This study demonstrates that an adjuvant consisting of Toll-like receptor and C-type lectin receptor agonist pairing significantly enhances immunoglobulin G (IgG) responses to polysaccharide antigens and supports the generation of IgG+ memory B cells that are responsive to boosting.
Keywords: Streptococcus pneumoniae, adjuvant, pneumococcal vaccine, B cell, T-cell–independent antigen
Abstract
Protection against encapsulated bacteria can be elicited using polysaccharide vaccines. These antigens often behave as T-cell–independent type 2 antigens (TI-2 Ags). However, TI-2 Ags, including pneumococcal polysaccharides, often elicit weak immunoglobulin G (IgG) responses and are refractive to boosting. Conjugate vaccines have not completely overcome this challenge and hence, alternative strategies are required to enhance polysaccharide vaccine responses. Herein, we describe an adjuvant consisting of a Toll-like receptor and C-type lectin receptor agonist pairing that significantly increases primary immunoglobulin M and IgG responses to TI-2 Ags as well as enables significant boosting when coadministered with polysaccharide vaccines. Consistent with this, the adjuvant significantly increased the generation of both TI-2 memory B cells and long-lived antibody secreting cells. Adjuvant effects were highly dependent on B-cell–intrinsic MyD88, but not Trif expression. Importantly, coadministration of the adjuvant with the Pneumovax vaccine significantly increased the protective efficacy of vaccination in a lethal challenge mouse model of pneumococcal respiratory infection. Collectively, these data provide evidence that B-cell–directed adjuvants have promise in significantly improving the quality and quantity of serologic and B-cell memory responses to clinically relevant polysaccharide vaccines.
Pneumococcal disease kills more people in the United States than all other vaccine-preventable diseases combined. Pneumococcal vaccines consist of Streptococcus pneumoniae–derived capsular polysaccharides (PPSs), which behave as T-cell–independent type 2 antigens (TI-2 Ags). One dose of Pneumovax 23, an adjuvant-free vaccine composed of PPS from 23 serotypes, provides protection against invasive pneumococcal disease in adults for ≤10 years, with an estimated efficacy of 60%–70% [1]. Achieving high levels of immunoglobulin G (IgG) against the 23 disease-causing serotypes is a major goal of vaccination, but this is not always achieved with 1 dose. Unfortunately, polysaccharide (Ps) Ags do not induce recall responses upon revaccination or even following infection [2–5]. The inability to boost IgG responses to Ps Ags is therefore a major barrier to enhancing protective antibody (Ab) titers through revaccination.
The inability to boost Ps Ab responses is a problem that extends far beyond pneumococcal vaccines, as there is rapidly growing interest in utilizing carbohydrate Ags from viruses, bacteria, parasites, fungi, and tumors as vaccine Ags [6]. Conjugation of proteins to PPS enables boosting in young children (except perhaps with serotype 3 PPS [PPS3] conjugates [3]). In adults, PPS conjugate vaccines do not boost well and overall Ab responses are similar to those elicited to native PPSs [7, 8–10]. Conjugate vaccines can nonetheless impact protection. However, because conjugate vaccines are costly, difficult to synthesize, provide limited serotype coverage, and do not seem to generate better responses than native Ps in adults, alternatives are needed.
TI-2 Ags (PPS, meningococcal Ps, haptenated Ficoll, etc) generate a limited number of memory B cells [11]. Ag-specific IgG and perhaps other inhibitory pathways suppress the ability of these memory cells to participate in secondary responses. Although some TI Ags encountered on gram(–) bacteria induce plasmablast-like memory cells with the capacity for expansion and increased Ab output upon pathogen reexposure [12, 13], this does not occur with native Ps. PPSs associated with live/killed pneumococci [14], and even some PPS-protein conjugates, do not elicit PPS Ab boosting [3, 7, 8–10]. The lack of boosting to S. pneumoniae may be due to weak pattern recognition receptor agonists [15–17] and/or suppressive factors (pneumolysin is a Toll-like receptor [TLR] 4 agonist and cytotoxin).
Adjuvants for native Ps vaccines have not been used in the clinic. Adjuvants that (1) boost isotype switching and Ab production and (2) promote generation and activation of functional memory B cells that optimally differentiate into antibody-secreting cells (ASCs) after boosting are needed. Alum does not boost primary Ab responses to TI-2 Ags [18] and poorly boosts responses to PPS conjugates [19]. However, TLR agonists may hold promise as they overcome programmed cell death-1–mediated suppression of B cell receptor (BCR)–induced proliferation [20], protect B cells from apoptosis [21], and promote activation of several signaling pathways induced by BCR activation. Nonetheless, TLR4 (including monophosphoryl lipid A [MPL]) and all other TLR1–9 agonists do not increase Ab responses when coadministered with PPS [22, 23], but do so if given several days after PPS vaccination. Earlier work with Ribi adjuvant, consisting of Salmonella typhimurium MPL and Mycobacterium cord factor (trehalose dimycolate) in squalene elicited increased primary PPS-specific Ab responses in mice [24, 25], but it is unsuitable for use in humans due to toxicity.
Given the above findings and the potential for CLR to synergize with TLR-induced signals [26], we examined the effect of low-toxicity Salmonella minnesota MPL and synthetic cord factor analogue emulsified in squalene (MTS) on Ps-specific Ab responses. Remarkably, MTS significantly increased Ag-specific immunoglobulin M (IgM) and IgG levels in response to PPS and haptenated-Ficoll and enabled increased memory responses through a pathway dependent upon B-cell–intrinsic MyD88 signaling. Our data suggest that combining these or other TLR and CLR agonists may be ideal for promoting enhanced IgG levels in response to Ps Ags and, moreover, support increased functional memory formation and overcome mechanisms that inhibit recall responses to Ps-specific Ags.
METHODS
Mice
WT, MyD88–/–, Trif–/–, muMT, and B1-8hi IgH knock-in mice on a C57BL/6 background (Jackson Laboratory) were bred in-house under specific pathogen free conditions. Mice were age- and sex-matched for studies. Studies were approved by the Wake Forest School of Medicine Animal Use Committee.
Immunizations, Enzyme-Linked Immunosorbent Assays, and Enzyme-Linked Immunospot Assays
Mice were immunized with the equivalent of approximately 1 μg each PPS contained within Pneumovax (Merck), or 1 μg 4-hydroxy-3-nitrophenyl acetyl (NP)40-Ficoll or 1–25 μg 2,4,6-trinitrophenyl (TNP)65-Ficoll [20]. Adjuvant containing 20 μg S. minnesota MPL, 20 μg synthetic cord factor (trehalose-6,6’-dicorynomycolate [TDCM] or trehalose-6,6-dibehenate) in 1% squalene/0.1% Tween-80 (for intraperitoneal administration) or 2% squalene/0.2% Tween-80 (for intramuscular administration) was mixed with Ags prior to injections (Sigma and Invivogen). A final volume of 200 μL was used for intraperitoneal injections and 50 μL for gastrocnemius muscle (hind leg) injections. Boosting was performed in the same muscle. Enzyme-linked immunosorbent assays and enzyme-linked immunospot assays were as previously described [20, 27, 28]. Additional information can be found in the Supplementary Materials and Methods.
B-Cell Phenotyping
Single-cell suspensions (2 × 107/mL) in phosphate-buffered saline containing 2% newborn calf serum were incubated with FcBlock (eBioscience) for 15 minutes, followed by staining with fluorochrome-conjugated Abs: CD19 (1D3), CD138 (281–2), (eBioscience), CD11b (M1/70), CD45.1 (BioLegend), pooled antimouse IgG1, IgG2b, and IgG3 (Southern Biotech), and NP-allophycocyanin (APC), for 30 minutes at room temperature. Fluorochrome-labeled isotype controls were used to determine background staining levels. Cells were analyzed using a FACSCantoII cytometer (BD Biosciences) with forward side scatter-area (FSC-A) and forward side scatter-height (FSC-H) doublet exclusion. Data were analyzed using FlowJo analysis software (Tree Star).
Adoptive Transfer Experiments
CD45.1+ splenic B cells were purified from B1-8hi IgH mice using CD43 bead depletion (Dynal). B cells were transferred intravenously into CD45.2+ mice (1 × 107/mouse) with mice immunized intramuscularly (1 μg NP-Ficoll) the next day. NP-specific CD45.1+CD19+ B cells were analyzed by flow cytometry as described above.
Radiation Chimera
Wild-type (WT) mice were lethally irradiated (950 rad) and reconstituted intravenously with bone marrow (BM) from IgM-mutant (muMT) mice mixed with WT or MyD88–/– marrow (90:10 ratio; 1 × 107 BM cells) [29]. Sulfamethoxazole (40 mg/mL) and trimethoprim (8 mg/mL) were supplied in drinking water 1 week prior to and 2 weeks following irradiation. Mice were rested for 4 weeks prior to immunization.
Streptococcus pneumoniae Respiratory Challenge
Mice were infected intranasally with serotype 3 WU2 strain S. pneumoniae by distributing 40 μL bacteria (1 × 107 colony-forming units) between 2 nares of isoflurane-anesthetized mice [28]. Mice were monitored daily for signs of distress and humanely killed.
Statistical Analyses
Data are shown as mean ± standard error of the mean. Differences between sample means were assessed using Student t test. Differences in Kaplan–Meier survival were assessed using the log-rank test.
RESULTS
An Adjuvant Composed of MPL and Cord Factor Analogue in Squalene Increases Primary PPS-Specific IgM and IgG Responses and Enables Recall Responses
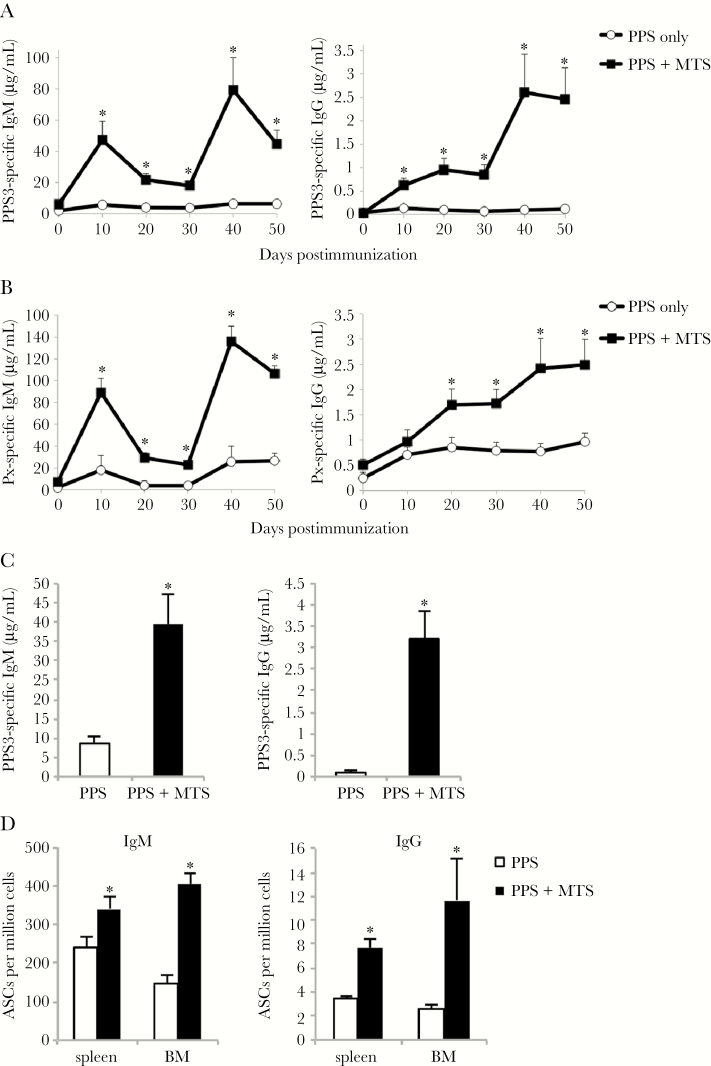
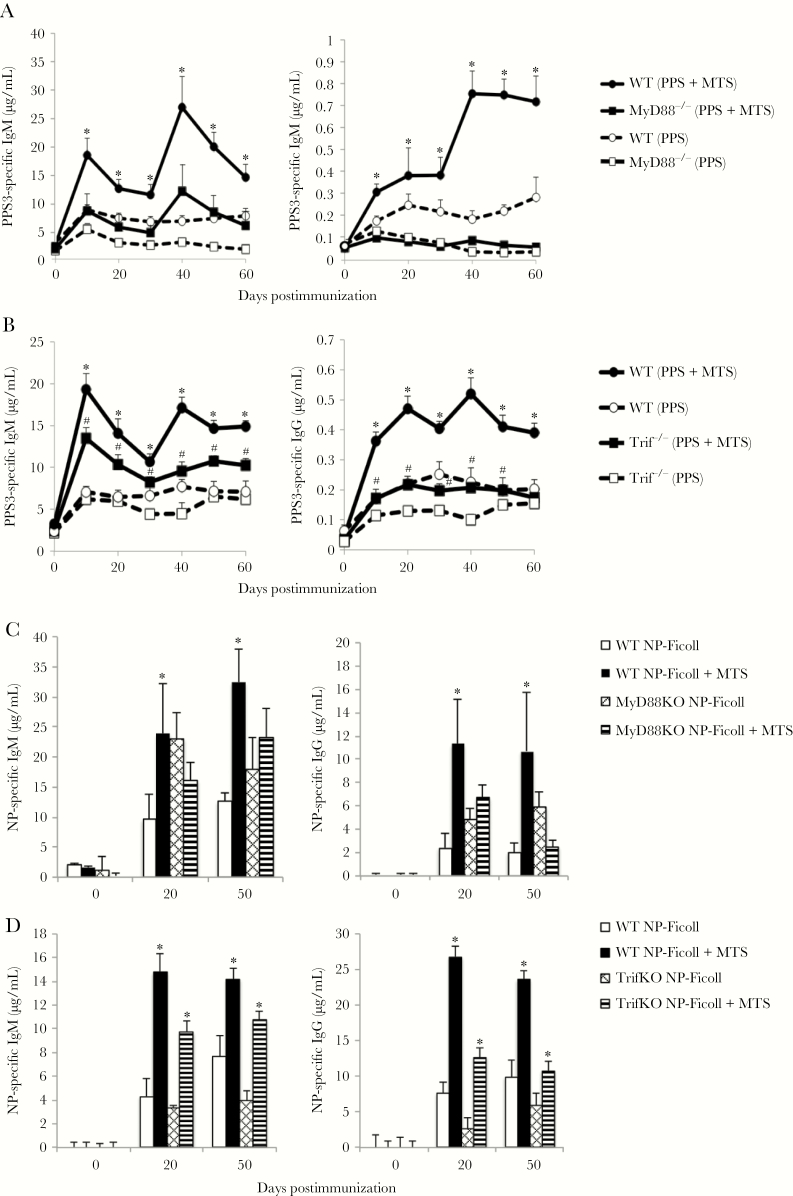
Serotype 3 S. pneumoniae remains an important cause of invasive pneumococcal disease and the PPS3 protein conjugate has performed poorly relative to other PPS conjugates [3, 30, 31], suggesting alternative vaccine strategies may be necessary for some Ps-based vaccines. As shown in Figure 1A, mice immunized with Pneumovax (intraperitoneal) mixed with an adjuvant consisting of MPL (a TLR4 agonist) and synthetic cord factor (TDCM; MCL/Mincle agonist) in 1% squalene (referred to as “MTS”) generated significantly higher primary PPS3-specific IgM and IgG levels compared to mice immunized with Pneumovax alone. Remarkably, these mice also showed significant PPS3-specific IgM and IgG boosting over primary levels (2.5-fold), similar to the increase observed when alum is used with haptenated proteins [32]. MTS significantly increased PPS3-specific IgG2b, IgG2c, and IgG3 levels, with IgG3 remaining the major isotype produced (Supplementary Figure 1). MTS also significantly increased total Pneumovax-specific Ab levels (Figure 1B). Notably, coadministration of MTS-adjuvanted PPS3 to mice that had only received PPS3 during the primary immunization enabled significant IgM, but not IgG, boosting (Supplementary Figure 2). These data suggest that MTS supports generation and activation of PPS-specific memory B cells. MTS effects on PPS3-specific IgM and IgG levels persisted at least 6 months, with IgM and IgG levels remaining significantly increased (5- to 25-fold) over mice that had been immunized without adjuvant (Figure 1C). Consistent with this, splenic and bone marrow PPS3-specific IgM and IgG ASCs were significantly increased in MTS-treated mice (Figure 1D).
Figure 1.
Monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS) increases primary and secondary pneumococcal polysaccharide (PPS)–specific immunoglobulin M (IgM) and immunoglobulin G (IgG) responses to Pneumovax. A–D, C57BL/6 wild-type mice were immunized with Pneumovax (~1 μg each PPS) alone or mixed with Sigma adjuvant system containing 20 μg MPL + 20 μg synthetic TDCM in 1% squalene oil (MTS) intraperitoneally (n = 4–5 mice/group) on days 0 and 30. Enzyme-linked immunosorbent assays to detect mean PPS3-specific (A and C) and Pneumovax (Px)–specific (B) serum IgM and IgG were performed. In C, PPS3-specific IgG levels were measured at 6 months postimmunization (n = 4–5 mice/group). D, Mean PPS3-specific IgM and IgG antibody-secreting cell frequencies in spleen and bone marrow (BM) 6 months postimmunization (n = 4–5 mice/group). *Significant differences between groups (P < .05). Results in A and B are representative of 3 independent experiments.
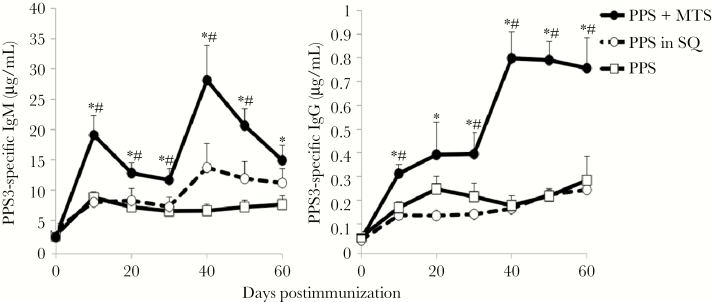
MTS-adjuvanted Pneumovax delivered imtramuscularly also significantly increased PPS3-specific IgM and IgG primary responses and promoted significant boosting, with IgM and IgG levels reaching 4-fold over that for mice immunized with Pneumovax alone (Figure 2). PPS3-specific IgM and IgG responses in mice immunized with Pneumovax mixed with 2% squalene were not significantly different from those obtained with Pneumovax alone. Thus, MTS significantly augments long-term IgM and IgG responses to PPS and enables recall responses, regardless of immunization route.
Figure 2.
Monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS) increases primary and secondary antibody responses to serotype 3 pneumococcal polysaccharide (PPS3) delivered via the intramuscular immunization route. Mice were immunized with Pneumovax (~1 μg each PPS) either alone, mixed with 20 μg monophosphoryl lipid A + 20 μg trehalose-6,6-dibehenate in 2% squalene oil (SQ), or with 2% SQ (n = 5–6 mice/group). All mice were boosted on day 30. Enzyme-linked immunosorbent assays were performed to detect PPS3-specific serum immunoglobulin M (IgM) and immunoglobulin G (IgG). *Significant differences between MTS group and PPS-only group. #Significant differences between MTS group and SQ group (P < .05). Results are representative of 3 independent experiments.
MTS Significantly Increases IgM and IgG Responses to Haptenated Ficoll
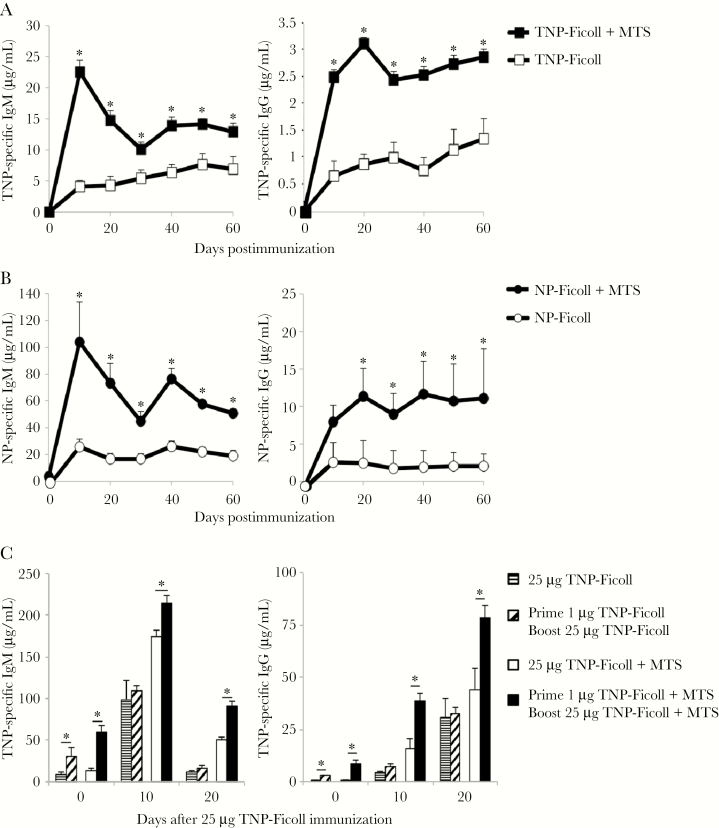
MTS also significantly increased IgM and IgG production (4- to 5-fold) in response to TNP-Ficoll (Figure 3A) and NP-Ficoll (Figure 3B). In contrast to the significant boosting observed with PPS + MTS (Figures 1 and 2), MTS promoted moderate boosting to haptenated Ficoll. This difference may be due to the significantly higher levels of IgG (and ratio of IgG to IgM) produced following primary immunization relative to that produced in response to PPS3, as TI-2 Ag-specific IgG can suppress secondary TI-2 Ab responses [11]. We therefore examined whether priming with 1 μg TNP-Ficoll in the presence of adjuvant would support boosting in response to high-dose (25 μg) TNP-Ficoll. Low-dose priming without adjuvant had no effect on the antibody response to 25 μg TNP-Ficoll (Figure 3C). However, mice primed with 1 μg TNP-Ficoll in the presence of adjuvant generated significantly increased IgG responses to TNP-Ficoll + MTS relative to mice administered 25 μg TNP-Ficoll + MTS for the first time. IgM levels were also increased, but not beyond that which was observed prior to immunization. Collectively, these data support that MTS significantly enhances primary and secondary IgM and IgG responses to clinically relevant (PPS) as well as model (haptenated Ficoll) TI-2 Ags.
Figure 3.
Monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS) significantly increases immunoglobulin M (IgM) and immunoglobulin G (IgG) responses to haptenated-Ficoll. A and B, Mice were immunized with 1 μg trinitrophenyl (TNP)65-Ficoll (A) or nitrophenyl (NP)40-Ficoll (B) either alone or mixed with MTS intramuscularly and boosted on day 30. C, Mice were immunized intraperitoneally with 1 μg TNP-Ficoll alone or mixed with MTS. Thirty days later, mice were immunized with 25 μg TNP-Ficoll ± MTS and TNP-specific IgM and IgG levels were measured by enzyme-linked immunosorbent assay. *Significant differences between groups (n = 5–6 mice/group, P < .05).
MTS Significantly Increases IgM and IgG ASCs and Memory B-Cell Formation
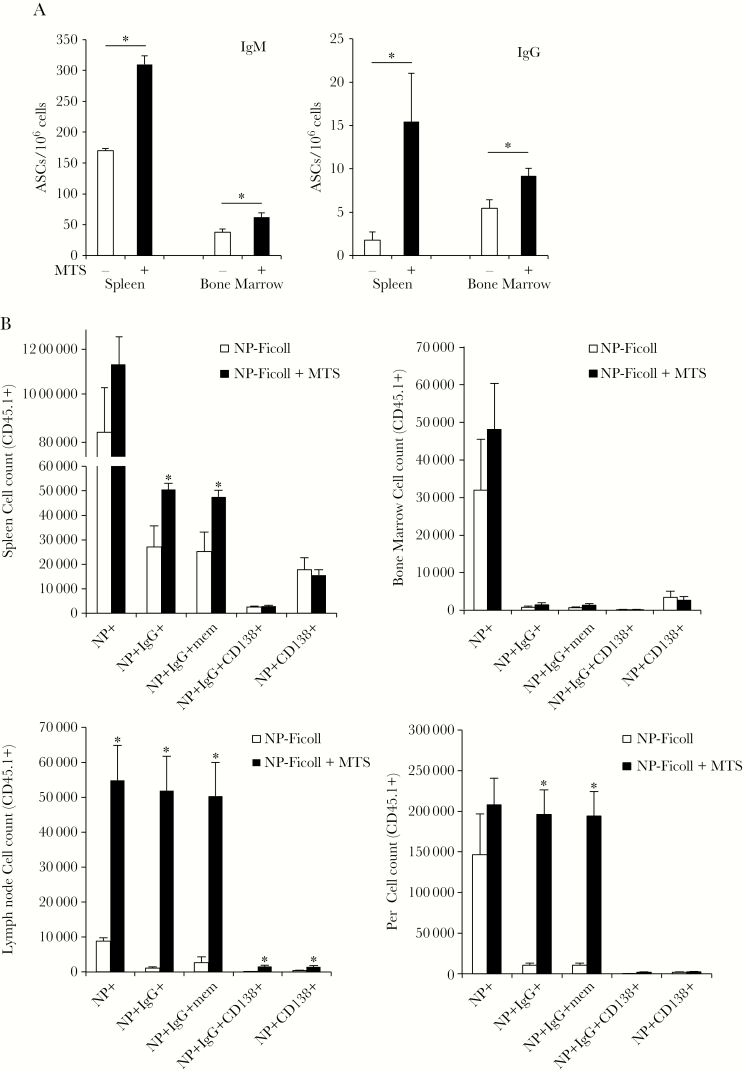
Three months postimmunization, NP-specific IgM and IgG ASCs were significantly increased in both bone marrow and spleen in mice immunized with NP-Ficoll + MTS relative to mice that received NP-Ficoll alone (Figure 4A). Thus, MTS significantly increases long-lived splenic and bone marrow ASCs generated in response to NP-Ficoll, similar to that which was observed for PPS3 (Figure 1D). Because MTS enabled significant IgG boosting, we assessed the extent to which MTS increased TI-2 memory B-cell formation. It is difficult to assess PPS3-specific memory B cells, so we took advantage of VHB1-8 transgenic (Tg) mice in which approximately 10% of B cells are specific for NP. CD45.1+ VHB1-8 Tg B cells were transferred into CD45.2+C57BL/6 mice. MTS significantly increased NP-specific Tg IgG+ B cells in spleen, draining (inguinal) lymph node, and peritoneal cavity 3 weeks postimmunization (Figure 4B). These increases were largely due to an increase in NP-specific IgG+CD138– (memory B cells), as opposed to NP-specific IgG+CD138+ plasmablasts, which were only significantly increased in lymph nodes of MTS-treated mice at this time point. NP-specific IgG+CD138– memory B cells were increased approximately 2-fold in spleen, and approximately 20-fold in lymph node and peritoneal cavity (Figure 4B). Remarkably, IgG-secreting plasmablasts were also selectively increased (>20-fold) in inguinal lymph nodes of MTS-treated mice, suggesting an additional, and yet unexpected, source of increased NP-specific serum IgG in adjuvant-treated mice. Notably, flow cytometric analysis could not account for MTS effects on increasing CD138+ plasma cells, which no longer bind NP, but are significantly increased by MTS (Figures 1C and 4A). To assess whether increased IgG+ memory cells elicited by MTS translated to increased IgG responses following secondary immunization without adjuvant, we transferred VHB1-8 Tg CD138neg memory cells into naive or immune recipients and assessed boost responses. Naive mice that received MTS-elicited memory B cells produced significantly more NP-specific IgG following immunization than mice that received memory cells from mice that were immunized without adjuvant (Supplementary Figure 3). However, transfer of memory cells into mice that were previously immunized exhibited significantly lower IgG responses, suggesting that MTS-elicited memory cells were suppressed in the immune environment. Thus, MTS significantly increases the generation of functional IgG+ class-switched memory B cells in response to haptenated Ficoll.
Figure 4.
Monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS) increases nitrophenyl (NP)–specific immunoglobulin M (IgM) and immunoglobulin G (IgG) antibody-secreting cell (ASC) frequencies in spleen and bone marrow (BM) and IgG+ memory cells. A, MTS increases long-lived NP-specific IgM and IgG ASCs. Mice were immunized with NP-Ficoll (1 μg) either alone or mixed MTS (n = 3 mice/group) and boosted on day 30. An enzyme-linked immunospot assay was performed to detect NP-specific IgM and IgG ASC frequencies in spleen and BM 3 months postimmunization. B, MTS significantly increases IgG switched memory cells following NP-Ficoll immunization. CD45.2+ wild-type mice received 1 × 107 VHB1-8 CD45.1+ transgenic CD43– spleen B cells intravenously. One day later, recipient mice were immunized with NP-Ficoll (1 μg) either alone or mixed with MTS, intramuscular (n = 4 mice/group). NP-specific CD45.1+ B cells were analyzed by flow cytometry 3 weeks postimmunization. *Significant differences between NP-Ficoll and NP-Ficoll + MTS (P < .05).
MTS Adjuvant Effects Require MyD88
MPL signals through TLR4/MD2, which utilizes MyD88 and/or Trif as signaling intermediates [33]. Cord factor analogues signal through Mincle/MCL and also have the potential to activate MyD88 signaling due to strong induction of interleukin (IL) 1, which signals through an IL-1R/MyD88–dependent pathway [34]. Squalene also activates MyD88-dependent signals [35]. Given this, we assessed the extent to which MyD88 was required for MTS adjuvant effects. MyD88–/– mice had reduced PPS3-specific IgM and IgG responses (2- to 4-fold) relative to WT mice immunized with Pneumovax alone (Figure 5A). However, in contrast to WT mice, MTS had an insignificant effect on increasing IgM or IgG responses in MyD88–/– mice, although some moderate increases in IgM were observed in the secondary response (Figure 5A). Thus, MyD88 is required for MTS-induced adjuvant effects on PPS-specific Ab responses.
Figure 5.
MyD88 is required for monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS)–induced increases in immunoglobulin M (IgM) and immunoglobulin G (IgG) responses to serotype 3 pneumococcal polysaccharide (PPS3) and nitrophenyl (NP)–Ficoll. A, Wild-type (WT) and MyD88–/– mice were immunized with Pneumovax (~1 μg each PPS) alone or mixed with MTS intramuscular (IM) (n = 3–5 mice/group). Mice were boosted on day 30. *Significant differences between WT groups (P < .05). B, WT and Trif–/– mice were immunized with Pneumovax (~1 μg each PPS) alone or mixed with MTS IM (n = 5–6 mice/group). Mice were boosted on day 30. *Significant differences between WT groups. #Significant differences between Trif–/– groups (P < .05). C and D, WT and MyD88–/– (C) or Trif–/– (D) mice were immunized with 1 μg NP-Ficoll alone or mixed with MTS IM. Mice were boosted on day 30. *Significant differences between WT groups (P < .05) or between Trif–/– groups (P < .05).
Trif–/– mice had lower PPS3-specific IgG responses to Pneumovax alone relative to WT mice. However, MTS significantly increased PPS3-specific IgM responses in Trif–/– mice and also significantly increased IgG responses over that of Trif–/– mice immunized with Pneumovax alone, albeit not to the same degree as WT mice (Figure 5B). Thus, Trif is not essential for MTS-induced increases in PPS-specific Ab responses, but is nonetheless required for optimal Ab responses to both nonadjuvanted and MTS-adjuvanted Pneumovax.
MyD88–/– mice generated normal, if not augmented IgM and IgG responses to NP-Ficoll, indicating a distinct requirement for MyD88 in promoting Ab responses to PPS, but not haptenated Ficoll (Figure 5C). However, MTS did not significantly increase these NP-specific IgM or IgG responses in MyD88–/– mice as observed in WT mice. In contrast to results with MyD88–/– mice, NP-specific IgM and IgG responses were significantly increased by MTS coadministration in Trif–/– mice (Figure 5D) with approximate 2- to 4-fold increases, similar to WT mice. Thus, MTS adjuvant effects on TI-2 Ags (PPS and haptenated Ficoll) are highly dependent upon MyD88, whereas Trif signaling is dispensable.
B-Cell–Intrinsic MyD88 Expression Is Required for MTS Adjuvant Effects on TI-2 Ab Responses
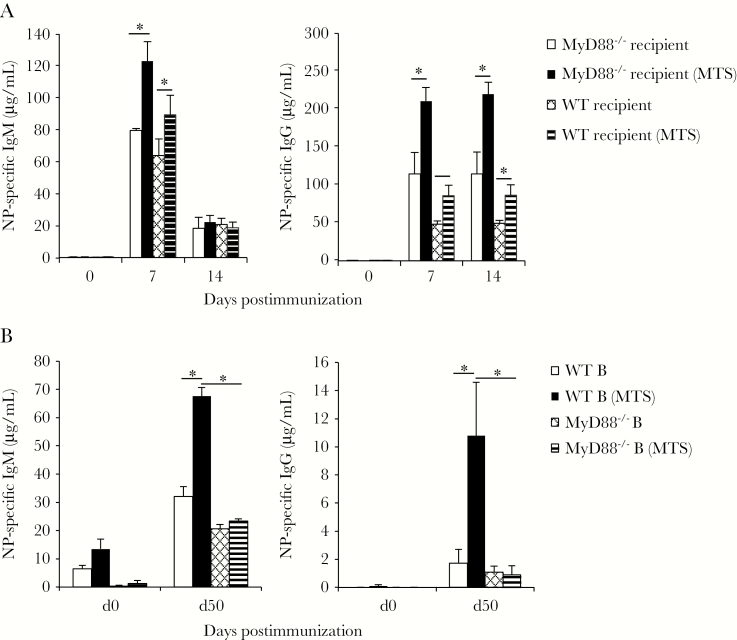
We next assessed whether MyD88 expression by B cells was required for MTS-induced adjuvant effects. To do this, we transferred VHB1-8Tg MyD88-sufficient B cells into WT and MyD88–/– mice. MyD88–/– recipient mice produced NP-specific IgM and IgG responses that were similar to or increased over responses of WT recipient mice (Figure 6A). Coadministration of MTS with NP-Ficoll significantly increased NP-specific IgM and IgG responses in both MyD88–/– and WT recipients, suggesting that B-cell–expressed MyD88 was needed for MTS responsiveness. Consistent with these results, MTS significantly increased IgM (2-fold) and IgG (5-fold) responses to NP-Ficoll in bone marrow chimeras reconstituted with WT B cells, but not in chimeras selectively lacking MyD88 in B cells (Figure 6B). Thus, B-cell–expressed MyD88 is required for MTS adjuvant effects on TI-2 Ab responses.
Figure 6.
B-cell intrinsic MyD88 expression is required for monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS)–induced effects on TI-2 antibody responses. A, VHB1-8 MyD88+/+ transgenic B cells are responsive to MTS following transfer into wild-type (WT) or MyD88–/– mice. WT and MyD88–/– received 1 × 107 VHB1-8 CD43– spleen B cells intravenously. Recipient mice were immunized with nitrophenyl (NP)–Ficoll (1 μg) either alone or mixed with MTS intramuscularly (IM) on day 1 (n = 4–9 mice/group). NP-specific immunoglobulin M (IgM)a and immunoglobulin G (IgG) were measured by enzyme-linked immunosorbent assay (ELISA). B, Irradiated WT mice were reconstituted with B-cell–deficient bone marrow (muMT) mixed with either WT or MyD88–/– bone marrow (90:10). Mice were immunized IM with 1 μg NP-Ficoll either alone or mixed with MTS on days 0 and 30. NP-specific IgM and IgG were measured by ELISA. *Significant differences between groups.
MTS Significantly Augments the Protective Efficacy of Pneumovax Against Respiratory Pneumococcal Infection
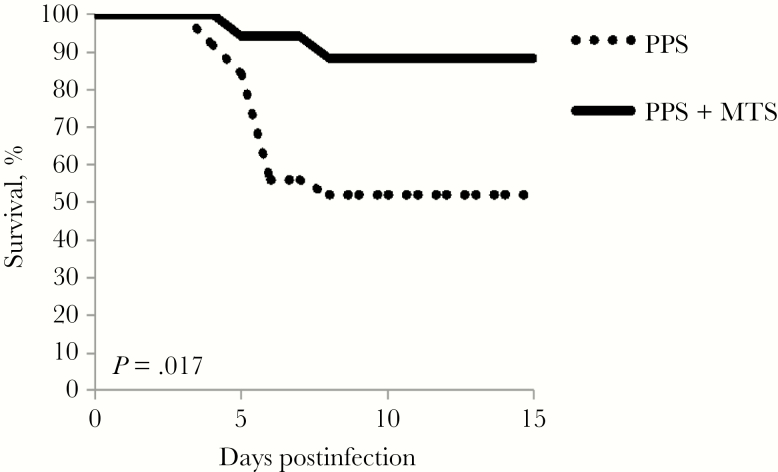
In humans, Pneumovax has approximately 65% efficacy against invasive pneumococcal disease [1]. Similarly, Pneumovax immunization has approximately 50%–60% efficacy in protecting normal C57BL/6 mice against lethal respiratory challenge with serotype 3 S. pneumoniae, strain WU2 [28]. We therefore assessed whether MTS-induced increases in PPS3-specific IgM and IgG influenced protection against respiratory infection with a lethal respiratory dose of WU2. Remarkably, immunization with MTS-adjuvanted Pneumovax yielded nearly 90% protection against lethal pneumococcal challenge (Figure 7). This level of protection was significantly higher than that observed for WT immunized with Pneumovax alone (50%). Mice that received MTS only were not protected (Supplementary Figure 4). Thus, MTS significantly increases the protective efficacy of Pneumovax against pneumococcal respiratory infection.
Figure 7.
Monophosphoryl lipid A (MPL) + trehalose-6,6’-dicorynomycolate (TDCM) + squalene oil (MTS) significantly augments the protective efficacy of Pneumovax against lethal respiratory pneumococcal infection. Wild-type mice were immunized with Pneumovax (~1 μg each pneumococcal polysaccharide [PPS]) either alone or mixed with MTS intramuscularly and boosted on day 30. On day 60, mice were challenged with a lethal intranasal dose (1 × 107) of WU2 (serotype 3 Streptococcus pneumoniae) and monitored for morbidity requiring humane euthanasia (results of 3 pooled experiments, with n = 25 mice immunized with Pneumovax; 17 mice immunized with Pneumovax + MTS; log-rank analysis P = .017).
DISCUSSION
Native polysaccharide vaccines have made a tremendous impact on global health. Nonetheless, polysaccharide vaccines elicit a limited amount of IgG, and boosting (ie, revaccinating) does not increase titers and, in some cases, reduces titers [2, 36]. This poses a problem, as it is not always possible to achieve optimal levels of protective serological titers with one vaccination. Interestingly, the conjugate vaccine enables boosting in children, but does so poorly in adults [8–10]. Thus, alternative strategies for improving native polysaccharide vaccines are needed. Our current study highlights an adjuvant that significantly increases protective serologic responses to polysaccharides, as well as significantly increases IgG+ memory B-cell formation and recall responses. To our knowledge this is the first study identifying an adjuvant that significantly increases IgG+ memory B-cell formation in response to TI-2 Ags and promotes boosting following secondary immunization. Our findings have implications for understanding the regulation of TI-2 memory B-cell formation and secondary responsiveness to Ags, both of which are essential to improving vaccine efficacy.
The lack of development of adjuvants suitable for polysaccharide vaccines may in part relate to the historical reliance on aluminum adjuvants for the use in human vaccines. Aluminum-based adjuvants do not enhance polysaccharide-specific Ab responses [18] and have a limited effect on responses to conjugate vaccines [10, 19, 37, 38]. Notably, clinical trials using recombinant IL-12, granulocyte-monocyte-colony stimulating factor, QS-21, indomethacin, Abatacept, and CpG have each failed to significantly promote IgG levels to native pneumococcal polysaccharides [38]. Pérez-Toledo et al have shown that primary Ab responses to Vi capsular polysaccharide in mice can be enhanced by purified Salmonella Typhi porin proteins [39]. Interestingly, other mouse studies have shown that TLR agonists alone only significantly increase primary Ab responses to PPS if they are administered several days following immunization [22, 23]. Although delayed adjuvant administration is not a practical vaccine strategy, these studies highlight the promise of using TLR agonists to provide Ps-activated B cells with an effective second stimulatory signal. That MPL delivered in conjunction with synthetic cord factor and squalene at the time of Ps immunization elicits significant adjuvant effects on Ps-specific humoral immunity, including memory formation, suggests the possibility that this combination may either prolong and/or intensify TLR-based costimulatory signaling [26, 40].
Our data reveal the adjuvant’s mechanism of action is largely dependent on B-cell–dependent MyD88 signaling. This is in contrast to findings for T-cell–dependent responses (TNP-KLH), whereby an MPL/TDCM-containing adjuvant functioned via a Myd88-independent pathway [41]. MTS adjuvant effects were dependent on TLR4 expression (Supplementary Figure 5); thus, it is likely that B cells must express this MyD88-signaling dependent receptor for the adjuvant to have efficacy in the context of polysaccharide-specific Ab responses. Nonetheless, additional pathways could be involved [42]. Transmembrane Activator and CAML Interactor (TACI) directly interacts with MyD88 after B-cell activating factor ligation to activate NF-κB, and coengagement of TACI and TLR promotes class-switch recombination [42]. It remains possible that IL-1R/IL-18R–dependent signaling contributes to the MyD88 dependency of this response, as IL-1 is elicited by Mincle signaling [34] and is known to support TI-2 Ab production [43]. Importantly, this particular adjuvant combination may not be ideal for humans given the low TLR4 expression by human B cells [44]. Future studies will identify the role(s) of Mincle and MCL and address the suitability of this or similar adjuvants for use in humans.
TLR and BCR signaling activates NF-kβ through distinct pathways and thus MyD88-dependent signaling may synergize with TI-2 Ag-induced BCR cross-linking to support proliferation, isotype switching, survival, and differentiation to both memory B cells and long-lived ASCs [45], especially in B-1 and MZ B cells [46], which typically contribute to TI-2 responses. Of note, it is presently unclear how the MTS adjuvant influences distinct B-cell subsets. Although previous work indicated a TLR3 agonist (poly:IC) promoted follicular B-cell responses to haptenated Ficoll via a type I interferon (IFN)–dependent mechanism [47], IFNAR–/– mice respond optimally to MTS adjuvant (unpublished observations), suggesting a distinct mechanism is involved. MyD88-dependent signals may also impact migration of B-1 and MZ B cells [48]. It is interesting that MTS induced striking increases in IgG+ memory B cells and IgG-secreting cells within the lymph nodes following intramuscular immunization. As this is not a typical site of innate-like B-cell localization or TI-2 Ab secretion, it suggests MTS either promotes altered trafficking and localization of Ag-activated MZ/B1b B cells and/or participation of follicular B cells in the TI-2 Ab response.
Our study reveals that pathogen associated molecular patterns (PAMPS) have the capacity to significantly increase the formation of IgG+ TI-2 memory B cells and support functional boosting with polysaccharides. In addition to promoting IgG boosting to PPS, MTS supported boosting of IgG responses to haptenated Ficoll when low-dose priming was used. The enhanced generation of functional IgG+ memory B cells by MTS was also evidenced by increased IgG production by the MTS-elicited memory B-cell pool upon Ag restimulation in naive recipients. Given these findings, it is evident that despite increased memory B-cell generation, there is still suppression that must be overcome in the memory environment to effectively boost TI-2 Ab responses. Notably, Ag-specific Ab-mediated inhibition of recall responses (to haptenated-Ficoll) is supported by early work [49]. A more recent study demonstrated IgM responses to NP-Ficoll boosted in AID–/– mice (IgG deficient), except when a high dose of NP-specific IgG1 was given during boosting [11], supporting that Ag-specific IgG1 suppresses IgM memory responses to haptenated Ficoll. The mechanism by which IgG elicits suppression to TI-2 Ags is unknown but is suggested to be independent of the inhibitory FcγIIb receptor [11] and Ag masking [11, 50].
That MTS supported boosting of PPS responses more effectively than haptenated Ficoll may be due to the increased level of IgG elicited by haptenated Ficoll relative to the level of IgM produced or other differences that exist between these Ags that we do not yet understand. Future work is required to determine this. In conclusion, in our murine model, MTS demonstrates promise as a polysaccharide-specific vaccine adjuvant through mechanisms involving increased and direct (MyD88-dependent) B-cell activation, increased IgG+ memory B-cell formation, and significant boosting by either relieving suppression and/or promoting activation of capsular Ps-specific memory B cells. Future work will assess the suitability of this and similar adjuvant combinations to boost functional polysaccharide-specific B-cell memory responses in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number R01AI18876). Additional support was provided by the National Cancer Institute (support grant number P30CA012197) issued to the Wake Forest Baptist Comprehensive Cancer Center.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ortqvist A. Pneumococcal vaccination: current and future issues. Eur Respir J 2001; 18:184–95. [DOI] [PubMed] [Google Scholar]
- 2. González-Fernández A, Faro J, Fernández C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine 2008; 26:292–300. [DOI] [PubMed] [Google Scholar]
- 3. Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines 2011; 10:307–22. [DOI] [PubMed] [Google Scholar]
- 4. Väkeväinen M, Soininen A, Lucero M, et al. . ARIVAC Consortium Serotype-specific hyporesponsiveness to pneumococcal conjugate vaccine in infants carrying pneumococcus at the time of vaccination. J Pediatr 2010; 157:778–83.e1. [DOI] [PubMed] [Google Scholar]
- 5. Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 2010; 201:1570–9. [DOI] [PubMed] [Google Scholar]
- 6. Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations?Nat Rev Drug Discov 2010; 9:308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldblatt D, Southern J, Andrews N, et al. . The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis 2009; 49:1318–25. [DOI] [PubMed] [Google Scholar]
- 8. Greenberg RN, Gurtman A, Frenck RW, et al. . Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60–64 years of age. Vaccine 2014; 32:2364–74. [DOI] [PubMed] [Google Scholar]
- 9. Musher DM, Rodriguez-Barradas MB. Why the recent ACIP recommendations regarding conjugate pneumococcal vaccine in adults may be irrelevant. Hum Vaccin Immunother 2016; 12:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juergens C, de Villiers PJ, Moodley K, et al. . Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine formulations with and without aluminum phosphate and comparison of the formulation of choice with 23-valent pneumococcal polysaccharide vaccine in elderly adults: a randomized open-label trial. Hum Vaccin Immunother 2014; 10:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med 2006; 203:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol 2009; 183:6359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 2004; 21:379–90. [DOI] [PubMed] [Google Scholar]
- 14. Snapper CM. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann N Y Acad Sci 2012; 1253:92–101. [DOI] [PubMed] [Google Scholar]
- 15. Han SH, Kim JH, Martin M, Michalek SM, Nahm MH. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect Immun 2003; 71:5541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schröder NW, Morath S, Alexander C, et al. . Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 2003; 278:15587–94. [DOI] [PubMed] [Google Scholar]
- 17. Arjunaraja S, Massari P, Wetzler LM, Lees A, Colino J, Snapper CM. The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain. J Immunol 2012; 188:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flebbe LM, Braley-Mullen H. Immunopotentiating effects of the adjuvants SGP and Quil A. I. Antibody responses to T-dependent and T-independent antigens. Cell Immunol 1986; 99:119–27. [DOI] [PubMed] [Google Scholar]
- 19. Wuorimaa T, Dagan R, Eskola J, et al. . Tolerability and immunogenicity of an eleven-valent pneumococcal conjugate vaccine in healthy toddlers. Pediatr Infect Dis J 2001; 20:272–7. [DOI] [PubMed] [Google Scholar]
- 20. Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol 2011; 187:5183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Souvannavong V, Lemaire C, Chaby R. Lipopolysaccharide protects primary B lymphocytes from apoptosis by preventing mitochondrial dysfunction and bax translocation to mitochondria. Infect Immun 2004; 72:3260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taillardet M, Haffar G, Mondière P, et al. . Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity in response to a plain polysaccharidic vaccine. J Infect Dis 2010; 202:470–9. [DOI] [PubMed] [Google Scholar]
- 23. Baker PJ, Hiernaux JR, Fauntleroy MB, Prescott B, Cantrell JL, Rudbach JA. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun 1988; 56:1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garg M, Subbarao B. Immune responses of systemic and mucosal lymphoid organs to Pnu-Imune vaccine as a function of age and the efficacy of monophosphoryl lipid A as an adjuvant. Infect Immun 1992; 60:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker PJ, Fauntleroy MB, Stashak PW, Hiernaux JR, Cantrell JL, Rudbach JA. Adjuvant effects of trehalose dimycolate on the antibody response to type III pneumococcal polysaccharide. Infect Immun 1989; 57:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 2009; 9:465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKay JT, Egan RP, Yammani RD, et al. . PD-1 suppresses protective immunity to Streptococcus pneumoniae through a B cell-intrinsic mechanism. J Immunol 2015; 194:2289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haas KM, Blevins MW, High KP, Pang B, Swords WE, Yammani RD. Aging promotes B-1b cell responses to native, but not protein-conjugated, pneumococcal polysaccharides: implications for vaccine protection in older adults. J Infect Dis 2014; 209:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKay JT, Haro MA, Daly CA, et al. . PD-L2 regulates B-1 cell antibody production against phosphorylcholine through an IL-5-dependent mechanism. J Immunol 2017; 199:2020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrews NJ, Waight PA, Burbidge P, et al. . Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014; 14:839–46. [DOI] [PubMed] [Google Scholar]
- 31. Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J 2012; 31:297–301. [DOI] [PubMed] [Google Scholar]
- 32. Haas KM, Hasegawa M, Steeber DA, et al. . Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity 2002; 17:713–23. [DOI] [PubMed] [Google Scholar]
- 33. Chilton PM, Embry CA, Mitchell TC. Effects of differences in lipid A structure on TLR4 pro-inflammatory signaling and inflammasome activation. Front Immunol 2012; 3:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desel C, Werninghaus K, Ritter M, et al. . The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One 2013; 8:e53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seubert A, Calabro S, Santini L, et al. . Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A 2011; 108:11169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis 2012; 205:422–30. [DOI] [PubMed] [Google Scholar]
- 37. Wuorimaa T, Dagan R, Väkeväinen M, et al. . Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J Infect Dis 2001; 184:1211–5. [DOI] [PubMed] [Google Scholar]
- 38. Søgaard OS. The clinical use of adjuvants in pneumococcal vaccination: current status and future perspectives. Hum Vaccin 2011; 7:276–80. [DOI] [PubMed] [Google Scholar]
- 39. Pérez-Toledo M, Valero-Pacheco N, Pastelin-Palacios R, et al. . Salmonella Typhi porins OmpC and OmpF are potent adjuvants for T-dependent and T-independent antigens. Front Immunol 2017; 8:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dennehy KM, Ferwerda G, Faro-Trindade I, et al. . Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol 2008; 38:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gavin AL, Hoebe K, Duong B, et al. . Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 2006; 314:1936–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He B, Santamaria R, Xu W, et al. . The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol 2010; 11:836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pike BL, Nossal GJ. Interleukin 1 can act as a B-cell growth and differentiation factor. Proc Natl Acad Sci U S A 1985; 82:8153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bekeredjian-Ding I, Jego G. Toll-like receptors—sentries in the B-cell response. Immunology 2009; 128:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pone EJ, Zhang J, Mai T, et al. . BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway. Nat Commun 2012; 3:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol 2007; 178:7779–86. [DOI] [PubMed] [Google Scholar]
- 47. Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med 2010; 207:1485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol 2012; 12:282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brodeur PH, Wortis HH. Regulation of thymus-independent responses: unresponsiveness to a second challenge of TNP-Ficoll is mediated by hapten-specific antibodies. J Immunol 1980; 125:1499–505. [PubMed] [Google Scholar]
- 50. Le Moal MA, Colle JH, Truffa-Bachi P. Study on B-memory generation by Tnp-Ficoll: induction but not expression is observed among various inbred mouse strains. Cell Immunol 1984; 87:110–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.