Abstract
Background
Computed tomography (CT) colonography's effectiveness, its associated patient advantages, and its potential role to increase colorectal cancer (CRC) screening rates have been demonstrated in previous research, but whether CT colonography has a cost advantage relative to optical colonoscopy for the commercially insured US population has not been assessed.
Objective
To compare the costs of CRC screening using CT colonography or optical colonoscopy for commercially insured people in the United States.
Methods
Using retrospective commercial healthcare claims data and peer-reviewed studies, we performed a simulated multiyear, matched-case comparison of the costs of CT and optical colonoscopies for CRC screening. We estimated commercial optical colonoscopy costs per screening based on the 2016 Truven Health MarketScan Commercial Database and ancillary services, such as bowel preparation, anesthesia, pathology, and complication costs. We developed 4 scenarios for CT colonography cost per screening using the ratio of commercial to Medicare fees, and calculated ancillary service and follow-up costs from payers' costs for these services when associated with optical colonoscopies. For comparison, we converted the costs per screening to the costs per screening year per person using real-world screening intervals that were obtained from peer-reviewed studies.
Results
In 2016, the average optical colonoscopy screening cost for commercial payers was $2033 (N = 406,068), or $340 per screening year per person. With our highest-cost CT colonography scenario, CT colonography costs 22% less, or $265 per screening year, than optical colonoscopy, mostly because of the advantages for patients of no anesthesia and the greatly reduced use of pathology services.
Conclusions
The use of CT colonography for CRC testing offers effective screening, patient-centered advantages, and lower costs compared with optical colonoscopy, and may be particularly appealing to the currently unscreened population with commercial health insurance. If the availability of CT colonography expands to meet the increased demand for it, CT colonography could cost up to 50% less than optical colonoscopy per screening year.
Keywords: colonoscopy, colorectal cancer, cost-effectiveness, CRC screening, CT colonography, optical colonoscopy
In the United States, colorectal cancer (CRC) is the fourth most common cancer diagnosed among men and women and the second leading cause of death from cancer.1 The majority of cases of CRC can be prevented by the detection and removal of noncancerous adenomatous polyps (adenomas).2 As in other types of cancer, survival is significantly better when CRC is diagnosed early, while the disease is still localized.2
Since 2002, the United States Preventive Services Task Force (USPSTF) has recommended routine CRC screening of asymptomatic average-risk adults aged 50 to 75 years.3 In 2018, the American Cancer Society (ACS) lowered the starting age for CRC screening to age 45 years for people with average risk.4 CRC screening has been a Healthcare Effectiveness Data and Information Set (HEDIS) quality-of-care measure since 2004 and is widely promoted as lifesaving.5
KEY POINTS
-
▸
Computed tomography (CT) colonography is effective, has patient-centered advantages, and can increase colorectal cancer (CRC) screening.
-
▸
This retrospective analysis compared the costs of CRC screening using CT colonography versus optical colonoscopy for patients with commercial insurance.
-
▸
Annual CT colonography screening is 22% to 55% less costly than optical colonoscopy because it does not use anesthesia and requires fewer pathology services.
-
▸
CT colonography for CRC testing offers more effective screening, more patient-centered advantages, and lower costs than optical colonoscopy.
-
▸
As awareness of and demand for CT colonography screening increases, fees will decline and more non–hospital outpatient sites will likely offer CT colonography.
-
▸
The use of CT colonography for CRC screening may appeal to currently unscreened individuals who have commercial health insurance.
-
▸
CT colonography could cost up to 50% less than optical colonoscopy per screening year if the availability of CT colonography increases to meet the growing demand.
Commercial payers (not Medicare or Medicaid) provide insurance to 75% of the approximately 61 million US adults aged 50 to 64 years,6 the ages that are appropriate for CRC screening. From 2000 to 2010, CRC screening rates improved from approximately 35% to almost 60%.7 However, in recent years, this increase has slowed dramatically, with the most recent estimate of 62.4% of men and women aged ≥50 years who report CRC testing that is consistent with the guideline recommendations.7 This results in a gap of approximately 18 million commercially insured adults aged 50 to 64 years who are noncompliant with the USPSTF's screening guidelines.3 Noncompliance may be related to access to screening, because noncompliant patients are disproportionately from rural areas.8
Although optical colonoscopy has been the dominant method for CRC screening in the United States to date, other methods that are also recommended by established guidelines include computed tomography (CT) colonography, guaiac-based fecal occult blood test, fecal immunochemical test, fecal immunochemical test-DNA (ie, Cologuard), flexible sigmoidoscopy, and flexible sigmoidoscopy with fecal immunochemical test.9,10 Offering patients choices for CRC screening appears to lead to higher screening rates and better screening compliance.10
In 2008, the ACS, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology recommended that clinicians make patients aware of the full range of CRC screening options, including CT colonography.11 The new recommendation for screening from the ACS regarding a starting age of 45 years is not reflected in this combined statement.4,11 In June 2016, the USPSTF recommended CRC screening by any of the methods mentioned above, including CT colonography.3 As a result, nongrandfathered Affordable Care Act (ACA) health plans must cover CT colonography, with no patient cost-sharing, and CT colonography screening is now incorporated into the HEDIS CRC screening quality-of-care measure.12–14
Optical colonoscopy and CT colonography are the only CRC screening methods that visualize the entire colon; for this reason, they have the highest sensitivity for the detection of precancerous and cancerous adenomas.15,16 Optical colonoscopy and CT colonography allow for the detection and subsequent removal of precancerous adenomas, which can prevent long-term and interscreening interval cancers. Patients who have a CT colonography screening that reveals a nondiminutive polyp (>6 mm) are referred to colonoscopy (and subsequent polypectomy). Although there is discussion about whether polypectomy is necessary for low-risk patients who present with small polyps (6–9 mm),15 Pickhardt and colleagues suggest that active surveillance of these otherwise healthy patients would be cost-effective and preferable to the patient.17
CT colonography also offers patient-centered advantages relative to optical colonoscopy and other CRC screening methods.16 Most optical colonoscopies include sedation and anesthesia, neither of which is routinely used for CT colonography. Because of anesthesia, patients who have had an optical colonoscopy must be accompanied home and will be absent from work the remainder of the day of the procedure, neither of which is required for patients who have CT colonography.18 The potential for less lost work time with CT colonography versus other testing methods may be especially important to the working-age population and to employers.
Moreover, CT colonography is less invasive than optical colonoscopy and has fewer complications.19 CT colonography can be performed by a radiology technologist with the scan interpreted by a radiologist at another site, which can be advantageous to patients in underserved areas. In addition, ethnic groups who are at high risk for CRC and who remain underscreened because of cultural objections to optical colonoscopy may prefer CT colonography.20
CT colonography is an abdominal CT scan that can detect abnormalities outside of the colon; it always exposes a patient to radiation. Experts have been unable to quantify patient harm from the reporting of extracolonic abnormalities or the radiation. Extracolonic reporting is beneficial if knowledge of the abnormality allows for timely intervention for conditions that are detrimental to a patient's health, and is harmful if the exploration of insignificant findings results in unnecessary patient stress, intervention, and costs.
The CT Colonography Reporting & Data System was established in 2005 as a mechanism of classifying CRC and extracolonic findings of CT colonography screenings, and consistent use of this system may reduce the reporting of nonsignificant extracolonic findings.21 In 2016, the USPSTF found insufficient evidence to assess the population consequences of reporting extracolonic findings.22 The task force also examined radiation exposure risk and concluded that ionizing radiation from a single CT colonography examination (1–5 mSv) repeated every 5 years is low compared with radiation from background sources (3 mSv annually).22 Furthermore, a 2017 American Association of Physicists in Medicine policy states that there is no convincing epidemiologic evidence of increased cancer incidence or mortality from low radiation doses (ie, <100 mSv).23
Our primary interest in this study is the cost of CT colonography screening. Because optical colonoscopy is considered a gold standard of CRC screening, and because the literature24 supports equivalent clinical outcomes for CT colonography and optical colonoscopy,3 we chose optical colonoscopy as the reference for this analysis of CT colonography cost. In published modeling of the Medicare population, the cost of CT colonography compares favorably to optical colonoscopy, but the comparison has not been made for the commercial population.25 The use of anesthesia in optical colonoscopy (but not in CT colonography) is a significant source of costs and, as such, is an important consideration for medical and pharmacy directors.
Methods
For historical cost and use patterns, we used the 4th quarter of the 2016 Truven Health MarketScan Commercial Database (hereafter MarketScan), which includes health plan membership and claims data for 2016 for approximately 28 million US employees and their dependents covered under fully insured and self-funded employer-sponsored health insurance. We also used the Medicare physician fee schedule for 2016.
Because the screening intervals for CT colonography and optical colonoscopy differ, we developed a cost-per-screening-year metric to compare the 2 services. We defined “cost per screening year” as the total cost of a screening service, divided by the interval years between screenings. This allowed a direct comparison of the cost of screening methods that have different screening intervals.
Our methodology had 3 steps. First, we estimated the cost per screening for optical colonoscopy using MarketScan 2016 US commercial claims data. Second, as a result of sparse commercial claims experience for CT colonography screening, we estimated CT colonography cost per screening using several fee scenarios. Finally, we converted optical colonoscopy and CT colonography costs per screening to respective costs per screening year using real-world screening intervals obtained from the literature.26–29
The cost data include the screening service and ancillary services. For example, a patient who had an optical colonoscopy may have several pathology services for biopsies performed during the colonoscopy. For cost, we used the allowed charges paid by the payer, which would also include any patient cost-sharing.
Optical Colonoscopy Cost per Screening
We restricted the optical colonoscopy cost data analysis to plan members who had medical and prescription drug coverage, as reported in the MarketScan database; members' age had to be between 45 years and <65 years. We included patients who were aged 45 to 49 years, because the US Multi-Society Task Force on Colorectal Cancer suggests that routine screening for people in this age range is appropriate for African Americans.30
We identified members with 1 facility or professional optical colonoscopy claim (service) that had either a Healthcare Common Procedure Coding System (HCPCS) code that indicates that the optical colonoscopy was for a screening procedure for an average-risk member, or, if the HCPCS code was ambiguous for screening, had an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code for the screening of an average-risk member. These HCPCS and ICD-10-CM code criteria avoided capturing screenings for high-risk individuals (such as those with a history of CRC) who are not candidates for CT colonography. We excluded optical colonoscopies with codes that indicate an incomplete colonoscopy or if an upper endoscopy was performed the same day. The latter exclusion is because we could not easily distinguish the additional costs related to the upper endoscopy.
For the remaining optical colonoscopies, we identified other same-day optical colonoscopy–related services and costs for the member. We summarized the optical colonoscopy–related costs, including anesthesia and pathology services that occurred on the same day, and categorized the costs as screening (ie, professional and facility services for the screening itself), anesthesia, or pathology.
For the bowel preparation costs, we identified the prescription drug costs for laxatives in the 30 days before the optical colonoscopy. We did not include nonprescription bowel preparation agents in our cost estimates.
Finally, we identified optical colonoscopy complication claim costs for the day of the procedure and the 30 days after the procedure by summing the costs with diagnosis codes that may be attributable to complications. We based the list of diagnosis codes on the article by Levin and colleagues (see Appendix Table A.1 at www.AHDBonline.com for all codes and descriptions).31
We divided the total costs for each service category by the number of screenings with any cost in the category to get the unit cost for each service category. In addition, we divided the costs by the number of unique screenings to get the cost per screening for each service category and for all services.
CT Colonography Cost per Screening
Nationally, fewer than 1% of CRC screenings in 2015 were a CT colonography.32 Within the MarketScan claims data, less than 0.1% of CRC screenings in 2015 were by CT colonography, which is an insufficient quantity for a credible claims analysis. We instead used data from several sources to construct our cost scenarios. The scenarios included assumptions as to whether the service is performed in an outpatient hospital department, because the outpatient hospital site of service is associated with significantly higher commercial cost.
We constructed 4 scenarios for the average cost per screening year for CT colonography. These scenarios varied the screening fee by setting (ie, hospital outpatient or non–hospital outpatient) and the distribution of screenings by setting (ie, outpatient hospital or non–outpatient hospital). In developing the fee scenarios, we considered Medicare fees for diagnostic CT colonography, because Medicare does not cover screening CT colonography, and we tested various ratios of commercial to Medicare fees. Commercial reimbursement for imaging is variable, with radiology benefit managers' fees being proximate to Medicare's, but hospital outpatient–based imaging is much higher. Therefore, there could be different scenarios to determine the cost of CT colonography. Nevertheless, we believe that our scenarios are a fair way to bracket the range of future commercial fees.
For the first scenario, we assumed that commercial screening CT colonography fees were higher than Medicare's diagnostic CT colonography by the ratio of commercial-to-Medicare diagnostic abdominal CT fees and that the portion of CT colonographies performed in the hospital outpatient and non–hospital outpatient settings would be the same as for commercial, nonemergency abdominal CTs.
For the second scenario, we used the same fees as the first scenario and the same observed site-of-service distribution as for commercial screening mammograms (the only widely adopted radiologic screening).
For the third scenario, we assumed that the commercial CT colonography fee has the same fee relativity to the Medicare CT colonography fee as commercial-to-Medicare screening mammograms and the same site-of-service distribution as commercial screening mammograms.
For the fourth scenario, we assumed that the commercial screening CT colonography fee is 200% of Medicare's diagnostic CT colonography fee, regardless of the site of service (Table 1).
Table 1.
CT Colonography Cost Scenarios
| Scenario | CT colonography fee by scan type | Hospital outpatient/non–hospital outpatient site-of-service mix |
|---|---|---|
| 1. Fees with same ratio to Medicare as diagnostic abdominal CTs with diagnostic abdominal CT site-of-service distribution | Medicare fee for diagnostic CT colonography × (commercial fee for diagnostic abdominal CT/Medicare fee for diagnostic abdominal CT) | Same mix as commercial diagnostic abdominal CTs |
| 2. Fees with same ratio to Medicare as diagnostic abdominal CTs with mammography site-of-service distribution | Medicare fee for diagnostic CT colonography × (commercial fee for diagnostic abdominal CT/Medicare fee for diagnostic abdominal CT) | Same mix as for commercial screening mammography |
| 3. Fees with same ratio to Medicare as mammography with mammography site-of-service distribution | Medicare fee for diagnostic CT colonography × (commercial fee for screening mammography/Medicare fee for screening mammography) | Same mix as commercial screening mammography |
| 4. Fees 200% of Medicare, regardless of site of service | Medicare fee for diagnostic CT colonography × 200% | NA |
CT indicates computed tomography; NA, not applicable.
We calculated the commercial fees for diagnostic abdominal CTs and mammograms using the same MarketScan data and restrictions as for optical colonoscopies (Appendix Table A.1). We obtained the 2015 Medicare fees from the Centers for Medicare & Medicaid Services website.
We assumed that the unit costs for bowel preparation, complications, and follow-up were the same for CT colonography and optical colonoscopy; therefore, we used the unit costs from the optical colonoscopy analysis, except that for follow-up optical colonoscopy screening we assumed that everyone would have a pathology service. We also assumed that the percent of patients with prescription bowel preparation would be the same for CT colonography and for optical colonoscopy. We relied on published estimates of the percent of CT colonographies that require follow-up (ie, 14.5%)26,33 and for the frequency of optical colonoscopy complications (ie, 0.4%).31,34 We multiplied the unit costs for each service by the frequency of each service to calculate the average cost by CT colonography.
Cost per Screening Year
Optical colonoscopy and CT colonography have different screening intervals. To compare the costs of optical colonoscopy with CT colonography, we divided our estimates of the cost of optical colonoscopy and CT colonography screenings by the estimated real-world screening intervals to calculate each cost per screening year.
People who have had an optical colonoscopy return for screening, on average, in 6 years (see Appendix Table A.2 at www.AHDBonline.com).27 This is earlier than is suggested by national optical colonoscopy guidelines, which recommend that people without adenomas (approximately 75% of people) should return in 10 years for their next screening and that the remaining 25% of people should return in 3 or 5 years, unless they have cancer.27–29 Studies suggests that approximately 75% of average-risk people who have an optical colonoscopy will have no adenomas, so the average screening return time when following the screening recommendations should be approximately 8.6 years.27–29
For CT colonography, we assumed that people will return, on average, in 4.9 years. The American College of Gastroenterology recommends a 5-year screening interval for people who have had a CT colonography and did not have a follow-up optical colonoscopy, and a 3-year screening interval for the approximately 6% of those who have had an optical colonoscopy after which a high-risk adenoma was found11,26,30,35 (see also Appendix Table A.2). We did not find evidence suggesting that the real-world return interval is less than 4.9 years, which differs from a study by Pickhardt and colleagues.26
All data analyses were performed using SAS version 9.4 (SAS Institute Inc; Cary, NC). The fee scenario models were created in Microsoft Excel 2016.
Results
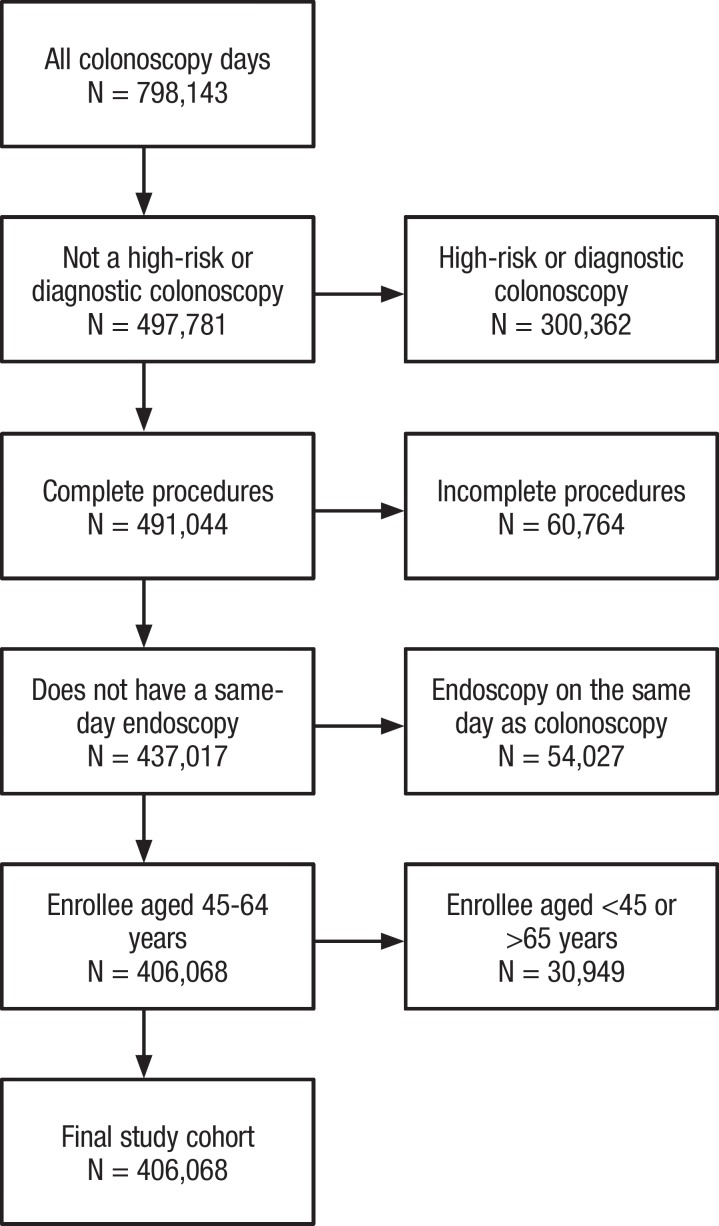
We identified 798,143 optical colonoscopies, of which (in order of exclusion) 300,362 were coded as diagnostic or high-risk; 60,764 were coded as incomplete; 54,027 were in plan members who had an upper endoscopy the same day; and 30,949 were for people aged <45 years or >65 years, which resulted in 406,068 screening optical colonoscopies for analysis (Figure).
Figure. Colonoscopy Days Identified in the 2016 Truven Health MarketScan Commercial Database.
We found that in 2016, the average screening optical colonoscopy cost was $2033. The cost of the screening itself was $1425, which is approximately 66% of the total fee associated with all screening services. Approximately 46% of the people had prescription drug bowel preparation costs that averaged $103; approximately 81% of the people had anesthesia fees that averaged $410; approximately 64% of the members had pathology fees that averaged $226; and approximately 1 of 270 people had optical colonoscopy–related complications that averaged approximately $22,400 (Table 2).
Table 2.
Total Commercial Costs of Optical Colonoscopy, 2016
| Screening parameter | Commercially insured people, N | Prevalence, % | Average unit cost, $ | Cost per screening, $ |
|---|---|---|---|---|
| Screening | 406,068 | 100.0 | 1425 | 1425 |
| Other services | ||||
| Bowel preparation | 189,322 | 46.6 | 103 | 48 |
| Anesthesia | 328,188 | 80.8 | 410 | 331 |
| Pathology | 261,324 | 64.4 | 226 | 146 |
| Complications | 1487 | 0.4 | 22,426 | 83 |
| Subtotal | 608 | |||
| Total cost | 2033 | |||
| Other variables | ||||
| Time to next screening (real-world), yrs | 6 | |||
| Cost per year until next screening, $ | 340 |
Combining the screening cost and ancillary costs, and dividing by the average return time of 6 years results in an optical colonoscopy cost of $340 per screening year (Table 2).
The non–CT colonography services associated with CT colonography, which were averaged per the screened members, include $48 for prescription bowel preparation (46.6% frequency), $4 for complications (0.02% frequency), and $307 for referral to colonoscopy (14.5% frequency; Table 3). The cost for the CT colonography itself ranged from $330 to $1081, depending on the scenario and the site of service; the total cost is inclusive of non–CT colonography services, referrals from CT colonography to optical colonoscopy, and complications, and ranged from $689 to $1440 (Table 4).
Table 3.
Cost of CT Colonography Other Services
| Fee | Unit cost, $ | Frequency, % | Cost, $ |
|---|---|---|---|
| Ancillary fees | |||
| Bowel preparation | 103 | 46.6 | 48 |
| Referral to colonoscopya | 2114 | 14.5 | 307 |
| Complications | 22,426 | 0.02 | 4 |
| Total | 359 |
Assumes all referrals have pathology services.
CT indicates computed tomography.
Table 4.
Cost Scenario of Estimated Total CT Colonography Cost
| Scenario | Site of service | Distribution by site, % | Commercial as percentage of Medicare,a % | Adjusted CT colonography screening fee,b $ | Ancillary fees,c $ | Predicted total cost, $ | Time until next screening, yrs | Cost per year until next screening, $ | Savings compared with optical colonoscopy,d % |
|---|---|---|---|---|---|---|---|---|---|
| 1. Fees with same ratio to Medicare as diagnostic abdominal CTs with diagnostic abdominal CT site-of-service distribution | Hospital outpatient | 74 | 459 | 1081 | 359 | 1440 | |||
| Non–hospital outpatient | 26 | 223 | 526 | 359 | 885 | ||||
| Composite | 100 | 398 | 937 | 359 | 1296 | 4.9 | 265 | 22 | |
| 2. Fees with same ratio to Medicare as diagnostic abdominal CTs with mammography site-of-service distribution | Hospital outpatient | 60 | 459 | 1081 | 359 | 1440 | |||
| Non–hospital outpatient | 40 | 223 | 526 | 359 | 885 | ||||
| Composite | 100 | 365 | 859 | 359 | 1218 | 4.9 | 249 | 27 | |
| 3. Fees with same ratio to Medicare as mammography with mammography site-of-service distribution | Hospital outpatient | 60 | 181 | 426 | 359 | 785 | |||
| Non–hospital outpatient | 40 | 140 | 330 | 359 | 689 | ||||
| Composite | 100 | 164 | 387 | 359 | 746 | 4.9 | 153 | 55 | |
| 4. Fees 200% of Medicare, regardless of site of service | Hospital outpatient | ||||||||
| Non–hospital outpatient | |||||||||
| Composite | 200 | 471 | 359 | 830 | 4.9 | 170 | 50 |
The ratio of the observed 2016 MarketScan commercial fee to the Medicare fee.
Commercial as a percentage of Medicare multiplied by the Medicare cost of a diagnostic CT colonography (HCPCS 74261, $235.60).
Ancillary fees include fees for prescription bowel preparation, pathology, and complications as in Table 3.
Optical colonoscopy cost per year until next screening is described in Table 2.
CT indicates computed tomography; HCPCS, Healthcare Common Procedure Coding System.
Using the CT colonography screening interval of 4.9 years resulted in CT colonography costs of $265, $249, $153, and $170 per screening year for scenarios 1 to 4, respectively, and savings of 22%, 27%, 55%, and 50%, respectively, compared with optical colonoscopy (Table 4).
Discussion
Our optical colonoscopy–related cost estimates and the high portion (ie, approximately 33%) of optical colonoscopy costs associated with ancillary services are consistent with numbers reported in the literature.36 In recent years, there have been increases in the use of anesthesia services, higher polyp detection rates, and, as a consequence, an increased use of pathology services.36 The increase in polyp detection rate is likely a result of the implementation of optical colonoscopy quality measures based on polyp detection rates and adenoma detection rates.37 The anesthesia and polypectomy trends may not necessarily be in the best interest of plan members, because anesthesia and polypectomies increase the risk for complications and add to the cost of optical colonoscopy that is paid by commercial payers.
The ACA, which requires coverage without cost-sharing of all USPSTF-recommended services, was followed by rules that also likely increased the cost to payers, including a 2010 requirement that CRC screening procedures that are initially coded as screening optical colonoscopies remain classified as screening optical colonoscopies even when polyps are found,38 and a 2016 ACA requirement that anesthesia be covered for all optical colonoscopies.39 Before 2016, some payers classified screening optical colonoscopies with polyps as diagnostic and did not cover anesthesia.39
Now that it is endorsed by the USPSTF, CT colonography has the potential to become a high-volume screening procedure. We present 4 commercial CT colonography fee scenarios to estimate the commercial costs for CT colonography screenings, and each scenario compares favorably to optical colonoscopy. We developed the CT colonography fee scenarios using the 2016 commercial fees for other radiology services that are similar to CT colonography, relative to Medicare fees for the same services.
In preparing the scenarios, we observed that commercial fees associated with the radiology procedures varied based on the site of service and volume. High-volume screening procedures performed in freestanding facilities have the lowest fees relative to Medicare, and lower-volume diagnostic procedures performed in hospital outpatient departments have the highest fees relative to Medicare. These dynamics suggest that expanding the use of screening CT colonographies can be attractive to payers and to providers.
Our 4 scenarios show that commercial CT colonography screening is 22% to 55% less expensive than optical colonoscopy per screening year. We anticipate that as awareness of and demand for CT colonography screening increases, fees will decline and more non–hospital outpatient sites will offer CT colonography. An increased availability of CT colonography procedures in non–hospital outpatient sites may be particularly attractive to people for their relative geographic convenience.
The potential for CT colonography expansion is large: if half of the 18 million guideline-nonadherent US adults aged 50 to 65 years with commercial health insurance use CT colonography for screening, there would be more than 1.8 million additional CT colonographies each year. An optical colonoscopy expansion could accompany a CT colonography expansion as well, because the current 60% compliance rate can accommodate increases for all methods of screening.
We noted several advantages for people to use CT colonography rather than optical colonoscopy, including convenience, time-savings, and fewer complications thanks to the screening technique itself and avoiding anesthesia. Offering CT colonography as a screening choice for CRC is a clear win on all 3 dimensions of the Triple Aim, including (1) CRC screening by any method improves the health of populations; (2) CT colonography improves the person's experience of care relative to optical colonoscopy; and (3) CT colonography offers cost advantages relative to optical colonoscopy. We suggest that payers and health systems that wish to improve population health and their CT colonography screening HEDIS scores should consider expanding the availability of CT colonography.
Our model assumes the USPSTF and other organizations' recommendation that screening begin at age 50 years. We did not comprehensively model the ACS's recent recommendation of a starting age of 45 years, although our testing shows that CT colonography's cost relativities would be preserved.11
Limitations
This study has several limitations. Our analysis compares CT colonography with optical colonoscopy, but we did not compare other methods. A comparison across all CRC screening methods for commercial payers would be useful and would need to consider the full follow-up required for a suspicious or indeterminate finding, which often involves optical colonoscopy. Such a comparison would need to account for the cost-benefit of the nondetection of earlier precancerous adenomas, which is not available for stool-based methods, such as the fecal occult blood test or fecal immunochemical test.
If optical colonoscopy were performed at recommended intervals instead of more frequently than recommended, the advantages of the CT colonography would be lower than we estimate. However, we are not aware of strong efforts to shift screening intervals to current recommendations. Conversely, if recommendations emerge for longer intervals than 5 years for CT colonography, the cost advantage of CT colonography would increase.
Furthermore, our analysis relies on claims from the 2016 Truven Health MarketScan Database, which is a national insurance database of commercially insured plan members and claims. Other national databases and time periods may potentially produce different results.
In addition, often there are significant regional, provider-specific, and payer-specific variations in costs as a result of differences in service-level fees and practice patterns (eg, the use of anesthesia and prescription bowel preparatory agents). These variations suggest that each care management organization should consider its circumstances in determining whether CT colonography offers cost-savings that are greater than optical colonoscopy. Future reimbursement changes could cause our estimates to be obsolete.
Finally, several areas warrant further research. In our analysis of US commercial claims, we excluded approximately 12% of the screening optical colonoscopies, because those people had a same-day upper endoscopy. This rate is similar to that reported in a 2015 Medicare analysis of CRC screening.36 Upper endoscopies are not recommended as a routine screening examination, and this practice could contribute to the overall invasiveness and potential complications associated with optical colonoscopy.40 More research into the short intervals for repeated optical colonoscopies could be conducted through longitudinal claims data sets. Furthermore, little is known about the potential advantages offered by screening centers that have the capability to perform immediately an optical colonoscopy on people who have had a CT colonography and require additional testing, which avoids delayed or missed follow-up appointments and the burden of additional bowel preparation.41
Conclusions
Our findings suggest that CT colonography for CRC screening offers effective screening, patient-centered advantages, and lower costs compared with optical colonoscopy. Therefore, CT colonography may be particularly appealing to the currently recommended but unscreened population of people who have commercial insurance.
Appendix
Funding Source
This study was funded by the National Electrical Manufacturers Association.
Author Disclosure Statement
Dr Goss Sawhney was, and Mr Pyenson, Dr Rotter, and Ms Berrios are employees of Milliman, which received funding for this study; Dr Yee has received research/grant support from EchoPixel and Philips.
Contributor Information
Tia Goss Sawhney, Healthcare Consultant and Actuary, Milliman, New York, NY.
Bruce S. Pyenson, Principal & Consulting Actuary, Milliman, and Commissioner of Medicare Payment Advisory Commission (MedPAC).
David Rotter, Senior Healthcare Analyst, Milliman.
Michele Berrios, Senior Healthcare Analytics Consultant, Milliman.
Judy Yee, Chair, Department of Radiology, Montefiore Medical Center, Bronx, NY.
References
- 1. National Cancer Institute. Surveillance, Epidemiology and End Results Program. Cancer stat facts: colorectal cancer. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed August 6, 2018.
- 2. American Society for Gastrointestinal Endoscopy. Colorectal cancer screening: facts about colorectal cancer. www.asge.org/home/about-asge/newsroom/media-backgrounders-detail/colorectal-cancer-screening. Accessed August 6, 2018.
- 3. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564–2575. Errata in: JAMA 2016;316:545; JAMA. 2017;317:2239. [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. American Cancer Society guideline for colorectal cancer screening. Revised May 30, 2018. www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed May 7, 2018.
- 5. National Committee for Quality Assurance. Colorectal cancer screening. www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2017-table-of-contents/colorectal-cancer. Accessed August 6, 2018.
- 6. United States Census Bureau. B27002 – Private health insurance status by sex by age. Universe: civilian noninstitutionalized population. 2016 version. 2012–2016 American Community Survey 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_16_5YR_B27002&prodType=table. Accessed January 18, 2018.
- 7. White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole AM, Jackson JE, Doescher M. Urban–rural disparities in colorectal cancer screening: cross-sectional analysis of 1998–2005 data from the Centers for Disease Control's Behavioral Risk Factor Surveillance study. Cancer Med. 2012;1:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subramanian S, Amonkar MM, Hunt TL. Use of colonoscopy for colorectal cancer screening: evidence from the 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2005;14:409–416. [DOI] [PubMed] [Google Scholar]
- 10. Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55–64. [DOI] [PubMed] [Google Scholar]
- 11. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Medicare & Medicaid Services. Affordable Care Act implementation FAQs – set 12. www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed January 8, 2018.
- 13. US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. [DOI] [PubMed] [Google Scholar]
- 14. National Committee for Quality Assurance. Colorectal cancer screening (COL). www.ncqa.org/hedis/measures/colorectal-cancer-screening/. Accessed September 18, 2018.
- 15. Pickhardt PJ, Hassan C, Laghi A, et al. Small and diminutive polyps detected at screening CT colonography: a decision analysis for referral to colonoscopy. AJR Am J Roentgenol. 2008;190:136–144. [DOI] [PubMed] [Google Scholar]
- 16. Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and meta-analysis. Radiology. 2011;259:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pickhardt PJ, Hassan C, Laghi A, et al. Clinical management of small (6- to 9-mm) polyps detected at screening CT colonography: a cost-effectiveness analysis. AJR Am J Roentgenol. 2008;191:1509–1516. [DOI] [PubMed] [Google Scholar]
- 18. Pickhardt PJ. CT colonography for population screening: ready for prime time? Dig Dis Sci. 2015;60:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL. CT colonography: performance and program outcome measures in an older screening population. Radiology. 2010;254:493–500. [DOI] [PubMed] [Google Scholar]
- 20. Bass SB, Gordon TF, Ruzek SB, et al. Perceptions of colorectal cancer screening in urban African American clinic patients: differences by gender and screening status. J Cancer Educ. 2011;26:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pooler BD, Kim DH, Lam VP, et al. CT Colonography Reporting and Data System (C-RADS): benchmark values from a clinical screening program. AJR Am J Roentgenol. 2014;202:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:2576–2594. Errata in: JAMA 2016;316:545; JAMA. 2016;316:1412. [DOI] [PubMed] [Google Scholar]
- 23. American Association of Physicists in Medicine. AAPM Position Statement on Radiation Risks from Medical Imaging Procedures. PP 25-C. April 10, 2018. www.aapm.org/org/policies/details.asp?id=318&type=PP¤t=true. Accessed August 13, 2018.
- 24. Geiger TM, Ricciardi R. Screening options and recommendations for colorectal cancer. Clin Colon Rectal Surg. 2009;22:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pickhardt PJ, Hassan C, Laghi A, Kim DH. CT colonography to screen for colorectal cancer and aortic aneurysm in the Medicare population: cost-effectiveness analysis. AJR Am J Roentgenol. 2009;192:1332–1340. [DOI] [PubMed] [Google Scholar]
- 26. Pickhardt PJ, Pooler BD, Mbah I, et al. Colorectal findings at repeat CT colonography screening after initial CT colonography screening negative for polyps larger than 5 mm. Radiology. 2017;282:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kruse GR, Khan SM, Zaslavsky AM, et al. Overuse of colonoscopy for colorectal cancer screening and surveillance. J Gen Intern Med. 2015;30:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy CC, Sandler RS, Grubber JM, et al. Underuse and overuse of colonoscopy for repeat screening and surveillance in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2016;14:436–444.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009. Am J Gastroenterol. 2009;104:739–750. Erratum in: Am J Gastroenterol. 2009;104:1613. [DOI] [PubMed] [Google Scholar]
- 31. Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–886. [DOI] [PubMed] [Google Scholar]
- 32. American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. 2017. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf. Accessed February 15, 2018.
- 33. Edwards JT, Mendelson RM, Fritschi L, et al. Colorectal neoplasia screening with CT colonography in average-risk asymptomatic subjects: community-based study. Radiology. 2004;230:459–464. [DOI] [PubMed] [Google Scholar]
- 34. Berrington de Gonzalez A, Kim KP, Yee J. CT colonography: perforation rates and potential radiation risks. Gastrointest Endosc Clin N Am. 2010;20:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cash BD, Riddle MS, Bhattacharya I, et al. CT colonography of a Medicare-aged population: outcomes observed in an analysis of more than 1400 patients. AJR Am J Roentgenol. 2012;199:W27–W34. [DOI] [PubMed] [Google Scholar]
- 36. Pyenson B, Pickhardt PJ, Sawhney TG, Berrios M. Medicare cost of colorectal cancer screening: CT colonography vs. optical colonoscopy. Abdom Imaging. 2015;40:2966–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc. 2011;73:498–506. [DOI] [PubMed] [Google Scholar]
- 38. Centers for Medicare & Medicaid Services Medicare Learning Network. Coding for polypectomy performed during screening colonoscopy or flexible sigmoidoscopy. MLN Matters Number SE0746. www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnmattersarticles/downloads/se0746.pdf. Accessed August 9, 2018.
- 39. The Center for Consumer Information & Insurance Oversight. Affordable Care Act Implementation FAQs-Set 12. February 20, 2013. www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed August 10, 2018.
- 40. Shaheen NJ, Weinberg DS, Denberg TD, et al; for the Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2012;157:808–816. [DOI] [PubMed] [Google Scholar]
- 41. Mayo Clinic. Virtual colonoscopy. www.mayoclinic.org/tests-procedures/virtual-colonoscopy/about/pac-20385156. Accessed February 15, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.