 min. After washing with PBS (–), the cells were subjected to antigen activation. Samples were boiled by micro wave for 30 min with a citric acid buffer (1.8 mM sodium citrate and 8.2 mM citric acid) followed by cooling for 30 min at room temperature.
After washing with PBS (–), the cells were blocked with 1% (w/v) BSA (Sigma-Andrich) in PBS-T for 1 h at room temperature. Washes with PBS (–), incubation with a primary rabbit polyclonal antibody for gamma H2A.X (ab11174, Abcam, Cambridge, MA, USA) diluted 1:500 with Can Get Signal® Immunostain Immunoreaction Enhance Solution A (Toyobo Life Science) or only vehicle-control at 4°C overnight, was performed for immunoreaction. The samples were washed three times with PBS (–) for 5 min each and incubated for 1 h with a fluorescein-conjugated secondary antibody (Alexa Fluor® 488 donkey anti-rabbit IgG, A21206, Thermo Fisher Scientific) diluted 1:500 with Can Get Signal® Immunostain Immunoreaction Enhance Solution A at room temperature. Cells were then washed with PBS (–) for 5 min, and 10 µl of the mounting solution (Fluoro-KEEPER Anti fade Reagent, Non-Hardening Type with DAPI, NacalaiTesque) was dropped on the samples, which were then covered with a cover glass. Then, gamma H2A.X staining was examined under a fluorescent microscope with FITC (Leica DMi8, Leica Camera AG, Wetzlar, Germany).
Statistical analysis
All data are shown as the mean ± standard error of the mean (SEM). The significance of differences was assessed by student’s t test in the R (version 3.3.0; https://www.r-project.org/). Data with P values of < 0.05 were considered statistically significant. P values of < 0.1 were assumed to indicate a tendency.
Results
Expression of apoptosis-related genes in pregnant and non-pregnant uterine endometrial tissues
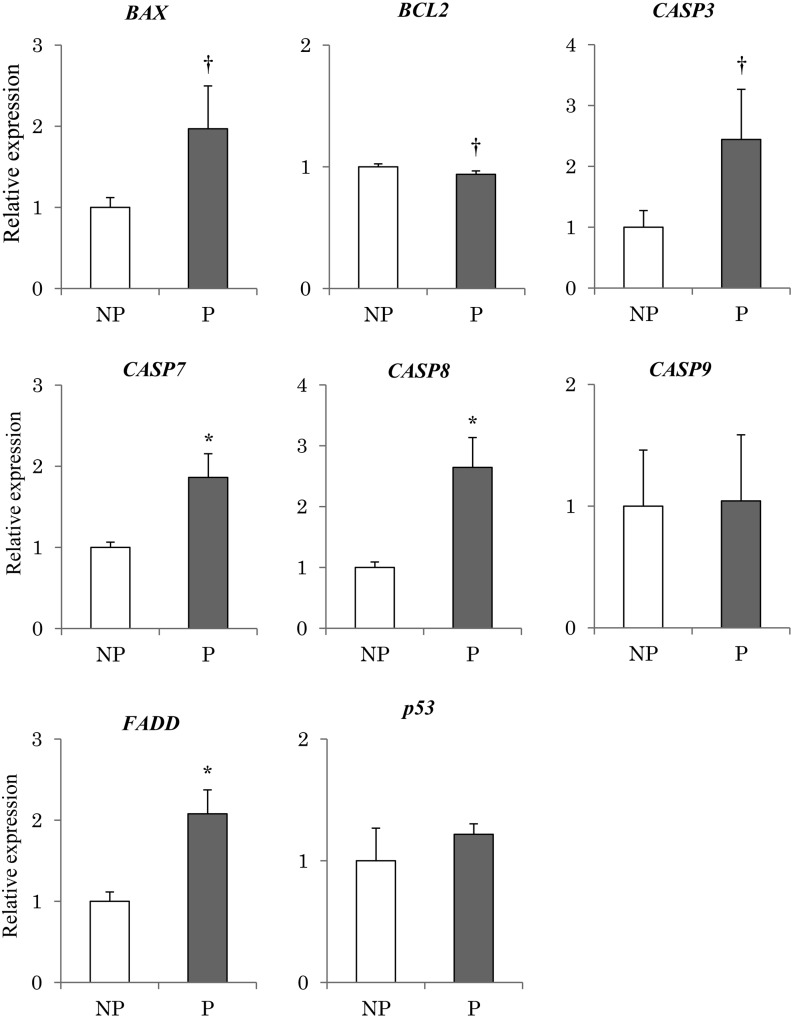
Expression of FADD, CASP7, and CASP8 was significantly higher (P < 0.05) in pregnant endometrial tissue than in non-pregnant tissue (Fig. 2). In Addition, expression of BAX and CASP3 tended to be higher in pregnant tissue compared with non-pregnant tissue. Expression of BCL2 tended to be lower (P < 0.1) in pregnant tissues than in non-pregnant tissue (Fig. 2). However, no significant differences were observed between the pregnant and non-pregnant tissues in terms of p53 and CASP9 expression.
Fig. 2.
Expression of apoptosis-related genes in the endometrium obtained from pregnant and non-pregnant cows. Expression levels of apoptosis-related genes (p53, BAX, BCL2, FADD, CASP3, CASP7, CASP8, CASP9) in the endometrial tissues obtained from the pregnant (P) (n = 3) and non-pregnant (NP) (n = 3) bovine uterus were analyzed using qRT-PCR normalized to H2AFZ as a reference gene. All data are shown as the means ± standard error of the mean (SEM). There was a statistically significant difference in mRNA levels (* P < 0.05), and tendency († P < 0.1) when pregnant and non-pregnant tissues were compared.
Expression of pyroptosis-related genes in pregnant and non-pregnant uterine endometrial tissues
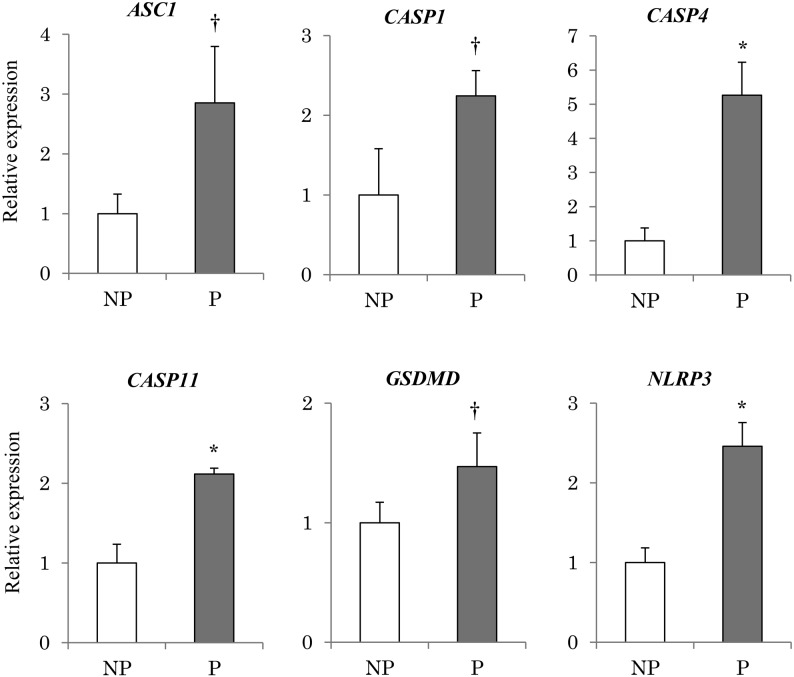
Expression of CASP4, CASP11, and NLRP3 was significantly higher (P < 0.05) in pregnant endometrial tissue compared with non-pregnant tissue (Fig. 3). In addition, expression of CASP1, ASC1, and GSDMD tended to be higher (P < 0.1) in pregnant tissues as well (Fig. 3).
Fig. 3.
Expression of pyroptosis-related genes in the endometrium obtained from pregnant and non-pregnant cows. Expression levels of pyroptosis-related genes (CASP1, CASP4, CASP11, NLRP3, ASC1, GSDMD) in the endometrial tissues obtained from the pregnant (P) (n = 3) and non-pregnant (NP) (n = 3) bovine uterus were analyzed using qRT-PCR normalized to H2AFZ as a reference gene. All data are shown as the means ± standard error of the mean (SEM). There was a statistically significant difference in mRNA levels (* P < 0.05), and tendency († P < 0.1) when pregnant and non-pregnant tissues were compared.
Expression of autophagy-related genes in pregnant and non-pregnant uterine endometrial tissues
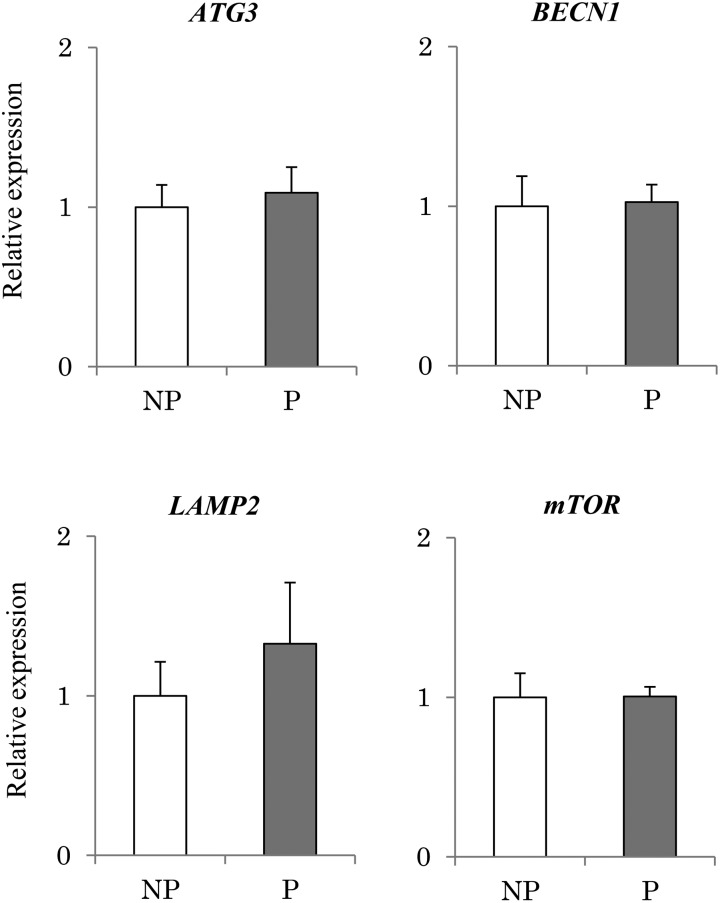
No significant differences were observed between pregnant and non-pregnant tissues in terms of autophagy-related gene expression (ATG3, BECN1, LAMP2 and mTOR; Fig. 4).
Fig. 4.
Expression of autophagy-related genes in the endometrium obtained from pregnant and non-pregnant cows. Expression levels of autophagy-related genes (mTOR, BECN1, ATG3, LAMP2) in the endometrial tissues obtained from the pregnant (P) (n = 3) and non-pregnant (NP) (n = 3) bovine uterus were analyzed using qRT-PCR normalized to H2AFZ as a reference gene. All data are shown as the means ± standard error of the mean (SEM). No significant difference was observed in genes expression (mTOR, BECN1, ATG3, LAMP2).
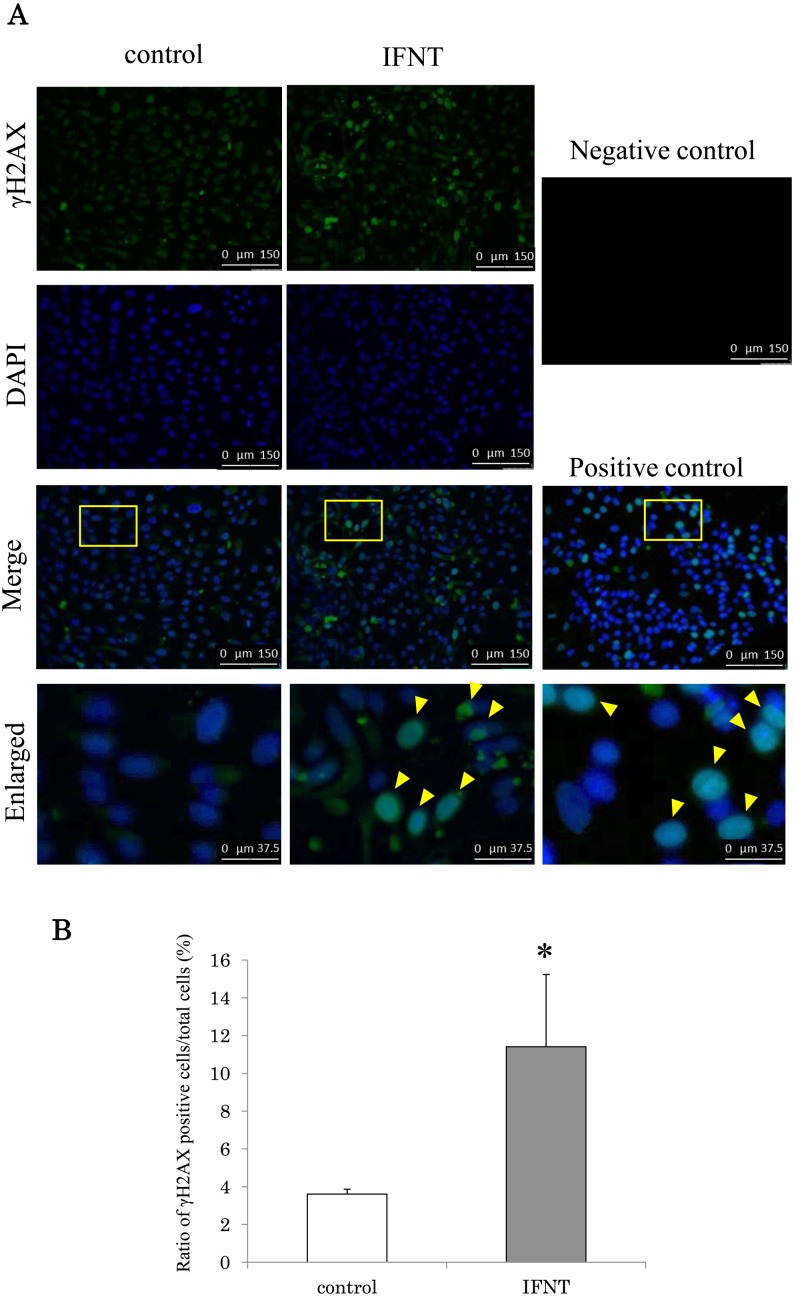
Effect of IFNT on DNA fragmentation of in vitro cultured bovine uterine endometrial epithelial cells
Figure 5 shows immunostaining for γH2AX (a marker of DNA fragmentation). Cycloheximide treatment increased DNA fragmentation in the epithelial cells (Fig. 5A). The addition of IFNT increased DNA fragmentation (Fig. 5A). The ratio of γH2AX-positive cells to DAPI-positive cells was significantly higher (P < 0.05) in the epithelial cells when cells were cultured with IFNT than in those cultured without IFNT (Fig. 5B).
Fig. 5.
Effect of IFNT on the induction of DNA fragmentation in the bovine uterine epithelial cells. (A) Immunostaining for γH2AX, images of DAPI, and merged images; the yellow square in epithelial cells from bovine luminal epithelium. γH2AX-positive cell was shown by yellow arrow. Positive control means cultured with cycloheximide. Negative control means incubation with vehicle instead of primary antibody. The experiment was repeated tree times. Immunostaining images are shown at magnification × 20. The white scale bar in enlarged shows 37.5 µm and the white scale bar in others shows 150 µm. (B) The vertical line shows the ratio of γH2AX positive cells to DAPI. IFNT and control mean cultured with and without IFNT, respectively. Data are shown as the means ± standard error of the mean (SEM). The asterisk (*) indicates significant difference between control and IFNT (P < 0.05).
Effect of IFNT on the expression of cell death pathway related genes in cultured uterine epithelial cells
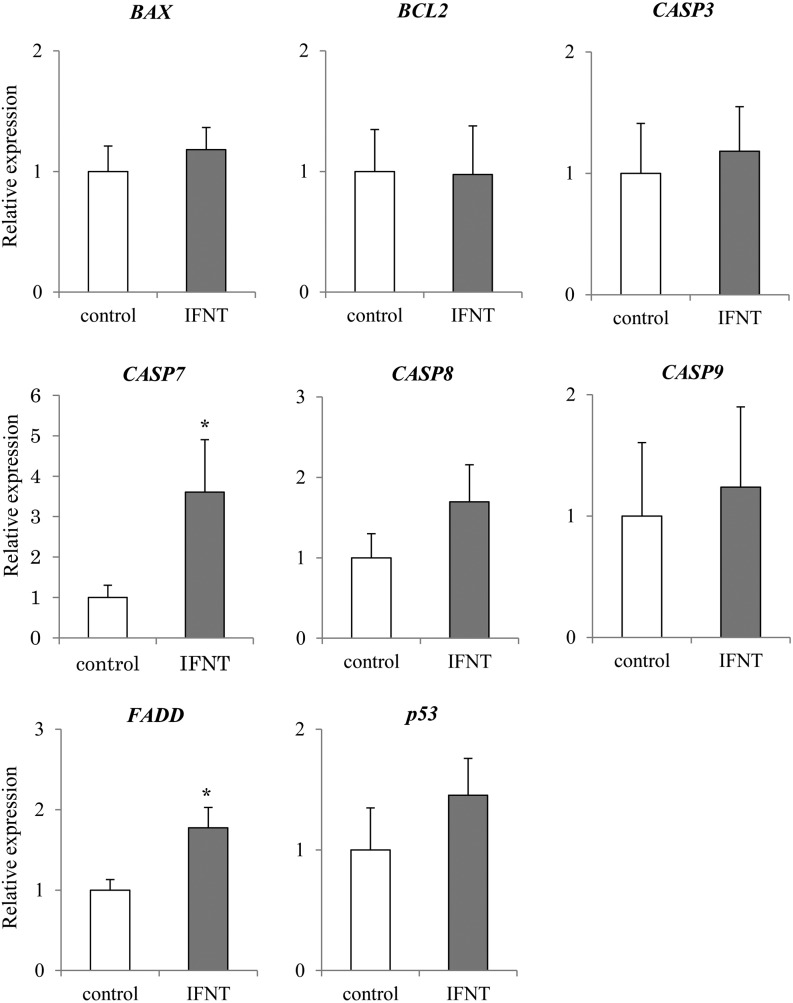
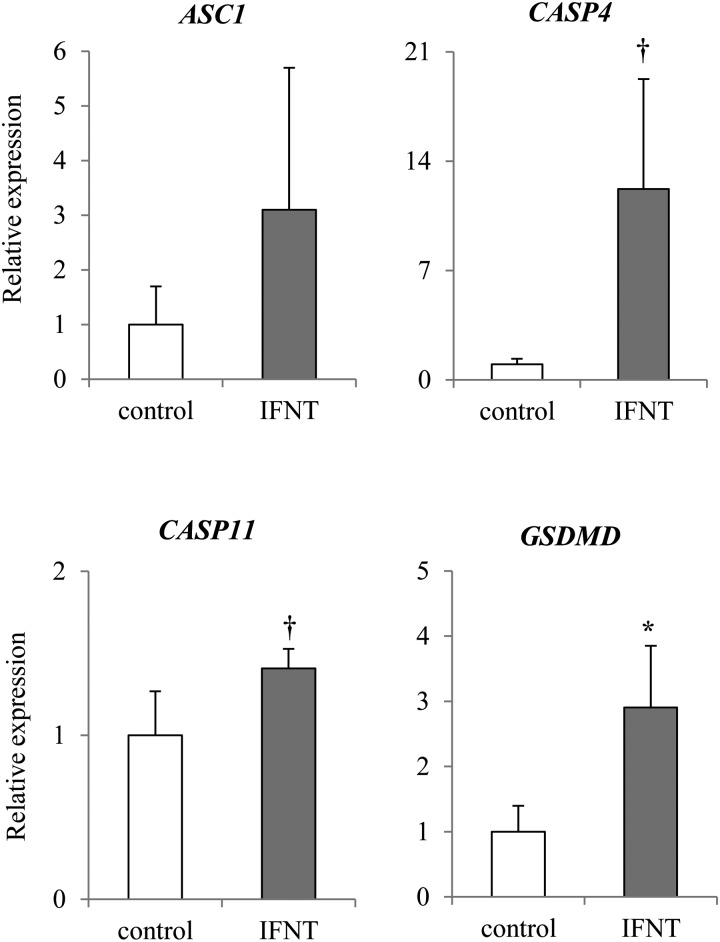
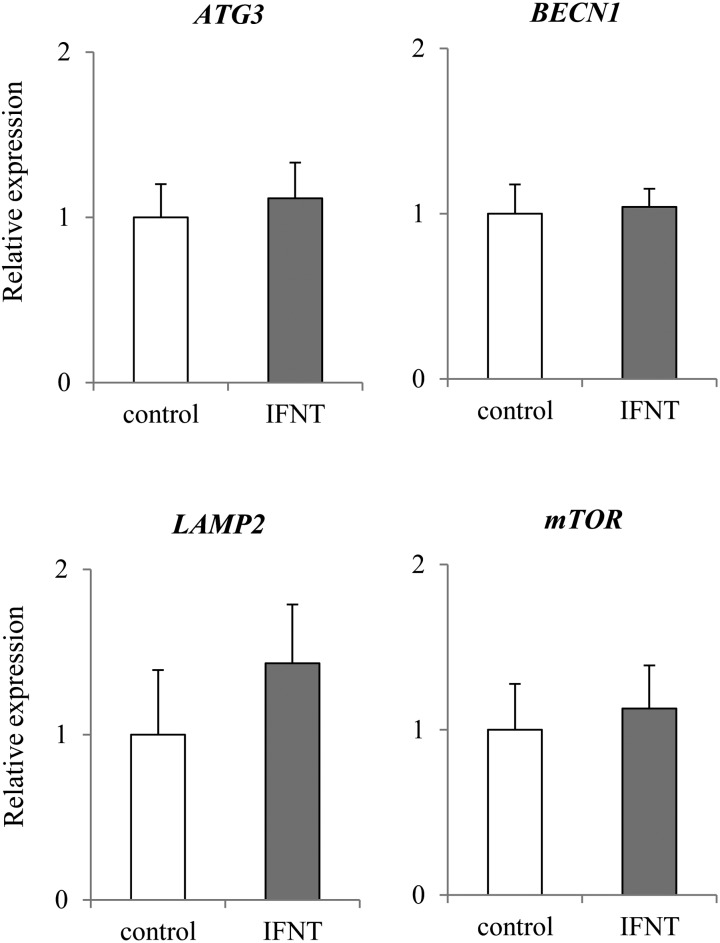
The expression of apoptosis-related genes CASP7 and FADD was significantly higher (P < 0.05) in the presence of IFNT than in control (Fig. 6). In addition, the expression of CASP8 seemed to be higher (P = 0.1011) in epithelial cells cultured with IFNT than in those cultured without IFNT (Fig. 6). However, no significant difference was observed in the expression of BCL2, BAX, CASP3, CASP9, and p53 in both cells cultured with and without IFNT. Expression of pyroptosis-related gene GSDMD was significantly higher (P < 0.05) in epithelial cells cultured with IFNT than in those cultured without IFNT (Fig. 7). Expression of CASP4 and 11 tended to be higher (P < 0.1) in epithelial cells cultured with IFNT than in those cultured without IFNT (Fig. 7). However, no significant difference was observed in the expression of ASC1 between cells cultured with and without IFNT (Fig. 7). Expression of pyroptosis-related genes CASP1 and NLRP3 was not detected (N. D.: Not Detected). No significant difference was observed in the expression of ATG3, BECN1, LAMP2, and mTOR (Fig. 8).
Fig. 6.
Effect of IFNT on the expression of apoptosis-related genes in the bovine uterine epithelial cells. Expression levels of apoptosis related genes (p53, BAX, BCL2, FADD, CASP3, CASP7, CASP8, CASP9) in the uterine epithelial cells cultured with or without IFNT were analyzed using qRT-PCR normalized to H2AFZ as a reference gene (n = 5). All data are shown as the means ± standard error of the mean (SEM). There was a statistically significant difference in mRNA levels (* P < 0.05) when compared between pregnant and non-pregnant tissues.
Fig. 7.
Effect of IFNT on the expression of pyroptosis-related genes in the bovine uterine epithelial cells. Expression levels of pyroptosis related genes (CASP4, CASP11, ASC1, GSDMD) in the uterine epithelial cells cultured with or without IFNT were analyzed using qRT-PCR normalized to H2AFZ as a reference gene (n = 5). All data are shown as the means ± standard error of the mean (SEM). The asterisk (*) indicates significant differences between control and IFNT (P < 0.05). The dagger (†) indicates the tendency (P < 0.1).
Fig. 8.
Effect of IFNT on the expression of autophagy-related genes in the bovine uterine epithelial cells. Expression levels of autophagy related genes (mTOR, BECN1, ATG3, LAMP2) in the bovine epithelial cells cultured with or without IFNT were analyzed using qRT-PCR normalized to H2AFZ as a reference gene (n = 5). All data are shown as the means ± standard error of the mean (SEM). No significant difference was observed in genes expression (mTOR, BECN1, ATG3, LAMP2).
Discussion
In the present study, we investigated the status of pregnancy-dependent cell-death pathways by analyzing several cell death-related gene expressions using in vivo collected uterine tissue and in vitro cultured uterine epithelial cells using IFNT.
In the first analysis, apoptosis-related gene expression was analyzed. In day 18 pregnant endometrial tissues, CASP7 and CASP8 were expressed at significantly higher levels, (with CASP3 expression also tending to be higher) in comparison to non-pregnant tissue (Fig. 2). To confirm that the increase in the expression of apoptosis-related genes was induced by IFNT secreted by the conceptus, we analyzed the expression of the same apoptosis-related genes in cultured uterine epithelial cells with IFNT administration. IFNT significantly increased CASP7 and FADD (Fig. 6). Moreover, the ratio of γH2AX-positive cells also increased with IFNT treatment (Fig. 5). In general, when DNA fragmentation occurs in the nucleus, it is repaired by enzymes using γH2AX and phosphorylated histone, as a scaffold. In fact, the increase in the expression of γH2AX gene was discovered in a human blood cell line at proapoptosis [37]. Next, we performed immunostaining for γH2AX to detect DNA fragmentation. The results in this study suggest that IFNT activates the apoptosis-related pathways in cultured bovine endometrial epithelial cells. This induction might be similarly occurring in bovine endometrial tissues because an abundance of IFNT is secreted in the preimplantation uterus [4]. An increase in FADD and CASP8 gene expression was detected in pregnant uterine tissues, although no significant difference was observed in the expression of p53 and CASP9 between pregnant and non-pregnant endometrial tissues. Similar results were observed in uterine epithelial cells cultured with IFNT. Thus, the induction of apoptosis-related factors at preimplantation may occur via a CASP8-mediated pathway and not a CASP9-mediated pathway. There are several apoptosis pathways mediated via caspases [16]. The apoptosis pathway via CASP8 is induced by extracellular ligands, such as TNF-alpha [19]. IFNT may act as a ligand to induce apoptosis-like TNF-alpha. On the other hand, in the present study, several factors related to the apoptosis pathway via CASP9 were increased during pregnancy or by IFNT treatment. In the previous study, BAX was up-regulated and BCL2 was suppressed by tBID, which is a BID molecule cleaved by CASP8 [38]. Likewise, the apoptotic pathway via CASP9 is induced by DNA fragmentation and mitochondrial mutation [39,40,41,42]. These factors might affect the pathway via CASP9 changing with time lag by DNA fragmentation and/or tBID .
We also investigated the pyroptosis pathway in vivo and in vitro. Expression of CASP4 and CASP11 was significantly increased, and expression of GSDMD tended to be increased in pregnant tissues (Fig. 3). In uterine epithelial cells cultured with IFNT, expression of CASP4, 11, and GSDMD was higher (Fig. 7). These results suggest that pyroptosis was induced in bovine endometrial epithelial cells by IFNT, which strongly suggests that a similar pathway is activated in the pregnant endometrial tissues by secreted IFNT. Expression of NLRP3 was significantly increased and expression of CASP1 and ASC1 tended to be increased in pregnant endometrial tissues. In contrast, expression of NLRP3 and CASP1 was not detected and no significant differences were observed in the expression of ASC1 in cultured endometrial epithelial cells. It was suggested that IFNT-induced pyroptosis in the cultured epithelial cells occurred via a pathway independent of CASP1. Pyroptosis has an important role of promptly communicating with surrounding cells regarding the presence of a pathogen [27, 43, 44].
No significant differences were observed in autophagy-related genes between pregnant tissue and non-pregnant tissue (Fig. 4), and in cultured uterine epithelial cells with or without IFNT. In the mouse uterus, autophagy was inhibited by P4 and/or E2 in the preimplantation endometrium [45]. It might be suggested that autophagy was regulated by steroid hormones, and maintained steady-state levels regardless of pregnancy status.
In the present study, the expression of cell-death-related genes increased in pregnant cow uterine endometrial tissues. This result is similar to a previous apoptotic study regarding mRNA expression levels in the bovine preimplantation endometrium [46]. However, it seemed that DNA fragmentation was not really induced based on the TUNEL analysis of the bovine preimplantation endometrium [46], although DNA fragmentation was obviously induced by IFNT in the present study. In a previous study, cell death caused by IFNT in the bovine endometrial epithelium was inhibited by P4 [7]. Furthermore, P4 concentration in the blood is high in pregnant cattle [47]. It was suggested that cell death by IFNT might be inhibited by P4 in downstream gene expression. Cell-death factors have other functions involved in cell proliferation [21]. Cell proliferation for implantation and/or enlargement of the uterus may be induced by inhibiting cell-death via P4. More detailed studies are necessary to elucidate the relationship of cell death, IFNT, and P4 in the preimplantation period.
In conclusion, the pregnancy-dependent cell death is activated in the bovine uterus through several pathways, such as apoptosis and pyroptosis, and such activation is possibly caused by IFNT. Further study is needed to clarify whether the cell-death pathway directly causes cell death of endometrial epithelial cells or affects indirect signal transduction to cause uterine modulation.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI, 15H04579) and by the Cooperative Research Program for Agriculture Science & Technology Development (RDAPJ01029305). The authors thank the members of Department of Animal Science of Hokkaido University.
References
- 1.Tassell W, Slater M, Barden JA, Murphy CR. Endometrial cell death during early pregnancy in the rat. Histochem J 2000; 32: 373–379. [DOI] [PubMed] [Google Scholar]
- 2.Okano A, Ogawa H, Takahashi H, Geshi M. Apoptosis in the porcine uterine endometrium during the estrous cycle, early pregnancy and post partum. J Reprod Dev 2007; 53: 923–930. [DOI] [PubMed] [Google Scholar]
- 3.Liu YX, Gao F, Wei P, Chen XL, Gao HJ, Zou RJ, Siao LJ, Xu FH, Feng Q, Liu K, Hu ZY. Involvement of molecules related to angiogenesis, proteolysis and apoptosis in implantation in rhesus monkey and mouse. Contraception 2005; 71: 249–262. [DOI] [PubMed] [Google Scholar]
- 4.Ealy AD, Yang QE. Control of interferon-tau expression during early pregnancy in ruminants. Am J Reprod Immunol 2009; 61: 95–106. [DOI] [PubMed] [Google Scholar]
- 5.Pontzer CH, Bazer FW, Johnson HM. Antiproliferative activity of a pregnancy recognition hormone, ovine trophoblast protein-1. Cancer Res 1991; 51: 5304–5307. [PubMed] [Google Scholar]
- 6.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003; 8: 237–249. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Xiao C, Goff AK. Progesterone-modulated induction of apoptosis by interferon-tau in cultured epithelial cells of bovine endometrium. Biol Reprod 2003; 68: 673–679. [DOI] [PubMed] [Google Scholar]
- 8.Baker PK, Pettitt AR, Slupsky JR, Chen HJ, Glenn MA, Zuzel M, Cawley JC. Response of hairy cells to IFN-alpha involves induction of apoptosis through autocrine TNF-alpha and protection by adhesion. Blood 2002; 100: 647–653. [DOI] [PubMed] [Google Scholar]
- 9.Steiner T, Junker U, Henzgen B, Nuske K, Durum SK, Schubert J. Interferon-alpha suppresses the antiapoptotic effect of NF-kB and sensitizes renal cell carcinoma cells in vitro to chemotherapeutic drugs. Eur Urol 2001; 39: 478–483. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, Pan P, Su X, Zhang L, Qin Q, Tan H, Huang L, Li Y. Interferon regulatory factor-1 mediates alveolar macrophage pyroptosis during LPS-induced acute lung injury in mice. Shock 2016; 46: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirozu T, Iwano H, Ogiso T, Suzuki T, Balboula AZ, Bai H, Kawahara M, Kimura K, Takahashi H, Rulan B, Kim SW, Yanagawa Y, Nagano M, Imakawa K, Takahashi M. Estrous cycle stage-dependent manner of type I interferon-stimulated genes induction in the bovine endometrium. J Reprod Dev 2017; 63: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011; 469: 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007; 27: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 2010; 16: 90–97. [DOI] [PubMed] [Google Scholar]
- 15.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi Y, Miura M. Programmed cell death in neurodevelopment. Dev Cell 2015; 32: 478–490. [DOI] [PubMed] [Google Scholar]
- 17.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donovan M, Cotter TG. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim Biophys Acta 2004; 1644: 133–147. [DOI] [PubMed] [Google Scholar]
- 19.Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet 1999; 33: 29–55. [DOI] [PubMed] [Google Scholar]
- 20.Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res 2007; 13: 5995–6000. [DOI] [PubMed] [Google Scholar]
- 21.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 2004; 7: 491–501. [DOI] [PubMed] [Google Scholar]
- 22.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol 2001; 9: 113–114. [DOI] [PubMed] [Google Scholar]
- 23.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 2006; 8: 1812–1825. [DOI] [PubMed] [Google Scholar]
- 25.Kenneth NS, Younger JM, Hughes ED, Marcotte D, Barker PA, Saunders TL, Duckett CS. An inactivating caspase 11 passenger mutation originating from the 129 murine strain in mice targeted for c-IAP1. Biochem J 2012; 443: 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014; 514: 187–192. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 2015; 265: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 2015; 25: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015; 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 30.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6: 1221–1228. [DOI] [PubMed] [Google Scholar]
- 32.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell 2007; 131: 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ 2012; 19: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakumoto R, Hayashi KG, Fujii S, Kanahara H, Hosoe M, Furusawa T, Kizaki K. Possible roles of CC- and CXC-chemokines in regulating bovine endometrial function during early pregnancy. Int J Mol Sci 2017; 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imakawa K, Anthony RV, Kazemi M, Marotti KR, Polites HG, Roberts RM. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature 1987; 330: 377–379. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira JF, Henkes LE, Ashley RL, Purcell SH, Smirnova NP, Veeramachaneni DN, Anthony RV, Hansen TR. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology 2008; 149: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 37.Wu XP, Xiong M, Xu CS, Duan LN, Dong YQ, Luo Y, Niu TH, Lu CR. Resveratrol induces apoptosis of human chronic myelogenous leukemia cells in vitro through p38 and JNK-regulated H2AX phosphorylation. Acta Pharmacol Sin 2015; 36: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene 2003; 22: 8543–8567. [DOI] [PubMed] [Google Scholar]
- 39.Yuan ZM, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci USA 1997; 94: 1437–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, Lu H, Kharbanda S, Weichselbaum R, Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 1999; 399: 814–817. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K, Komatsu K, Wang HG, Kufe D. c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage. Mol Cell Biol 2002; 22: 3292–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida K, Yamaguchi T, Natsume T, Kufe D, Miki Y. JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat Cell Biol 2005; 7: 278–285. [DOI] [PubMed] [Google Scholar]
- 43.Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol 2007; 9: 2543–2551. [DOI] [PubMed] [Google Scholar]
- 44.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 2010; 11: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi S, Shin H, Song H, Lim HJ. Suppression of autophagic activation in the mouse uterus by estrogen and progesterone. J Endocrinol 2014; 221: 39–50. [DOI] [PubMed] [Google Scholar]
- 46.Groebner AE, Schulke K, Unterseer S, Reichenbach HD, Reichenbach M, Büttner M, Wolf E, Meyer HH, Ulbrich SE. Enhanced proapoptotic gene expression of XAF1, CASP8 and TNFSF10 in the bovine endometrium during early pregnancy is not correlated with augmented apoptosis. Placenta 2010; 31: 168–177. [DOI] [PubMed] [Google Scholar]
- 47.Mann GE, Lamming GE. The influence of progesterone during early pregnancy in cattle. Reprod Domest Anim 1999; 34: 269–274. [Google Scholar]