A prospective cohort study on acute respiratory infections of children in the Philippines found that the risk for subsequent respiratory infections was significantly enhanced after infections with adenovirus, influenza A virus, parainfluenza virus type 4, and rhinovirus species C.
Keywords: acute respiratory infection, viral infection, Philippines, risk factor, prospective cohort study
Abstract
Background
Acute respiratory infection (ARI) is of great concern in public health. It remains unclear whether viral infections can affect the host’s susceptibility to subsequent ARIs.
Methods
A prospective cohort study on ARIs of children below 5 years old was conducted in the Philippines from 2014 to 2016. The respiratory symptoms were recorded daily, and nasopharyngeal swabs were collected at both household and health facilities. The specimens were tested for respiratory viruses. We then determined whether viral etiology was associated with the severity of the present ARI and whether previous viral infections was associated with subsequent ARIs.
Results
A total of 3851 children and 16337 ARI episodes were enrolled and recorded, respectively. Samples were collected from 24% of all ARI episodes; collection rate at the healthcare facilities was 95%. Enterovirus D68, rhinovirus species C, and respiratory syncytial virus were significantly associated with severe ARIs. The risk for subsequent ARIs was significantly enhanced after infections with adenovirus, influenza A virus, parainfluenza virus type 4, and rhinovirus species C.
Conclusions
This study revealed that viral etiology plays a significant role in the severity of the present ARI and that viral infection affects the host’s susceptibility to subsequent ARIs.
Acute respiratory infection (ARI) remains a major global health problem, with pneumonia being the leading cause of mortality among young children in low- and middle-income countries [1]. Adenovirus (AdV), enterovirus D68 (EV-D68), human metapneumovirus (hMPV), influenza virus (IFV), parainfluenza virus (PIV), and respiratory syncytial virus (RSV), which are commonly detected in hospitalized children with ARIs, are considered to play important etiological roles in the severity of infections [2–5].
Serological studies have shown that most children experience infections with common respiratory viruses such as hMPV, IFV, and RSV during their early years [6, 7]. Therefore, children are likely to experience multiple ARIs caused by a variety of viruses [8]. Previous respiratory viral infections could alter host susceptibility to subsequent ARIs. Viral infections could affect the host’s immune status, including the production of cytokines, such as interferons, and the number and function of immune cells [9, 10], thus possibly altering the host’s susceptibility to subsequent infections. Tissue damage and impairment of the epithelial barrier function of the respiratory tract by viral infections may also affect the host susceptibility to subsequent infections [9, 11]. Furthermore, viral infections have been found to alter the composition of respiratory tract microbiome [12, 13], the profile of which has been reported to be associated with the risk of respiratory infections [14–16].
Jartti et al [8] reported that children with recurrent respiratory infections have often been infected with a series of different viruses. However, whether viral infections affect the risk for subsequent ARIs has not been fully elucidated. The present study analyzes data from a pediatric ARI cohort study in the Philippines and assesses how prior viral infections can lead to an enhanced or decreased risk for subsequent ARIs.
METHODS
Cohort Design
A prospective cohort study on ARIs was conducted in Biliran Island, Philippines, from March 2014 to June 2016. Biliran Province consists of a main island (Biliran Island, 556 km2) and small islands, with a total of 161760 inhabitants [17]. The province consists of 8 municipalities, which are further divided into barangays. In the Philippines, the barangay is the smallest administrative unit under municipalities and is equivalent to a village in other countries. The healthcare system within the study area includes the Barangay Health Station, Rural Health Unit, and the Biliran Provincial Hospital. Barangay Health Stations provide pre- and postnatal care, health education, and public health services such as mass drug administration and immunization. Rural Health Units are primary healthcare facilities located in each municipality where a qualified doctor is assigned to provide outpatient care in addition to public health services. Biliran Provincial Hospital, a secondary level referral hospital with 20 beds allocated for the pediatric ward, is the only hospital in Biliran Province.
Children younger than 5 years old from 25 barangays of 2 municipalities (Kawayan and Caibiran) in Biliran Island were recruited for this study. Children in the study area were identified by a census and household visit. An eligible child for the cohort was defined as a person who had lived in the household for at least 1 month or since birth at the time of consideration of enrollment, with no plans to move in the next month. Newborns and children who moved into the study site during the study period were also enrolled. Children whose guardians declined to participate were excluded. Children were monitored until the study ended, they reached 5 years old, or they moved out of the study area.
Demographic and other information, including date of birth, gender, family structure, socioeconomic (SES) score [18], and birthweight, were obtained through a questionnaire given upon recruitment to the cohort. Socioeconomic score was grouped by tertile for analysis: 0–24, 25–36, and 37–93. Height and weight were measured upon recruitment and updated at least every 6 months. Nutritional status was assessed using the height-for-age Z-score (HAZ) [19].
Follow-Up of Respiratory Symptoms and Sampling
The guardians or caregivers of cohort children were asked to record respiratory symptoms (a cough and difficulty breathing) and presence of fever daily. Every other week, when each household was visited, the records were collected and reviewed by trained nurses. When children developed respiratory symptoms with fever within 7 days of the visit, nasopharyngeal swabs were collected for viral detection.
All healthcare facilities where there is a medical doctor (Rural Health Units and Biliran Provincial Hospital) that the cohort children could access were included in the present study. Nurses or a medical doctor employed and trained for this study conducted physical examinations to check for chest indrawing and respiratory rate, measured blood oxygen saturation level (SpO2) using a pulse oximeter (PalmSAT 2800; Nonin Medical, Plymouth, MN), and obtained nasopharyngeal swabs for viral detection from the cohort children who visited the healthcare facilities because of respiratory symptoms.
Definition of Acute Respiratory Infection
Acute respiratory infection was defined as the presence of a cough or difficulty breathing determined using the daily records of respiratory symptoms. Coughing or difficulty breathing that lasted >28 days were not considered an ARI in this study. At least 7 asymptomatic (no cough or difficulty breathing) days were required to distinguish each ARI episode. Therefore, the end of an ARI was defined as the last day when a child had a cough or difficulty breathing followed by 7 asymptomatic days.
Acute respiratory infection severity was assessed at the healthcare facilities during patient visit. A severe ARI was defined as an ARI that caused “tachypnea (respiratory rate of >60/min, >50/min, and >40/min for children below two months old, below one year old, and between one and four years old, respectively, according to the Integrated Management of Childhood Illness [20]) or SpO2 < 95%” and “chest indrawing or SpO2 < 93%” based on the proposal for definition of severe lower respiratory tract infection by the World Helath Organization [21].
Laboratory Tests
Nasopharyngeal swabs collected at the participant’s home and healthcare facilities were placed in a viral transport medium, stored at 4°C in refrigerators at Rural Health Units and Biliran Provincial Hospital for 1–4 days, and transported with ice packs twice a week to the Research Institute for Tropical Medicine, Metro Manila, Philippines, for sample processing. Virological tests were performed to detect the following: AdV [22]; EV including EV-D68 [23]; hMPV [24]; IFV-A and IFV-B [25]; PIV-1, PIV-2, PIV-3, and PIV-4 [26]; rhinovirus species A, B, and C (RhV-A, RhV-B, and RhV-C) [3]; and RSV [27].
In brief, after nucleic acid extraction using the QIAamp MinElute Virus Spin Kit (QIAGEN, Hilden, Germany), polymerase chain reaction (PCR), reverse transcription-PCR, and reverse transcription-quantitative PCR were conducted. Random primer was used for reverse transcription and sequence information of primers and probes are listed in Supplementary Table 1. The PCR amplicon was sequenced to confirm PCR results and to determine the viral classification. Adenovirus serotypes were identified by both sequencing and neutralization tests with viral isolates using HEp-2 cells. When multiple viruses were detected, the ARI episodes were regarded as positive for each virus during further analyses.
Subsequent Acute Respiratory Infections
To investigate whether previous viral infection affects the incidence of subsequent ARIs in the cohort, children who had experienced ARI with viral detection were identified (“children with preceding infection”). Subsequent ARIs were defined as the next ARI that occurred after the preceding infection. When children with preceding infection had multiple ARI episodes with the same virus, only the first episode was used to identify subsequent ARIs.
We selected matched controls among children who had not experienced infections with a virus of interest by the time their counterpart had an ARI positive for the virus. The matched controls were selected even if they had experienced infection with virus other than the virus of interest. Controls were selected from the cohort and matched for gender (male or female), number of siblings (the difference in the number of siblings should be 0 or 1), HAZ (≥−2 or <−2), prematurity (birth weight ≥2500 grams or <2500 grams), SES score (same tertile), residential area (same or neighboring barangays), and age (those with the closest date of birth among children who met all of the above criteria for matching). When the age difference between children with preceding infection and their matched controls was greater than 6 months for those below 1 year old, or the difference was greater than 1 year for those between 1 and 4 years old, the criterion for either gender, number of siblings, HAZ, prematurity, or SES score was overlooked to find another matched control whose age was closer to children with preceding infection. To minimize the effect of intrinsic susceptibility to ARI and healthcare utilization pattern between children with preceding infection and their matched controls, the number of ARIs and healthcare facility visits by the time of sampling for viral detection (in children with preceding infection) was also matched. When an appropriate control could not be found, the case was excluded from further analyses.
Follow-up for subsequent ARIs in children with preceding infection and their matched control was performed at the same time to minimize the effect of seasonal ARI fluctuations and viral circulation. The follow-up began 8 days after the last day of an ARI positive for a virus of interest in children with preceding infection because 7 asymptomatic days are required to distinguish each ARI episode, as described above. The risk for subsequent ARIs was calculated using the time from the beginning of follow-up for subsequent ARIs to the onset of the first subsequent ARI of children with preceding infection and their matched controls by Cox proportional hazards regression analysis.
Statistical Analysis
Binomial logistic regression analyses with generalized estimating equations were conducted to determine the odds ratio for demographic factors in severe vs nonsevere ARIs identified at healthcare facilities. The same was also used to determine the odds ratio for the detection of each virus in severe vs nonsevere ARIs adjusted for age, gender, HAZ, and SES score. Cox proportional hazards regression analysis was conducted to determine the hazard ratio (HR) for a subsequent ARI after a preceding ARI with viral detection (in children with preceding infection), which was compared with the first ARI in matched controls during the corresponding period. Statistical tests were performed using SPSS version 24 (IBM, Armonk, NY). Confidence intervals (CIs) of 95% were computed to test statistical significance.
Ethics Approval
Informed consent was obtained from the guardians of all participants. The study protocol was approved by the Ethics Committee of Tohoku University Graduate School of Medicine, Japan, and the Institutional Review Board of the Research Institute for Tropical Medicine, Philippines.
RESULTS
We recruited 4012 children, but 161 were excluded because of missing or incomplete (shorter than 28 days) daily records of respiratory symptoms. As such, 3851 children were enrolled and analyzed in this study (Table 1). The median follow-up period was 392 days (interquartile range, 168–714) with 4547 person-years of follow-up. According to the HAZ [19], 41% (1568 of 3851) of the children were classified as having moderate or severe malnutrition at the time of enrollment. Among the cohort of children, 16337 ARI episodes were recorded, the incidence rate being 359 per 100 person–years (95% CI, 354–365). Seventeen percent of the ARI episodes (2851 of 16337) prompted a visit to a health facility. Among these episodes, 11% (327 of 2851) and 89% (2524 of 2851) were classified as severe and nonsevere ARIs, respectively (Table 2). The incidence rate of severe ARIs was 7.2 per 100 person-years (95% CI, 6.5–8.0). All hospitalized cases (n = 85) were classified as severe ARIs, 3 of which were fatal. A comparison between severe and nonsevere ARIs revealed that the ARI episodes occurring in males and children of younger age were significantly associated with severe ARIs (Table 2).
Table 1.
Summary of Characteristics of Children Included in the Cohort
| Variable | Category | Number of Children |
|---|---|---|
| Number of Children | 3851 | |
| Gender | Female | 1848 (48%) |
| Male | 2003 (52%) | |
| Age group (at enrollment) | 0–5 M | 1053 (27%) |
| 6–11 M | 391 (10%) | |
| 1 Y | 650 (17%) | |
| 2 Y | 643 (17%) | |
| 3 Y | 575 (15%) | |
| 4 Y | 539 (14%) | |
| Height-for-age Z-score (at enrollment) | ≥−2 | 2283 (59%) |
| ≥−3, <−2 (moderate malnutrition) | 846 (22%) | |
| <−3 (severe malnutrition) | 722 (19%) | |
| Low birth weight | <2500 grams | 455 (15%) |
| Socioeconomic status scorea | 37–93 | 1285 (33%) |
| 25–36 | 1248 (32%) | |
| 0–24 | 1318 (34%) | |
| Incidence of ARIs per year | 0 | 599 (16%) |
| >0, ≤1 | 180 (5%) | |
| >1, ≤2 | 462 (12%) | |
| >2, ≤3 | 587 (15%) | |
| >3, ≤4 | 532 (14%) | |
| >4, ≤5 | 410 (11%) | |
| >5, ≤6 | 344 (9%) | |
| >6 | 737 (19%) | |
| Incidence of severe ARIs per year | 0 | 3581 (93%) |
| >0, ≤1 | 178 (5%) | |
| >1, ≤2 | 62 (2%) | |
| >2, ≤3 | 19 (0.5%) | |
| >3, ≤4 | 4 (0.1%) | |
| >4, ≤5 | 3 (0.1%) | |
| >5, ≤6 | 3 (0.1%) | |
| >6 | 1 (0.03%) |
Abbreviations: ARIs, acute respiratory infections; M, month; Y, year.
aSocioeconomic status score is grouped by tertile.
Table 2.
Demographic Factors and Severity of ARIsa
| Variable | Category | Nonsevere | Severe | Odds Ratio (Unadjusted) | 95% Confidence Interval |
|---|---|---|---|---|---|
| Number | 2524 (89%) | 327 (11%) | - | - | |
| Gender | Female | 1270 (90%) | 143 (10%) | Ref | - |
| Male | 1254 (87%) | 184 (13%) | 1.3 | 1.0–1.7 | |
| Age | 0–5 M | 342 (83%) | 70 (17%) | 3.4 | 2.0–5.8 |
| 6–11 M | 460 (88%) | 64 (12%) | 2.3 | 1.3–3.9 | |
| 1 Y | 625 (84%) | 116 (16%) | 3.1 | 1.8–5.1 | |
| 2 Y | 473 (92%) | 41 (8%) | 1.4 | 0.81–2.5 | |
| 3 Y | 344 (95%) | 19 (5%) | 0.91 | 0.49–1.7 | |
| 4 Y | 280 (94%) | 17 (6%) | Ref | - | |
| Height-for-age Z-score | ≥−2 | 1735 (88%) | 226 (12%) | Ref | - |
| ≥−3, <−2 | 453 (89%) | 254 (11%) | 0.91 | 0.66–1.3 | |
| <−3 | 336 (88%) | 47 (12%) | 1.1 | 0.76–1.5 | |
| Low birth weight | No | 1815 (88%) | 242 (12%) | Ref | - |
| (<2500 grams) | Yes | 289 (91%) | 28 (9%) | 0.73 | 0.44–1.2 |
| Socioeconomic status score | 37–93 | 706 (89%) | 87 (11%) | Ref | - |
| 25–36 | 933 (91%) | 96 (9%) | 0.84 | 0.60–1.2 | |
| 0–24 | 885 (86%) | 144 (14%) | 1.3 | 0.96–1.8 |
Abbreviations: ARIs, acute respiratory infections; M, month; Ref, reference; Y, year.
aOdds ratios for severe ARIs were calculated by binomial logistic regression analyses with generalized estimating equations.
Nasopharyngeal swabs were taken from 3971 ARI episodes (24%); samples were collected from 95% of ARI episodes at the healthcare facilities. Patients had fever in 26% of ARI episodes in households, and samples were collected from 34% of the ARI episodes with fever. There was no significant difference in patients’ characteristics between ARI episodes with sample collection and those without sample collection (data not shown). At least 1 respiratory virus was detected in 56% of the samples (2204 of 3971). The most frequently detected virus was RhV (n = 867, 22%), followed by RSV (n = 489, 12%), PIV (n = 378, 10%), IFV (n = 320, 8%), hMPV (n = 129, 3%), AdV (n = 95, 2%), and EV (n = 93, 2%) (Supplementary Figure 1). Among the 93 EV-positive samples, 42 (45%) were EV-D68. Given that the serotype for some EV-positive samples could not be determined and that its etiological role in ARIs was unclear, we separated EV-D68 from other EVs (EVs other than D68) during further analyses. We identified the serotype in 62 (65%) of the 95 AdV-positive samples, serotype 7 being the most common (n = 18). Among 54 PIV-4-positive samples, 43 and 11 were types 4a and 4b, respectively. We collected samples from 2 fatal patients of the 3. One was positive for AdV serotype 7 and the other was virus negative. The codetection of multiple viruses was found in 8% of virus-positive samples (169 of 2204) (Supplementary Figure 1). Acute respiratory infection episodes with codetection of multiple viruses were not excluded for further analyses but regarded as positive for each virus.
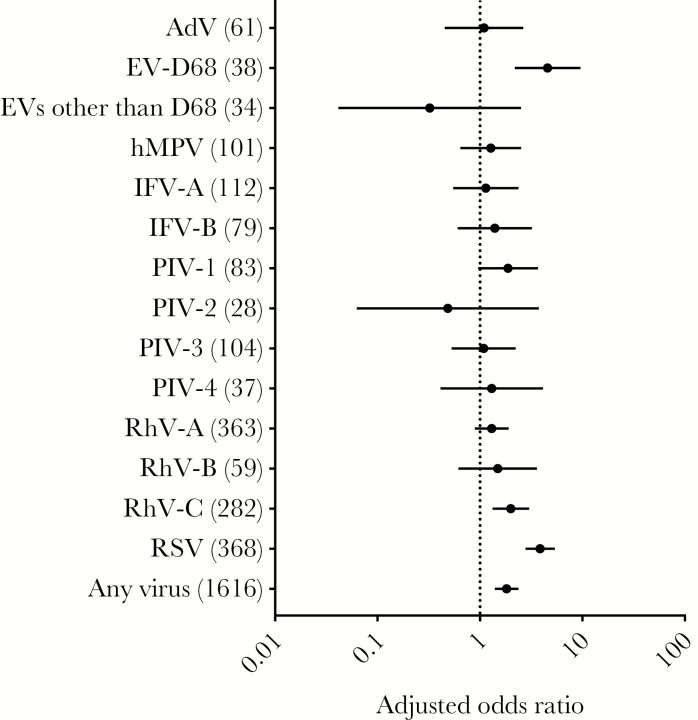
We initially calculated the association between viral detection and disease severity. Enterovirus D68 (adjusted odds ratio [aOR], 4.6; 95% CI, 2.2–9.6), RhV-C (aOR, 2.0; 95% CI, 1.3–3.0), and RSV (aOR, 3.9; 95% CI, 2.8–5.4) were frequently detected in severe ARIs, with statistical significance (Figure 1). The codetection of different viruses was insignificantly associated with severe ARIs (aOR 1.3 compared with single-virus detection; 95% CI, 0.82–2.1).
Figure 1.
Viral detection and severity of acute respiratory infections (ARIs). Odds ratio and 95% confidence intervals by binomial logistic regression analyses with generalized estimating equations are shown for viral detection of severe vs nonsevere ARIs adjusted for age, gender, height-for-age Z-score (HAZ), and socioeconomic (SES) score. The reference to calculate odds ratio was virus-negative ARIs. The numbers in parentheses indicate the number of ARI episodes positive for each virus. A log scale was used for the odds ratio.
We then investigated whether prior viral infections could affect the risk for subsequent ARIs. Risk for the next ARI in children with preceding infection was calculated by comparison of risk for the first ARI during the corresponding time in their matched controls. Matching was successful in over 89% of children with preceding infection. The characteristics of children with preceding infection and matched controls are summarized in Supplementary Table 2.
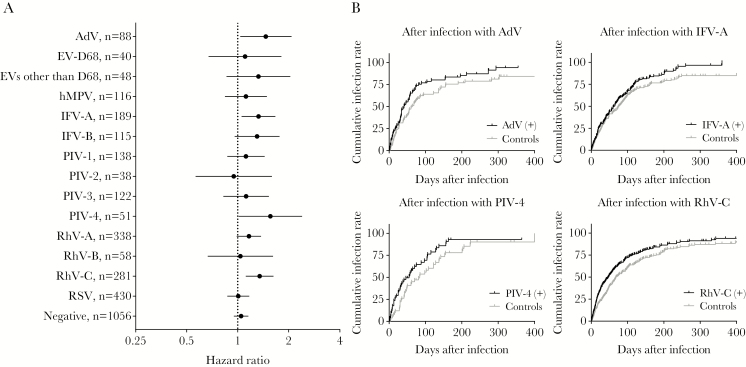
The Cox proportional hazards model showed that children who had preceding infections with AdV (HR, 1.5; 95% CI, 1.0–2.1), IFV-A (HR, 1.3; 95% CI, 1.1–1.7), PIV-4 (HR, 1.6; 95% CI, 1.0–2.4), and RhV-C (HR, 1.3; 95% CI, 1.1–1.6) had a significantly high risk for subsequent ARIs (Figure 2 and Supplementary Figure 2). None of preceding viral infections significantly decreased the risk for subsequent ARIs. The risk for subsequent severe ARIs was not enhanced significantly after preceding infections with any viruses tested (Supplementary Figure 3).
Figure 2.
Preceding viral infections and subsequent acute respiratory infections (ARIs). (A) Hazard ratios and 95% confidence intervals determined using Cox proportional hazards regression analysis are shown for subsequent ARIs after preceding infections compared with the first ARIs during the corresponding time for matched controls. A log scale was used for hazard ratios. (B) Cumulative infection rates by Kaplan-Meier curves are shown for subsequent ARIs after preceding infections and ARIs in matched controls during the corresponding period. The curves were shown for preceding infections with adenovirus, influenza A virus, parainfluenza virus type 4, and rhinovirus species C. Results for all viruses can be found in Supplementary Figure 2.
Next, we analyzed the risk for subsequent ARIs after preceding viral infections by age group to see whether the observed enhanced risk was age-dependent. The effect of age on the risk for subsequent ARIs varied among viruses (Supplementary Figure 4). The age-dependent enhanced risk for subsequent ARIs was evident for AdV, whereas the enhanced risk for subsequent ARIs was not evident for children in a specific age group for other viruses.
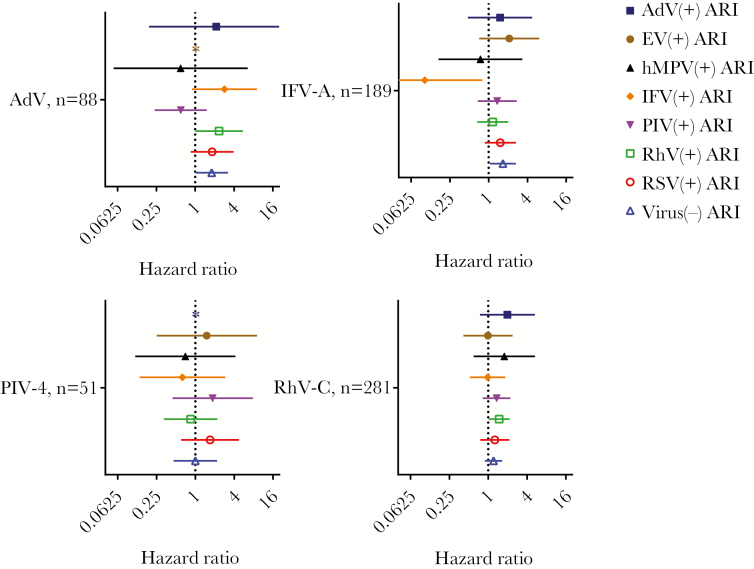
Finally, we determined the effect of viral etiology in subsequent ARIs. In particular, we analyzed the risk for subsequent ARIs positive for respiratory viruses in those with preceding infections with viruses that enhanced the risk for subsequent ARIs, namely, AdV, IFV-A, PIV-4, and RhV-C (Figures 2 and 3). The risk for subsequent infections with RhV increased significantly after preceding infections with AdV (HR, 2.3; 95% CI, 1.0–5.5). The risk for subsequent virus-negative ARIs was also high after preceding infections with AdV (HR, 1.8; 95% CI, 1.0–3.2). Furthermore, preceding infections with RhV-C increased the risk for subsequent RhV-positive ARIs (HR, 1.5; 95% CI, 1.0–2.1), and preceding infections with IFV-A increased the risk for subsequent virus-negative ARIs (HR, 1.7; 95% CI, 1.0–2.6). The risk for subsequent infection with specific virus did not increase after preceding infection with PIV-4. Children who had preceding infections with IFV-A were less likely to have subsequent ARIs positive for the homologous virus (HR, 0.10; 95% CI, 0.013–0.80).
Figure 3.
Risk for subsequent virus-positive and virus-negative acute respiratory infections (ARIs) after preceding viral infections. Hazard ratios and 95% confidence intervals determined using Cox regression analysis are shown for subsequent virus (+) and virus (−) ARIs after preceding adenovirus (AdV), influenza virus (IFV)-A, parainfluenza virus (PIV)-4, and rhinovirus (RhV)-C infections compared with matched controls. *, Not calculated because of the limited number of events. A log scale was used for the hazard ratios.
DISCUSSION
This study revealed the association between viral etiology and symptom severity in children with ARIs who sought care from primary and secondary healthcare facilities in the Philippines. Enterovirus D68, RhV-C, and RSV were more prevalent in severe ARIs than in nonsevere ARIs. In addition, we evaluated the effect of viral infections on subsequent ARIs. We found that preceding infections with AdV, IFV-A, PIV-4, and RhV-C were associated with enhanced risk for acquiring subsequent ARIs.
Respiratory syncytial virus is a well known pathological agent of lower respiratory tract infections among infants and young children [28]. Besides, a surge of infections with EV-D68 and their severe clinical manifestation have been reported all over the world since 2010 [4]. The present study confirmed high positivity rate for these viruses and a strong association with severe diseases in children below 5 years old (Supplementary Figure 1 and Figure 1). Although RSV and EV-D68 played an important role in the severity of the present ARIs, preceding infections with those viruses did not significantly increase the risk for subsequent ARIs (Figure 2).
The pathological role of RhV remains unclear. The virus has been detected in respiratory specimens from healthy individuals [29–31], whereas others reported an association between the virus and severe symptoms [32–34]. In this study, infection with RhV-C was associated with severe symptoms in the present ARI (Figure 1), and the infection was a significant risk factor for subsequent ARIs (Figure 2). Rhinoviruses, especially RhV-C, are reported to trigger the development of recurrent wheezing and asthma [35–37]. This association might contribute to our finding of high susceptibility for subsequent ARIs after RhV-C infections; underlying hypersensitivity, such as allergic asthma, may be triggered by an otherwise asymptomatic respiratory infection. Still, the enhanced risk for subsequent ARIs after preceding infections with AdV and PIV-4 (Figure 2) has yet to be reported, and the mechanisms remain unclear. A high risk for subsequent ARIs after viral infections was observed a matter of months after the infections (Figure 2). This result suggests that not only temporal changes in immune status [38] but also other long-lasting factors, such as persistent modulation of immune cells [10], gene regulation [39], alterations in the respiratory tract microbiome [12–16], and tissue damage [11, 40, 41], might be responsible for the higher risk for subsequent ARIs. It is interesting to note that increased risk for subsequent ARIs after viral infections does not seem to be caused by susceptibility to a specific virus (Figure 3).
Influenza is known to be associated with severe secondary bacterial infections [42, 43]. In this study, we found an enhanced risk for subsequent ARIs after preceding infection with IFV-A (Figure 2). It is notable that the risk was especially high for virus-negative ARIs (Figure 3). Although we did not test bacterial pathogens in this study, secondary bacterial infections may be attributed to the high risk for subsequent ARIs after influenza in the cohort.
A possible concern is that the high risk for subsequent ARIs after preceding viral infections resulted from children who are highly vulnerable to ARIs. Such children may have developed ARIs on multiple occasions, leading to a high possibility of virus detection from the collected samples and a high likelihood of suffering from subsequent ARIs. To reduce this possibility, we matched the incidence of ARI by the time of the preceding infection between each pair of child with preceding infection and the matched control (see Methods for detail). Seasonality of respiratory viruses reported in the study area could also affect the risk for subsequent infection [3]. To address the issue, we compared ARIs in child with preceding infection and the matched control during the same time to calculate the risk (see Methods for detail).
A limitation of this study is that the sampling of nasopharyngeal swabs was only conducted when children developed respiratory symptoms with fever within 7 days of household visit or were brought to the healthcare facilities. Undetected viral infections could confound the interpretation of the impact of specific viruses identified in our sampling on the risk for subsequent ARIs. Still, we collected samples from 95% of ARI episodes at healthcare facilities and 34% of ARI episodes with fever in households. We assume that our sampling included most of the clinically important ARI episodes. Another limitation is that matching strategy was compromised for some cases (see Methods for detail). This could cause biases in the results although matching rate was high and difference in characteristics between the case and matched control was small (Supplementary Table 2).
CONCLUSIONS
In this study, we revealed that viral etiology plays a significant role in the severity of ARIs and, more importantly, that preceding viral infection was associated with the host’s susceptibility to subsequent ARIs. Interactions may exist among ARIs with different pathological agents [44–47]. Further study would be required to reveal the risk not only for subsequent ARIs but also for the development of asthma in children after viral infections. Moreover, close attention should be paid to vaccines for respiratory viruses to determine their effect on not only infection with the targeted pathogens but also other infections because the present study found high susceptibility for ARIs after natural viral infections. A better understanding of the etiological role of viral infections and the risk for subsequent ARIs is needed for the prevention and management of childhood ARIs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the research staff at the cohort project and medical and administrative staff at the Rural Health Units in Kawayan and Caibiran, Biliran Provincial Hospital, and Biliran Provincial Health Office. We also thank Dr. Clyde Dapat for critical reading of the manuscript and helpful comments and Dr. Emiko Nakagawa and Dr. Yoshio Koyanagi for support.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded by Science and Technology Research Partnership for Sustainable Development (Grant Number JP16jm0110001) from the Japan International Cooperation Agency and the Japan Agency for Medical Research and Development (AMED), the Japan Initiative for Global Research Network on Infectious Diseases (Grant Number JP16fm0108013) from the Ministry of Education, Culture, Sport, Science & Technology in Japan (MEXT) and AMED, KAKENHI (Grant Number JP16H02642) from Japan Society for the Promotion of Science (JSPS), Creative Interdisciplinary Research Program funding from Tohoku University, and the Leading Initiative for Excellent Young Researchers from the MEXT and JSPS.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: International Union of Microbiological Societies 2017, 20 July 2017, Singapore.
References
- 1. Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Heal Organ 2004; 82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor S, Lopez P, Weckx L, et al. . Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect 2017; 74:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki A, Lupisan S, Furuse Y, et al. . Respiratory viruses from hospitalized children with severe pneumonia in the Philippines. BMC Infect Dis 2012; 12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis 2016; 16:e64–75. [DOI] [PubMed] [Google Scholar]
- 5. Self WH, Williams DJ, Zhu Y, et al. . Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016; 213:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sauerbrei A, Langenhan T, Brandstädt A, et al. . Prevalence of antibodies against influenza A and B viruses in children in Germany, 2008 to 2010. Eurosurveillance 2014; 19: pii: 20687. [DOI] [PubMed] [Google Scholar]
- 7. Lu G, Gonzalez R, Guo L, et al. . Large-scale seroprevalence analysis of human metapneumovirus and human respiratory syncytial virus infections in Beijing, China. Virol J 2011; 8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J 2008; 32:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellinghausen C, Rohde GG, Savelkoul PH, Wouters EF, Stassen FR. Viral-bacterial interactions in the respiratory tract. J Gen Virol 2016; 97:3089–102. [DOI] [PubMed] [Google Scholar]
- 10. Roquilly A, McWilliam HE, Jacqueline C, et al. . Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity 2017; 47:135–147.e5. [DOI] [PubMed] [Google Scholar]
- 11. Jamieson AM, Pasman L, Yu S, et al. . Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 2013; 340:1230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosas-Salazar C, Shilts MH, Tovchigrechko A, et al. . Differences in the nasopharyngeal microbiome during acute respiratory tract infection with human rhinovirus and respiratory syncytial virus in infancy. J Infect Dis 2016; 214:1924–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yi H, Yong D, Lee K, Cho YJ, Chun J. Profiling bacterial community in upper respiratory tracts. BMC Infect Dis 2014; 14:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Lastours V, Malosh R, Ramadugu K, Srinivasan U. Co-colonization by Streptococcus pneumoniae and Staphylococcus aureus in the throat during acute respiratory illnesses. Epidemiol Infect 2016; 18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolter N, Tempia S, Cohen C, et al. . High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis 2014; 210:1649–57. [DOI] [PubMed] [Google Scholar]
- 16. de Steenhuijsen Piters WA, Heinonen S, Hasrat R, et al. . Nasopharyngeal microbiota, host transcriptome and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med 2016; 194:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Philippine Statistics Authority. Total Population by Province, City, Municipality and Barangay: as of May 1, 2010 Available at: http://psa.gov.ph/sites/default/files/attachments/hsd/pressrelease/Eastern%20Visayas.pdf. Accessed 2 September 2018. [Google Scholar]
- 18. Kosai H, Tamaki R, Saito M, et al. . Incidence and risk factors of childhood pneumonia-like episodes in Biliran Island, Philippines--a community-based study. PLoS One 2015; 10:e0125009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization, United Nations Childrens Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children. World Heal Organ 2009; 11 Available at: http://apps.who.int/iris/bitstream/10665/44129/1/9789241598163_eng.pdf. Accessed 21 June 2018. [PubMed] [Google Scholar]
- 20. World Health Organization. Integrated Management of Childhood Illness Chart Booklet. 2014. Available at: http://apps.who.int/iris/bitstream/10665/104772/16/9789241506823_Chartbook_eng.pdf. Accessed 21 June 2018. [Google Scholar]
- 21. Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS. WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine 2016; 34:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heim A, Ebnet C, Harste G, Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol 2003; 70:228–39. [DOI] [PubMed] [Google Scholar]
- 23. Imamura T, Suzuki A, Lupisan S, et al. . Molecular evolution of enterovirus 68 detected in the Philippines. PLoS One 2013; 8:e74221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuzaki Y, Takashita E, Okamoto M, et al. . Evaluation of a new rapid antigen test using immunochromatography for detection of human metapneumovirus in comparison with real-time PCR assay. J Clin Microbiol 2009; 47:2981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Institute for Infectious Diseases. Influenza Diagnosis Manual [Japanese]. 2014. Available at: https://www.niid.go.jp/niid/images/lab-manual/Influenza2014.pdf. Accessed 31 Jul 2018. [Google Scholar]
- 26. Bellau-Pujol S, Vabret A, Legrand L, et al. . Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods 2005; 126:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malasao R, Okamoto M, Chaimongkol N, et al. . Molecular characterization of human respiratory syncytial virus in the Philippines, 2012–2013. PLoS One 2015; 10:e0142192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi T, McAllister DA, O’Brien KL, et al. . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douros K, Kotzia D, Kottaridi C, et al. . Many children aged two to five years have a persistent presence of respiratory viruses in their nasopharynx. Acta Paediatr 2016; 105:e89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health 2015; 5:010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Byington CL, Ampofo K, Stockmann C, et al. . Community surveillance of respiratory viruses among families in the utah better identification of germs-longitudinal viral epidemiology (BIG-LoVE) study. Clin Infect Dis 2015; 61:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Renwick N, Schweiger B, Kapoor V, et al. . A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J Infect Dis 2007; 196:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Louie JK, Roy-Burman A, Guardia-Labar L, et al. . Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J 2009; 28:337–9. [DOI] [PubMed] [Google Scholar]
- 34. Fuji N, Suzuki A, Lupisan S, et al. . Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS One 2011; 6:e27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller EK. New human rhinovirus species and their significance in asthma exacerbation and airway remodeling. Immunol Allergy Clin North Am 2010; 30:541–52, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson DJ, Gangnon RE, Evans MD, et al. . Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008; 178:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turunen R, Vuorinen T, Bochkov Y, Gern J, Jartti T. Clinical and virus surveillance after the first wheezing episode. Pediatr Infect Dis J 2017; 36:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong SS, Oshansky CM, Guo XJ, et al. . Severe influenza is characterized by prolonged immune activation: results from the SHIVERS cohort study. J Infect Dis 2017; 217:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mejias A, Dimo B, Suarez NM, et al. . Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 2013; 10:e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kilani MM, Mohammed KA, Nasreen N, et al. . Respiratory syncytial virus causes increased bronchial epithelial permeability. Chest 2004; 126:186–91. [DOI] [PubMed] [Google Scholar]
- 41. Kash JC, Walters KA, Davis AS, et al. . Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. MBio 2011; 2:pii: e00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis 2010; 202:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mina MJ, Klugman KP. The role of influenza in the severity and transmission of respiratory bacterial disease. Lancet Respir Med 2014; 2:750–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laurie KL, Guarnaccia TA, Carolan LA, et al. . Interval between infections and viral hierarchy are determinants of viral interference following influenza virus infection in a ferret model. J Infect Dis 2015; 212:1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karppinen S, Toivonen L, Schuez-Havupalo L, Waris M, Peltola V. Interference between respiratory syncytial virus and rhinovirus in respiratory tract infections in children. Clin Microbiol Infect 2016; 22:208.e1–208.e6. [DOI] [PubMed] [Google Scholar]
- 46. Casalegno JS, Ottmann M, Bouscambert Duchamp M, et al. . Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect 2010; 16:326–9. [DOI] [PubMed] [Google Scholar]
- 47. Gröndahl B, Ankermann T, von Bismarck P, et al. . The 2009 pandemic influenza A(H1N1) coincides with changes in the epidemiology of other viral pathogens causing acute respiratory tract infections in children. Infection 2014; 42:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.