Abstract
Background
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia due to either insulin deficiency or resistance or both. Hyperglycemia induces tissue damage through mitochondrial superoxide production, affecting retina, glomerulus, and neurons. It requires continuing medical care and ongoing self-care management to prevent and delay acute and long-term complications. Therefore, our study was designed to assess glycemic control and diabetes complications among diabetes patients attending at University of Gondar Hospital.
Materials and methods
A cross-sectional study was conducted among DM patients attending at University of Gondar Hospital diabetes follow-up clinic during February–March 2017. Five milliliters of blood was collected using aseptic technique. Levels of fasting blood sugar (FBS), triglycerides, and cholesterol were measured using MINDRAY BS-200E machine. FBS ≥152 mg/dL was taken as poor glycemic control. Binary and multivariable logistic regression models were used to evaluate associated risk factors for the outcome variable. A P-value of <0.05 was considered as statistically significant.
Result
Three hundred sixty-seven diabetes patients were included in this study. About 222 (60.5%) of them had poor glycemic control (FBS ≥152 mg/dL). The proportion of poor glycemic control was slightly higher among type 1 DM patients (61.4%) than type 2 DM patients (59.8%). Age ≥65 years (adjusted odds ratio [AOR]: 0.070; 95% CI: 0.016–0.308), being divorced (AOR: 0.226; 95% CI: 0.064–0.8000), and increased waist circumference (AOR: 0.361: 95% CI: 0.181–0.720) were factors that significantly reduce poor glycemic control. Diabetes complications were slightly higher in insulin- and tablet-only users, 72.5% and 64.5%, respectively. DM complications were also higher in patients who had poor glycemic control (61/222) and type 2 diabetes (78 [37.3%]).
Conclusion
Prevalence of poor glycemic control and DM complications was high, which indicate that appropriate intervention is required to improve glycemic control and prevent or control complications among DM patients.
Keywords: diabetes mellitus, glycemic control, diabetes complications, Ethiopia
Introduction
Diabetes mellitus (DM) is a group of metabolic disorders characterized by hyperglycemia due to failure in secretion, action, or both of insulin.1 Chronic noncommunicable diseases are becoming considerable 21st century global epidemic and have already become the leading causes of death and disability worldwide.2 A total of 415 million adults were estimated to live with diabetes in 2015 and the number is expected to reach 640 million by the year 2040 worldwide. In seven International Diabetes Federation regions, the highest regional unadjusted prevalence was seen in the North America and Caribbean (11.5%), and Africa has the lowest (3.8%) prevalence. However, Africa is projected to have the largest proportional increase by 2040, with 147% increment. In Ethiopia, the prevalence of diabetes is expected to be 5.1% in 2035 from 4.4% in 2013.3
Chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of different organs.1
Based on the pathophysiology, DM complications are classified as microvascular (small blood vessels damage) and macrovascular (arterial damage) complications.1,4 Thus, diabetes is the leading cause of blindness, end-stage renal disease, and stroke, which are common among diabetic patients.5
Hyperglycemia is a biochemical parameter seen in all types of diabetes patients, which induces tissue damage through mitochondrial superoxide production.4,6 Capillary endothelial cells in the retina, mesangial cells in the renal glomerulus, and neurons and Schwann cells in peripheral nerves are cells commonly damaged due to hyperglycemia. These cells are particularly at high risk for damage because they are unable to regulate uptake of glucose during hyperglycemia.7,8 Diabetic polyneuropathy develops in the case of prolonged hyperglycemia, which is associated with metabolic imbalances, accumulation of advanced glycation end products, oxidative stress, and lipid alterations.9
Diabetes management targets on maintenance of optimum glucose level and prevention and early diagnosis of complications.10 According to the American Diabetes Association (ADA) Standards of Medical Care in Diabetes 2017, fasting blood sugar (FBS) measurement can be used for glycemic control assessment and individuals having FBS ≥152 mg/dL are said to have poor glycemic control.11
Chronic diabetes complications like retinopathy, nephropathy, neuropathy, self-reported chest pain, vision decrement, painful paresthesias, and psychiatric events result in decrement of health-related quality of life.12 Appropriate glycemic control and management is fundamental to prevent and delay DM complications. Poor glycemic control is highly correlated with high burden of diabetes complications. However, data are scanty in Ethiopia, particularly in Gondar, regarding factors for poor glycemic control and the relationship between glycemic control and DM complications. Hence, this study will be used as a baseline data for further researches regarding factors associated with poor glycemic control and DM complications among diabetes patients. Therefore, the aim of this study was to assess glycemic control and DM complications among DM patients attending at University of Gondar Hospital, Northwest Ethiopia.
Materials and methods
Study area
The study was conducted at University of Gondar Hospital, which is a teaching and referral hospital located 738 km away from Addis Ababa in northwest Ethiopia. The city is a well-known tourist site with its collection of Royal castles and very ancient churches. The hospital gives a referral service with >400 beds and serving for >5 million people in northwest Ethiopia. The hospital has chronic illness follow-up clinic providing service for >8,000 diabetes patients.
Study design and subjects
Institution-based cross-sectional study was conducted from February 1 to March 30, 2017 to assess glycemic control and diabetes complications among diabetes patients attending at University of Gondar Hospital. All DM patients who visited chronic illness follow-up clinic during the study period were included until the required sample size was attained. Severely ill patients were excluded because they were unable to answer questions.
Sample size and sampling technique
The required sample size was calculated via Open Epi software using single population proportion formula by considering the following assumptions: prevalence of poor glycemic control in Gondar, Ethiopia (P=64.7%),13 95% confidence level, and 5% margin of error. Therefore, the total calculated sample size was 351. DM patients who had at least 12 months follow-up in the hospital during the study period were included in this study.
Data collection and laboratory analysis
Sociodemographic data were collected by trained nurses working in the diabetes clinic of University of Gondar Hospital using a pretested semi-structured questionnaire. All study subjects were approached during their respective appointment schedule for follow-up. After interview and detailed review of their medical records, the study subjects were sent to laboratory where blood was collected for determination of FBS, total cholesterol (TC), and triglycerides (TG). Five milliliters of blood was collected using anticoagulant-free and clot-activated test tube for biochemical parameters determination. Those biochemical parameters were determined using MINDRAY BS-200E (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) using its MINDRAY reagents. The manufacturer instructions were followed for each parameter.14 Glucose, TG, and TC were determined using glucose oxidase, glycerokinase peroxidase, and cholesterol oxidase peroxidase methods, respectively. Glycemic control level was categorized as poor if FBS was >152 mg/dL, which is comparable with 7% HbA1C according to ADA Standards of Medical Care in Diabetes.11
Anthropometric measurements
All measurements were taken using standardized techniques and calibrated equipment. Weight was measured by balance with light indoor clothing and bare foot. Height was measured using a stadiometer. Body mass index (BMI) was calculated as weight/height.2 It was classified as underweight (BMI <18.5), normal (18.5≤ BMI ≤25.0), overweight (25.0≤ BMI ≤30.0), and obese (BMI ≥30).
Waist circumference (WC) was measured by placing a plastic tape to the nearest 0.5 cm horizontally, midway between the 12th rib and the iliac crest on the mid axillary line. WHO recommended three health risk categories: low risk (men, WC ≤93.9 cm; women, WC ≤79.9 cm); increased risk (men, WC =94.0–101.9 cm; women, WC =80.0–87.9 cm); and high risk (men, WC ≥102.0 cm; women, WC ≥88.0 cm or more) for DM patients.15 Blood pressure (BP) was measured using calibrated automated sphygmomanometer. Cutoff point for BP was taken as >135 mmHg for systolic blood pressure and >85 mmHg for diastolic blood pressure.
Data analysis and interpretation
Data were entered into Epi Info™ version 7 software and then exported to Microsoft Excel 2013 to check its completeness and cleanness. Finally, the data were exported to SPSS version 20 software for analysis. Frequency distributions of sociodemographic, clinical, and behavioral characteristics of study subjects were explored. Continuous variables were expressed as mean ± SD and categorical variables were expressed as percentage. Binary and multivariable logistic regression models were fitted to evaluate associated risk factors for the outcome variable. All variables with a P-value of ≤0.2 were entered into a multivariable model to control the possible effect of confounders. A P-value of <0.05 was considered as statistically significant.
Ethical consideration
Ethical clearance was obtained from School of Biomedical and Laboratory Sciences Research and Ethics committee and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the study participants before the commencement of data collection. There was no financial compensation or provision for the study participants. To ensure confidentiality of data, the study participants were identified using codes, and unauthorized persons had no access to the collected data. Furthermore, all findings were utilized for proper management of the patients.
Results
Sociodemographic characteristics
A total of 367 individuals participated in this study. The majority of the T2DM patients were within 45–64 years old (59.8%) having a mean age of 48.6±16.6. Among the T2DM patients, majority were females (124 [59.3%]). Majority of the study participants were urban residents (269 [73.3%]), married (213 [58.0%]), orthodox Christians (325 [88.6%]), and unable to read and write (157 [42.8%]) (Table 1).
Table 1.
Sociodemographic characteristics of DM patients attending at University of Gondar Hospital, northwest Ethiopia, 2017 (N=367)
| Variables | N (%) | Type 1 DM N (%) | Type 2 DM N (%) | |
|---|---|---|---|---|
|
| ||||
| Age in years | ≤24 | 37 (10.1) | 37 (23.4) | 0 (0.0) |
| 25–44 | 100 (27.2) | 71 (44.9) | 29 (13.9) | |
| 45–64 | 163 (44.4) | 38 (24.1) | 125 (59.8) | |
| ≥65 | 67 (18.3) | 12 (7.6) | 55 (26.3) | |
| Sex | Male | 173 (47.1) | 88 (55.7) | 85 (40.7) |
| Female | 194 (52.9) | 70 (44.3) | 124 (59.3) | |
| Residence | Urban | 269 (73.3) | 83 (52.5) | 186 (89.0) |
| Rural | 98 (26.7) | 75 (47.5) | 23 (11.0) | |
| Marital status | Married | 213 (58.0) | 82 (51.9) | 131 (62.7) |
| Single | 48 (13.1) | 44 (27.8) | 4 (1.9) | |
| Divorced | 52 (14.2) | 22 (13.9) | 30 (14.4) | |
| Widowed | 53 (14.4) | 10 (6.3) | 43 (20.6) | |
| Separated | 1 (0.3) | 0 (0.0) | 1 (0.5) | |
| Religion | Orthodox | 325 (88.6) | 145 (91.8) | 180 (86.1) |
| Muslim | 38 (10.4) | 12 (7.6) | 26 (12.4) | |
| Protestant | 2 (0.5) | 1 (0.6) | 1 (0.5) | |
| Others | 2 (0.5) | 0 (0.0) | 2 (1.0) | |
| Occupational status | Unemployed | 127 (34.6) | 57 (36.1) | 70 (33.5) |
| Self-employed | 177 (48.2) | 87 (55.1) | 90 (43.1) | |
| Government employed | 63 (17.2) | 14 (8.9) | 49 (23.4) | |
| Educational status | Unable to read and write | 157 (42.8) | 66 (41.8) | 91 (43.5) |
| Primary school | 87 (23.7) | 48 (30.4) | 39 (18.7) | |
| Secondary school | 55 (15.0) | 25 (15.8) | 30 (14.4) | |
| Certificate | 3 (0.8) | 2 (1.3) | 1 (0.5) | |
| Diploma | 42 (11.4) | 12 (7.6) | 30 (14.4) | |
| Degree and above | 23 (6.3) | 5 (3.2) | 18 (8.6) | |
Abbreviation: DM, diabetes mellitus.
Prevalence of poor glycemic control
The mean FBS level of study participants was 174.25±57.14 mg/dL. The overall prevalence of poor glycemic control was 60.5% (222/367) (95% CI: 55.6–65.7). The proportion of poor glycemic control was 61.4% among type 1 and 59.8% among type 2 DM patients. Poor glycemic control was predominant among 45–64 years age group (99 [60.7%]), in females (116 [59.8%]), in urban dwellers (157 [58.4%]), in self-employed individuals (101 [57.1%]), and among participants who cannot read and write (99 [63.1%]) (Table 2).
Table 2.
Prevalence of poor glycemic control among DM patients attending at University of Gondar Hospital, northwest Ethiopia, 2017 (N=367)
| Variables | Good glycemic control N (%) | Poor glycemic control N (%) | |
|---|---|---|---|
|
| |||
| Age in years | ≤24 | 7 (18.9) | 30 (81.1) |
| 25–44 | 37 (37.0) | 63 (63.0) | |
| 45–64 | 64 (39.3) | 99 (60.7) | |
| ≥65 | 37 (55.2) | 30 (44.8) | |
| Sex | Male | 67 (38.7) | 106 (61.3) |
| Female | 78 (40.2) | 116 (59.8) | |
| Residence | Urban | 112 (41.6) | 157 (58.4) |
| Rural | 33 (33.7) | 65 (66.3) | |
| Marital status | Married | 84 (39.4) | 129 (60.6) |
| Single | 17 (35.4) | 31 (64.6) | |
| Divorced | 19 (36.5) | 33 (63.5) | |
| Widowed | 24 (45.3) | 29 (54.7) | |
| Separated | 1 (100.0) | 0 (0.0) | |
| Occupational status | Unemployed | 44 (34.6) | 83 (65.4) |
| Self-employed | 76 (42.9) | 101 (57.1) | |
| Government employed | 25 (39.7) | 38 (60.3) | |
| Educational status | Unable to read and write | 58 (36.9) | 99 (63.1) |
| Primary school | 38 (43.7) | 49 (56.3) | |
| Secondary school | 15 (27.3) | 40 (72.7) | |
| Certificate | 3 (100.0) | 0 (0.0) | |
| Diploma | 22 (52.4) | 20 (47.6) | |
| Degree and above | 9 (39.1) | 14 (60.9) | |
| Type of DM | Type 1 | 61 (38.6) | 97 (61.4) |
| Type 2 | 84 (40.2) | 125 (59.8) | |
Abbreviation: DM, diabetes mellitus.
Clinical and anthropometric measurements
Higher prevalence of poor glycemic control was reported among study participants with DM duration <7 years (152 [62.8%]), non-glucometer users (188 [60.6%]), and insulin-(113 [62.1%]) and tablet-only users (90 [58.1%]) (Table 3).
Table 3.
Clinical and anthropometric measurements of DM patients attending at University of Gondar Hospital, northwest Ethiopia, 2017 (N=367)
| Variables | Glycemic control
|
Diabetes complications
|
|||
|---|---|---|---|---|---|
| Good N (%) | Poor N (%) | Yes | No | ||
|
| |||||
| Family history of DM | Yes | 24 (39.3) | 37 (60.7) | 24 (39.3) | 37 (60.7) |
| No | 121 (39.5) | 185 (60.5) | 91 (29.7) | 215 (70.3) | |
| DM duration, years | ≥7 | 55 (44.0) | 70 (56.0) | 49 (39.2) | 76 (60.8) |
| <7 | 90 (37.2) | 152 (62.8) | 66 (27.3) | 176 (72.7) | |
| Glucometer usage | Yes | 23 (40.4) | 34 (59.6) | 15 (26.3) | 42 (73.7) |
| No | 122 (39.4) | 188 (60.6) | 100 (32.3) | 210 (67.7) | |
| BMI | Underweight | 7 (30.4) | 16 (69.6) | 4 (17.4) | 19 (82.6) |
| Normal | 75 (36.1) | 133 (63.9) | 58 (27.9) | 150 (72.1) | |
| Overweight | 44 (48.9) | 46 (51.1) | 35 (38.9) | 55 (61.1) | |
| Obese | 19 (41.3) | 27 (58.7) | 18 (39.1) | 28 (60.9) | |
| Systolic BP | ≤135 | 109 (38.2) | 176 (61.8) | 82 (28.8) | 203 (71.2) |
| >135 | 36 (43.9) | 46 (56.1) | 33 (40.2) | 49 (59.8) | |
| Diastolic BP | ≤85 | 129 (40.3) | 191 (59.7) | 95 (29.7) | 225 (70.3) |
| >85 | 16 (34.0) | 31 (66.0) | 20 (42.6) | 27 (57.4) | |
| Drug regimen | Not started yet | 1 (33.3) | 2 (66.7) | 0 (0.0) | 3 (100.0) |
| Insulin | 69 (37.9) | 113 (62.1) | 50 (27.5) | 132 (72.5) | |
| Insulin and tablets | 10 (37.0) | 17 (63.0) | 10 (37.0) | 17 (63.0) | |
| Tablets only | 65 (41.9) | 90 (58.1) | 55 (35.5) | 100 (64.5) | |
| Waist circumference | Low risk | 51 (30.5) | 116 (69.5) | 43 (25.7) | 124 (74.3) |
| Intermediate risk | 35 (55.6) | 28 (44.4) | 18 (28.6) | 45 (71.4) | |
| High risk | 59 (43.1) | 78 (56.9) | 54 (39.7) | 83 (60.6) | |
| TC level | ≤150 mg/dL | 95 (40.6) | 139 (59.4) | 72 (30.8) | 162 (69.2) |
| >150 mg/dL | 50 (37.6) | 83 (62.4) | 43 (32.3) | 90 (67.7) | |
| TG level | ≤200 mg/dL | 120 (39.2) | 186 (60.8) | 96 (31.4) | 210 (68.6) |
| >200 mg/dL | 25 (41.0) | 36 (59.0) | 19 (31.1) | 42 (68.9) | |
Abbreviations: BMI, body mass index, BP, blood pressure, DM, diabetes mellitus, TC, total cholesterol; TG, triglyceride.
Factors associated with poor glycemic control
In multivariable logistic regression model, age ≥65 years (adjusted odds ratio [AOR]: 0.070; 95% CI: 0.016–0.308), divorce (AOR: 0.226; 95% CI: 0.064–0.800), and increased WC (AOR: 0.361; 95% CI: 0.181–0.720) were factors significantly associated with poor glycemic control (Table 4).
Table 4.
Multivariable logistic regression analysis of factors associated with poor glycemic control among DM patients attending at University of Gondar Hospital, northwest Ethiopia, 2017 (N=367)
| Variables | N | Poor glycemic control N (%)
|
COR | AOR | P-value | ||
|---|---|---|---|---|---|---|---|
| Yes N (%) | No N (%) | ||||||
|
| |||||||
| Age, years | ≤24 | 37 | 30 (81.1) | 7 (18.9) | 1.00 | 1.00 | |
| 25–44 | 100 | 63 (63.0) | 37 (37.0) | 0.397 (0.159–0.994) | 0.151 (0.041–0.555) | 0.004 | |
| 45–64 | 163 | 99 (60.7) | 64 (39.3) | 0.361 (0.150–0.871) | 0.127 (0.031–0.514) | 0.004 | |
| ≥65 | 67 | 30 (44.8) | 37 (55.2) | 0.189 (0.073–0.491) | 0.070 (0.016–0.308) | 0.000* | |
| Occupational status | Unemployed | 127 | 83 (65.4) | 44 (34.6) | 1.241 (0.665–2.314) | 1.100 (0.547–2.213) | 0.790 |
| Self-employed | 177 | 101 (57.1) | 76 (42.9) | 0.874 (0.487–1.571) | 0.734 (0.381–1.412) | 0.354 | |
| Government employed | 63 | 38 (60.3) | 25 (39.7) | 1.00 | 1.00 | ||
| Marital status | Married | 213 | 129 (60.6) | 84 (39.4) | 1.00 | 1.00 | |
| Single | 48 | 31 (64.6) | 17 (35.4) | 1.187 (0.619–2.280) | 0.966 (0.486–1.924) | 0.923 | |
| Divorced | 52 | 33 (63.5) | 19 (36.5) | 1.131 (0.604–2.119) | 0.226 (0.064–0.800) | 0.021* | |
| Widowed | 54 | 29 (53.7) | 25 (46.3) | 0.755 (0.414–1.378) | 1.091 (0.465–2.559) | 0.841 | |
| BMI | Underweight | 23 | 16 (69.6) | 7 (30.4) | 1.00 | 1.00 | |
| Normal | 208 | 133 (63.9) | 75 (36.1) | 0.776 (0.305–1.971) | 0.810 (0.293–2.241) | 0.685 | |
| Overweight | 90 | 46 (51.1) | 44 (48.9) | 0.457 (0.172–1.218) | 0.526 (0.161–1.715) | 0.287 | |
| Obese | 46 | 27 (58.7) | 19 (41.3) | 0.622 (0.214–1.803) | 0.804 (0.223–2.893) | 0.738 | |
| Triglycerides | ≤150 mg/dL | 234 | 139 (59.4) | 95 (40.6) | 1.00 | 1.00 | |
| >150 mg/dL | 133 | 83 (62.4) | 50 (37.6) | 1.135 (0.733–1.757) | 1.442 (0.796–2.614) | 0.227 | |
| Cholesterol | ≤200 mg/dL | 306 | 186 (60.8) | 120 (39.2) | 1.00 | 1.00 | |
| >200 mg/dL | 61 | 36 (59.0) | 25 (41.0) | 0.929 (0.531–1.626) | 0.932 (0.477–1.819) | 0.836 | |
| Diastolic BP | ≤85 | 320 | 191 (59.7) | 129 (40.3) | 1.00 | 1.00 | |
| >85 | 47 | 31 (66.0) | 16 (34.0) | 1.309 (0.688–2.490) | 1.785 (0.808–3.947) | 0.152 | |
| Systolic BP | ≤135 | 285 | 176 (61.8) | 109 (38.2) | 1.00 | 1.00 | |
| >135 | 82 | 46 (56.1) | 36 (43.9) | 0.791 (0.481–1.301) | 0.812 (0.419–1.575) | 0.538 | |
| DM duration years | ≤7 | 242 | 152 (62.8) | 90 (37.2) | 1.00 | 1.00 | |
| >7 | 125 | 70 (56.0) | 55 (44.0) | 0.754 (0.486–1.169) | 0.854 (0.528–1.381) | 0.519 | |
| Waist circumference | Low risk | 167 | 116 (69.5) | 51 (30.5) | 1.00 | 1.00 | |
| Increased risk | 63 | 28 (44.4) | 35 (55.6) | 0.352 (0.194–0.638) | 0.361 (0.181–0.720) | 0.004* | |
| High risk | 137 | 78 (56.9) | 59 (43.1) | 0.581 (0.363–0.932) | 0.726 (0.355–1.485) | 0.380 | |
| DM complications | Yes | 115 | 61 (53.0) | 54 (47.0) | 1.00 | 1.00 | |
| No | 252 | 161 (63.9) | 91 (36.1) | 0638 (0.408–0.999) | 0.716 (0.432–1.186) | 0.195 | |
Note:
Statistically significant association.
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; BP, blood pressure; COR, crude odds ratio; DM, diabetes mellitus; N, number.
Diabetes complications
Diabetes complications were found in 115 (31.33%) of study participants. The prevalence of DM complications was higher in DM patients with >7 years DM duration (39.2%), overweight (38.9%), high-risk WC (39.7%), and TG level ≤150 mg/dL (30.8%) (Table 2).
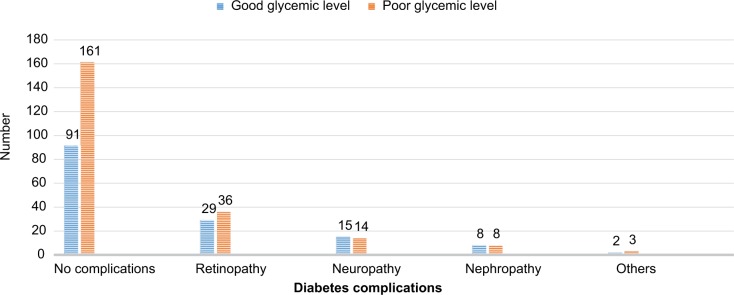
DM complications by their type account as follows: retinopathy (65 [17.7%]), neuropathy (29 [7.9%]), nephropathy (16 [4.4%]), and others (5 [1.4%]). The prevalence of diabetes complications was higher in diabetic patients having poor glycemic control (61/222) than those with good glycemic control (Figure 1).
Figure 1.
Diabetes complications based on glycemic level of diabetic mellitus patients attending at University of Hospital, northwest Ethiopia, 2017.
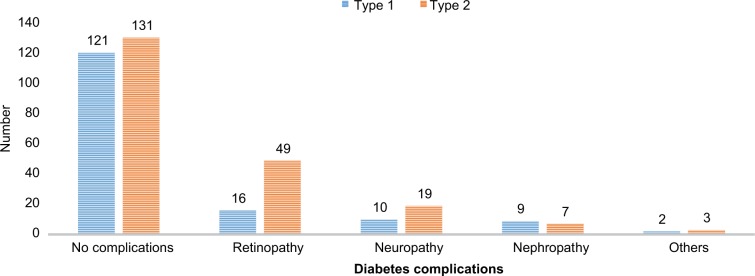
Diabetes complications were higher among T2DM patients (78 [37.32%]) than T1DM study participants (37 [23.42%]) (Figure 2).
Figure 2.
Diabetes complications based on the type of DM among DM patients attending at University of Gondar Hospital, northwest Ethiopia, 2017.
Abbreviation: DM, diabetes mellitus.
Discussion
The main target in the management of DM is to maintain good glycemic control, which is very important for controlling diabetes and preventing and delaying diabetes complications.16 Glucose measurement is the main tool for assessing glycemic control. In this study, glucose level >152 mg/dL was taken as poor glycemic control level according to ADA Standards of Medical Care in Diabetes 2017.11
In this study, the mean FBS level was 174.25±57.14 mg/dL. Similar study from Jimma, Ethiopia found an average FBS of 163±45 mg/dL.17 The overall prevalence of poor glycemic control was 60.5% (95% CI: 55.6–65.7) in the current study. This finding is similar to the previous studies conducted in Gondar (64.7%), Kenya (60.5%), and Jordan (65.1%).18–20
Prevalence of poor glycemic control (60.5%) is lower compared to the studies conducted in Jimma, Ethiopia (81.7%), Venezuela (76%), and Hawaii (68.5%).17,21,22 This variation would have happened due to the difference in the method of glucose measurement, cutoff points, socioeconomic status, culture, genetics, environmental factors, urbanization, and lifestyle, which predispose individuals to different risk factors of poor glycemic control among the study participants.
The prevalence of poor glycemic control was slightly higher among type 1 DM patients (61.4%) than among type 2 DM patients (59.8%). This is similar to the studies done in Gondar and Venezuela.13,21 This may be related to the fact that type 1 DM patients are commonly treated by insulin or combination therapy in more severe cases that require more aggressive treatment to control their disease, while type 2 DM patients with milder disease are more easily controlled by diet or oral hypoglycemic agents.
The result of our study revealed that age ≥65 years, divorced marital status, and increased WC have significant negative association with poor glycemic control. Possible reason of poorer glycemic control in younger populations compared to elders can be associated with the fact that the young people may not adhere to their treatments as elders.
In the current study, poor glycemic control was predominant among 25- to -year-old study subjects with 63% prevalence (AOR: 0.151; 95% CI: 0.041–0.555), which is consistent with a similar study in Gondar,18 which showed higher prevalence among younger DM patients. Diabetic patients aged ≥65 years are less likely to have poor glycemic control (AOR: 0.070; 95% CI: 0.016–0.308) compared to other age groups similar to another study done here in Gondar13 and in USA, which indicated that elderly people had better diabetes control.22 Exposure to stressful conditions associated with puberty may aid to the poor glycemic control through stimulation of the autonomic nervous system to induce hyperglycemia.23 Several studies have demonstrated that insulin sensitivity decreases early in puberty, which returns to normal once somatic growth and sexual maturation are completed.24 However, the result contradicts to a study in Kenya,19 in which patients aged >40 years were at risk for poor glycemic control (COR: 1.08; 95% CI: 0.60–1.95). Variation in age group cut point may be the possible reason for the discrepancy.
Study participants who were single showed higher (64.6%; AOR: 0.966; 95% CI: 0.486–1.924) poor glycemic control compared to other groups. Divorced DM patients were less likely to have poor glycemic control (AOR: 0.226; 95% CI: 0.064–0.800) compared to married DM patients. Poor glycemic control was also higher in unemployed individuals (65.4%; AOR: 1.100; 95% CI: 0.547–2.213) compared to employed DM patients. Unemployed DM patients may not be economically good to buy DM medications. Work type had no significant association according to a study in Kenya.19
In our study, individuals having high-risk WC are less likely to have poor glycemic control (AOR: 0.361; 95% CI: 0.181–0.720) compared to individuals having low-risk WC (≤93.9 cm for males and ≤79.9 cm for females). Most of the low-risk WC participants were type 1 DM patients treated mostly with insulin; therefore, if they have strict adherence to insulin, it may increase body weight and WC.25
Even if it was not significant, ≤7 years diabetes duration was protective for poor glycemic level (AOR: 0.854; 95% CI: 0.528–1.381) compared to ≥7 years diabetes duration. Besides, studies in Venezuela, Jordan, and Hawaii20–22 showed that poor glycemic control was more likely associated with long duration of the disease. The association between poor glycemic control and long diabetes duration may be due to progressive impairment of insulin secretion through time because of β cell failure and the difficulty for the patients to continue monitoring the blood glucose level and adjust with the regimen of treatment, diet, and exercise.26
Diabetic complications were high in diabetic patients having poor glycemic control (61 [53.0%]) than their counterparts (54 [47.0%]). A study in Arbaminch, Ethiopia27 also found higher level of glucose (177±35.45 mg/dL) among DM patients with foot ulcer. Similarly, diabetic retinopathy was predominant among poor glycemic control level DM patients according to a case–control study in Brazil.28 The relationship between the level of glucose and diabetic retinopathy was also showed by a follow-up study in USA, that is, regulating glucose level using intensive treatment resulted in delayed slow progression of diabetic retinopathy.29 Increased level of HbA1c also showed significant association with urinary tract infection in women with type 1 DM.30 The main limitations of our study were the use of FBS instead of HbA1c to determine the glycemic status, unable to measure the role of inflammatory cytokines on DM complications, and the cross-sectional nature of the study, which does not show cause–effect relationship of the independent variables to the outcome variable.
Conclusion
The prevalence of poor glycemic control and DM complications is considerably high among diabetes patients. Poor glycemic control showed significant and negative association with study participants aged ≥65 years old, increased WC, and divorced diabetes patients. DM complications are found to be higher among patients with poor glycemic control and type 2 DM.
Data sharing statement
The data sets used and analyzed during the current study are available from the corresponding author on a reasonable request.
Acknowledgments
Our deepest gratitude goes to all study participants, and nurses working at University of Gondar Hospital Chronic Illness Clinic. We would also like to extend our appreciation to University of Gondar, School of Biomedical and Laboratory Sciences, Department of Clinical Chemistry for their multidimensional support. No funding is received from any funding organization for this study.
Footnotes
Author contributions
AF designed and implemented the study, collected data, undertook statistical analysis, performed data interpretation, and drafted the manuscript. AF, MA and BB participated in data analysis and data interpretation and reviewed the manuscript. All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Gizaw M, Harries AD, Ade S, et al. Diabetes mellitus in Addis Ababa, Ethiopia: admissions, complications and outcomes in a large referral hospital. Public Health Action. 2015;5(1):74–78. doi: 10.5588/pha.14.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 5.Feleke Y, Enquselassie F. An assessment of the health care system for diabetes in Addis Ababa, Ethiopia. Ethiop J Health Dev. 2005;19(3):203–210. [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Heilig C, Concepcion L, Riser B, Freytag S, Zhu M, Cortes P. Over-expression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest. 1995;96(4):1802. doi: 10.1172/JCI118226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser N, Sasson S, Feener EP, et al. Toronto Diabetic Neuropathy Expert Group Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42(1):80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 9.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Standards of medical care in diabetes-2017: summary of revisions. Diabetes Care. 2017;40(Suppl 1):S4–S5. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME, DCCT/EDIC Research Group The long-term effects of type 1 diabetes treatment and complications on health-related quality of life. Diabetes Care. 2013;36(10):3131–3138. doi: 10.2337/dc12-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abebe SM, Berhane Y, Worku A, Alemu S, Mesfin N. Level of sustained glycemic control and associated factors among patients with diabetes mellitus in Ethiopia: a hospital-based cross-sectional study. Diabetes Metab Syndr Obes. 2015;8:65. doi: 10.2147/DMSO.S75467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biochemistry Handbook for Mindray. [Accessed January 10, 2017]. BS-200E-Operation-Manual-V4-0-En.pdf. Available from: https://www.scribd.com/document/341537236/BS-200E-Operation-Manual-V4-0-En-pdf.
- 15.Patry-Parisien J, Shields M, Bryan S. Comparison of waist circumference using the World Health Organization and National Institutes of Health protocols. Health Rep. 2012;23(3):53–60. [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 17.Angamo MT, Melese BH, Ayen WY. Determinants of glycemic control among insulin treated diabetic patients in Southwest Ethiopia: hospital based cross sectional study. PLoS One. 2013;8(4):e61759. doi: 10.1371/journal.pone.0061759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abebe SM, Berhane Y, Worku A, Alemu S, Mesfin N. Level of sustained glycemic control and associated factors among patients with diabetes mellitus in Ethiopia: a hospital-based cross-sectional study. Diabetes Metab Syndr Obes. 2015;8:65–71. doi: 10.2147/DMSO.S75467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otieno CF, Kariuki M, Ng’ang’a L. Quality of glycaemic control in ambulatory diabetics at the out-patient clinic of Kenyatta National Hospital, Nairobi. East Afr Med J. 2003;80(8):406–410. doi: 10.4314/eamj.v80i8.8731. [DOI] [PubMed] [Google Scholar]
- 20.Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24(2):84–89. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Moreira ED, Neves RC, Nunes ZO, et al. Venezuelan Diabetes Investigators’ Group Glycemic control and its correlates in patients with diabetes in Venezuela: results from a nationwide survey. Diabetes Res Clin Pract. 2010;87(3):407–414. doi: 10.1016/j.diabres.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Juarez DT, Sentell T, Tokumaru S, et al. Factors associated with poor glycemic control or wide glycemic variability among diabetes patients in Hawaii, 2006-2009. Prev Chronic Dis. 2012;9:120065. doi: 10.5888/pcd9.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moussa MA, Alsaeid M, Abdella N, Refai TM, Al-Sheikh N, Gomez JE. Social and psychological characteristics of Kuwaiti children and adolescents with type 1 diabetes. Soc Sci Med. 2005;60(8):1835–1844. doi: 10.1016/j.socscimed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 25.Castaneda C, Janssen I, Castaneda C, Janssen I. Ethnic comparisons of sarcopenia and obesity in diabetes. Ethn Dis. 2005;15(4):664–670. [PubMed] [Google Scholar]
- 26.Mohammad HA, Farghaly HS, Metwalley KA, Monazea EM, Abd El-Hafeez HA. Predictors of glycemic control in children with Type 1 diabetes mellitus in Assiut-Egypt. Indian J Endocrinol Metab. 2012;16(5):796. doi: 10.4103/2230-8210.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deribe B, Woldemichael K, Nemera G. Prevalence and factors influencing diabetic foot ulcer among diabetic patients attending Arbaminch Hospital, South Ethiopia. J Diabetes Metab. 2014;05(01):1–7. [Google Scholar]
- 28.Lima VC, Cavalieri GC, Lima MC, Nazario NO, Lima GC. Risk factors for diabetic retinopathy: a case-control study. Int J Retina Vitreous. 2016;2(1):21. doi: 10.1186/s40942-016-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiello LP, DCCT/EDIC Research Group Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):17–23. doi: 10.2337/dc13-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenherr SM, Clemens JQ, Braffett BH, et al. DCCT/EDIC Research Group Glycemic control and urinary tract infections in women with type 1 diabetes: results from the DCCT/EDIC. J Urol. 2016;196(4):1129–1135. doi: 10.1016/j.juro.2016.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]