Abstract
Background
Immunotherapies and targeted therapies have drastically improved survival in metastatic melanoma, but they can cause a range of adverse events (AEs). Understanding the costs of these events would facilitate an accurate comparison of melanoma treatments.
Objective
To compare the costs and frequency of AEs associated with immunotherapy and with targeted therapy in elderly patients with metastatic melanoma.
Methods
We conducted a retrospective cohort study using Medicare claims data from 2011 to 2014. Patients included had to have ≥1 diagnoses of metastatic melanoma and ≥1 claims for an immunotherapy or targeted therapy. We compared the 30-day expenditures of patients with and without each AE using a generalized linear model to determine the incremental cost per AE in patients who received immunotherapy or targeted therapy. The baseline demographic and clinical differences were adjusted for using propensity score with inverse probability of treatment. We also compared the mean costs of AEs associated with immunotherapy and targeted therapy.
Results
A total of 844 patients were included in the study (mean age, 75 years; standard deviation, 14 years). The mean baseline Charlson Comorbidity Index score was 8.4, and 65% of the patients were male. The mean cost for AEs was highest for respiratory events (ie, $24,150). Gastrointestinal, respiratory, and hematologic AEs were more common in patients who received immunotherapy, whereas general and administration-site AEs and other AEs (eg, fatigue, infections, muscular weakness) were more frequent in patients who received targeted therapy. AE-related costs with immunotherapy were highest for gastrointestinal, respiratory, and pain-related AEs; AEs with targeted therapy were highest for cardiovascular and general and administration-site events.
Conclusion
These findings suggest that incremental costs associated with treatment-related AEs among elderly patients with metastatic melanoma were substantial, but the risks for and costs of the various types of AEs differed by therapy. Understanding the risks for and costs of AEs associated with the various therapeutic options can inform treatment decision-making in elderly patients with metastatic melanoma.
Keywords: adverse events, economic burden, elderly patients, immunotherapy, metastatic melanoma, targeted therapy, treatment-related costs
Melanoma is the fifth most common malignancy among men and the sixth most common malignancy among women in the United States.1 The American Cancer Society estimated that in 2018, 91,270 new cases of invasive melanoma will be diagnosed in the United States, and the disease will result in approximately 9320 deaths.2 Approximately 4% of melanoma cases are diagnosed at an advanced stage, when the cancer has already metastasized.3
The prognosis for patients with metastatic melanoma has historically been poor, with a median overall survival of only 6 to 10 months and a 5-year survival rate of only 5% to 10% before 2011.4 However, recent advances have completely changed the treatment landscape for metastatic melanoma. The 2 most important novel therapeutic strategies for metastatic melanoma are immune checkpoint blockade and selective kinase inhibition.
Immunotherapies and targeted therapies are associated with improved disease control and significantly increased survival times in metastatic melanoma, which has dramatically improved the outlook for this patient population.5
KEY POINTS
-
▸
Immunotherapies and targeted therapies improve survival in metastatic melanoma but are associated with adverse events (AEs).
-
▸
This retrospective study compared the frequency and costs of AEs associated with immunotherapy versus targeted therapy in elderly patients with metastatic melanoma.
-
▸
The highest costs were for respiratory AEs ($24,150), CNS/psychiatric disorders ($21,932), metabolic/nutritional disorders ($19,776), and skin/subcutaneous tissue disorders ($19,183).
-
▸
The costs of immunotherapy-related AEs were highest for gastrointestinal, respiratory, and pain; for targeted therapy these included cardiovascular and general and administration-site AEs.
-
▸
Gastrointestinal, respiratory, and hematologic AEs were more common with immunotherapy; general and administration-site AEs, as well as other AEs (eg, fatigue, infections, muscular weakness), were more common with targeted therapy.
-
▸
Understanding the risks for and costs associated with the treatment-related AEs can help to inform treatment decision-making in elderly patients with metastatic melanoma.
For the first-line treatment of metastatic melanoma, the National Comprehensive Cancer Network guidelines now recommend targeted therapy with combination BRAF/MEK inhibition (for patients with BRAF mutations), or immunotherapy with anti–PD-1 monotherapy or combined anti–PD-1/CTLA-4 antibodies (regardless of BRAF status).6
Despite their efficacy, these novel therapies are associated with adverse events (AEs) and the potential for considerable toxicity. Immunotherapy is associated with several severe, mostly immune-related AEs, involving the gastrointestinal (GI), liver, skin, endocrine, nervous, ocular, and other organ systems.7–12 BRAF inhibitors are associated with increased rates of cutaneous AEs, including squamous-cell carcinoma and keratoacanthoma, as well as with pyrexia, rash, arthralgia, and fatigue; MEK inhibitors have been associated with hypertension, ocular toxicities, diarrhea, fatigue, and rash.9,13–15 With combined BRAF/MEK inhibition, the rate of cutaneous AEs is attenuated, but this combination's AEs profile is similar to that seen in clinical trials with the single agents.9,16–18
Studies have shown that the management of treatment-related AEs in patients with metastatic melanoma is associated with substantial healthcare resource utilization and high costs.19–23 The National Cancer Institute estimates that the direct cost of melanoma care in the United States was $2.36 billion in 2010.24
As new therapies for metastatic melanoma enter the marketplace, it is important to fully understand the healthcare expenditures associated with the use of these agents. Previous studies have assessed the costs of AEs associated with the treatment of metastatic melanoma, but the majority of those studies have included patients who used older forms of treatment, such as chemotherapy. To our knowledge, no study to date has specifically compared the AE-related healthcare expenditures associated with immunotherapy and targeted therapy. The objective of this study was to compare the real-world costs and frequency of treatment-related AEs among elderly patients with metastatic melanoma who received immunotherapy or targeted therapy.
Methods
Melanoma is most common among people aged 65 to 74 years, with a median age of 64 years at diagnosis and a median age of 70 years at death from the disease.3 The Medicare database includes data on 97% of the US population aged ≥65 years and contains near-complete information on healthcare utilization.25 Thus, we believed that the Medicare database was the most appropriate source for this retrospective cohort study.
We used Medicare enrollment information linked to claims data, which were generated from 100% research-identifiable files, to identify the study population, using 100% of the outpatient Medicare data. The study period was from January 1, 2011, to December 31, 2014. The start date reflects the US Food and Drug Administration (FDA) approval of the first targeted therapy for melanoma (ie, vemurafenib) on August 17, 2011.26 The end date reflects the fact that the annual Medicare files are available in February every year, with a 14-month lag. The data for this study were requested in late 2016, and were thus the most up-to-date data available at the time of the analysis. The enrollment files included demographic characteristics, as well as monthly indicators of participation in Medicare Parts A, B, and D. Claims for Medicare Parts A, B, and D included detailed information on service dates, medical diagnoses, and services provided based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes, Healthcare Common Procedure Diagnosis Coding System codes, and/or National Drug Codes.
Patient Selection
All patients with at least 1 diagnosis of malignant melanoma (ICD-9-CM 172.0–9) during the study period, a diagnosis of metastasis (ICD-9-CM 196.xx, 197.xx, 198.xx, 199.xx) within 30 days before or 60 days after receiving a malignant melanoma diagnosis, and at least 1 pharmacy or medical claim for an immunotherapy or targeted therapy that was approved by the FDA during the study period, were eligible for inclusion. The approved drugs at that time were the immunotherapies ipilimumab and pembrolizumab, and the targeted therapies dabrafenib, trametinib, vemurafenib, and the combination of dabrafenib and trametinib; thus, these were the drugs included in our study.
Since 2014, the immunotherapies nivolumab and nivolumab plus ipilimumab and the targeted therapy combinations vemurafenib plus cobimetinib and encorafenib plus binimetinib have also been approved by the FDA for the treatment of patients with metastatic melanoma. These drugs were not included in the study, because they were approved outside of the study period.
We based the study design on the 2015 study by Arondekar and colleagues, whose study of the economic burden of AEs in metastatic melanoma was the first of its kind in this patient population.20 The index diagnosis date was defined as the date of the first diagnosis of malignant melanoma accompanied by metastasis. The index date was defined as the date of the first prescription for a study drug (ie, the index treatment). The 6-month period before the index date was defined as the baseline period. The observation (ie, follow-up) period began on the index date and continued to the end of continuous enrollment in the database, the end of the study period (2011–2014), or death, and thus varied between patients.
Eligible patients had to be continuously enrolled in the database during the 6 months before the index date to ensure that the index diagnosis date was the date of the patient's first diagnosis of metastatic melanoma and that the index date was the date of their first study drug prescription. Patients also had to be continuously enrolled for 3 months after the index date to ensure that they had adequate follow-up data for analysis.
A diagnosis of nonmelanoma primary malignancy (ICD-9-CM 140.xx-165.xx, 170.xx-171.xx, 173.xx-195.xx, 200.xx-208.xx) during the 6 months before the index date, pregnancy (ICD-9-CM 630.xx-679.xx, V22.xx-V24.xx, V27.xx-V28.xx) at any point during the study period, and the presence of more than 1 index drug (ie, >1 study drug prescribed on the index date, not including dabrafenib plus trametinib combination therapy), were the exclusion criteria.
Outcomes and Measures
The primary study outcomes were the costs (inpatient and outpatient) of the treatment-related AEs that the patients had during the observation period. We performed a comprehensive literature search in late 2016 to determine the treatment-related AEs that were known to be associated with the study drugs. These AEs were grouped as follows:
Cardiovascular (CV): secondary hypertension, hypertension complications, hypotension, tachycardia (including supraventricular)
Central nervous system (CNS) and psychiatric: anxiety or depression, confusion, convulsions, hemiparesis, somnolence, encephalopathy
GI: abdominal pain, colitis, constipation, diarrhea, mucositis and stomatitis, nausea or vomiting
Hematologic and lymphatic: anemia, leukopenia, lymphopenia, neutropenia, pulmonary embolism, thrombocytopenia
Metabolic and nutritional: acute renal failure; abnormal renal or liver function test; bilirubinemia; elevation of transaminase, lactate dehydrogenase, phosphatase, amylase, or lipase; hyponatremia; hypophysitis; peripheral edema
Pain: headache; myalgia, arthralgia, musculoskeletal, back, or other pain; peripheral neuropathy
Skin and subcutaneous tissue: alopecia, diaphoresis (sweating), hyperkeratosis, benign neoplasms of the skin (including papilloma), photosensitivity reaction, pruritus (itching), rash, squamous-cell carcinoma, keratoacanthoma
Respiratory: dyspnea, pneumonitis
General disorder and administration-site conditions: fever (pyrexia) and/or chills
Other: anaphylaxis, anuria or oliguria, asthenia or fatigue, decreased appetite or anorexia, infections (including folliculitis), decreased ejection fraction, muscular weakness, retinal detachment.
The AEs were identified by a primary or secondary diagnosis on any nondiagnostic inpatient or outpatient claim within the observation period. In the first part of the analysis, the total incremental costs of the 10 categories of AEs were assessed in the whole study population by comparing pairs of cohorts, including an AE cohort, with patients who had the treatment-related AE in question, and a control cohort, with patients who received the same treatment and did not have that AE during follow-up. The incremental AE-related costs were calculated as the average difference in 30-day healthcare expenditures between patients with and without the AE.
The date of the first specific AE claim served as the beginning of the 30-day cost period. A shadow AE date was assigned for patients without the specific AE by randomly sampling from the distribution of the number of days from the index date to the event for patients with the AE, and then adding that number of days to the control's index date. This ensured that the controls received the study drug for an amount of time comparable with that of the patients who had the AE. The costs associated with AEs included the total adjudicated amount paid to all providers for inpatient and outpatient services and drugs, with the exception of the study drugs and other cancer therapies. This amount included payments made by the insurer, patient (deductible, copayment, coinsurance), and any coordination of benefits, as indicated on the claim. All costs were inflation-adjusted to 2017 US dollars.
In the second part of the analysis, the AE-related costs were compared between 2 cohorts: the immunotherapy cohort, which included patients whose index drug was ipilimumab or pembrolizumab, and the targeted therapy cohort, which included patients whose index drug was dabrafenib, trametinib, vemurafenib, or the combination therapy of dabrafenib plus trametinib.
Statistical Methods
The baseline demographic (eg, age, sex) and clinical (eg, Charlson Comorbidity Index [CCI] score, comorbidities) characteristics were analyzed descriptively and compared for the immunotherapy and targeted therapy cohorts. For the binary and categorical variables, the between-group differences were assessed using χ2 tests. For the continuous variables, the between-group differences were assessed using the Wilcoxon rank-sum test. Descriptive analysis also included the assessment of unadjusted AE costs, as well as comparative analysis between the immunotherapy and targeted therapy cohorts. The Kruskal-Wallis test was used to compare the AE-related costs between the 2 cohorts. A P value <5% was considered statistically significant throughout the analyses.
The adjusted incremental cost of each category of AE was computed by using multivariate regressions to estimate the costs during the 30 days after the AE. To address the skewness of the cost data and the large number of $0 costs, we applied Blough and Ramsey's formulation of a 2-part cost model to the data.27 Logistic regression was first estimated to examine the determinants and to predict the probability of any healthcare expenditures during the 30 days after the AE. We modeled the costs for patients with positive (more than $0) healthcare expenditures using a generalized linear model with a log link and gamma distribution of variance to account for the skewed distribution of costs. The propensity score method with inverse probability of treatment weighting was used to adjust for the patients' baseline characteristics.
The predicted costs were estimated by using the generalized linear model coefficients for the AE and control cohorts, and recycled predictions were adopted. In this way, the regression model was used to calculate a predicted 30-day cost for every patient that was based on the covariate values that assumed that the patient had an AE (ie, the case) and again assuming that the patient did not (ie, the control). We estimated incremental cost by calculating the difference at the patient level, followed by averaging the incremental cost across patients. Again, this aspect of the study design was based on the 2015 study by Arondekar and colleagues.20
Results
A total of 844 patients met all the eligibility criteria, of whom 316 (37.4%) received targeted therapies and 528 (62.6%) received immunotherapies (Table 1); the Appendix Table (at www.AHDBonline.com) depicts the sample selection steps, and the Appendix Figure illustrates the distribution of treatments received by the patients. Overall, 64.8% of patients were male, 94.9% were white, and the average age at cohort entry was approximately 75 years. The baseline demographic characteristics were well-balanced between the groups, although a greater proportion of targeted therapy patients were aged >85 years.
Table 1.
Patients' Baseline Demographic and Clinical Characteristics
| Patient demographics | Overall patients (N = 844) | Patients receiving immunotherapy (N = 528) | Patients receiving targeted therapy (N = 316) | P valuea |
|---|---|---|---|---|
| Age, yrs, mean (SD) | 74.72 (13.7) | 74.69 (13.5) | 74.78 (14.0) | .051 |
| Age-group, yrs, N (%) | <.01 | |||
| 65–74 | 441 (52.3) | 286 (54.2) | 155 (49.1) | |
| 75–84 | 285 (33.8) | 186 (35.2) | 99 (31.3) | |
| 85+ | 118 (14.0) | 56 (10.6) | 62 (19.6) | |
| Male, N (%) | 547 (64.8) | 352 (66.7) | 195 (61.7) | .041 |
| Race, N (%) | .991 | |||
| White | 801 (94.9) | 500 (94.7) | 301 (95.3) | |
| Asian | 8 (0.9) | 4 (0.8) | 4 (1.3) | |
| Black | 21 (2.5) | 16 (3.0) | 5 (1.6) | |
| Hispanic | 6 (0.7) | 3 (0.6) | 3 (0.9) | |
| Other | 8 (0.9) | 5 (0.9) | 3 (0.9) | |
| Year of index treatment, N (%) | .025 | |||
| 2011 | 85 (10.1) | 28 (5.3) | 57 (18.0) | |
| 2012 | 275 (32.6) | 193 (36.6) | 82 (25.9) | |
| 2013 | 203 (24.1) | 132 (25.0) | 71 (22.5) | |
| 2014 | 281 (33.3) | 175 (33.1) | 106 (33.5) | |
| Charlson Comorbidity Index, mean (SD) | 8.39 (2.22) | 8.39 (2.23) | 8.40 (2.20) | .58 |
| Baseline cancer therapy, N (%) | ||||
| Excision surgery | 448 (53.1) | 275 (52.1) | 173 (54.7) | .24 |
| Chemotherapy or biologic therapy | 398 (47.2) | 243 (46.0) | 155 (49.1) | .02 |
| Interferon alpha | 28 (3.3) | 13 (2.5) | 15 (4.7) | .04 |
| Baseline hospitalization, N (%) | 253 (30.0) | 151 (28.6) | 102 (32.3) | .01 |
| Baseline emergency department visit, N (%) | 257 (30.5) | 153 (29.0) | 104 (32.9) | .01 |
| Baseline comorbidities, N (%) | ||||
| Anxiety | 81 (9.6) | 63 (11.9) | 18 (5.7) | <.01 |
| Cardiovascular disease | 396 (46.9) | 254 (48.1) | 142 (44.9) | .42 |
| Cerebrovascular disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Chronic obstructive pulmonary disease | 62 (7.3) | 39 (7.4) | 23 (7.3) | .75 |
| Diabetes | 126 (14.9) | 83 (15.7) | 43 (13.6) | <.01 |
| Depression | 51 (6.0) | 35 (6.6) | 16 (5.1) | <.01 |
For binary and categorical variables, the between-group differences were assessed using χ2 tests. For continuous variables, the between-group differences were assessed using the Wilcoxon rank-sum test. P <5% was considered statistically significant.
SD indicates standard deviation.
The mean CCI score was high, at approximately 8.4 in both groups. Compared with the patients who received immunotherapy, the patients who received targeted therapy were more likely to have been hospitalized (approximately 29% vs 32%, respectively; P <.05) or have visited the emergency department during the baseline period (approximately 29% vs 33%, respectively; P <.01). On the other hand, the targeted therapy group had a lower incidence of all comorbidities compared with the immunotherapy group, which suggests that patients who receive targeted therapy may have had better overall health at baseline. Interestingly, the patients who received immunotherapy were significantly more likely to have anxiety at baseline than those who received targeted therapy. Because of the nature of claims data, the reasons for this are unclear, but all baseline covariates were controlled for in the analyses. The detailed baseline characteristics are listed in Table 1.
The unadjusted costs in patients with and without AEs among the overall study population are shown in Table 2. As can be expected, the mean cost was higher for patients with any type of AEs, ranging from $17,570 to $30,534, compared with $5962 to $7840 in patients without the AEs. A multivariate analysis showed that the adjusted incremental costs were significantly higher for patients with all categories of AEs compared with patients without the AE (Table 3).
Table 2.
Unadjusted Costs, by Adverse Event Category
| Adverse event categorya | Patients with adverse event | Patients without adverse event | ||
|---|---|---|---|---|
| Patients, N | Mean cost, $ | Patients, N | Mean cost, $ | |
| Respiratory | 295 | 30,534 | 549 | 7438 |
| General disorder/administration-site condition | 199 | 27,790 | 645 | 6705 |
| CNS/psychiatric | 261 | 27,137 | 583 | 6159 |
| Pain | 383 | 23,108 | 461 | 7157 |
| Metabolic/nutritional | 377 | 21,984 | 467 | 5962 |
| Skin/subcutaneous tissue | 447 | 21,726 | 397 | 6374 |
| Cardiovascular | 510 | 21,469 | 334 | 6877 |
| Hematologic/lymphatic | 296 | 20,563 | 548 | 6712 |
| Gastrointestinal | 359 | 19,909 | 485 | 6130 |
| Other | 394 | 17,570 | 450 | 7840 |
Details of adverse events, by category, are listed in the text.
CNS indicates central nervous system.
Table 3.
Adjusted Incremental Costs, by Adverse Event Category
| Adverse event category | Adjusted mean costa | Incremental cost | 95% CI for adjusted incremental cost, $ | ||
|---|---|---|---|---|---|
| Patients with adverse events, $ | Patients without adverse events, $ | Observed, $ | Adjusted, $ | ||
| Respiratory | 31,927 | 7777 | 23,096 | 24,150 | 17,630–30,671 |
| CNS/psychiatric | 28,372 | 6440 | 20,977 | 21,932 | 16,011–27,854 |
| Metabolic/nutritional | 27,136 | 7360 | 16,022 | 19,776 | 14,239–25,314 |
| Skin/subcutaneous tissue | 27,147 | 7964 | 15,352 | 19,183 | 14,195–24,170 |
| General disorder/administration-site condition | 25,011 | 6035 | 21,084 | 18,976 | 13,663–24,289 |
| Pain | 26,666 | 8259 | 15,951 | 18,406 | 13,805–23,008 |
| Cardiovascular | 24,119 | 7726 | 14,592 | 16,393 | 12,131–20,655 |
| Hematologic/lymphatic | 23,531 | 7681 | 13,851 | 15,850 | 11,888–19,813 |
| Gastrointestinal | 19,794 | 6095 | 13,779 | 13,699 | 10,138–17,261 |
| Other | 17,613 | 7859 | 9730 | 9754 | 7315–12,192 |
Propensity score with inverse probability of treatment weighting was used to adjust for baseline demographic and clinical characteristics. The predicted costs were estimated by using the generalized linear model coefficients for the adverse event and control cohorts, adopting recycled predictions.
CI indicates confidence interval; CNS, central nervous system.
The adjusted 30-day incremental cost was highest for respiratory AEs, at $24,150 (95% confidence interval [CI], $17,630-$30,671), followed by CNS and psychiatric disorders ($21,932; 95% CI, $16,011-$27,854), metabolic and nutritional disorders ($19,776; 95% CI, $14,239-$25,314), and skin and subcutaneous tissue disorders ($19,183; 95% CI, $14,195-$24,170).
In the comparative analysis, the patients who received immunotherapy had a greater proportion of GI (N = 282:359; 79%), respiratory (N = 223:295; 76%), and hematologic and lymphatic AEs (N = 211:296; 71%), whereas patients who received targeted therapy had more of the general and administration-site AEs (N = 151:199; 76%) and other AEs (N = 260:394; 66%; Table 4).
Table 4.
Comparing 30-Day Costs in Patients with Adverse Events Receiving Immunotherapy or Targeted Therapy
| Adverse event category | Overall patients, N | Immunotherapy cohort | Targeted therapy cohort | P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, N | Patients with adverse event, % | Mean cost, $ | SD, $ | Patients, N | Patients with adverse event, % | Mean cost, $ | SD, $ | |||
| Cardiovascular | 510 | 295 | 58 | 18,023 | 27,145 | 215 | 42 | 26,208 | 32,468 | .05 |
| Skin/subcutaneous tissue | 447 | 249 | 56 | 21,535 | 32,648 | 198 | 44 | 21,967 | 33,464 | .23 |
| Other | 394 | 134 | 34 | 17,176 | 27,118 | 260 | 66 | 17,774 | 28,064 | <.01 |
| Pain | 383 | 197 | 52 | 24,152 | 34,404 | 186 | 48 | 21,997 | 33,501 | <.01 |
| Metabolic/nutritional | 377 | 181 | 48 | 22,034 | 32,690 | 196 | 52 | 21,938 | 33,404 | .05 |
| Gastrointestinal | 359 | 282 | 79 | 21,887 | 30,302 | 77 | 21 | 12,617 | 20,580 | <.01 |
| Hematologic/lymphatic | 296 | 211 | 71 | 21,041 | 32,881 | 85 | 29 | 19,366 | 28,974 | .32 |
| Respiratory | 295 | 223 | 76 | 31,179 | 38,703 | 72 | 24 | 28,525 | 35,650 | <.01 |
| CNS/psychiatric | 261 | 127 | 49 | 27,979 | 39,041 | 134 | 51 | 26,334 | 32,625 | .15 |
| General disorder/administration-site condition | 199 | 48 | 24 | 23,431 | 33,137 | 151 | 76 | 29,192 | 36,564 | .02 |
The Kruskal-Wallis test was used to assess the between-group differences in adverse event costs, with P value <5% considered statistically significant.
CNS indicates central nervous system; SD, standard deviation.
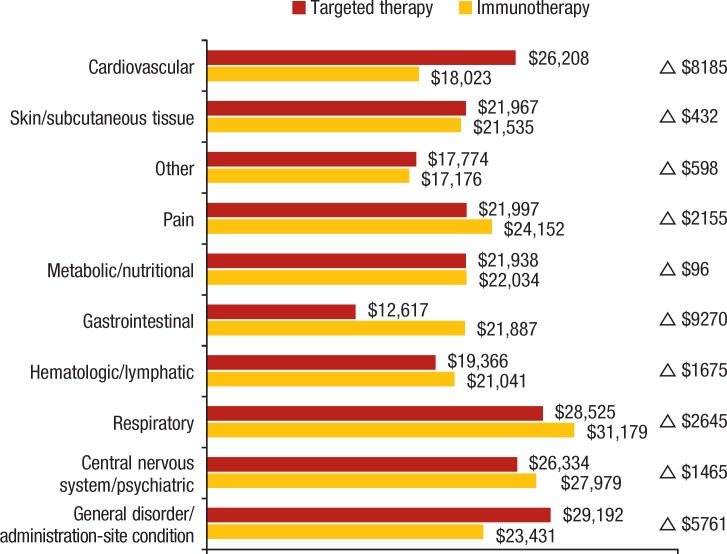
The targeted therapy cohort had higher costs than the immunotherapy cohort for CV ($26,208 vs $18,023, respectively; P <.05), general and administration-site ($29,192 vs $23,431, respectively; P <.02), and other AEs ($17,774 vs $17,176, respectively; P <.01; Figure). By contrast, patients who received targeted therapy had lower costs than patients who received immunotherapy for GI ($12,617 vs $21,887, respectively; P <.01), respiratory ($28,525 vs $31,179, respectively; P <.01), and pain-related AEs ($21,997 vs $24,152, respectively; P <.01).
Figure. Comparative Adverse Event Costs: Immunotherapy versus Targeted Therapy Cohorts.
Discussion
As can be expected, all categories of treatment-related AEs significantly increased the incremental healthcare costs in elderly patients with metastatic melanoma in the United States. However, the incidence and costs of the various types of AEs differed according to the class of therapy received.
To our knowledge, this is the first study to specifically compare the costs of AEs associated with immunotherapy and targeted therapy in metastatic melanoma. The comparative part of our analysis was descriptive; therefore, additional statistical methods may provide further insight into the cost impact of AEs of immunotherapy versus targeted therapy in future studies. Nonetheless, our data provide some novel observations that may inform evaluations of the efficacy, safety, and cost-effectiveness of the treatment options for patients with metastatic melanoma.
The high AE-related costs observed in this study are consistent with the results of previous studies, which have consistently shown that systemic therapies for metastatic melanoma are associated with treatment-related AEs that lead to increased healthcare resource utilization and expenditures.19–23 Although the majority of previous studies have examined older treatments, several more recent studies include patients who received some of the targeted therapies or immunotherapies in our study.
For example, Bilir and colleagues conducted a cost-estimation study from 2012 to 2013 of trametinib, dabrafenib, vemurafenib, and ipilimumab, alongside chemotherapies and interleukin-2, by obtaining AE rates and costs via a literature review and interviews with US melanoma specialists.19 Their study showed that cutaneous squamous-cell carcinoma, keratoacanthoma, rash, and elevated liver enzymes were the most frequent AEs seen with vemurafenib treatment; squamous-cell carcinoma and pyrexia were the most frequent AEs with dabrafenib treatment; and hypertension and rash were the most frequent with trametinib treatment. In contrast, the most common AEs with ipilimumab treatment were immune-related diarrhea or colitis, dyspnea, anemia, vomiting, and hypophysitis. The highest mean AE treatment costs in the outpatient setting were for neutropenia ($2092), headache ($609), and peripheral neuropathy ($539), whereas the highest inpatient AE-related costs were for acute myocardial infarction, sepsis, and coma ($31,682-$47,069).19
Our study expands on the existing literature by looking at more recent data and by including more patients who received novel therapies. As a result, our findings show higher costs for all types of AEs. Targeted and immunotherapy agents, although effective treatments for metastatic melanoma, are associated with a wide spectrum of AEs. To some extent, the incidence and costs of AEs in our study correlate. Patients who received immunotherapy were more likely to have GI, respiratory, and pain-related AEs, and also incurred higher costs for these AEs. Patients who received targeted therapy were more likely to have general and administration-site AEs (this included pyrexia, which is known to be associated with BRAF inhibitors) and other AEs (including infections, which are also known to be associated with these therapies); again, these patients incurred higher costs after having these AEs. However, substantial variation in frequency and costs of AEs was also evident.
The cost of an AE reflects the complexity and cost of its management. As expected, Bilir and colleagues' study showed that the costliest AEs were those that required hospitalization or expensive outpatient procedures.19 Because we grouped the AEs in our study into categories, it is likely that the between-treatment differences in cost could be at least in part explained by the differences in the specific AEs the patients had, and by the differences in the severity of those AEs.
For example, respiratory events were the most costly AEs in the adjusted analysis (Table 3). In the comparative analysis (Table 4), 76% of these AEs occurred in patients who received immunotherapy, and respiratory AEs were also significantly more expensive in these patients. This likely reflects the increased risk for dyspnea or pneumonitis with immunotherapy and the high cost of treating these respiratory AEs.19,28,29 Similarly, GI AEs were more common and substantially more expensive in the immunotherapy than the targeted therapy cohort (Table 4), which may reflect the known association between immunotherapy and a variety of grade 3 or 4 GI AEs.7,8
By contrast, CV AEs were more frequent in the immunotherapy cohort but more costly in the targeted therapy cohort (Table 4). Trametinib, dabrafenib, and the combination of trametinib plus dabrafenib have been associated with an increased rate of CV AEs.15,16 It is therefore possible that the CV AEs in our targeted therapy cohort were more severe or more likely to lead to hospitalization compared with those in the immunotherapy cohort.
Conversely, skin and subcutaneous tissue AEs were not more frequent in the targeted therapy group, or more costly, despite the known association of BRAF inhibitor therapy with increased rates of skin toxicity, including squamous-cell carcinoma,9,13 and the conclusion by Bilir and colleagues and others that squamous-cell carcinoma is a particularly costly AE.19,21,23
Immunotherapy is also associated with a variety of skin toxicities. Because the cost of this category of AE was high in both cohorts in our study, the similarity in cost may reflect similarly severe skin-related AEs with the 2 forms of therapy (ie, targeted therapy and immunotherapy).8,9
It is worth noting that the mean age in our study was high (ie, 75 years). The mean CCI score was high in both treatment cohorts (ie, 8.4), although the immunotherapy cohort had more comorbidities than the targeted therapy cohort at baseline. It is possible that despite the use of an advanced multivariate analysis to adjust the incremental costs in the overall population, the costs of the AEs we analyzed were influenced by the relative frailty of the population in our study.
Although our findings require confirmation in larger studies, they have some potential implications for clinicians who are considering the choice of systemic therapy for an individual patient. For example, based on the high rate and cost of respiratory complications with immunotherapy, clinicians may prefer to prescribe targeted therapy to patients who have a history of respiratory problems if they have BRAF-positive metastatic melanoma. Respiratory complications are extremely rare with BRAF inhibitors,28 although interstitial lung disease or pneumonitis occurred in 2.4% of patients who received trametinib monotherapy in the phase 3 trial.15 Similarly, in patients with a known history of CV disease, with uncorrectable electrolyte abnormalities, with a long QT syndrome, or who are receiving other medications that are known to prolong the QT interval, targeted therapy may not be the first choice because of the more costly (and thus potentially more severe) CV AEs associated with this type of therapy.28
Limitations
This study has several limitations. Because of the time lag on the availability of Medicare data, we were not able to include the newer immunotherapies of nivolumab the combination of and ipilimumab plus nivolumab, or the newer targeted therapy combinations of vemurafenib plus cobimetinib and encorafenib plus binimetinib. However, the AE profiles of these therapeutic approaches are not radically different from those included in our study,9–11,17,18,30–32 and some of the components of the newer approaches (eg, ipilimumab and vemurafenib) were included in our analysis as monotherapies.
Furthermore, the aim of our analysis was to compare immunotherapy and targeted therapy with regard to AEs as therapy classes rather than to analyze the AEs that patients had with each specific drug. Although we would have preferred to include the newer therapies, their exclusion does not invalidate this study.
The comparison of AE costs between the 2 therapeutic cohorts was conducted descriptively. Future studies are required to test our findings in larger groups of patients who receive novel therapies, and advanced statistical analyses, such as propensity score matching, should be considered to remediate baseline variation between the 2 cohorts.
We used ICD-9-CM codes to identify AEs, and these AEs may not directly correspond to those used in clinical trials.
In addition, claims data are dependent on physicians and hospitals accurately recording AEs. Measurement bias might have been introduced if AEs were undercoded or miscoded on administrative claims, and mild AEs might have been underreported. Therefore, the costs of AEs might have been underestimated in our study.
The way we calculated the costs of AEs is another limitation. Claims data do not allow for the calculation of specific AE episode costs, so we used a 30-day window as a proxy and compared the all-cause 30-day costs between patients with and without each AE in the adjusted analysis. It is possible that these costs do not correlate exactly with the actual costs that resulted from AEs.
In addition, although we imposed a time requirement to identify treatment-related AEs and included comorbidities in the multivariate analysis, the AEs that were included might not have resulted from the specific treatment but rather from chronic conditions or treatments received before the study period.
Moreover, some AEs, in particular immune-mediated AEs (eg, hypophysitis or hypothyroidism), have a prolonged impact on patients, and thus, again, the 30-day cost difference might have underestimated the true cost of the treatment-related AEs.
Conclusions
The incremental costs associated with treatment-related AEs among elderly patients with metastatic melanoma were substantial, but the risks for and costs of the various types of AEs differed by therapy. No randomized controlled trial has yet compared targeted therapy and immunotherapy directly; therefore, clinicians must use the best available data, their clinical judgment, and patient and disease characteristics when making treatment decisions.
Treatment options in the elderly may be limited by reduced toleration of medication side effects, comorbid medical conditions, and increased likelihood of drug interactions. Understanding the risks and costs of AEs associated with the various therapeutic options in this population can help clinicians to make informed choices in the treatment of elderly patients with metastatic melanoma.
Acknowledgment
The authors thank Clare Byrne, PhD, for her writing assistance.
Funding Source
This study was funded by Novartis Pharmaceuticals.
Author Disclosure Statement
Dr Ghate is an employee of Novartis; Mr Li is a consultant to Novartis; Mr Tang is a consultant to Novartis; Dr Nakasato is an employee of Novartis.
Contributor Information
Sameer R. Ghate, Director, Health Economics and Outcomes Research, Novartis Oncology, East Hanover, NJ.
Zhiyi Li, Partner, Asclepius Analytics, New York, NY.
Jackson Tang, Partner, Asclepius Analytics, New York, NY.
Antonio Reis Nakasato, Medical Director, Novartis Oncology.
References
- 1. American Cancer Society. Cancer Facts & Figures 2018. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed August 13, 2018.
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3. National Cancer Institute. SEER cancer stat facts: melanoma of the skin. http://seer.cancer.gov/statfacts/html/melan.html. Accessed August 13, 2018.
- 4. Leung AM, Hari DM, Morton DL. Surgery for distant melanoma metastasis. Cancer J. 2012;18:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies–update 2017. Eur J Cancer. 2017;83:247–257. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Melanoma. Version 3.2018. July 12, 2018. www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed August 13, 2018.
- 7. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. Erratum in: N Engl J Med. 2010;363:1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the Ipilimumab Network. PLoS One. 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–482. [DOI] [PubMed] [Google Scholar]
- 10. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. [DOI] [PubMed] [Google Scholar]
- 14. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flaherty KT, Robert C, Hersey P, et al; for the METRIC study group. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. [DOI] [PubMed] [Google Scholar]
- 16. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dréno B, Ribas A, Larkin J, et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann Oncol. 2017;28:1137–1144. [DOI] [PubMed] [Google Scholar]
- 18. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. [DOI] [PubMed] [Google Scholar]
- 19. Bilir SP, Ma Q, Zhao Z, et al. Economic burden of toxicities associated with treating metastatic melanoma in the United States. Am Health Drug Benefits. 2016;9(4):203–213. [PMC free article] [PubMed] [Google Scholar]
- 20. Arondekar B, Curkendall S, Monberg M, et al. Economic burden associated with adverse events in patients with metastatic melanoma. J Manag Care Spec Pharm. 2015;21:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vouk K, Benter U, Amonkar MM, et al. Cost and economic burden of adverse events associated with metastatic melanoma treatments in five countries. J Med Econ. 2016;19:900–912. [DOI] [PubMed] [Google Scholar]
- 22. Chang CL, Schabert VF, Munakata J, et al. Comparative healthcare costs in patients with metastatic melanoma in the USA. Melanoma Res. 2015;25:312–320. [DOI] [PubMed] [Google Scholar]
- 23. Wehler E, Zhao Z, Pinar Bilir S, et al. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ. 2017;18:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Cancer Institute. Cancer prevalence and cost of care projections: national costs for cancer care. www.costprojections.cancer.gov/expenditures.html. Accessed June 9, 2017.
- 25. Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV–3–IV-18. [DOI] [PubMed] [Google Scholar]
- 26. Drugs.com. FDA approves Zelboraf and companion diagnostic test for late-stage skin cancer. August 17, 2011. www.drugs.com/newdrugs/fda-approves-zelboraf-companion-diagnostic-test-late-stage-skin-cancer-2814.html. Accessed September 17, 2018.
- 27. Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcomes Res Methodol. 2000;1:185–202. [Google Scholar]
- 28. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis. 2016;10:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen P, Chen F, Zhou B. Therapeutic efficacy and safety of combined BRAF and MEK inhibition in patients with malignant melanoma: a meta-analysis. Onco Targets Ther. 2017;10:5391–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hao C, Tian J, Liu H, et al. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pasquali S, Chiarion-Sileni V, Rossi CR, Mocellin S. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: a network meta-analysis. Cancer Treat Rev. 2017;54:34–42. [DOI] [PubMed] [Google Scholar]