Abstract
Background
Clinical guidelines recommend psychosocial interventions for cocaine and/or amphetamine addiction as first-line treatment, but it is still unclear which intervention, if any, should be offered first. We aimed to estimate the comparative effectiveness of all available psychosocial interventions (alone or in combination) for the short- and long-term treatment of people with cocaine and/or amphetamine addiction.
Methods and findings
We searched published and unpublished randomised controlled trials (RCTs) comparing any structured psychosocial intervention against an active control or treatment as usual (TAU) for the treatment of cocaine and/or amphetamine addiction in adults. Primary outcome measures were efficacy (proportion of patients in abstinence, assessed by urinalysis) and acceptability (proportion of patients who dropped out due to any cause) at the end of treatment, but we also measured the acute (12 weeks) and long-term (longest duration of study follow-up) effects of the interventions and the longest duration of abstinence. Odds ratios (ORs) and standardised mean differences were estimated using pairwise and network meta-analysis with random effects. The risk of bias of the included studies was assessed with the Cochrane tool, and the strength of evidence with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. We followed the PRISMA for Network Meta-Analyses (PRISMA-NMA) guidelines, and the protocol was registered in PROSPERO (CRD 42017042900). We included 50 RCTs evaluating 12 psychosocial interventions or TAU in 6,942 participants. The strength of evidence ranged from high to very low. Compared to TAU, contingency management (CM) plus community reinforcement approach was the only intervention that increased the number of abstinent patients at the end of treatment (OR 2.84, 95% CI 1.24–6.51, P = 0.013), and also at 12 weeks (OR 7.60, 95% CI 2.03–28.37, P = 0.002) and at longest follow-up (OR 3.08, 95% CI 1.33–7.17, P = 0.008). At the end of treatment, CM plus community reinforcement approach had the highest number of statistically significant results in head-to-head comparisons, being more efficacious than cognitive behavioural therapy (CBT) (OR 2.44, 95% CI 1.02–5.88, P = 0.045), non-contingent rewards (OR 3.31, 95% CI 1.32–8.28, P = 0.010), and 12-step programme plus non-contingent rewards (OR 4.07, 95% CI 1.13–14.69, P = 0.031). CM plus community reinforcement approach was also associated with fewer dropouts than TAU, both at 12 weeks and the end of treatment (OR 3.92, P < 0.001, and 3.63, P < 0.001, respectively). At the longest follow-up, community reinforcement approach was more effective than non-contingent rewards, supportive-expressive psychodynamic therapy, TAU, and 12-step programme (OR ranging between 2.71, P = 0.026, and 4.58, P = 0.001), but the combination of community reinforcement approach with CM was superior also to CBT alone, CM alone, CM plus CBT, and 12-step programme plus non-contingent rewards (ORs between 2.50, P = 0.039, and 5.22, P < 0.001). The main limitations of our study were the quality of included studies and the lack of blinding, which may have increased the risk of performance bias. However, our analyses were based on objective outcomes, which are less likely to be biased.
Conclusions
To our knowledge, this network meta-analysis is the most comprehensive synthesis of data for psychosocial interventions in individuals with cocaine and/or amphetamine addiction. Our findings provide the best evidence base currently available to guide decision-making about psychosocial interventions for individuals with cocaine and/or amphetamine addiction and should inform patients, clinicians, and policy-makers.
Using network meta-analysis, Andrea Cipriani and colleagues compare individual and combined psychosocial interventions aimed at treating cocaine and amphetamine addictions, to identify which strategies are acceptable to patients and effective, both short and long-term.
Author summary
Why was this study done?
Cocaine and amphetamines are the most commonly abused stimulants in people aged 15–64 years, and they are linked to significant physical and mental illness as well as a substantial burden for society.
Currently, clinical guidelines recommend the use of psychosocial interventions as first-line treatment for people with cocaine and/or amphetamine addiction.
However, it is still unclear which psychosocial intervention, if any, is the most effective treatment for such patients.
What did the researchers do and find?
We used network meta-analysis to analyse 50 clinical studies (6,943 participants) on 12 different psychosocial interventions for cocaine and/or amphetamine addiction.
We found that the combination of 2 different psychosocial interventions, namely contingency management and community reinforcement approach, was the most efficacious and most acceptable treatment both in the short and long term.
What do these findings mean?
To our knowledge, this is the best evidence base to guide decision-making about psychosocial interventions for cocaine and/or amphetamine addiction. Clinical guidelines should be updated to reflect these results, and policy-makers are encouraged to invest accordingly.
Future trials should use contingency management plus community reinforcement approach as the reference treatment.
Introduction
Drug use disorders are the 15th leading cause of disability-adjusted life years in high-income countries [1]. Cocaine and amphetamines are the most commonly abused stimulants in people aged 15–64 years, with an annual prevalence of misuse of 0.38% and 1.20%, respectively [2]. Patients addicted to stimulants experience a range of psychological and physical sequelae including psychosis and other mental illnesses, neurological disorders and cognitive deficits, cardiovascular dysfunctions, sexually transmitted diseases, and blood-borne viral infections such as HIV and hepatitis B and C [3], and are at increased risk of all-cause mortality [4]. Moreover, the social burden of stimulant abuse is worsened by its association with crime, violence, and sexual abuse [5].
Currently, international clinical guidelines recommend the use of psychosocial interventions for cocaine and/or amphetamine addiction as first-line treatment, and there is little evidence supporting pharmacotherapy or brain stimulation treatments [6–8]. In the absence of approved pharmacotherapies, several structured psychosocial and self-help approaches are available, such as contingency management (CM) (a behavioural approach that consists in providing stimulant users with rewards upon drug-free urine samples), community reinforcement approach (a multi-layered intervention involving functional analysis, coping-skills training, and social, familial, recreational, and vocational reinforcements), and 12-step programme (a set of guiding principles outlining a course of action for self-help recovery from addiction) [8]. International guidelines are unclear on whether any specific intervention should be considered first [9,10]; for example, the National Institute for Health and Care Excellence (NICE) recommends CM alone, cognitive behavioural therapy (CBT) alone, or self-help groups based on 12-step programme alone for the treatment of individuals with stimulant use disorders [8]. However, a recent systematic review showed that CM and CBT were well accepted and moderately efficacious at the end of treatment, but not at follow-up after treatment completion [11].
Previous pairwise meta-analyses relied on a limited number of studies with direct comparisons between different interventions [12,13]. From a clinical perspective, it is important to assess whether psychosocial interventions are effective and acceptable in both the short and long term, and also whether the combination of 2 approaches can produce a significant benefit. We therefore performed a network meta-analysis to compare and rank the efficacy and acceptability of individual and combined psychosocial interventions for the treatment of cocaine and/or amphetamine addiction at different time-points.
Methods
Search strategy and selection criteria
This network meta-analysis was conducted following a pre-established protocol registered on PROSPERO (CRD 42017042900), and is reported according to the PRISMA for Network Meta-Analyses (PRISMA-NMA) guidelines [14]. We searched the Cochrane Drugs and Alcohol Group Specialised Register, PubMed, Embase, CINAHL, ISI Web of Science, and PsycINFO from the date of database inception to 8 April 2018. We also screened international registers, hand-searched the reference lists of retrieved articles, and looked at key conference proceedings (for the full search strategy, see S1 Text). When needed, we contacted the investigators and relevant trial authors to obtain information about unpublished or incomplete trials. All searches included non-English language literature.
We included randomised controlled trials (RCTs) comparing any structured psychosocial intervention against a control intervention—another psychosocial intervention or treatment as usual (TAU)—for the treatment of individuals with cocaine and/or amphetamine addiction, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) III, III-R, IV, IV-TR, or V or the International Classification of Diseases–10th revision (ICD-10) criteria. CBT, CM, community reinforcement approach, meditation-based therapies, non-contingent rewards, supportive-expressive psychodynamic therapy, 12-step programme, and their combinations were all identified as structured psychosocial interventions. We excluded studies on occasional users not actively seeking treatment and RCTs with study duration less than 4 weeks. We did not exclude studies on individuals with a comorbid substance use disorder (including opioid, alcohol, or cannabis use) or with a comorbid psychiatric disorder.
Data extraction and quality assessment
Four authors (FDC, GLD, MC, RDG) independently screened the references retrieved by the search, selected the studies, and extracted the data, using a predefined data-extraction sheet. The same reviewers discussed any uncertainty regarding study eligibility and data extraction until consensus was reached; conflicts of opinion were resolved with another member of the review team (AC). 2 authors (GLD, MC) independently assessed the risk of bias of the included studies with the Cochrane tool [15], Three authors (FDC, CDG, AC) used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [16], through the Confidence in Network Meta-Analysis Software (CINeMA) [17], to evaluate the strength of evidence for results at the end of treatment from the network meta-analysis (S2 Text).
Outcomes
We considered as primary outcomes the efficacy and the acceptability of the interventions at the end of treatment [18]. Efficacy was measured as the proportion of individuals abstinent (assessed by urinalysis), and acceptability as the proportion of individuals who dropped out from the study due to any cause. As secondary outcomes, we also measured efficacy and acceptability at 12 weeks from the start of treatment and at the longest follow-up (with follow-up starting at the end of treatment, independent of the duration of the intervention). If 12-week data were not available, we used data ranging between 4 and 20 weeks (giving preference to the time-point closest to 12 weeks). Other secondary outcomes were the longest duration of abstinence measured both at 12 weeks and at the end of treatment.
Statistical analysis
We performed first pairwise meta-analyses using a random-effects model to estimate pooled odds ratios (ORs) for dichotomous outcomes and standardised mean differences (SMDs) for continuous outcomes with their 95% confidence intervals (CIs) using STATA [19]. We assessed statistical heterogeneity in each pairwise comparison with τ2, I2 statistic, and P value [15]. If at least 10 studies were available, we used the funnel plot and Egger’s test to detect publication bias [15].
We incorporated indirect comparisons with direct comparisons using random-effects network meta-analyses within a frequentist framework using STATA (network package), and results are presented with the network graphs package [20]. We report the results of network meta-analyses for both primary and secondary outcomes in league tables with effect sizes (OR or SMD) and their 95% CIs. When dichotomous outcome data were missing, we assumed that patients who dropped out after randomisation had a negative outcome. Missing continuous outcome data were analysed on an endpoint basis, including only participants with a final assessment, as reported by the original study authors. We also calculated the number needed to treat (NNT), which is the number of patients that need to be treated in order for 1 to benefit from the intervention compared with TAU.
We assessed incoherence between direct and indirect sources of evidence using local and global approaches. Coherence (or consistency) is an important assumption to check in network meta-analyses because it is the manifestation of transitivity in the data from a network of interventions: coherence exists when treatment effects from direct and indirect evidence are in agreement (subject to the usual variation due to heterogeneity in the direct evidence) [21]. Local incoherence was measured by using a loop-specific approach (which identified inconsistent loops of evidence) [22] and a side-splitting approach (which separated evidence on a particular comparison into direct and indirect evidence) [23]. Global incoherence was measured with the between-studies standard deviation (SD) (heterogeneity parameter) by using both a coherence and incoherence model and by measuring the chi-squared incoherence, with its P value. We estimated the presence of publication bias by plotting comparison-adjusted funnel plots for the network meta-analyses with a linear regression line [24]. We also estimated the ranking probabilities for all treatments, i.e., their probability of being at each possible rank for each intervention. We report the treatment hierarchy as the surface under the cumulative ranking curve (SUCRA) and as the mean rank (S2 Text) [24].
To determine whether the results were affected by study characteristics, we performed subgroup network meta-analyses for abstinence and dropout at the end of treatment according to the following variables: year of publication, sex ratio, mean age group, intensity of the treatment, type of stimulant, risk of bias, opioid therapy, sample size, and comorbid alcohol misuse. Additionally, we performed sensitivity network meta-analyses for the primary outcomes by considering (a) only trials on individuals addicted to cocaine and no other stimulant and (b) only trials on individuals addicted to stimulants and on opioid substitution therapy.
Results
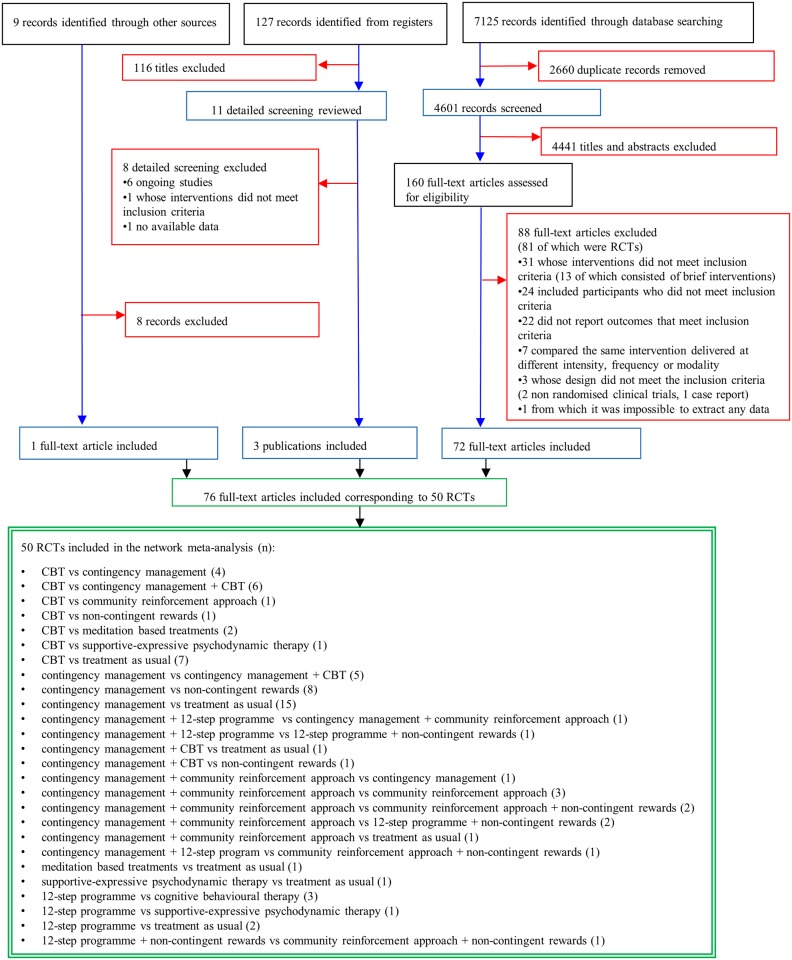
From the initially identified 7,261 citations, we retrieved 160 potentially eligible articles in full text (Fig 1). We excluded 88 reports, but then included 4 additional studies (3 from trial registers and 1 from screening the references), resulting in 76 publications (S3 Text) describing 50 RCTs (6,942 participants), published between 1993 and 2016 (Fig 2; Table 1), comparing 12 psychosocial interventions or TAU (listed and defined in S4 Text). Overall, 5,158 participants were randomly assigned to psychosocial treatments, and 1,784 to TAU. Full clinical and demographic characteristics are reported in Table 1. The mean study sample size was 139 participants, ranging between 19 and 487 participants. The median duration of treatment was 12 weeks (range 6–36). Dropout rates varied between 15.1% (CM + CBT) and 60.2% (meditation-based therapies) (S1 Table). A total of 37 studies were followed up after study completion, for a mean duration of 41.4 weeks (range 16–96). A total of 42 (84%) trials recruited patients from North America, 6 from Europe, 1 from Latin America, and 1 from Oceania. About a third of the population was women (35.9%), and the mean age was 36.8 years. A total of 76% of trials (38 of 50) enrolled participants with cocaine addiction, 8% of trials (4 of 50) with amphetamine addiction, and 16% (8 of 50) with both. About one-third of trials (18 of 50) enrolled participants on methadone maintenance. The mean addiction severity was moderate/high (S2 Table). In terms of risk of bias, 22 (44%) trials were rated low risk, 13 (26%) moderate, and 15 (30%) high (S3 Table; S1 Fig).
Fig 1. Study selection.
The flowchart shows the records identified through database searching (black boxes), the records screened (blue boxes), the records excluded (red boxes), and the studies included (green boxes). CBT, cognitive behavioural therapy; RCT, randomised controlled trial.
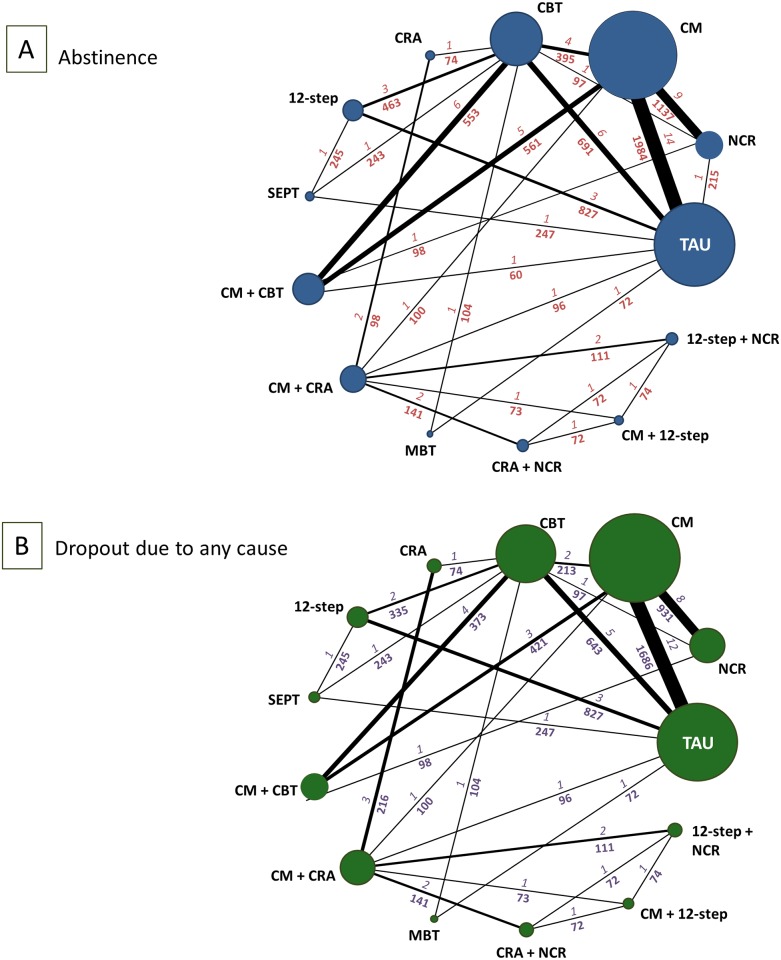
Fig 2. Network of eligible comparisons for abstinence and dropout due to any cause at the end of treatment.
The figure plots the network of eligible direct comparisons for abstinence at the end of treatment (46 trials) (A) and dropout due to any cause (43 studies) (B). The width of the lines is proportional to the number of trials comparing every pair of treatments, and the size of every node is proportional to the number of randomised participants. The numbers above each connection relate to the numbers of trials and the numbers below each connection relate to the number of patients for each direct comparison. 12-step, 12-step programme; CBT, cognitive behavioural therapy; CM, contingency management; CRA, community reinforcement approach; MBT, meditation-based treatments; NCR, non-contingent rewards; SEPT, supportive-expressive psychodynamic therapy; TAU, treatment as usual.
Table 1. Characteristics of included randomised controlled trials.
| Study, year | Country | Diagnostic criteria | Intervention (n) | Duration of intervention (weeks) | Follow-up (weeks) | Setting | Age mean (SD) (years) | Female n (%) | Stimulant abused | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|
| Carroll, 1994 [25] | US | DSM-III-R | CBT (29) CBT (29) TAU (25) TAU (27) |
12 | NA | Outpatient | 28.8 (5.8) | 30 (27.3) | Cocaine | Alcohol (<50%) |
| Carroll, 1998 [26] | US | DSM-III-R | 12-step (25) 12-step (25) CBT (26) CBT (19) TAU (27) |
12 | 52 | Outpatient | 30.5 (5.5) | 26 (26.9) | Cocaine | Alcohol (100%) |
| Carroll, 2012 [27] | US | DSM-IV | 12-stepc (27) 12-stepd (29) TAUc (26) TAUd (30) |
12 | 48 | Outpatient | 38.3 (7.6) | 46 (41.1) | Cocaine | Alcohol (≥50%), Methadone (100%) |
| Carroll, 2014 [28] | US | DSM-IV | CBT (47) TAU (54) |
8 | 48 | Outpatient | 41.2 (9.6) | 61 (60.4) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Carroll, 2016 [29] | US | DSM-IV | CBTc (26) CBTd (28) CBT + CMc (22) CBT + CMd (23) |
12 | 48 | Outpatient | 39.3 (7.5) | 27 (27.3) | Cocaine | Alcohol (≥50%) |
| Chen, 2013 [30] | US | DSM-IV | MBT (37) TAU (35) |
12 | NA | Outpatient | 45.1 (6.5) | 31 (42.9) | Cocaine | NA |
| Crits-Cristoph, 1999 [31] | US | DSM-IV | 12-step (121) CBT (119) SEPT (124) TAU (123) |
36 | 48 | Outpatient | 33.9 (6.3) | 113 (23.2) | Cocaine | NA |
| Donovan, 2013 [32] | US | DSM-IV | 12-step (234) TAU (237) |
8 | 24 | Outpatient | 38.3 (9.7) | 277 (58.8) | Amphetamine and cocaine | Alcohol (<50%) |
| Dursteler-MacFarland, 2013 [33] | Switzerland | DSM-IV | CBTc (17) CBTe (15) TAUc (15) TAUe (15) |
12 | NA | Outpatient | 35.9 (6.1) | 22 (35.6) | Cocaine | Methadone (100%) |
| Epstein, 2003 [34] | US | DSM-III-R | CBT (48) CBT + CM (49) CM (47) NCR (49) |
12 | 52 | Outpatient | 39.0 (6.8) | 90 (47.0) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Festinger, 2014 [35] | US | DSM-IV | CMf (71) CMg (73) TAU (78) |
12 | NA | Outpatient | 37.1 (9.9) | 73 (32.9) | Cocaine | Methadone (100%) |
| Garcia-Fernandez, 2011 [36] | Spain | DSM-IV | CM + CRA (29) CRA (29) |
24 | 48 | Outpatient | 29.9 (5.7) | 7 (12.1) | Cocaine | Alcohol (≥50%) |
| Garcia-Rodriguez, 2007 [37] | Spain | DSM-IV | CM + CRA (44) TAU (52) |
24 | NA | Outpatient | 29.1 (5.5) | 9 (9.2) | Cocaine | Alcohol (≥50%) |
| Ghitza, 2007 [38] | US | DSM-IV | CM (76) NCR (40) |
12 | 24 | Outpatient | 37.3 (8.4) | 51 (44.3) | Cocaine | Methadone (100%) |
| Hagedorn, 2013 [39] | US | NR | CM (71) TAU (68) |
8 | 48 | Outpatient | NA | 2 (1.4) | Amphetamine and cocaine | Alcohol (≥50%) |
| Higgins, 1993 [40] | US | DSM-III-R | 12-step + NCR (19) CM + CRA (19) |
24 | 52 | Outpatient | 29.3 (5.2) | 0 (0) | Cocaine | Alcohol (≥50%) |
| Higgins, 1994 [41] | US | DSM-III-R | CM + CRA (20) CRA (20) |
24 | 52 | Outpatient | 31.4 (5.1) | 13 (32.5) | Cocaine | Alcohol (≥50%) |
| Higgins, 2000 [42] | US | DSM-III-R | CM + CRA (36) CRA + NCR (34) |
24 | 78 | Outpatient | 30.4 (5.0) | 19 (27.1) | Cocaine | Alcohol (≥50%) |
| Higgins, 2003 [43] | US | DSM-III-R | CM (51) CM + CRA (49) |
24 | 96 | Outpatient | 33.9 (6.3) | 41 (41.0) | Cocaine | Alcohol (≥50%) |
| Kirby, 1998 [44] | US | DSM-III-R | CM (44) TAU (46) |
12 | NA | Outpatient | NA | NA | Cocaine | NA |
| Landovitz, 2015 [45] | US | NR | CM (70) NCR (100) |
8 | 24 | Outpatient | 36.7 (11) | 0 (0) | Amphetamine and cocaine | NA |
| Ledgerwood, 2006 [46] | US | DSM-IV | CM (104) TAU (38) |
12 | NA | Outpatient | 36.6 (7.3) | 77 (54.3) | Cocaine | Alcohol (<50%) |
| Maude-Griffin, 1998 [47] | US | DSM-III-R | 12-step (69) CBT (59) |
12 | 26 | Outpatient | NA | NA | Cocaine | Alcohol (≥50%) |
| McDonell, 2013 [48] | US | DSM-IV | CM (91) NCR (85) |
12 | 24 | Outpatient | 41.7 (9.6) | 61 (34.5) | Amphetamine and cocaine | Alcohol (<50%) |
| McKay, 1997 [49] | US | DSM-III-R | CBT (46) TAU (52) |
24 | NA | Outpatient | 40.1 (7.1) | NA | Cocaine | Alcohol (<50%) |
| Menza, 2010 [50] | US | NR | CM (70) TAU (57) |
6 | 24 | Outpatient | 38.7 (NA) | 0 (0) | Amphetamine | NA |
| Miguel, 2016 [51] | Brazil | DSM-IV | CM (33) TAU (32) |
12 | NA | Outpatient | 35.3 (8.5) | 9 (14.3) | Cocaine | Alcohol (≥50%) |
| Milby, 2008 [52] | US | DSM-IV | CBT + CM (103) CM (103) |
24 | 52 | Outpatient | 40.1 (7.2) | 56 (27.2) | Cocaine | Alcohol (<50%) |
| Peirce, 2006 [53] | US | DSM-IV | CM (198) TAU (190) |
12 | 24 | Outpatient | 42.0 (8.6) | 171 (44.1) | Amphetamine and cocaine | Alcohol (<50%), Methadone (100%) |
| Petitjean, 2014 [54] | Switzerland | DSM-IV | CBT (31) CBT + CM (29) |
24 | 48 | Outpatient | 34.5 (7.7) | 12 (20.0) | Cocaine | Methadone (<50%) |
| Petry, 2002 [55] | US | DSM-IV | CM (19) TAU (23) |
12 | 24 | Outpatient | 38.5 (4.6) | 30 (71.3) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Petry, 2005 [56] | US | DSM IV | CM (209) TAU (206) |
12 | 24 | Outpatient | 35.8 (8.7) | 230 (55.4) | Amphetamine and cocaine | Alcohol (<50%) |
| Petry, 2005 [57] | US | DSM-IV | CM (40) TAU (37) |
12 | 24 | Outpatient | 39.5 (1.1) | 56 (72.7) | Cocaine | Methadone (100%) |
| Petry, 2007 [58] | US | DSM-IV | CMf (27) CMg (30) TAU (19) |
12 | 36 | Outpatient | 41.6 (8.2) | 43 (56.6) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Petry, 2012 [59] | US | DSM-IV | CM (71) TAU (59) |
12 | 36 | Outpatient | 36.6 (9.4) | 61 (46.9) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Petry, 2012 [60] | US | DSM-IV | CMh,i (118) CMh,j (35) CMj,k (40) NCR,i (107) TAU,i (108) TAUj (34) |
6 | 36 | Outpatient | 36.5 (9.1) | 176 (52.8) | Cocaine | Alcohol (<50%) |
| Petry, 2013 [61] | US | DSM-IV | CM (10) TAU (9) |
8 | NA | Outpatient | NA | NA | Cocaine | Methadone (≥50%) |
| Poling, 2006 [62] | US | DSM-IV | CMl (27) CMc (25) NCRl (30) NCRc (24) |
25 | NA | Outpatient | 34.6 (NA) | 32 (30.2) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Rawson, 2002 [63] | US | DSM-IV | CBT (30) CBT + CM (30) CM (30) TAU (30) |
16 | 52 | Outpatient | 43.6 (NA) | 54 (45.0) | Cocaine | Methadone (100%) |
| Rawson, 2006 [64] | US | DSM-IV | CBT (58) CBT + CM (59) CM (60) |
16 | 52 | Outpatient | NA | 42 (23.7) | Amphetamine and cocaine | NA |
| Roll, 2013 [65] | US | DSM-IV | CMm (30) CMn (30) CMo (29) TAU (29) |
16 | 48 | Outpatient | 32.2 (9.5) | 53 (44.9) | Amphetamine | NA |
| Sánchez-Hervás, 2010 [66] | Spain | DSM-IV | CBT (34) CRA (40) |
24 | 52 | Outpatient | 31.2 (6.3) | 10 (13.5) | Cocaine | Alcohol (≥50%) |
| Schottenfeld, 2011 [67] | US | DSM-IV | 12-step + CM (37) 12-step + NCR (37) CM + CRA (36) CRA + NCR (35) |
24 | 48 | Outpatient | 31.1 (30.7) | 145 (100) | Cocaine | Alcohol (<50%) |
| Secades-Villa, 2013 [68] | Spain | DSM-IV | CM + CRA (50) CRA (68) |
24 | NA | Outpatient | 31.2 (6.6) | 17 (14.4) | Cocaine | Alcohol (≥50%) |
| Shoptaw, 2005 [69] | US | DSM-IV | CBT (40) CBT + CM (40) CM (42) CBTp (40) |
16 | 52 | Outpatient | 37.2 (7.4) | 0 (0) | Amphetamine | NA |
| Shoptaw, 2008 [70] | US | NR | CBT (64) TAU (64) |
16 | 52 | Outpatient | 37.1 (7.7) | 0 (0) | Amphetamine and cocaine | Alcohol (<50%) |
| Silverman, 1996 [71] | US | DSM-III-R | CM (19) NCR (18) |
12 | 16 | Outpatient | 36.1 (1.5) | NA | Cocaine | Alcohol (<50%), Methadone (100%) |
| Silverman, 1998 [72] | US | DSM-III-R | CMf (20) CMq (20) NCR (19) |
12 | 18 | Outpatient | 37.8 (5.1) | 20 (33.9) | Cocaine | Alcohol (<50%), Methadone (100%) |
| Smout, 2010 [73] | Australia | DSM-IV | CBT (53) MBT (51) |
12 | 24 | Outpatient | NA | NA | Amphetamine | NA |
| Umbricht, 2014 [74] | US | DSM-IV | CM (39) CMr (40) NCR (47) NCRr (45) |
12 | NA | Outpatient | 42.0 (7.0) | 82 (47.9) | Cocaine | Alcohol (<50%), Methadone (100%) |
aFor full reference list, see S3 Text
bWith desipramine
cWith placebo
dWith disulfiram
eWith methylphenidate
fWith voucher
gWith cash/prize
h$250 reward
iCocaine negative at intake
j$560 reward
kCocaine negative at intake
lWith bupropion hydrochloride
mOne-month duration of treatment
nTwo-month duration of treatment
oFour-month duration of treatment
pGay-specific CBT
qWith voucher and $50 bonus
rWith topiramate.
CBT, cognitive behavioural therapy; CM, contingency management; CRA, community reinforcement approach; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; MBT, meditation-based treatments; NA, not assessed; NCR, non-contingent rewards; NR, not reported; SEPT, supportive-expressive psychodynamic therapy; TAU, treatment as usual; 12-step, 12-step programme.
We present all the networks for specific outcomes in S2 Fig. Eight psychosocial interventions had at least 1 trial versus TAU, and all of them were directly compared with at least another psychosocial intervention. We obtained unpublished or supplementary information for 5 of the included studies [45,49,59–61].
The pairwise meta-analyses are presented in S4 Table, while data on heterogeneity are presented in S5 Table. The pairwise meta-analyses showed some statistically significant results in terms of abstinence and dropout. CM plus CBT, CM, and 12-step programme were superior to TAU in terms of abstinence at the end of treatment, while CBT, CM, and the combination of CM plus community reinforcement approach were superior to TAU in terms of dropout at the end of treatment (S4 Table).
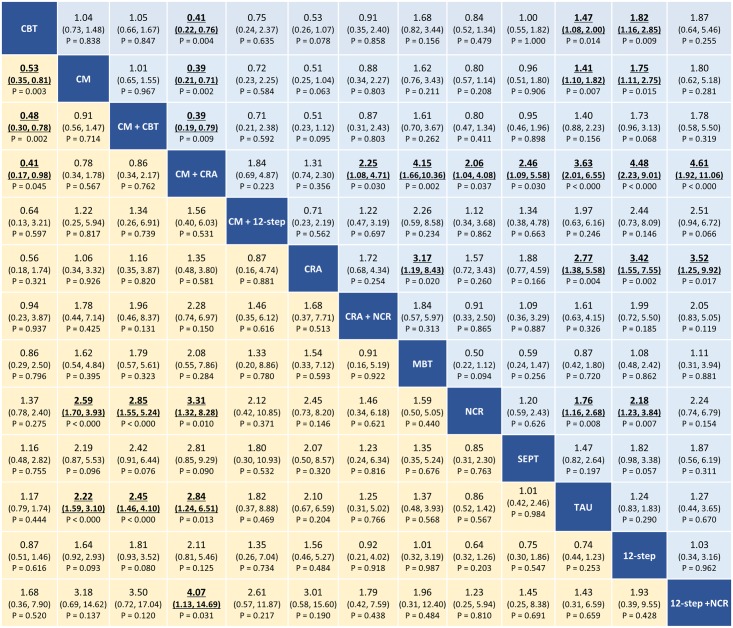
The results of the network meta-analysis are presented in Fig 3. In terms of abstinence at the end of treatment, the combination of CM plus community reinforcement approach, the combination of CM plus CBT, and CM alone were superior to non-contingent rewards (OR ranging between 2.59 [95% CI 1.70–3.93], P < 0.001, and 3.31 [95% CI 1.32–8.28], P = 0.010) and to TAU (OR ranging between 2.22 [95% CI 1.59–3.10], P < 0.001, and 2.84 [95% CI 1.24–6.51], P = 0.013). Moreover, the combination of CM plus community reinforcement approach was also superior to the combination 12-step programme plus non-contingent rewards and to CBT (OR 4.07 [95% CI 1.13–14.69], P = 0.031, and 2.43 [95% CI 1.02–5.88], P = 0.045, respectively), while CM alone and the combination of CM plus CBT were superior to CBT (OR 1.88 [95% CI 1.52–2.85], P = 0.003, and 2.08 [95% CI 1.28–3.33], P = 0.002, respectively). In terms of dropouts at the end of treatment, the combination of CM plus community reinforcement approach, community reinforcement approach alone, non-contingent rewards, CM alone, and CBT were better accepted than TAU (OR ranging between 1.41 [95% CI 1.10–1.82], P = 0.007, and 3.63 [95% CI 2.01–6.55], P < 0.001). Moreover, the combination of CM plus community reinforcement approach was better accepted than CBT, CM alone, CM plus CBT, community reinforcement approach plus non-contingent rewards, meditation-based therapies, non-contingent rewards, supportive-expressive psychodynamic therapy, 12-step programme alone, and 12-step programme plus non-contingent rewards (OR ranging between 2.06 [95% CI 1.04–4.08], P = 0.037, and 4.61 [95% CI 1.92–11.06], P < 0.001).
Fig 3. Network meta-analysis of efficacy (yellow) and acceptability (blue) at the end of treatment.
Psychosocial treatments are reported in alphabetical order. Comparisons should be read from left to right. Abstinence and dropout estimates are located at the intersection between the column-defining and the row-defining treatment. For abstinence, ORs above 1 favour the column-defining treatment. For dropout, ORs above 1 favour the row-defining treatment. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. Significant results are in bold and underlined. CBT, cognitive behavioural therapy; CM, contingency management; CRA, community reinforcement approach; MBT, meditation-based therapies; NCR, non-contingent rewards; OR, odds ratio; SEPT, supportive-expressive psychodynamic therapy; TAU, treatment as usual; 12-step, 12-step programme.
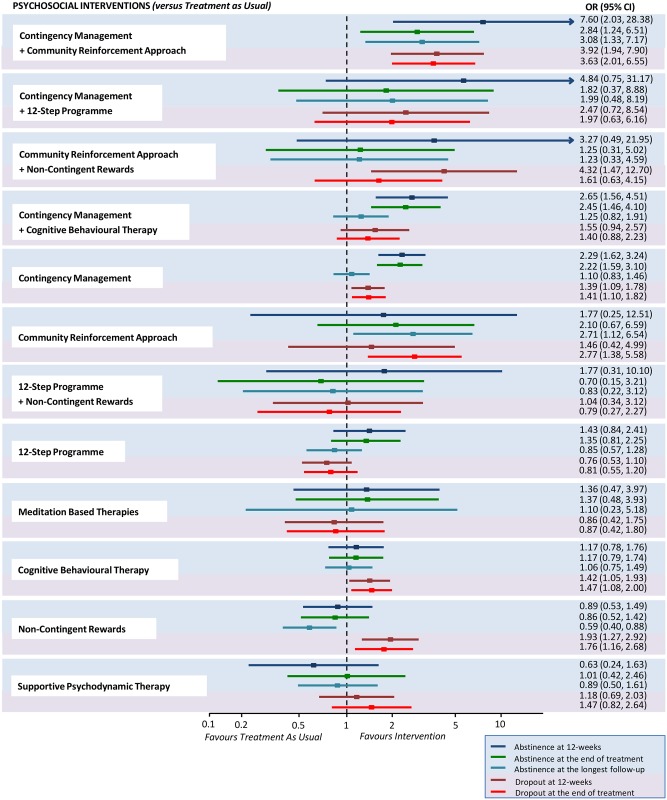
Fewer studies reported results for abstinence measured at 12 weeks of treatment (S3 Fig) and at the longest follow-up after treatment completion (S4 Fig), but findings were in line with the outcome data at the end of treatment. Comparative abstinence and dropout at different time-points for each psychosocial intervention versus TAU are presented in Fig 4.
Fig 4. Abstinence and dropout at different time-points for each psychosocial intervention versus treatment as usual.
Estimates are reported by ORs, where an OR above 1 favours the psychosocial intervention indicated on the left side over treatment as usual. For each intervention, efficacy outcomes are reported in the blue-shaded area, while acceptability outcomes are reported in the pink-shaded area. OR, odds ratio.
In terms of the longest duration of abstinence measured at 12 weeks (S5 Fig), we found that the combination of CM plus CBT and CM alone were superior to TAU (SMD 0.75 [95% CI 0.31–1.19] and 0.62 [95% CI 0.43–0.80], respectively). Likewise, for the longest duration of abstinence measured at the end of treatment (S6 Fig), we found that the combination of CM plus CBT and CM alone were superior to TAU (SMD 0.74 [95% CI 0.43–1.06] and 0.60 [95% CI 0.43–0.76], respectively).
The common heterogeneity SD for the coherence model was 0.46 and 0.21 for abstinence and dropout at the end of treatment, respectively (it was 0.47 and 0.19 for abstinence and dropout at 12 weeks, respectively). The global incoherence was not significant for all the outcomes considered (S6 Table). Tests of local incoherence did not show any inconsistent loops for abstinence and dropout at the end of treatment, although in some cases the ratio of the odds ratios (RoR) from direct and indirect evidence was large (i.e., RoR > 2), and we cannot definitely exclude the presence of incoherence [22]. We found only 1 inconsistent loop for abstinence measured at 12 weeks and no other inconsistent loops for the other outcomes considered at 12 weeks (S7 Table; S7 Fig). The test of incoherence from the side-splitting model did not show significant differences for abstinence at the end of treatment but found some differences between some comparisons for dropout at the end of treatment (S8 Table). The comparison-adjusted funnel plots of the network meta-analysis for abstinence and dropout at the end of treatment were not suggestive for significant publication bias (S8 Fig).
The ranking of psychosocial interventions based on cumulative probability plots and SUCRAs is presented in S9 Table and S9 Fig. We also performed subgroup analyses for abstinence and dropout at the end of treatment to study the effect of several potential moderator variables, the findings of which did not substantially differ from those of the primary analysis for most of the comparisons (S10 Table). Pre-planned sensitivity analysis on individuals addicted to cocaine only did not affect the main results (S10 Fig), while pre-planned sensitivity analysis on individuals on opioid substitution therapy showed a superiority of CM alone and CM plus CBT over TAU and non-contingent rewards, and a superiority of CM alone over CBT (S11 Fig). Predictivity intervals of mixed estimates are presented in S11 Table, while the overall limitations per comparison are presented in S12 and S13 Figs.
We found that 4 patients needed to be treated with CM plus community reinforcement approach to have 1 additional patient abstinent at the end of treatment compared toTAU (NNT 4.07, 95% CI 2.29–21.95), with consistent results at longest follow-up after treatment completion (NNT 3.68, 95% CI 2.36–14.24). Similarly, 3 patients needed to be treated with CM plus community reinforcement approach to have 1 fewer patient dropping out at the end of treatment compared to TAU (NNT 3.25, 95% CI 2.42–5.79). For abstinence at the end of treatment, the NNT for the combination of CM plus CBT and for CM alone versus TAU was 4.80 (95% CI 2.99–12.12) and 5.44 (95% CI 3.74–9.75), respectively. For dropout at the end of treatment, the NNT ranged from 4.02 (95% CI 2.58–12.62) for CRA to 7.15 (95% CI 4.15–27.66) for non-contingent rewards, 10.52 (95% CI 5.83–53.65) for CBT, and 11.82 (95% CI 6.74–43.26) for CM.
The overall strength of evidence according to GRADE is summarised in S12 Table for abstinence and for dropout at the end of treatment. For CM plus community reinforcement approach versus TAU, the strength of evidence was rated as “moderate” for abstinence and as “high” for dropout due to any cause. For CM plus CBT and CM alone versus TAU, the strength of evidence for abstinence was rated as “moderate” for both, while the strength of evidence for dropout due to any cause was rated as “very low” and “moderate”, respectively.
Discussion
This network meta-analysis is based on 50 studies including 6,942 individuals randomly assigned to 12 different psychosocial interventions or TAU. To our knowledge, it is the most comprehensive synthesis of data for all available psychosocial interventions in individuals with cocaine and/or amphetamine addiction.
We found that CM alone or in combination with either community reinforcement approach or CBT had superior efficacy and acceptability compared to TAU at 12 weeks and at the end of treatment. This effect was not significantly influenced by clinical modifiers in the subgroup analyses and remained significant in the sensitivity analyses. Moreover, CM in combination with community reinforcement approach and community reinforcement approach alone were more effective than TAU at the longest follow-up after treatment completion. The clinical relevance of this finding is key, because achieving long-term abstinence is the main treatment goal for individuals with cocaine and/or amphetamine addiction [18].
Several major guidelines recommend the use of either CBT or CM alone for the treatment of cocaine and/or amphetamine addiction [6,8,10]. Self-help groups following the 12-step programme are also recommended [8]. Our results do not support these recommendations. We found that CBT alone was more acceptable than TAU (NNT 10.5, 95% CI 5.8–53.6), but it was not superior for abstinence on any dichotomous or continuous outcome measured and was less effective than CM alone. CM alone showed greater efficacy (NNT 5.2, 95% CI 3.6–9.3) and acceptability (NNT 12.5, 95% CI 7.0–48.8) than TAU at 12 weeks of treatment and at the end of treatment (NNT 5.4, 95% CI 3.8–9.8, and 11.9, 95% CI 6.8–43.3, respectively), but the effect was not sustained at the longest follow-up after treatment completion. Both CBT and CM were inferior to CM in combination with community reinforcement approach at the longest follow-up after treatment completion. Community reinforcement approach alone was not different from TAU for abstinence at 12 weeks of treatment or at the end of treatment, but showed increased abstinence at the longest follow-up after treatment completion (NNT 4.1, 95% CI 2.4–36.2). CM in combination with community reinforcement approach was superior to TAU for abstinence at 12 weeks of treatment (NNT 2.1, 95% CI 1.6–6.2), at the end of treatment (NNT 4.1, 95% CI 2.3–21.9), and at the longest follow-up after treatment completion (NNT 3.7, 95% CI 2.4–14.2), as well as for acceptability at 12 weeks of treatment (NNT 3.1, 95% CI 2.2–6.1) and at the end of treatment (NNT 3.3, 95% CI 2.3–6.3), as shown in Fig 4. CM plus community reinforcement approach was also superior to 12-step programme for abstinence and dropout at 12 weeks of treatment, for dropout at the end of treatment, and for abstinence at the longest follow-up after study completion.
Behavioural interventions have proved effective for the treatment of other forms of addiction [75–77]. There is growing evidence that reducing punishment—such as incarceration—and adopting positive reinforcement for people with substance use improves their access to services, their reintegration into society, and, ultimately, public safety [78–80]. Recent experimental data emphasise the potential of interventions that focus on improving goal-directed behaviour and positive reinforcement rather than punishment in people with cocaine addiction [81]. The efficacy of a purely behavioural intervention—such as CM alone—shows that financial rewards can compete with biological rewards mediated by cocaine and amphetamine cues and incentives [82]. This seems to be true only if rewards are contingent upon the provision of drug-free urine samples, as non-contingent rewards were not shown to be effective (Fig 4). Indeed, CM strategies help individuals to overcome apathy or resistance to the recovery process. In this study we found that, although CM was efficacious at the end of treatment, the effect of CM alone was not sustained at longest follow-up after treatment completion (Fig 4). Cocaine and amphetamine addiction is conceptualised as a chronic and recurrent brain disease, which entails behavioural and psychological abnormalities (primarily reward-processing deficits) following the learned or conditioned pairing of situational and social cues with the reinforcing effects of drug use [83,84]. It seems unlikely that a behavioural strategy alone could address in the long term the whole complexity of biological, psychological, and behavioural factors that underlie addiction. The addition of community reinforcement approach to CM potentiates an otherwise purely behavioural intervention with psychological and social components that may enhance its effect. Notably, community reinforcement approach alone performs no differently from TAU in the short term, but its effect is more sustained at longest follow-up. The combined intervention, CM plus community reinforcement approach, overall achieves the best outcomes.
Cocaine and amphetamine addiction is highly prevalent in the world and is incredibly costly economically. In 2015, illicit drugs cost tens of millions of disability-adjusted life years, with Europeans proportionately experiencing more, but with the greatest mortality rate in low- and middle-income countries [85]. We did not do a formal cost-effectiveness analysis. Indeed, recent cost-effectiveness analyses on psychosocial interventions for substance use are encouraging [8,86], but without a full economic model our recommendation cannot be made unequivocally.
This study has some limitations. Some comparisons were appraised as having low or very low quality, potentially restricting the validity of those results. All RCTs of psychosocial interventions for cocaine and/or amphetamine addiction are not blinded, which increases the risk of performance bias for self-reported outcomes. For this reason, we only reported data based on objective outcomes (abstinence on urinalysis and data on attrition), which are less likely to be influenced by the lack of blinding. The risk of selective study reporting was minimised as we contacted study authors to retrieve unpublished data, but we cannot exclude that some unpublished studies remain missing or that published reports overestimated the efficacy of treatments. Finally, some interventions are designed to last more than 12 weeks, namely, CM in combination with community reinforcement approach (24 weeks) [40], community reinforcement approach alone (time unlimited, but in the studies included the intervention lasted always 24 weeks), and supportive-expressive psychodynamic therapy (which could be either time-limited or unlimited and lasted 36 weeks in the only study included) [87]. For these interventions, the evaluation at 12 weeks was extracted before the end of treatment, which was a disadvantage over other interventions requiring shorter duration.
Conclusions
The results of this network meta-analysis support the use of combined CM plus community reinforcement approach as the most effective and acceptable intervention for both short- and long-term treatment of individuals with cocaine and/or amphetamine addiction. The provision of evidence-based psychosocial treatments for stimulant use disorders is all the more important because of the lack of validated pharmacological or brain-stimulation-based treatment for cocaine and/or amphetamine addiction. These findings may influence clinical guidelines; however, further studies are warranted to confirm these results and evaluate cost-effectiveness.
Supporting information
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Nancy M. Petry from UConn Health Center in Farmington and Raphael J. Landovitz from UCLA Center for Clinical AIDS Research & Education in Los Angeles for providing unpublished data for this network meta-analysis. We thank James R. McKay from Treatment Research Center at University of Pennsylvania in Philadelphia and Emilio Sánchez-Hervás from Agencia Valenciana de Salud in Valencia for providing advice on their studies. We also thank Laura Amato, Silvia Minozzi, Zuzana Mitrova, and Rosella Saulle from the Cochrane Drugs and Alcohol Group for their help on the search strategy.
The views expressed are those of the authors and not necessarily those of the UK National Health Service, the National Institute for Health Research, or the UK Department of Health.
Abbreviations
- 12-step
12-step programme
- CBT
cognitive behavioural therapy
- CM
contingency management
- CRA
community reinforcement approach
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- MBT
meditation-based treatments
- NCR
non-contingent rewards
- NNT
number needed to treat
- OR
odds ratio
- RCT
randomised controlled trial
- SEPT
supportive-expressive psychodynamic therapy
- SMD
standardised mean difference
- SUCRA
surface under the cumulative ranking curve
- TAU
treatment as usual
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by National Institute for Health Research (NIHR) Oxford Health Biomedical Research Centre (BRC-1215-20005, https://oxfordhealthbrc.nihr.ac.uk/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–1344. 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Office on Drugs and Crime. World drug report 2017. Vienna: United Nations Office on Drugs and Crime; 2017.
- 3.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. 10.1016/S0140-6736(11)61138-0 [DOI] [PubMed] [Google Scholar]
- 4.Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189 10.3389/fpsyt.2015.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson A, Anderson Z, Hughes K, Bellis MA, Sumnall H, Syed Q. Interpersonal violence and illicit drugs. Liverpool: Liverpool John Moores University Centre for Public Health; 2009 [cited 2018 May 24]. http://www.who.int/violenceprevention/interpersonal_violence_and_illicit_drug_use.pdf.
- 6.Kleber HD, Weiss RD, Anton RF Jr, George TP, Greenfield SF, Kosten TR, et al. Treatment of patients with substance use disorders, second edition. American Psychiatric Association. Am J Psychiatry. 2007;164(4 Suppl):5–123. [PubMed] [Google Scholar]
- 7.World Health Organization. mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings. Version 2.0 Geneva: World Health Organization; 2016. [cited 2018 May 25]. http://apps.who.int/iris/bitstream/10665/250239/1/9789241549790-eng.pdf?ua=1. [Google Scholar]
- 8.National Institute for Health and Care Excellence. Drug misuse in over 16s: psychosocial interventions (CG51). London: National Institute for Health and Care Excellence; 2016.
- 9.European Monitoring Centre for Drugs and Drug Addiction. Standards and guidelines. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2018 [cited 2018 May 18]. http://www.emcdda.europa.eu/best-practice/guidelines.
- 10.Center for Substance Abuse Treatment. Treatment for stimulant use disorders. Rockville (MD): Substance Abuse and Mental Health Services Administration; 1999. [PubMed] [Google Scholar]
- 11.Minozzi S, Saulle R, De Crescenzo F, Amato L. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst Rev. 2016;9:CD011866. 10.1002/14651858.CD011866.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farronato NS, Dursteler-Macfarland KM, Wiesbeck GA, Petitjean SA. A systematic review comparing cognitive-behavioral therapy and contingency management for cocaine dependence. J Addict Dis. 2013;32(3):274–87. 10.1080/10550887.2013.824328 [DOI] [PubMed] [Google Scholar]
- 13.Fischer B, Blanken P, Da Silveira D, Gallassi A, Goldner EM, Rehm J, et al. Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: a comprehensive narrative overview of English-language studies. Int J Drug Policy. 2015;26(4):352–63. 10.1016/j.drugpo.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 14.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 London: Cochrane Collaboration; 2011. [cited 2018 Nov 20]. https://handbook-5-1.cochrane.org/. [Google Scholar]
- 16.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9(7):e99682 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.University of Bern Institute of Social and Preventive Medicine. CINeMA: Confidence in Network Meta-Analysis. Bern: University of Bern Institute of Social and Preventive Medicine; 2017 [cited 2018 Nov 20]. http://cinema.ispm.ch.
- 18.Kiluk BD, Carroll KM, Duhig A, Falk DE, Kampman K, Lai S, et al. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug Alcohol Depend. 2016;158:1–7. 10.1016/j.drugalcdep.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stata statistical software. Release 13.1. College Station (TX): StataCorp; 2014.
- 20.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8(10):e76654 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130–7. 10.7326/0003-4819-159-2-201307160-00008 [DOI] [PubMed] [Google Scholar]
- 22.Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health. 2014;17(4):111–6. 10.1136/eb-2014-101967 [DOI] [PubMed] [Google Scholar]
- 23.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 24.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 25.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51(12):989–97. 10.1001/archpsyc.1994.03950120061010 [DOI] [PubMed] [Google Scholar]
- 26.Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–27. 10.1046/j.1360-0443.1998.9357137.x [DOI] [PubMed] [Google Scholar]
- 27.Carroll KM, Nich C, Shi JM, Eagan D, Ball SA. Efficacy of disulfiram and twelve step facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug Alcohol Depend. 2012;126(1–2):224–31. 10.1016/j.drugalcdep.2012.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy GA, Marino DR, et al. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171(4):436–44. 10.1176/appi.ajp.2013.13070987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA. A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug Alcohol Depend. 2016;160:135–42. 10.1016/j.drugalcdep.2015.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen KW, Berger CC, Gandhi D, Weintraub E, Lejuez CW. Adding integrative meditation with ear acupressure to outpatient treatment of cocaine addiction: a randomized controlled pilot study. J Altern Complement Med. 2013;19(3):204–10. 10.1089/acm.2011.0311 [DOI] [PubMed] [Google Scholar]
- 31.Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry. 1999;56(6):493–502. [DOI] [PubMed] [Google Scholar]
- 32.Donovan DM, Daley DC, Brigham GS, Hodgkins CC, Perl HI, Garrett SB, et al. Stimulant abuser groups to engage in 12-step: a multisite trial in the National Institute on Drug Abuse Clinical Trials Network. J Subst Abuse Treat. 2013;44(1):103–14. 10.1016/j.jsat.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dursteler-MacFarland KM, Farronato NS, Strasser J, Boss J, Kuntze MF, Petitjean SA, et al. A randomized, controlled, pilot trial of methylphenidate and cognitive-behavioral group therapy for cocaine dependence in heroin prescription. J Clin Psychopharmacol. 2013;33(1):104–8. [DOI] [PubMed] [Google Scholar]
- 34.Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychol Addict Behav. 2003;17(1):73–82. 10.1037/0893-164X.17.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Festinger DS, Dugosh KL, Kirby KC, Seymour BL. Contingency management for cocaine treatment: cash vs. vouchers. J Subst Abuse Treat. 2014;47(2):168–74. 10.1016/j.jsat.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Fernandez G, Secades-Villa R, Garcia-Rodriguez O, Sanchez-Hervas E, Fernandez-Hermida JR, Higgins ST. Adding voucher-based incentives to community reinforcement approach improves outcomes during treatment for cocaine dependence. Am J Addict. 2011;20(5):456–61. 10.1111/j.1521-0391.2011.00154.x [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Rodriguez O, Secades-Villa R, Alvarez Rodriguez H, Río Rodríguez A, Fernández-Hermida JR, Carballo JL, et al. [Effect of incentives on retention in an outpatient treatment for cocaine addicts.] Psicothema. 2007;19(1):134–9. [PubMed] [Google Scholar]
- 38.Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin JL, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. J Consult Clin Psychol. 2007;75(5):765–74. 10.1037/0022-006X.75.5.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagedorn HJ, Noorbaloochi S, Simon AB, Bangerter A, Stitzer ML, Stetler CB, et al. Rewarding early abstinence in Veterans Health Administration addiction clinics. J Subst Abuse Treat. 2013;45(1):109–17. 10.1016/j.jsat.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 40.Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150(5):763–9. 10.1176/ajp.150.5.763 [DOI] [PubMed] [Google Scholar]
- 41.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51(7):568–76. 10.1001/archpsyc.1994.03950070060011 [DOI] [PubMed] [Google Scholar]
- 42.Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68(1):64–72. 10.1037/0022-006X.68.1.64 [DOI] [PubMed] [Google Scholar]
- 43.Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, et al. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60(10):1043–52. 10.1001/archpsyc.60.9.1043 [DOI] [PubMed] [Google Scholar]
- 44.Kirby KC, Marlowe DB, Festinger DS, Lamb RJ, Platt JJ. Schedule of voucher delivery influences initiation of cocaine abstinence. J Consult Clin Psychol. 1998;66(5):761–7. 10.1037/0022-006X.66.5.761 [DOI] [PubMed] [Google Scholar]
- 45.Landovitz RJ, Fletcher JB, Shoptaw S, Reback CJ. Contingency management facilitates the use of postexposure prophylaxis among stimulant-using men who have sex with men. Open Forum Infect Dis. 2015;2(1):ofu114. 10.1093/ofid/ofu114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend. 2006;83(1):65–72. 10.1016/j.drugalcdep.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 47.Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: main and matching effects. J Consult Clin Psychol. 1998;66(5):832–7. 10.1037/0022-006X.66.5.832 [DOI] [PubMed] [Google Scholar]
- 48.McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am J Psychiatry. 2013;170(1):94–101. 10.1176/appi.ajp.2012.11121831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKay JR, Alterman AI, Cacciola JS, Rutherford MJ, O’Brien CP, Koppenhaver J. Group counseling versus individualized relapse prevention aftercare following intensive outpatient treatment for cocaine dependence: initial results. J Consult Clin Psychol. 1997;65(5):778–88. 10.1037/0022-006X.65.5.778 [DOI] [PubMed] [Google Scholar]
- 50.Menza TW, Jameson DR, Hughes JP, Colfax GN, Shoptaw S, Golden MR. Contingency management to reduce methamphetamine use and sexual risk among men who have sex with men: a randomized controlled trial. BMC Public Health. 2010;10:774 10.1186/1471-2458-10-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miguel AQ, Madruga CS, Cogo-Moreira H, Yamauchi R, Simões V, da Silva CJ, et al. Contingency management is effective in promoting abstinence and retention in treatment among crack cocaine users in Brazil: a randomized controlled trial. Psychol Addict Behav. 2016;30(5):536–43. 10.1037/adb0000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milby JB, Schumacher JE, Vuchinich RE, Freedman MJ, Kertesz S, Wallace D. Toward cost-effective initial care for substance-abusing homeless. J Subst Abuse Treat. 2008;34(2):180–91. 10.1016/j.jsat.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63(2):201–8. 10.1001/archpsyc.63.2.201 [DOI] [PubMed] [Google Scholar]
- 54.Petitjean SA, Dursteler-MacFarland KM, Krokar MC, Strasser J, Mueller SE, Degen B, et al. A randomized, controlled trial of combined cognitive-behavioral therapy plus prize-based contingency management for cocaine dependence. Drug Alcohol Depend. 2014;145:94–100. 10.1016/j.drugalcdep.2014.09.785 [DOI] [PubMed] [Google Scholar]
- 55.Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70(2):398–405. 10.1037/0022-006X.70.2.398 [DOI] [PubMed] [Google Scholar]
- 56.Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62(10):1148–56. 10.1001/archpsyc.62.10.1148 [DOI] [PubMed] [Google Scholar]
- 57.Petry NM, Martin B, Simcic F Jr. Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. J Consult Clin Psychol. 2005;73(2):354–9. 10.1037/0022-006X.73.2.354 [DOI] [PubMed] [Google Scholar]
- 58.Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol. 2007;75(6):983–91. 10.1037/0022-006X.75.6.983 [DOI] [PubMed] [Google Scholar]
- 59.Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol. 2012;80(2):286–98. 10.1037/a0026826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80(2):276–85. 10.1037/a0026883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petry NM, Alessi SM, Rash CJ. A randomized study of contingency management in cocaine-dependent patients with severe and persistent mental health disorders. Drug Alcohol Depend. 2013;130(1–3):234–7. 10.1016/j.drugalcdep.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219–28. 10.1001/archpsyc.63.2.219 [DOI] [PubMed] [Google Scholar]
- 63.Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59(9):817–24. 10.1001/archpsyc.59.9.817 [DOI] [PubMed] [Google Scholar]
- 64.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiberet C, al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–74. 10.1111/j.1360-0443.2006.01312.x [DOI] [PubMed] [Google Scholar]
- 65.Roll JM, Chudzynski J, Cameron JM, Howell DN, McPherson S. Duration effects in contingency management treatment of methamphetamine disorders. Addict Behav. 2013;38(9):2455–62. 10.1016/j.addbeh.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sánchez-Hervás E, Secades-Villa R, Romaguera FZ, García Fernández G, Santonja Gómez FJ, García-Rodríguez O. [Behavioral therapy for cocaine addicts: outcomes of a follow-up six month study.] Rev Mex Psicol. 2010;27(2):159–67. [Google Scholar]
- 67.Schottenfeld RS, Moore B, Pantalon MV. Contingency management with community reinforcement approach or twelve-step facilitation drug counseling for cocaine dependent pregnant women or women with young children. Drug Alcohol Depend. 2011;118(1):48–55. 10.1016/j.drugalcdep.2011.02.019 [DOI] [PubMed] [Google Scholar]
- 68.Secades-Villa R, Garcia-Fernandez G, Pena-Suarez E, Garcia-Rodriguez O, Sanchez-Hervas E, Fernandez-Hermida JR. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat. 2013;44(3):349–54. 10.1016/j.jsat.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 69.Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fullera E, Larkinset S, al. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78(2):125–34. 10.1016/j.drugalcdep.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 70.Shoptaw S, Reback CJ, Larkins S, Wang PC, Rotheram-Fuller E, Danget J, et al. Outcomes using two tailored behavioral treatments for substance abuse in urban gay and bisexual men. J Subst Abuse Treat. 2008;35(3):285–93. 10.1016/j.jsat.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 71.Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53(5):409–15. 10.1001/archpsyc.1996.01830050045007 [DOI] [PubMed] [Google Scholar]
- 72.Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66(5):811–24. 10.1037/0022-006X.66.5.811 [DOI] [PubMed] [Google Scholar]
- 73.Smout MF, Longo M, Harrison S, Minniti R, Wickes W, White JM. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98–107. 10.1080/08897071003641578 [DOI] [PubMed] [Google Scholar]
- 74.Umbricht A, DeFulio A, Winstanley EL, Tompkins A, Peirce J, Mintzeret MZ, et al. Topiramate for cocaine dependence during methadone maintenance treatment: a randomized controlled trial. Drug Alcohol Depend. 2014;140:92–100. 10.1016/j.drugalcdep.2014.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ainscough TS, McNeill A, Strang J, Calder R, Brose LS. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318–39. 10.1016/j.drugalcdep.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carroll KM, Weiss RD. The role of behavioral interventions in buprenorphine maintenance treatment: a review. Am J Psychiatry. 2017;174(8):738–47. 10.1176/appi.ajp.2016.16070792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, Grabinski MJ. Nationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction. 2017;112(5):875–83. 10.1111/add.13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Busch SH, Epstein AJ, Harhay MO, Fiellin DA, Un H, Leader D Jr, et al. The effects of federal parity on substance use disorder treatment. Am J Manag Care. 2014;20(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- 79.Hui K, Angelotta C, Fisher CE. Criminalizing substance use in pregnancy: misplaced priorities. Addiction. 2017;112(7):1123–5. 10.1111/add.13776 [DOI] [PubMed] [Google Scholar]
- 80.Clark N, Dolan K, Farabee D. Public health alternatives to incarceration for drug offenders. East Mediterr Health J. 2017;23(3):222–30. [DOI] [PubMed] [Google Scholar]
- 81.Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LH, Luijten M, et al. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352(6292):1468–71. 10.1126/science.aaf3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Ann N Y Acad Sci. 2014;1327:94–111. 10.1111/nyas.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luijten M, Schellekens AF, Kuhn S, Machielse MW, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74(4):387–98. 10.1001/jamapsychiatry.2016.3084 [DOI] [PubMed] [Google Scholar]
- 84.Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363–71. 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113(10):1905–26. 10.1111/add.14234 [DOI] [PubMed] [Google Scholar]
- 86.Washington State Institute for Public Policy. Benefit-cost results. Olympia: Washington State Institute for Public Policy; 2017 [cited 2017 Aug 7]. http://www.wsipp.wa.gov/BenefitCost.
- 87.Mark D, Luborsky L. Manual for the use of supportive-expressive psychotherapy in the treatment of cocaine abuse. Philadelphia (PA): University of Pennsylvania Department of Psychiatry; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.