Abstract
17α-Ethinyl estradiol (EE), a synthetic analog of natural estrogen 17β-estradiol (E2), is extensively used in hormonal contraceptives and estrogen replacement therapy, and it has also been found in sewage effluents. Given that E2 is a well-known immunomodulator, surprisingly there has been only limited information on the cellular and molecular immunologic consequences of exposure to EE. To address this fundamental gap, we directly compared the effects of EE with E2 on splenic leukocytes of New Zealand Black × New Zealand White F1 progeny (NZB/WF1) mice during the preautoimmune period. We found that EE and E2 have common, as well as distinctive, immunologic effects, with EE exposure resulting in more profound effects. Both EE and E2 increased numbers of splenic neutrophils, enhanced neutrophil serine proteases and myeloperoxidase expression, promoted the production of nitric oxide and monocyte chemoattractant protein-1, and altered adaptive immune T cell subsets. However, activation of splenic leukocytes through the T cell receptor or Toll-like receptor (TLR)4 revealed not only common (IL-10), but also hormone-specific alterations of cytokines (IFNγ, IL-1β, ΤΝFα, IL-2). Furthermore, in EE-exposed mice, TLR9 stimulation suppressed IFNα, in contrast to increased IFNα from E2-exposed mice. EE and E2 regulated common and hormone-specific expression of immune-related genes. Furthermore, EE exposure resulted in more marked alterations in miRNA expression levels than for E2. Only EE was able to reduce global DNA methylation significantly in splenic leukocytes. Taken together, our novel data revealed that EE and E2 exposure confers more similar effects in innate immune system–related cell development and responses, but has more differential regulatory effects in adaptive immune–related cell development and responses.
Estrogen has previously been shown to regulate the delicate balance of the immune system (1–5). 17β-Estradiol (E2) is a naturally produced estrogen, and serum levels fluctuate during menstrual cycles and pregnancy and decline during menopause. E2 is able to alter the development, differentiation, function, and responses of all types of immune cells, including both innate and adaptive cells (6–9). Among estrogens, E2 is the most extensively studied compound with respect to its regulatory effects on the immune system (5, 7, 9, 10). These E2-induced immunoregulatory effects have been shown to influence disease presentation and clinical signs in such categories as autoimmune and allergic diseases (11–13). The potential effects of estrogen on autoimmunity are suggested by clinical observations that lupus flares increase after puberty, when estrogen levels are increased compared with prepubertal age, and decrease following menopause, when estrogen levels drop (14, 15). Exposure to estrogenic compounds may mimic or block the action of natural hormones by binding to their receptor or interfering with receptor activities (1, 16). Estrogens have been shown to alter various functions of the immune system and regulate the responses to stimuli in both normal and autoimmune individuals through estrogen receptor–dependent or –independent mechanisms (13, 17–20). E2 treatment, both in vivo and in vitro, of splenic leukocytes has been shown to influence the production of multiple proinflammatory cytokines and chemokines and promote B cell survival following stimulation (8, 15, 21–25). Increased B cell survival following estrogen treatment has been associated with a break in B cell tolerance, as well as promotion of autoantibody production and lupus-related pathology in nonautoimmune mouse strains (12). Increased circulating E2 was also associated with increased autoimmune activity in human males (26).
E2 and other estrogenic compounds, such as bisphenol A (BPA), exert modulatory effects on the epigenetic regulatory mechanisms such as miRNA and DNA methylation, impacting cellular responses and gene expression (11, 27–31). We have reported that E2 regulated miRNA expression in both orchidectomized male C57BL/6 (B6) and New Zealand Black × New Zealand White F1 progeny (NZB/WF1) mice (27, 32). A subset of E2-regulated miRNAs, including miR-146a, miR-148a, miR-125a, and miR-126, has been implicated in human lupus pathogenesis by targeting lupus-related signaling pathways (33–39). These data suggest the potential involvement of miRNAs in E2-mediated effects on lupus. While the roles of E2 in the regulation of multiple aspects of the immune system and exacerbation of autoimmune inflammatory processes have been well documented, the effects of 17α-ethinyl estradiol (EE), a synthetic analog of E2, on immune development, differentiation, and function have been minimally documented. Also, to date, no studies have directly compared the effects of E2 and EE on immune response and/or epigenetic regulation in an autoimmune-prone immune system.
EE is a primary component in common contraceptives, both transdermal and oral, used by females and has been implemented in estrogen replacement therapy. Other common indications for EE treatment include breast cancer, vasomotor symptoms in menopause, female hypogonadism, hirsutism, acne vulgaris, and dysmenorrhea (https://www.drugbank.ca/drugs/DB00977). Detection of EE as an aquatic pollutant and in sewage effluents shows an increase in the potential exposure to human populations in addition to pharmaceutical administration and is regarded as an environmental endocrine-disrupting chemical (40). EE was shown to have a 100-fold stronger potency than E2 for estrogen receptor α nuclear translocation (41). However, resultant immunological functional assays were not performed in that study, and few studies have been published detailing the specific effects of EE on immune system development and function.
In this study, we investigated whether EE can induce similar or dissimilar immunologic and epigenetic effects compared with those of E2. Furthermore, because estrogen modulation of the immune system can affect responses to infections, we investigated the effects of E2 and EE exposure on cytokine responses through T cell receptor/CD3 complex, Toll-like receptor (TLR)4 [a receptor of bacterial lipopolysaccharide (LPS)], and TLR9 (recognizes unmethylated double-stranded DNA commonly found in bacteria and viruses) stimulation. To understand the transcriptional and epigenetic regulatory effects of E2 and EE exposure in splenic leukocytes, we also profiled the expression patterns of immune-related genes and miRNAs. Our study shows that exposure to E2 or EE resulted in distinct differences in action between cellular responses and cell populations generally considered innate vs those generally considered part of the adaptive immune response.
Materials and Methods
Mice and subcutaneous implants
Genetically lupus-prone NZB/WF1 (NZBWF1/J, stock no. 100008) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Wild-type B6 mice were bred in the Virginia-Maryland College of Veterinary Medicine vivarium. All mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care International–certified animal facility at the Virginia-Maryland College of Veterinary Medicine. Since historically, predominantly females are exposed to these estrogenic compounds through contraceptive use and estrogen replacement therapy, and lupus exhibits a strong female sex bias, for this study we investigated the effects of E2 and EE on female NZB/WF1 mice only. Mice were fed the 7013 NIH-31 modified 6% mouse/rat sterilizable diet (Harlan Laboratories, Madison, WI). Mice were implanted with a silastic capsule of the same size containing either E2 or EE, or an empty control capsule for the placebo group, at 6 to 7 weeks of age. For the experiments investigating DNA methylation levels, in addition to intact NZB/WF1 female mice, orchidectomized male B6 mice were implanted with a silastic capsule containing E2 or an empty control capsule for the placebo group. Mice were euthanized at 16 to 17 weeks of age in the early disease stage, constituting 9 to 10 weeks of treatment. Care was taken to ensure that all groups were subjected to the same housing, local environment, and handling conditions. All animal procedures and experiments were performed in accordance with guidelines of the Institutional Animal Care and Use Committee at Virginia Tech. CO2 was used for euthanasia as required by the approved Institutional Animal Care and Use Committee protocol.
Tissue preparation and cellular culture
Whole splenic leukocytes and thymocytes were isolated using standard laboratory procedures described in detail previously (24, 25, 27, 42). Briefly, the spleens and thymus were dissociated by gently scraping through a steel screen, and the cell suspension was passed through a 70-μm cell strainer to remove undissociated tissue debris. The splenic leukocytes and thymocytes were isolated by lysing red blood cells with ACK lysis buffer/Tris/NH4Cl and then washed with complete RPMI 1640 medium (Mediatech, Manassas, VA) that was supplemented with 10% charcoal-stripped fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 2 mM l-glutamine (HyClone Laboratories, Logan, UT), 100 IU/mL penicillin and 100 μg/mL streptomycin (HyClone Laboratories), and 1% nonessential amino acids (HyClone Laboratories). The cells were adjusted to 5 × 106/mL in complete medium for seeding into cell culture plates. Briefly, the cells were plated onto a 48-well cell culture plate (0.25 mL per well) and stimulated with LPS (500 ng/mL; Sigma-Aldrich, St. Louis, MO), concanavalin A (ConA; 5 μg/mL; Sigma-Aldrich), or TLR9 agonist oligodeoxynucleotide (ODN)-1585 (0.5 μM; synthesized by Integrated DNA Technologies, Skokie, IL) for the designated time by adding an equal volume of 2× concentration of stimulation medium. For anti-CD3 plus anti-CD28 stimulation, the cells were plated onto anti-CD3 (5 μg/mL; eBioscience, San Diego, CA) precoated cell culture plates and then equal volumes of medium containing anti-CD28 (eBioscience) at 4 μg/mL (final at 2 μg/mL) were added for 24 hours.
Flow cytometry
The relative percentages of CD4-, CD8-, CD25-, and CD69-expressing cells in the thymus and spleen, as well as B220-, CD19-, IgG-, IgM-, IgG2a-, CD11b+-, and Ly6G+-expressing cells in the spleen, were quantified by flow cytometric analysis. Stained cells were visualized using a FACSAria flow cytometer (BD Biosciences, San Jose, CA) and data were analyzed using FlowJo version 7 software as described in our previous studies (43–45).
Detection of inducible nitric oxide synthase and nitric oxide
Griess assays were used to detect nitric oxide (NO) levels in culture supernatants as described (25). Western blots were used to analyze inducible NO synthase (iNOS) protein expression in whole-cell extracts as described before (25). The whole-cell extracts were prepared by lysing the cell pellet with CelLytic M cell lysis reagent (Sigma-Aldrich). The anti-iNOS [M19, sc-650; RRID: AB_631831 (46)] antibody was purchased from Santa Cruz Biotechnology (Paso Robles, CA). The protein loading control antibody, anti–β-actin antibody [ab8227; RRID: AB_2305186 (47)], was obtained from Abcam (Cambridge, MA). The blot images were captured using a Kodak Image Station 440.
Cytokine/chemokine ELISAs
The levels of monocyte chemoattractant protein-1 (MCP-1) in ConA, anti-CD3 plus anti-CD28, or LPS-stimulated culture supernatants were determined with a mouse MCP-1 ELISA MAX Deluxe kit (BioLegend, San Diego, CA). The levels of IFNα in TLR9 against ODN 1585–stimulated splenic leukocytes culture supernatants were determined with an eBioscience mouse IFNα Platinum ELISA kit (Fisher Scientific, Hampton, NH). The ELISAs were performed per the manufacturers’ protocols. Ciraplex® chemiluminescent assay kits (Aushon BioSystems, Billerica, MA) were used to quantify the levels of IFNγ, IL-1β, IL-2, IL-6, IL-10, and TNFα in cell culture supernatants per the manufacturer’s instructions (28, 44). The images of chemiluminescent array plates were captured with a Cirascan image system (Aushon BioSystems), and the image data were processed with Cirasoft software.
DNA isolation and global DNA methylation analysis
The whole genomic DNA was isolated from splenic cells with a DNeasy blood and tissue kit (Qiagen, Valencia, CA). The DNA concentration was measured with a NanoDrop 2000 spectrophotometer. The 5-methylcytosine DNA ELISA kit (Zymo Research, Irvine, CA) was used to measure the global DNA methylation level as we previously reported (28, 44). Briefly, 100 ng of DNA of each sample was brought up to 100 μL of volume with 5-mC coating buffer, denatured at 98°C, and then coated onto a 96-well assay plate. After washing, the coated DNA was incubated with an antibody mix consisting of anti–5-methylcytosine antibody and secondary antibody. After antibody incubation, the plate was washed and HRP developer solution was added to develop color signal. The absorbance was measured by reading the plate at 405 nm on a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA). The percentage of 5-mC in each DNA sample was quantified with a standard curve that was generated with kit-provided positive control (100% methylation) and negative control (0% methylation).
mRNA and immune-related miRNA gene expression profile analysis
Total RNA, containing small RNA, was isolated from whole splenic leukocytes using a miRNeasy mini kit (Qiagen) as described in our previous publications (27, 28, 32). Briefly, on-column DNase digestion with RNase-free DNase (Qiagen) was performed to remove residual amounts of DNA contamination in the isolated RNA. The RNA concentration was quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE). miRNA and immune-related mRNA expression profiles of freshly isolated splenic leukocytes from EE-, E2-, and placebo-treated NZB/WF1 mice were analyzed with a NanoString nCounter gene expression system (NanoString Technologies, Seattle, WA). Briefly, ∼150 ng of total RNA (containing small RNA) for each sample was sent to NanoString Technologies for mRNA and miRNA expression analysis with an nCounter GX mouse immunology kit (contains 547 immune-related genes) and an nCounter mouse miRNA v1.5 assay kit (contains 578 miRNA targets). The acquired raw data were uploaded to nSolver NanoString software 3.0 and processed with default quality control settings, and then for data normalization and expression analysis. The data normalization took two steps. First, raw counts were normalized to the six internal positive controls to adjust for systematic variation from assay to assay. Second, the positive control normalized counts were further normalized with the geometric mean of 14 reference genes in the CodeSet (for immune-related genes) or with the geometric mean of the top 100–expressed miRNAs to adjust for the variation of RNA contents. The scatterplot expression data for mRNA and miRNA were generated in nSolver using group expression values. Ratios for the comparison of treatment groups and associated P values were generated by the nSolver software. The normalized expression values of the mRNA genes that demonstrated ≥1.5-fold change with P < 0.1 in either the EE- or E2-treated group and of the miRNAs that demonstrated ≥1.5-fold change in either the EE- or E2-treated group were exported from nSolver to Excel and were selected to generate the Venn diagram. To visualize the differential expression intensity levels of mRNA and miRNAs in different treatment groups, the normalized expression intensity values of exported genes (mRNAs or miRNAs) from individual samples were log2 transformed, centered by genes, and then used for cluster analysis to generate the heat map with Java TreeView (48). The NanoString mRNA and miRNA gene expression analysis data sets have been submitted to Gene Expression Omnibus database under accession no. GSE121745.
Quantitative RT-PCR analysis of miRNA and mRNA expression
As we described in detail previously (27, 32, 42), TaqMan miRNA assay reagents (Applied Biosystems, Grand Island, NY) were used to quantify the miRNA expression per the manufacturer’s instructions. The iScript one-step RT-PCR with SYBR Green kit (Bio-Rad Laboratories, Hercules, CA) was used for quantifying the mRNA expression levels of neutrophil serine proteinases, including neutrophil elastase (NE), proteinase 3 (PR3), cathepsin G (CG), and myeloperoxidase (MPO), as we previously reported (43). QuantiTect 10× PCR primer mixes for mouse NE, PR3, CG, MPO, and β-actin genes were purchased from Qiagen. The expression levels of miRNAs and mRNAs were normalized to endogenous small RNA control small nucleolar RNA 202 and housekeeping gene β-actin, respectively. The data are shown as relative expression level to an appropriate control by using the 2−ΔΔCT formula (Livak method).
Statistical analysis
All values in the graphs are given as mean ± SEM or as otherwise stated in the figure legend. To assess statistical significance, one-way ANOVA followed by the least significant difference method was performed for the group comparisons, unless otherwise specified in the figure legend. JMP software (Pro13 version) was used for all statistical analyses. Graphical presentations of data were performed in GraphPad Prism software (v7.04).
Results
E2 and EE treatment affect spleen weight
Compared with placebo-treated NZB/WF1 mice, in both E2- and EE-exposed mice, a significant increase of absolute spleen weight and the spleen/body weight ratio was observed (49). Although similar splenomegaly was present in E2-exposed NZB/WF1 mice, there was no evidence of reduced splenic leukocyte numbers, as we previously observed in E2-exposed orchidectomized male B6 mice (43, 49). We did observe an increase in splenic leukocyte cell counts in EE-exposed mice, although the value did not reach statistical significance, consistent with increased spleen weight in these mice (49). This suggests that most of the increased spleen weight may be due to accumulation of erythrocytes and/or stromal cell populations rather than leukocytes in the estrogenic compound–exposed NZB/WF1 female mice. We then examined the phenotypic and functional effects of EE and E2 exposure on innate immunity and adaptive immune cells.
Both E2 and EE treatments increase splenic neutrophil numbers and increase neutrophil serine protease and MPO mRNA expression
In this study, we demonstrated that both E2 and EE exposure significantly increased the percentage of neutrophils (CD11b+Ly6G+) in the splenic leukocytes of gonadal intact female NZB/WF1 mice (Fig. 1A). However, the increase of the absolute splenic CD11b+Ly6G+ counts in both E2- and EE-treated mice were not significant (Fig. 1B). Further real-time RT-PCR analysis revealed that neutrophil serine proteases, including NE, CG, PR3, and neutrophil-specific MPO, were all significantly increased in the splenic leukocytes from both E2- and EE-treated NZB/WF1 mice at a similar level when compared with placebo controls (Fig. 1C).
Figure 1.
Exposures to both E2 and EE increase splenic neutrophil percentages and neutrophil serine protease (NSP) mRNAs in NZB/WF1 mice. (A and B) Flow cytometric analysis results of splenic leukocytes from mice exposed to placebo (n = 4), E2 (n = 5), and EE (n = 4). Cells were stained with neutrophil surface markers CD11b and Ly6G antibodies. Summary graphs show (A) the percentages (mean ± SEM) of neutrophils in splenic leukocytes, and (B) total CD11b+Ly6G+ splenic leukocyte counts from placebo, E2-, and EE-exposed mice. Samples were initially gated for live singlets, followed by gating for CD11b and Ly6G double-positive cells. (C) Real-time RT-PCR analysis of the relative mRNA expression levels of neutrophil serine proteases (NE, CG, and PR3) and MPO in splenic leukocytes from placebo-, E2-, and EE-exposed mice (n = 2). ANOVA with a post hoc Tukey test was used for statistical analysis of NSP and MPO expression data. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01.
E2 and EE exposures promote the production of NO and iNOS
iNOS and nitric oxide NO production following LPS or ConA stimulation in splenic leukocytes has previously been shown to be sensitive to estrogen exposure. There was a significant increase of NO production in ConA-activated splenic leukocytes from EE-exposed mice when compared with placebo control mouse splenic leukocytes (Fig. 2A). Although it did not reach statistical significance (P = 0.055), there was a clear trend of increased NO in ConA-activated splenic leukocytes from E2-exposed mice when compared with placebo control (Fig. 2A). Compared with ConA-activated splenic leukocytes, there was a much lower production of NO in LPS-activated splenic leukocytes (Fig. 2B). Consistent with the NO data, there was an increase of iNOS protein expression in E2- and EE-exposed splenic leukocytes (Fig. 2C). Western blot analysis also showed only a marginal increase of iNOS protein in LPS-activated splenic leukocytes of EE-exposed mice (Fig. 2D).
Figure 2.
E2 and EE exposure increased NO, iNOS, and MCP-1 production by activated splenic leukocytes. Splenic leukocytes were activated by (A) ConA (5 μg/mL) or (B) LPS (500 ng/mL) for 48 h. The NO level was measured in cell culture supernatants by Griess assay. Splenic leukocytes were activated with either (C) ConA (5 μg/mL) or (D) LPS (500 ng/mL) for 24 h and then collected for western blot analysis for the expression of iNOS and β-actin (loading control). Splenic leukocytes were activated by (E) ConA (5 μg/mL), (F) LPS (500 ng/mL), or (G) anti-CD3 plus anti-CD28 (anti-CD3, 5 µg/mL for plate coating; anti-CD28, 2 μg/mL) for 24 h. MCP-1 levels in cell culture supernatants were measured using ELISA. Data are expressed as mean ± SEM (n ≥ 4). *P < 0.05; **P < 0.01; ***P < 0.001.
Both E2 and EE exposure increased MCP-1 production following stimulation, but to different degrees
MCP-1 production levels were remarkably increased in ConA-, LPS-, or anti-CD3 plus anti-CD28–activated splenic leukocytes from E2- and EE-exposed NZB/WF1 mice, and there was only a minimal level of MCP-1 production in either LPS- or ConA-activated splenic leukocytes from placebo-treated NZB/WF1 mice (Fig. 2E–2G). EE exposure consistently led to more pronounced MCP-1 production compared with E2-exposed mice with different stimulants. The MCP-1 data, together with above iNOS/NO (Fig. 2A–2D) and neutrophils/neutrophil serine proteases (Fig. 1), suggested that both E2 and EE affect innate immune cell development and functions, and that EE appears to have a more profound effect than does E2 with regard to the production of NO and MCP-1.
E2 and EE treatments alter the T cell subsets (composition) and activation phenotype in the spleen and thymus
Flow cytometric analysis was performed to evaluate the T cell composition in the spleen and thymus of NZB/WF1 mice following in vivo E2 and EE treatment. As indicated, E2 and EE treatment reduced the percentage of CD4+ single-positive (SP) T cells in the spleen. The reductive effect of EE on CD4+ SP T cells was more prominent than of E2 (Fig. 3A and 3E). We further analyzed the expression of T cell activation markers CD25 and CD69 in splenic leukocytes. Both E2 and EE exposure led to reduced CD4+CD25+ and minimal changes to CD4+CD69+ subsets (Fig. 3B, 3C, 3F, and 3G) in NZB/WF1 mice when compared with placebo controls. Gating only on the splenic CD4+ cells, the percentage of CD25+CD69+ (CD4+/CD25+CD69+) was significantly increased (Fig. 3D and 3H).
Figure 3.
E2 and EE exposure alters splenic CD4+ cell subsets. Flow cytometric analysis results of splenic leukocytes from mice exposed to placebo (n = 4), E2 (n = 5), and EE (n = 4). Cells were stained with antibodies against the surface markers CD4, CD25, and CD69. Shown are the representative (A–D) FACS plots accompanied by (E–H) summary graphs showing the percentages (mean ± SEM) of CD4+ subsets in splenic leukocytes. Samples were initially gated for live singlets, followed by gating for (A) CD4+ cells. (B and C) Samples were further gated on singlets for CD4+ and CD25+, as well as for CD4+ and CD69+. (D) Samples were gated on the CD4+ population followed by CD25+ and CD69+ cells. Data are presented as (A–C and E–G) percentage of total splenic leukocytes or (D and H) percentage of CD4+ splenic leukocytes from placebo-, E2-, and EE-exposed mice. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
E2 had only minimal effects on splenic CD8+ cell populations and activation. However, EE exposure led to significant differences in CD8+ subsets when compared with both placebo and E2 groups (Fig. 4A and 4E). When compared with placebo controls, in EE-treated, but not E2-treated, splenic leukocytes, the percentages of splenic CD8+CD25+ cells were increased with minimal effects on CD8+CD69+ subsets in EE-treated, but not E2-treated, splenic leukocytes (Fig. 4B, 4C, 4F, and 4G). The percentage of the CD8+CD25+CD69+ cell population was also only significantly increased in EE-exposed, but not E2-exposed, NZB/WF1 mice (Fig. 4D and 4H).
Figure 4.
E2 and EE exposure alters splenic CD8+ cell subsets. Flow cytometric analysis results of splenic leukocytes from mice exposed to placebo (n = 4), E2 (n = 5), and EE (n = 4). Cells were stained with antibodies against the surface markers CD8, CD25, and CD69. Shown are the representative (A–D) FACS plots accompanied by (E–H) summary graphs showing the percentages (mean ± SEM) of CD8+ subsets in splenic leukocytes. Samples were initially gated for live singlets, followed by gating for (A) CD8+ cells. (B and C) Samples were further gated on singlets for CD8+ and CD25+, as well as CD8+ and CD69+. (D) Samples were gated on the CD8+ population followed by CD25+ and CD69+ cells. Data are presented as (A–C and E–G) percentage of total splenic leukocytes or (D and H) percentage of CD8+ splenic leukocytes from placebo-, E2-, and EE-exposed mice. Data are expressed as mean ± SEM. **P < 0.01; ***P < 0.001.
Thymic T cell populations were also altered due to EE exposure. The effect of E2 on thymic T cell populations was minimal. No differences were identified in CD4+ SP cells in both E2- and EE-treated mice when compared with placebo controls (49). Thymic CD8+ SP and double-positive CD4+CD8+ cells were significantly decreased in EE-exposed mice (49). EE, but not E2, exposure led to increased CD4+CD25+ cells in the thymus compared with the placebo and E2 groups (49). Similar to the findings in the splenic cells, the percentage of CD4+CD69+ was not altered, but the percentage of CD25+CD69+ cells in CD4+ T cells (CD4+/CD25+CD69+) was significantly increased in EE-treated mice (49). The percentage of CD8+CD25+, but not CD8+CD69+, in splenic cells and the percentage of CD25+CD69+ double-positive cells in CD8+ SP T cells were increased in EE-exposed mice (49). Taken together, our data indicated that EE exposures significantly affect T cell composition in the thymus, and they promote T cell activation. Although E2 exposure on thymic T cell populations had similar trends as for EE exposure, the effects were not as pronounced as for EE.
The effect of E2 and EE treatment on B cell numbers and immunoglobulin levels is marginal
We also examined whether estrogen and EE exposure affected B cell development and function in NZB/WF1 mice. There were no obvious changes in the percentages of CD19+, B220+, IgG+, and IgM + cells in the spleens of E2- and EE-exposed mice (49). We only observed a significant increase in IgG2a+ splenic cells in EE-exposed NZB/WF1 mice when compared with placebo controls (49).
E2 and EE exposure leads to differential effects on the induction of various inflammatory cytokines from in vitro–activated splenic leukocytes
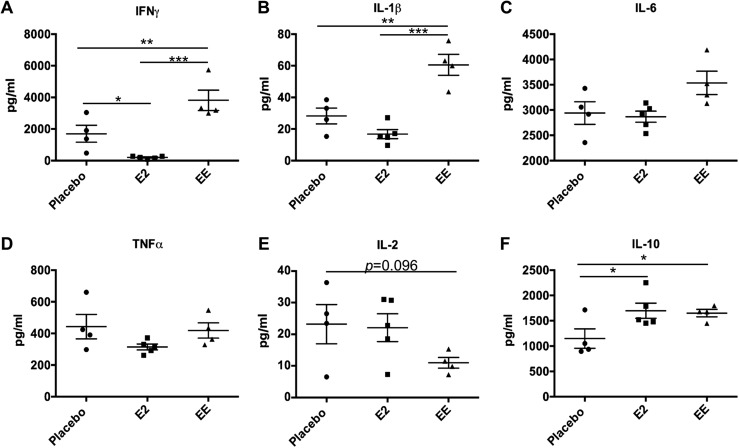
Our previous studies have demonstrated that E2 significantly promoted the induction of IFNγ and IL-1β, as well as IL-6 in LPS-stimulated splenic leukocytes from male B6 mice (24, 27, 43). To our surprise, we observed a significant reduction of IFNγ, and a similar trend in IL-1β, in LPS-activated splenic leukocytes from E2-exposed NZB/WF1 mice when compared with placebo controls. However, IFNγ and IL-1β were significantly upregulated in LPS-activated splenic leukocytes from EE-exposed NZB/WF1 mice when compared with placebo control NZB/WF1 mice (Fig. 5A and 5B). There were only minimal changes in IL-6 and TNFα production in LPS-activated splenic leukocytes from both E2- and EE-exposed mice when compared with placebo controls, whereas there was a trend of reduction of LPS-induced IL-2 (P = 0.096) in EE-treated mice (Fig. 5C–5E). LPS activation increased IL-10 levels in both EE- and E2-exposed mice similarly when compared with placebo controls (Fig. 5F).
Figure 5.
Differential effect of E2 and EE exposure on the production of different cytokines from LPS-activated splenic leukocytes from NZB/WF1 mice. Splenic leukocytes were activated by LPS (500 ng/mL) for 24 h. Cell culture supernatants were used for ELISA to evaluate the production of cytokines (A) IFNγ, (B) IL-1β, (C) IL-6, (D) TNFα, (E) IL-2, and (F) IL-10 with Ciraplex® multiplex cytokine assay. Data are expressed as mean ± SEM (n ≥ 4). *P < 0.05; **P < 0.01; ***P < 0.001.
Owing to the decreased CD4+ and CD8+ SP T cells in splenic leukocytes, we evaluated a more specific T cell response using anti-CD3 plus anti-CD28, instead of ConA, to stimulate the splenic leukocytes in this study. Surprisingly, even with reduced CD4+ T cells in splenic leukocytes, we observed a significant increase of IFNγ and IL-1β in anti-CD3 plus anti-CD28–activated splenic leukocytes from EE-exposed mice (Fig. 6A and 6B). We did observe a significant reduction of IL-6, TNFα, and IL-2 in anti-CD3 plus anti-CD28–activated splenic leukocytes from EE-exposed mice (Fig. 6C–6E). E2 exposure had no obvious effect on the induction of IFNγ, IL-1β, IL-6, TNFα, or IL-2 in anti-CD3 plus anti-CD28–activated splenic leukocytes (Fig. 6A–6E). E2 and EE exposure both significantly increased IL-10 production, whereas E2 showed a stronger magnitude of effect in splenic leukocytes following anti-CD3 plus anti-CD28 stimulation (Fig. 6F).
Figure 6.
Differential effect of E2 and EE exposure on the production of different cytokines from anti-CD3 plus CD28–activated splenic leukocytes from NZB/WF1 mice. Splenic leukocytes were activated by anti-CD3 (5 µg/mL for plate coating) plus anti-CD28 (2 µg/mL) for 24 h. Cell culture supernatants were used for ELISA to evaluate the production of cytokines (A) IFNγ, (B) IL-1β, (C) IL-6, (D) TNFα, (E) IL-2, and (F) IL-10. Data are expressed as mean ± SEM (n ≥ 4). *P < 0.05; **P < 0.01; ***P < 0.001.
TLR-driven IFNα plays a critical role in the pathogenesis of lupus (50–52). We therefore analyzed the production of IFNα in splenic leukocytes from E2- and EE-exposed mice in response to TLR9 agonist class A CpG ODN-1585. As shown in Fig. 7A, E2 exposure significantly increased IFNα production in ODN-1585–activated splenic leukocytes. IFNα production from EE-exposed mice was significantly lower than that of E2-exposed mice, but the reduction did not reach significance when compared with the placebo-exposed mice. As a class A TLR9 agonist, ODN-1585 also has a weak stimulatory effect on TLR9-dependent NF-κB signaling to drive the expression of inflammatory cytokines such as IL-6. We observed that EE exposure also significantly reduced IL-6 production, whereas E2 had no obvious effect on the IL-6 in ODN-1585–activated cells (Fig. 7B).
Figure 7.
Differential effect of E2 and EE exposure on the production of cytokines following TLR9 stimulation. Splenic leukocytes were activated by class A ODN-1585 (0.5 µM) for 24 h. Cell culture supernatants were used for ELISA to evaluate the production of cytokines (A) IFNα and (B) IL-6. Data are expressed as mean ± SEM (n ≥ 4). *P < 0.05; **P < 0.01; ***P < 0.001.
E2 and EE exposure has similar and yet distinct effects on the regulation of immune-related gene expression
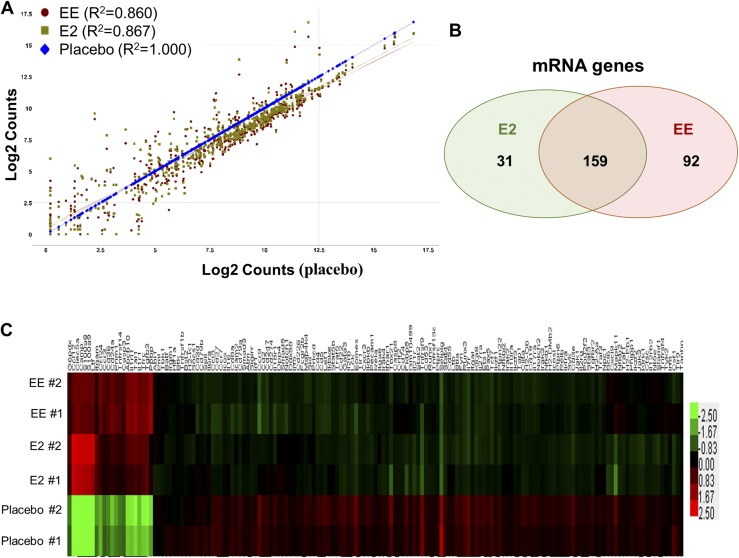
To better understand the immunoregulatory role of E2 and EE, we performed gene expression analysis in splenic leukocytes from NZB/WF1 mice following placebo, E2, or EE exposure with a NanoString nCounter GX mouse immunology code set. The scatter plot showed that E2 and EE treatment altered numerous immune-related genes at a similar pattern (Fig. 8A). The regression analysis revealed that the R2 value of gene expression between placebo and E2 is 0.867, and the R2 value between placebo and EE was 0.860. We identified that 190 genes were altered by E2 and 251 immune-related genes were altered by EE at a fold change of ≥1.5 with P < 0.1, of which there were 159 genes were commonly regulated by E2 and EE treatment (Fig. 8B) (49). A heat map was generated to visualize the similar expression change of these 159 genes induced by E2 and EE treatment (Fig. 8C). Taken together, we demonstrated that E2 and EE exposure has a similar effect on the regulation of immune-related gene expression.
Figure 8.
E2 and EE exposure has similar effect in the regulation of immune-related gene expression in splenic leukocytes from NZB/WF1 mice. (A–C) NanoString gene expression analysis of splenic leukocytes of placebo-, E2-, and EE-treated NZB/WF1 mice. The raw data were processed and analyzed using nSolver software 3.0. (A) Scatter plot shows the means of normalized log intensities of individual gene probes. R2 indicates the correlation level of the expression of all analyzed genes between placebo and E2 or EE treatment groups. (B) The Venn diagram shows the number of mRNAs that were regulated by both E2 and EE or specifically by E2 or EE. The mean normalized intensity of the mRNA genes that demonstrated at least 1.5-fold change with P < 0.1 in either EE- or E2-treated group when compared with placebo-treated group were selected to generate the Venn diagram. (C) Hierarchical cluster heat map shows the expression levels of mRNAs that were regulated by both E2 and EE (159 genes) in different treatment groups.
EE has a more profound effect than E2 on the expression of miRNA genes
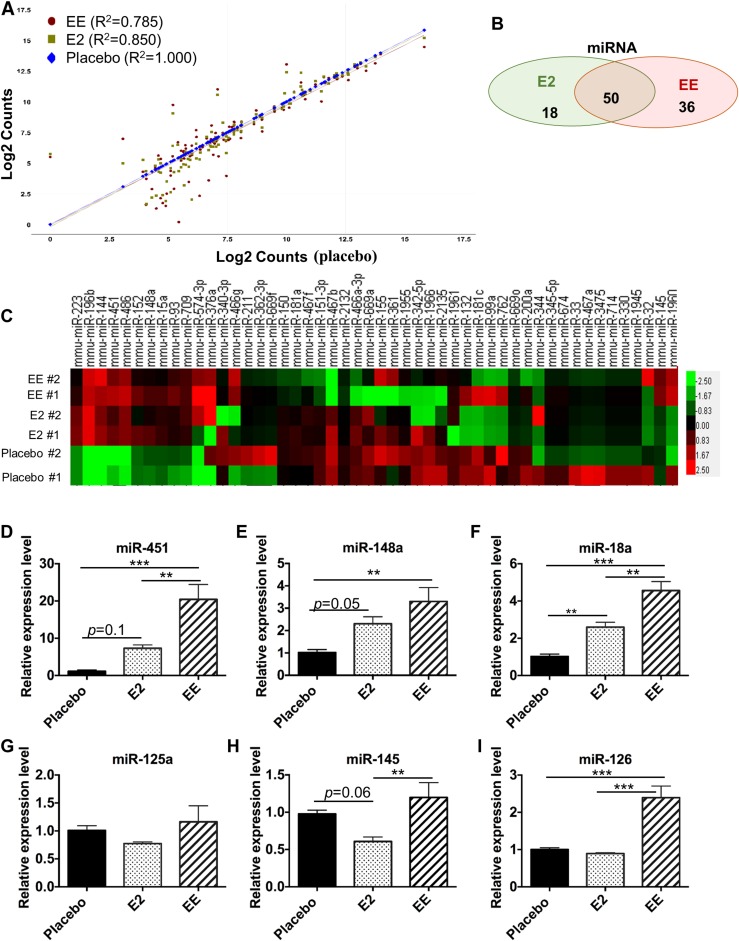
We have reported that estrogen regulated miRNA expression in splenic leukocytes from E2-treated orchidectomized male B6 mice (27). There are limited data with regard to EE regulation of miRNAs, and these data were collected in tissues/cells other than immune cells (53, 54). To understand the epigenetic regulatory role of E2 and EE in NZB/WF1 mice, we profiled the miRNA expression in placebo-, EE-, and E2- exposed splenic leukocytes. The scatter plot showed correlation of miRNA expression between E2- or EE-exposed groups and placebo group (Fig. 9A). It is apparent that EE had more profound effects than E2 on the miRNA expression, as the R2 value of miRNA expression between placebo and E2 was 0.852, but the R2 value of miRNA expression between placebo and EE was only 0.777. The R2 between E2 and EE groups for miRNA expression was 0.902. Compared with the R2 of 0.962 for immune-related gene expression between the E2 and EE groups, E2 and EE have more differential effects on the miRNA expression than on mRNA gene expression. Further analysis demonstrated that 68 miRNAs were altered by E2 and 86 miRNAs were altered by EE at a fold change of at least 1.5 (Fig. 9B) (49). A heat map was generated to show the miRNA expression level changes of 50 miRNAs that are commonly regulated by E2 and EE in the individual samples of different treatment groups (Fig. 9C). It is noteworthy that there was greater biological variation in the same group for miRNA profiling assays when compared with mRNA profiling analysis data. We then performed TaqMan assays to confirm the expression changes of selected miRNAs in E2 and EE treatment NZB/WF1 mice (Fig. 9D–9I). These specific miRNAs (miR-451, miR-148a, miR-18a, miR-125a, miR-145, and miR-126) were selected because they have previously been shown to be regulated by estrogen (27).
Figure 9.
EE exposure has a more profound effect than E2 in the regulation of miRNA expression in splenic leukocytes from NZB/WF1 mice. (A–C) NanoString nCounter miRNA expression analysis of splenic leukocytes of placebo-, E2-, and EE- treated NZB/WF1 mice. Acquired raw data were processed and analyzed using nSolver software 3.0. (A) Scatter plot show the means of normalized log intensities of individual miRNA probe. R2 is reported to show the miRNA expression correlation levels between the placebo and E2 or EE treatment groups. (B) The Venn diagram shows the number of miRNAs that were regulated by both E2 and EE or specifically by E2 or EE. The mean normalized intensity of the miRNAs that demonstrated at least 1.5-fold change in either the EE- or E2-treated group when compared with placebo-treated group were selected to generate the Venn diagram. (C) Hierarchical cluster heat map shows differential expression levels of miRNAs (52) that were commonly regulated by E2 and EE in individual samples of different treatment groups. (D–I) Real-time RT-PCR analysis of the relative expression levels of miRNAs in unstimulated splenic leukocytes from placebo-, E2-, or EE-exposed mice. Data are expressed as mean ± SEM (n = 4 per group). **P < 0.01; ***P < 0.001.
Consistent with the previous data in E2-treated B6 mice, in this study, we showed the upregulation of miR-451, miR-148a, and miR-18a in splenic leukocytes of E2-treated NZB/WF1 mice, whereas the upregulation of miR-451 (P = 0.1) and miR-148a (P = 0.05) did not reach statistical significance (Fig. 9D–9F). EE treatment significantly upregulated the expression of miR-451, miR-148a, and miR-18a at a higher magnitude than did E2 (Fig. 9D–9F). Unlike the downregulation effect of E2 on miR-125a, miR-145, and miR-126 in orchidectomized male B6 mice (27), there were only trends of reduction of these three miRNAs in splenic leukocytes of E2-treated NZB/WF1 mice (Fig. 9G–9I). More surprisingly, there was a significant upregulation of miR-145 and miR-126 in splenic leukocytes of EE-treated NZB/WF1 mice when compared with placebo controls (Fig. 9H and 9I). Taken together, our data indicated that E2 and EE exposure regulated numerous miRNA expressions in splenic leukocytes of NZB/WF1 mice and that EE has more dramatic effects on miRNA expression than does E2. Also, EE and E2 have opposite effects on the expression of some miRNAs such as miR-145 and miR-126 (Fig. 9H and 9I).
EE, but not E2, significantly reduces DNA methylation of splenic leukocytes of NZB/WF1 mice
DNA methylation is an important epigenetic mechanism linking environmental exposure and human disease risk (55–60). Estrogen has been shown to regulate DNA methylation in different types of cancer cells (31, 61, 62). However, there are limited data with regard to estrogen regulation of DNA methylation in immune cells. By surveying the DNA samples prepared from splenic leukocytes of placebo- and estrogen-treated orchidectomized B6 mice, we found that estrogen treatment significantly reduced global DNA methylation levels in splenic leukocytes (49). In this study, we observed a slight, but not significant, reduction of global DNA methylation in splenic leukocytes of E2-treated NZB/WF1 mice (Fig. 10A). However, we observed a dramatic reduction of DNA methylation in splenic leukocytes of EE-treated NZB/WF1 mice when compared with placebo controls (Fig. 10A). Given that DNA hypomethylation plays an important role in lupus pathogenesis, environmental estrogen exposure may promote the disease development by decreasing the DNA methylation.
Figure 10.
EE, but not E2, exposure significantly reduced global DNA methylation and increased DNMT1 in splenic leukocytes from NZB/WF1 mice. (A) DNA was extracted from unstimulated splenic leukocyte cell pellets. The extracted DNA was used for a 5-methylcytosine ELISA kit to measure global DNA methylation levels from placebo-, E2-, or EE-exposed mice. Real-time RT-PCR analysis of the relative mRNA expression levels of (B–D) DNMT1, DNMT3a, and DNMT3b and (E–G) TET1, TET2, or TET3 in unstimulated splenic leukocytes from placebo-, E2-, and EE-exposed mice. Data are expressed as mean ± SEM (n ≥ 4 each group). **P < 0.01; ***P < 0.001. DNMT, DNA methyltransferase; TET, ten-eleven translocation protein.
To further explore the underlying mechanism of EE exposure–induced DNA hypomethylation, we evaluated the expression levels of multiple DNA methyltransferases (DNMTs) because DNA hypomethylation in lupus has been suggested to be associated with decreased expression of DNMTs (63, 64). Interestingly, we observed that DNMT1 expression was significantly elevated in EE-exposed mice compared with both placebo- and E2-exposed mice (Fig. 10B). No other DNMTs were altered due to E2 or EE exposure (Fig. 10C and 10D). Recent studies suggested the involvement of ten-eleven translocation proteins (TETs), active demethylation enzymes in the regulation of DNA methylation and gene expression (65–69). We therefore checked the expression of TETs in splenic leukocytes of EE-, E2-, and placebo-treated mice. No significant differences were observed in the expression TET1, TET2, or TET3 among different treatment groups (Fig. 10E–10G).
Discussion
The immune system is one of the main nonreproductive target organs for estrogenic compounds. There is now overwhelming evidence that estrogens modulate the immune system (6–8, 11, 12, 25, 43, 70). Estrogens modulate the immune system through estrogen receptor–dependent and –independent mechanisms (5, 13, 17, 20). Several studies have shown that the cells of the immune system possess estrogen receptors, and that genetically engineered mice with depletion of estrogen receptor α, β, or both had specific altered development and/or immune capability of the immune system (5, 71–76). The estrogen modulation of the immune system, in part, is thought to contribute to sex-based differences in immune capabilities and susceptibility of autoimmune diseases. It is conceivable that estrogen modulation of the immune system can influence responses to external agents such as infectious agents, vaccines, and allergens (9, 11–13, 77). Despite the vast body of knowledge on estrogen-modulation of the immune system, it is surprising that there is a paucity of literature on EE modulation of the immune system, in comparison with E2 or even BPA.
A very limited number of studies have shown that exposure to EE modulated the immune system through alterations in circulating cytotoxic T cells, B cells, and NK cells, reduced NK cell activity, and altered cytokine, chemokine, and adhesion molecule gene expression (78). However, reports comprehensively comparing and contrasting the effects of E2 and EE exposure on immune response, gene expression, and epigenetic modulation in autoimmune-prone mouse models are lacking. Previous studies have demonstrated an inconsistency between the effects of two estrogenic compounds on TLR9, but not TLR4, responses (79) and sex differences in behavioral response following EE or BPA exposure (80). Our study asked a fundamental question: Are the immunomodulatory effects of EE comparable to E2 or does EE exposure have unique features?
Evaluation of E2 and EE effects on lymphoid organ cell populations revealed differences in splenic neutrophils (Fig. 1), splenic and thymic CD4+ (Fig. 3), and CD8+ (Fig. 4) subsets (49). The neutrophil data in this study are consistent with our previous report showing that estrogen treatment in B6 mice increased splenic neutrophils and neutrophil serine proteases (43). The mechanisms underlying mildly increased neutrophils in spleens of E2- and EE-exposed mice remain unclear. It is possible that neutrophil migratory behavior was altered, increased neutrophil chemoattractants were present in the spleen, to promote neutrophil accumulation, or that myeloid cell maturation and development were promoted at sites of extramedullary hematopoiesis through direct and indirect estrogenic influence. It is plausible that the increased presence of neutrophils in the spleen may interact with T and B cells to influence their function. Because defining the mechanisms underlying neutrophil accumulation and aberration related to estrogen exposure and lupus disease phenotype are beyond the scope of this study, we will investigate these mechanisms in a separate detailed study.
Consistent with our previous report and those of others (25, 81–83), E2 increased NO, iNOS, and MCP-1 production following stimulation of splenic leukocytes (Fig. 2). EE promoted effects that are similar to E2 in response patterns generally considered part of the innate immune branch. This does not appear to hold true for adaptive immune responses. Although EE reduced CD4+ and CD8+ cells in both spleen and thymus, the remaining cells exhibited a more robust activation profile (Figs. 3 and 4) (49). This was also evident in the cytokine response following stimulation (Figs. 5 and 6). To simulate exposure to infections, we stimulated splenic leukocytes with ligands for TLR4 (bacterial LPS), TLR9 (ODN-1585 to simulate viral activation), and T cell receptors (through activating CD3 and costimulatory CD28 molecules). Remarkably, differential production of cytokines in response to different stimuli and estrogenic exposure were found in multiple cytokines typically considered “pro-inflammatory,” such as IFNγ, IL-1β, and TNFα, among the E2 and EE exposure groups (Figs. 5 and 6). EE increased IFNγ and IL-1β and decreased IL-2, whereas E2 did not, in NZB/WF1 mice. Furthermore, in EE-exposed mice, TLR9 stimulation suppressed IL-6 and IFNα, in contrast to increased IFNα in splenic leukocytes of E2-exposed NZB/WF1 mice, suggesting that these hormones have differential ability to modulate splenic leukocytes to release cytokines (Fig. 7). These studies show that these two estrogenic hormones may differentially alter the signaling through TLR4, TLR9, or T cell receptor/CD3 to regulate cytokine production. In our previous study using gonadectomized B6 mice given E2, we found that IFNγ and IL-1β were significantly upregulated from LPS or ConA-activated splenic leukocytes (24). It is noteworthy that in this study (unlike our previous study) the mice were gonadally intact. It is likely that the existence of endogenous estrogen levels may affect the extraneous estrogen effects, or the difference in strains may have contributed to the differences observed between studies. The common increased production of IL-10 in LPS- and anti-CD3 plus anti-CD28–activated splenic leukocytes from both E2- and EE-treated mice is consistent with previous reports (24, 43, 84).
E2 exposure has been shown to regulate numerous gene expression levels and epigenetic regulation in various cell types (27, 85, 86). In this study, we profiled the immune-related gene expression and miRNA in splenic leukocytes of NZB/WF1 mice following E2 and EE exposure, respectively (Figs. 8 and 9). Although both EE and E2 induced a large number of common immune-related gene expression changes, EE and E2 also induced hormone-specific immune-related gene expression. With regard to miRNA gene expression, both E2 and EE exposure induced expression change of numerous miRNAs in splenic leukocytes of NZB/WF1 mice (Fig. 9). However, the magnitude of EE in regulation of miRNAs is more profound than E2, as noted for miR-451, miR-18a, miR-145, and miR-126. Additionally, although E2 and EE induced immune-related gene expression changes in the same direction, E2 and EE exposures also demonstrated differential effects on selected miRNAs. Whether and how the differences in miRNA expression levels contribute to E2- and EE-induced differential immune responses are beyond the scope of this present study, an aspect that will be investigated in separate studies.
DNA methylation is a well-acknowledged epigenetic mechanism linking environmental exposure and risk of human diseases, including autoimmune disorders (55–60). However, most studies of estrogenic regulation of epigenetics have been focused on nonlymphoid organs/tissues. Estrogen has been reported to regulate DNMTs and DNA methylation in different types of cancer cells (31, 61, 87, 88). We found that E2 exposure had a negligible effect on DNA methylation of splenic leukocytes from gonadal-intact NZB/WF1 mice, whereas EE exposure significantly reduced global DNA methylation (Fig. 10A). It is possible that the reduced methylation led to increased expression of specific genes related to inflammation and cytokine production. miR-148a and miR-126 have been shown to target DNMT1, leading to DNA hypomethylation in lupus CD4+ T cells (63). We observed a strong increase of both miR-148a and miR-126 in EE-, but not E2-, exposed splenic leukocytes (Fig. 9E and 9I). We therefore hypothesized that increased miR-148a and miR-126 may contribute to EE-induced DNA hypomethylation via suppressing DNMT1. However, surprisingly, we observed a significant increase of DNMT1 in splenic leukocytes from EE-exposed samples (Fig. 10B). No differences were seen in DNMT3a/b or TET proteins (known to be involved in altering DNA methylation). Therefore, EE-induced DNA hypomethylation is unlikely to have contributions from either reduced DNMTs or increased demethylation enzymes (TETs). Further investigation is warranted to understand the mechanism of EE-mediated reduction of global DNA methylation in splenic leukocytes from NZB/WF1 mice. Future studies should investigate the DNA methylation changes on the promoters of specific genes and correlate the methylation status with gene expression changes following E2 or EE treatment.
Our study focused on investigating whether the immunomodulatory and epigenetic effects of EE are comparable to E2 in a genetically lupus-prone mouse model of disease. To address this central issue, we limited our studies to only female mice based on the fact that there is a significant female bias of lupus in NZB/WF1 mice, and that EE is pharmaceutically administered to females. Now that we have established the effects of EE on the innate and adaptive immune systems in female mice, we are also interested in sex differences in response to E2 and EE exposure. We have begun experiments to elucidate these differences between the male and female mouse immune response when exposed to estrogenic chemicals in a separate project. Future studies should also compare these two chemicals in intact and ovariectomized females or orchidectomized males. This current study only included a single dose implant of each of the two chemicals. The dose of human exposure to estrogenic chemicals is highly variable, and future studies incorporating multiple doses of either E2 or EE, as well as combinations of chemicals, may help identify a safe dose of exposure, or potentially reveal an exaggerated response to chronic low-dose exposure in genetically lupus-prone animals. It would also be relevant to study the immunomodulatory effects of these chemicals at pre-disease and developing disease ages.
In summary, in this study, we comprehensively investigated and compared E2 and EE exposure-induced immunological, molecular, and epigenetic changes in the spontaneous lupus-prone murine model of NZB/WF1 mice. Both E2 and EE exposure leads to similar changes in neutrophil serine protease expression as well as gene expression. Our studies show that the two very similarly chemically structured estrogenic compounds are able to exert vastly different effects on cytokine production, cell populations, and epigenetic regulatory mechanisms. These data provide an insightful perspective in understanding the similarity and also the difference of natural estrogen and its synthetic analog, EE, in the regulation of immunity and autoimmunity. Our findings are further evidence that each estrogenic compound should be evaluated in a context-specific manner, and no assumptions should be made that any particular estrogenic compound will act in a similar manner as other compounds in the same classification.
Acknowledgments
We thank Melissa Makris for assistance with flow cytometry results. We thank Karen Hall, Betsy S. Midkiff, and other animal care staff members at the Virginia-Maryland College of Veterinary Medicine for their assistance.
Financial Support: This work was supported in part by Virginia-Maryland College of Veterinary Medicine Intramural Research Competition Grant 175185 (interdepartmental funds to S.A.A.) and by National Institutes of Health T32 Training Grant 5T32OD010430-09. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Virginia-Maryland College of Veterinary Medicine.
Author Contributions: All authors made substantial, direct, and intellectual contributions to the work and approved it for publication.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- B6
C57BL/6
- BPA
bisphenol A
- CG
cathepsin G
- ConA
concanavalin A
- DNMT
DNA methyltransferase
- E2
17β-estradiol
- EE
17α-ethinyl estradiol
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MPO
myeloperoxidase
- NE
neutrophil elastase
- NO
nitric oxide
- NZB/WF1
New Zealand Black × New Zealand White F1 progeny
- ODN
oligodeoxynucleotide
- PR3
proteinase 3
- SP
single-positive
- TET
ten-eleven translocation protein
- TLR
Toll-like receptor
References
- 1. Pellegrini M, Bulzomi P, Lecis M, Leone S, Campesi I, Franconi F, Marino M. Endocrine disruptors differently influence estrogen receptor β and androgen receptor in male and female rat VSMC. J Cell Physiol. 2014;229(8):1061–1068. [DOI] [PubMed] [Google Scholar]
- 2. Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227(4684):257–261. [DOI] [PubMed] [Google Scholar]
- 3. Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, Straub RH. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13(9):635–638. [DOI] [PubMed] [Google Scholar]
- 4. Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol. 2013;375(1–2):121–129. [DOI] [PubMed] [Google Scholar]
- 5. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Panchanathan R, Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Mol Immunol. 2013;53(1–2):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Priyanka HP, Krishnan HC, Singh RV, Hima L, Thyagarajan S. Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes. Mol Immunol. 2013;56(4):328–339. [DOI] [PubMed] [Google Scholar]
- 8. Asaba J, Bandyopadhyay M, Kindy M, Dasgupta S. Estrogen receptor signal in regulation of B cell activation during diverse immune responses. Int J Biochem Cell Biol. 2015;68:42–47. [DOI] [PubMed] [Google Scholar]
- 9. Dragin N, Nancy P, Villegas J, Roussin R, Le Panse R, Berrih-Aknin S. Balance between estrogens and proinflammatory cytokines regulates chemokine production involved in thymic germinal center formation [published correction appears in Sci Rep. 2018;8(1):8118] Sci Rep. 2017;7(1):7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17(4):369–384. [DOI] [PubMed] [Google Scholar]
- 11. Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A, Nottin R, Klatzmann D, Cumano A, Barkats M, Le Panse R, Berrih-Aknin S. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest. 2016;126(4):1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed SA, Verthelyi D. Antibodies to cardiolipin in normal C57BL/6J mice: induction by estrogen but not dihydrotestosterone. J Autoimmun. 1993;6(3):265–279. [DOI] [PubMed] [Google Scholar]
- 13. Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environ Health Perspect. 1999;107(Suppl 5):681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kassi E, Moutsatsou P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:317452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 16. Rogers JA, Metz L, Yong VW. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol. 2013;53(4):421–430. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed SA, Aufdemorte TB, Chen JR, Montoya AI, Olive D, Talal N. Estrogen induces the development of autoantibodies and promotes salivary gland lymphoid infiltrates in normal mice. J Autoimmun. 1989;2(4):543–552. [DOI] [PubMed] [Google Scholar]
- 18. Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, Conaway M, Wang H, Korach KS, Bocchinfuso W, Santen R. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161–170. [DOI] [PubMed] [Google Scholar]
- 20. Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113(3):224–230. [DOI] [PubMed] [Google Scholar]
- 21. Herblot S, Aplan PD, Hoang T. Gradient of E2A activity in B-cell development. Mol Cell Biol. 2002;22(3):886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr Opin Rheumatol. 2006;18(5):456–461. [DOI] [PubMed] [Google Scholar]
- 23. Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17β-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176(5):2703–2710. [DOI] [PubMed] [Google Scholar]
- 24. Dai R, Phillips RA, Ahmed SA. Despite inhibition of nuclear localization of NF-κB p65, c-Rel, and RelB, 17-β estradiol up-regulates NF-κB signaling in mouse splenocytes: the potential role of Bcl-3. J Immunol. 2007;179(3):1776–1783. [DOI] [PubMed] [Google Scholar]
- 25. Dai R, Phillips RA, Karpuzoglu E, Khan D, Ahmed SA. Estrogen regulates transcription factors STAT-1 and NF-κB to promote inducible nitric oxide synthase and inflammatory responses. J Immunol. 2009;183(11):6998–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. The relationship between circulating estradiol and thyroid autoimmunity in males. Eur J Endocrinol. 2013;170(1):63–67. [DOI] [PubMed] [Google Scholar]
- 27. Dai R, Phillips RA, Zhang Y, Khan D, Crasta O, Ahmed SA. Suppression of LPS-induced interferon-γ and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood. 2008;112(12):4591–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai R, Lu R, Ahmed SA. The upregulation of genomic imprinted DLK1-Dio3 miRNAs in murine lupus is associated with global DNA hypomethylation. PLoS One. 2016;11(4):e0153509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Wan Y, Ji Q, Fang Y, Wu Y. The role of microRNAs in B-cell development and function. Cell Mol Immunol. 2013;10(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klinge CM. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol Cell Endocrinol. 2015;418(Pt 3):273–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adjakly M, Ngollo M, Lebert A, Dagdemir A, Penault-Llorca F, Boiteux JP, Bignon YJ, Guy L, Bernard-Gallon D. Comparative effects of soy phytoestrogens and 17β-estradiol on DNA methylation of a panel of 24 genes in prostate cancer cell lines. Nutr Cancer. 2014;66(3):474–482. [DOI] [PubMed] [Google Scholar]
- 32. Dai R, McReynolds S, Leroith T, Heid B, Liang Z, Ahmed SA. Sex differences in the expression of lupus-associated miRNAs in splenocytes from lupus-prone NZB/WF1 mice. Biol Sex Differ. 2013;4(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32(3–4):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, Huang X, Zhou H, de Vries N, Tak PP, Chen S, Shen N. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60(4):1065–1075. [DOI] [PubMed] [Google Scholar]
- 35. Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan YH, Xu ZM, Yin YB. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus. 2007;16(12):939–946. [DOI] [PubMed] [Google Scholar]
- 36. Te JL, Dozmorov IM, Guthridge JM, Nguyen KL, Cavett JW, Kelly JA, Bruner GR, Harley JB, Ojwang JO. Identification of unique microRNA signature associated with lupus nephritis. PLoS One. 2010;5(5):e10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otaegui D, Baranzini SE, Armañanzas R, Calvo B, Muñoz-Culla M, Khankhanian P, Inza I, Lozano JA, Castillo-Triviño T, Asensio A, Olaskoaga J, López de Munain A. Differential micro RNA expression in PBMC from multiple sclerosis patients. PLoS One. 2009;4(7):e6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D. Altered expression of microRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58(4):1001–1009. [DOI] [PubMed] [Google Scholar]
- 39. Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–1259. [DOI] [PubMed] [Google Scholar]
- 40. King OC, van de Merwe JP, McDonald JA, Leusch FD. Concentrations of levonorgestrel and ethinylestradiol in wastewater effluents: is the progestin also cause for concern? Environ Toxicol Chem. 2016;35(6):1378–1385. [DOI] [PubMed] [Google Scholar]
- 41. Dickson RB, Eisenfeld AJ. 17α-Ethinyl estradiol is more potent than estradiol in receptor interactions with isolated hepatic parenchymal cells. Endocrinology. 1981;108(4):1511–1518. [DOI] [PubMed] [Google Scholar]
- 42. Dai R, Zhang Y, Khan D, Heid B, Caudell D, Crasta O, Ahmed SA. Identification of a common lupus disease-associated microRNA expression pattern in three different murine models of lupus. PLoS One. 2010;5(12):e14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dai R, Cowan C, Heid B, Khan D, Liang Z, Pham CT, Ahmed SA. Neutrophils and neutrophil serine proteases are increased in the spleens of estrogen-treated C57BL/6 mice and several strains of spontaneous lupus-prone mice. PLoS One. 2017;12(2):e0172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edwards MR, Dai R, Heid B, Cecere TE, Khan D, Mu Q, Cowan C, Luo XM, Ahmed SA. Commercial rodent diets differentially regulate autoimmune glomerulonephritis, epigenetics and microbiota in MRL/lpr mice. Int Immunol. 2017;29(6):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan D, Dai R, Karpuzoglu E, Ahmed SA. Estrogen increases, whereas IL-27 and IFN-γ decrease, splenocyte IL-17 production in WT mice. Eur J Immunol. 2010;40(9):2549–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RRID:AB_631831.
- 47.RRID:AB_2305186.
- 48. Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. [DOI] [PubMed] [Google Scholar]
- 49. Dai R, Edwards MR, Heid B, Ahmed SA. Data from: 17-β estradiol and 17α-ethinyl estradiol exhibit immunologic and epigenetic regulatory effects in NZB/WF1 female mice. VTechData Repository 2018. Deposited 26 October 2018. 10.7294/W4-A15D-1W64. [DOI]
- 50. Mortezagholi S, Babaloo Z, Rahimzadeh P, Namdari H, Ghaedi M, Gharibdoost F, Mirzaei R, Bidad K, Salehi E. Evaluation of TLR9 expression on PBMCs and CpG ODN-TLR9 ligation on IFN-α production in SLE patients. Immunopharmacol Immunotoxicol. 2017;39(1):11–18. [DOI] [PubMed] [Google Scholar]
- 51. Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192(12):5459–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu Z, Shen WJ, Cortez Y, Tang X, Liu LF, Kraemer FB, Azhar S. Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland. PLoS One. 2013;8(10):e78040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Porseryd T, Volkova K, Reyhanian Caspillo N, Källman T, Dinnetz P, Porsh Hällström I. Persistent effects of developmental exposure to 17α-ethinylestradiol on the zebrafish (Danio rerio) brain transcriptome and behavior. Front Behav Neurosci. 2017;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Balada E, Ordi-Ros J, Vilardell-Tarrés M. DNA methylation and systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1108(1):127–136. [DOI] [PubMed] [Google Scholar]
- 56. Sawalha AH, Jeffries M. Defective DNA methylation and CD70 overexpression in CD4+ T cells in MRL/lpr lupus-prone mice. Eur J Immunol. 2007;37(5):1407–1413. [DOI] [PubMed] [Google Scholar]
- 57. Patel DR, Richardson BC. Epigenetic mechanisms in lupus. Curr Opin Rheumatol. 2010;22(5):478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Absher DM, Li X, Waite LL, Gibson A, Roberts K, Edberg J, Chatham WW, Kimberly RP. Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet. 2013;9(8):e1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coit P, Jeffries M, Altorok N, Dozmorov MG, Koelsch KA, Wren JD, Merrill JT, McCune WJ, Sawalha AH. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. J Autoimmun. 2013;43:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O’Hanlon TP, Rider LG, Jacinto FV, Lopez-Longo FJ, Dopazo J, Forn M, Peinado MA, Carreño L, Sawalha AH, Harley JB, Siebert R, Esteller M, Miller FW, Ballestar E. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20(2):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li L, Lee KM, Han W, Choi JY, Lee JY, Kang GH, Park SK, Noh DY, Yoo KY, Kang D. Estrogen and progesterone receptor status affect genome-wide DNA methylation profile in breast cancer. Hum Mol Genet. 2010;19(21):4273–4277. [DOI] [PubMed] [Google Scholar]
- 62. Benevolenskaya EV, Islam AB, Ahsan H, Kibriya MG, Jasmine F, Wolff B, Al-Alem U, Wiley E, Kajdacsy-Balla A, Macias V, Rauscher GH. DNA methylation and hormone receptor status in breast cancer. Clin Epigenetics. 2016;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184(12):6773–6781. [DOI] [PubMed] [Google Scholar]
- 64. Qin HH, Zhu XH, Liang J, Yang YS, Wang SS, Shi WM, Xu JH. Associations between aberrant DNA methylation and transcript levels of DNMT1 and MBD2 in CD4+T cells from patients with systemic lupus erythematosus. Australas J Dermatol. 2013;54(2):90–95. [DOI] [PubMed] [Google Scholar]
- 65. Ichiyama K, Chen T, Wang X, Yan X, Kim BS, Tanaka S, Ndiaye-Lobry D, Deng Y, Zou Y, Zheng P, Tian Q, Aifantis I, Wei L, Dong C. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells [published correction appears in Immunity. 2015;42(6):1214]. Immunity. 2015;42(4):613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsagaratou A, Rao A. TET proteins and 5-methylcytosine oxidation in the immune system. Cold Spring Harb Symp Quant Biol. 2013;78(0):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, Chen C, Liu S, Liu D, Chen Y, Zandi E, Chen W, Zhou Y, Shi S. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory t cell differentiation and maintain immune homeostasis. Immunity. 2015;43(2):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao M, Wang J, Liao W, Li D, Li M, Wu H, Zhang Y, Gershwin ME, Lu Q. Increased 5-hydroxymethylcytosine in CD4+ T cells in systemic lupus erythematosus. J Autoimmun. 2016;69:64–73. [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Xiao M, Chen X, Chen L, Xu Y, Lv L, Wang P, Yang H, Ma S, Lin H, Jiao B, Ren R, Ye D, Guan KL, Xiong Y. WT1 recruits TET2 to regulate its target gene expression and suppress leukemia cell proliferation. Mol Cell. 2015;57(4):662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ahmed SA. The immune system as a potential target for environmental estrogens (endocrine disrupters): a new emerging field. Toxicology. 2000;150(1–3):191–206. [DOI] [PubMed] [Google Scholar]
- 71. Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Horm Behav. 2012;62(3):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kawashima I, Seiki K, Sakabe K, Ihara S, Akatsuka A, Katsumata Y. Localization of estrogen receptors and estrogen receptor-mRNA in female mouse thymus. Thymus. 1992;20(2):115–121. [PubMed] [Google Scholar]
- 74. Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180(2):727–738. [DOI] [PubMed] [Google Scholar]
- 75. Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors α and β in immune organ development and in oestrogen-mediated effects on thymus. Immunology. 2001;103(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. Estrogen receptor α is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 1999;163(8):4168–4174. [PubMed] [Google Scholar]
- 77. Ishikawa S. Possible roles of B1 cells and environmental estrogens (endocrine disruptors) in the development of autoimmune diseases. Allergol Int. 2005;54(4):499–505. [Google Scholar]
- 78. Auerbach L, Hafner T, Huber JC, Panzer S. Influence of low-dose oral contraception on peripheral blood lymphocyte subsets at particular phases of the hormonal cycle. Fertil Steril. 2002;78(1):83–89. [DOI] [PubMed] [Google Scholar]
- 79. Chakhtoura M, Sriram U, Heayn M, Wonsidler J, Doyle C, Dinnall JA, Gallucci S, Roberts RA. Bisphenol A does not mimic estrogen in the promotion of the in vitro response of murine dendritic cells to Toll-like receptor ligands. Mediators Inflamm. 2017;2017:2034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johnson SA, Painter MS, Javurek AB, Ellersieck MR, Wiedmeyer CE, Thyfault JP, Rosenfeld CS. Sex-dependent effects of developmental exposure to bisphenol A and ethinyl estradiol on metabolic parameters and voluntary physical activity. J Dev Orig Health Dis. 2015;6(6):539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Azenabor AA, Yang S, Job G, Adedokun OO. Expression of iNOS gene in macrophages stimulated with 17β-estradiol is regulated by free intracellular Ca2+. Biochem Cell Biol. 2004;82(3):381–390. [DOI] [PubMed] [Google Scholar]
- 82. Han G, Magee T, Khorram O. Regulation of nitric oxide synthase isoforms by estrogen in the human endometrium. Fertil Steril. 2005;84(Suppl 2):1220–1227. [DOI] [PubMed] [Google Scholar]
- 83. Hong M, Zhu Q. Macrophages are activated by 17β-estradiol: possible permission role in endometriosis. Exp Toxicol Pathol. 2004;55(5):385–391. [DOI] [PubMed] [Google Scholar]
- 84. Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-α, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cohen A, Shmoish M, Levi L, Cheruti U, Levavi-Sivan B, Lubzens E. Alterations in micro-ribonucleic acid expression profiles reveal a novel pathway for estrogen regulation. Endocrinology. 2008;149(4):1687–1696. [DOI] [PubMed] [Google Scholar]
- 86. Kovalchuk O, Tryndyak VP, Montgomery B, Boyko A, Kutanzi K, Zemp F, Warbritton AR, Latendresse JR, Kovalchuk I, Beland FA, Pogribny IP. Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications and aberrant microRNA expression. Cell Cycle. 2007;6(16):2010–2018. [DOI] [PubMed] [Google Scholar]
- 87. Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, Taketani T, Matsuoka A, Tamura H, Sugino N. DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Hum Reprod. 2009;24(5):1126–1132. [DOI] [PubMed] [Google Scholar]
- 88. Cui M, Wen Z, Yang Z, Chen J, Wang F. Estrogen regulates DNA methyltransferase 3B expression in Ishikawa endometrial adenocarcinoma cells. Mol Biol Rep. 2009;36(8):2201–2207. [DOI] [PubMed] [Google Scholar]