Abstract
Background
Despite the widespread use of medication reviews, many older adults are still exposed to the risks of polypharmacy.
Objectives
To quantify and describe the drug therapy problems identified and interventions undertaken by pharmacists before and after implementation (on July 1, 2015) of collaborative medication review for high-risk older adult patients (> 80 years of age).
Methods
A retrospective single-centre pre–post cohort study was conducted between July 1, 2014, and July 31, 2016, to characterize the impact of collaborative medication reviews—consisting of a thorough medication review by a pharmacist and care conferences with the hospitalist and family physician—on prescribing patterns in an Acute Care for Elders unit. A standardized template was used to conduct medication reviews for the post-implementation group, whereas a chart review was conducted for the pre-implementation group. The primary outcomes were the number of drug therapy problems identified by the clinical pharmacists and the associated interventions by the pharmacists, which were categorized as clinical or compliance interventions. Secondary outcomes included the number of medications at discharge, the rate of hospital readmission within 30 days, and the length of hospital stay.
Results
A total of 137 patients were identified for inclusion in either the pre-implementation group (n = 70) or the post-implementation group (n = 67). After implementation of collaborative medication reviews, there were statistically significant increases in the mean number of drug therapy problems identified (p < 0.001), the mean number of interventions undertaken (p = 0.004), and the median length of hospital stay (p < 0.001). There was no difference between the 2 groups in the number of medications at discharge, the proportion of patients taking more than 5 medications at discharge, or readmission within 30 days.
Conclusion
At the study institution, implementation of a quality improvement program that included pharmacist-led medication reviews and collaborative care conferences involving community and hospital care providers helped to improve documentation by clinical pharmacists of potential medication-related problems and led to more interventions to optimize patients’ medication regimens.
Keywords: geriatrics, senior or older adult, medication review, clinical pharmacists, intervention, polypharmacy
RÉSUMÉ
Contexte
Malgré l’utilisation répandue des revues des médicaments, bon nombre de personnes âgées sont encore exposées à des risques causés par la polypharmacie.
Objectif
Quantifier et décrire les problèmes pharmacothérapeutiques repérés et les interventions effectuées par les pharmaciens avant et après la mise en place (le 1er juillet 2015) d’une revue collaborative des médicaments chez les patients âgés (de plus de 80 ans) à haut risque.
Méthodes
Une étude de cohorte rétrospective avant-après menée dans un seul centre entre le 1er juillet 2014 et le 31 juillet 2016 dans le but d’offrir un portrait de l’influence des revues collaboratives des médicaments (qui se résument en une évaluation complète des médicaments par un pharmacien et des discussions sur les soins avec le médecin hospitalier et le médecin de famille) sur les habitudes de prescription dans une unité de soins de courte durée pour aînés. Un modèle standardisé a servi pour effectuer les revues des médicaments auprès du groupe d’après mise en place alors qu’une analyse des dossiers médicaux a été menée auprès du groupe d’avant mise en place. Les principaux critères d’évaluation étaient le nombre de problèmes pharmacothérapeutiques décelés par les pharmaciens cliniciens et les interventions connexes effectuées par les pharmaciens, qui ont été classées de type soit clinique soit conformité. Les critères d’évaluation secondaires comprenaient le nombre de médicaments au congé, les taux de réadmission dans les 30 jours suivant le congé et la durée du séjour à l’hôpital.
Résultats
Au total, 137 patients répondaient aux critères d’admissibilité pour le groupe d’avant mise en place (n = 70) ou pour le groupe d’après mise en place (n = 67). Après la mise en place des revues collaboratives des médicaments, on a observé une augmentation statistiquement significative dans le nombre moyen de problèmes pharmacothérapeutiques décelés (p < 0,001), le nombre moyen d’interventions effectuées (p = 0,004) et la durée médiane du séjour à l’hôpital (p < 0,001). Aucune différence n’a été remarquée entre les deux groupes quant au nombre de médicaments au congé, à la proportion de patients prenant plus de cinq médicaments au congé et au taux de réadmission dans les 30 jours suivant le congé.
Conclusion
À l’établissement où s’est déroulée l’étude, on a mis en place un programme d’amélioration de la qualité comprenant des revues des médicaments dirigées par des pharmaciens et des discussions sur les soins en collaboration avec des fournisseurs de soins communautaires et hospitaliers. Le programme a aidé à améliorer la consignation par les pharmaciens cliniciens de potentiels problèmes liés à la pharmacothérapie et a mené à un plus grand nombre d’interventions visant à optimiser la pharmacothérapie des patients.
Mots clés: gériatrie, aîné ou personne âgée, revue des médicaments, pharmaciens cliniciens, intervention, polypharmacie
INTRODUCTION
In 2012, the American Board of Internal Medicine launched the Choosing Wisely Campaign to promote discussion between health care providers and patients to ensure that medical tests, treatments, and procedures were supported by evidence, were not duplicative, were free from harm, and were necessary.1 To date, more than 70 medical specialty associations in the United States have joined the campaign to identify relevant tests and treatments in their areas of specialty that are overused and have limited clinical benefit.1 The American Geriatrics Society, one partner in the Choosing Wisely campaign, has put forward the recommendation that providers should not “prescribe a medication without conducting a drug regimen review”.1
Older adults tend to use more prescription and nonprescription medications than other age groups.1 Polypharmacy, defined as the use of multiple medications, has been associated with increased inappropriate use of medications.2 Older adults are also more prone to the adverse effects of medications, because of the pharmacokinetic and pharmacodynamic changes that occur with aging.3 Medication reviews may help to identify unnecessary, ineffective, and unsafe medications, while uncovering the need for additional medications from which the patient might benefit. They can also help to identify the need for strategies to improve adherence, such as blister packaging or weekly dispensing. Medication reviews may be done differently at different institutions; however, they are typically carried out by pharmacists, who systematically review the medications that patients are taking to ensure that they are necessary, effective, and safe and that they are being taken correctly. Within the older adult population, a pharmacist’s assessment of a patient’s drug therapy during a medication review can help to identify medications on the Beers list, a list of potentially inappropriate medications to be avoided or used with caution in older adults in general or in those with certain comorbidities.4 In addition to a medication review, a collaborative care conference involving the patient’s family physician is thought to help improve continuity of care. In a cohort study of 105 patients who had at least one change in their drug regimen during a hospital stay, a clinical pharmacist followed up with each patient’s general practitioner 4 to 5 months after discharge.5 The study showed that 46.3% of the patients stopped a drug that had been started during their hospitalization, and 24.1% restarted a drug that had been discontinued during the hospital stay.5 The reasons for these postdischarge changes were not documented, but the authors hypothesized that they were related to poor communication between hospital and community care providers.5 Through the care conference, a patient’s family physician can provide valuable input and can be informed of any medication changes, so that there will be appropriate postdischarge follow-up.
Despite the widespread use of medication reviews, the impact on clinical outcomes, such as hospital admissions and mortality, is unknown.6 Medication reviews can be time-consuming, leaving many pharmacists unsure whether it is worthwhile to conduct them, given the apparent lack of benefit in terms of meaningful patient outcomes.6 There is currently limited evidence concerning medication reviews and their effects on clinically important outcomes, with most studies being of short duration and underpowered for clinical outcomes. A systematic review of pharmacist-led medication reviews showed no significant effect on clinical outcomes, such as all-cause hospital admission or mortality, and only a slight reduction in the number of drugs prescribed.6 However, the review authors included studies that took place in settings with limited multidisciplinary collaboration and younger patients, so the results may not be applicable to settings involving older adults where pharmacists work collaboratively with other members of the health care team. A Cochrane review evaluating the use of medication reviews for hospital inpatients showed that the type of medication review and the degree of pharmacist involvement did not affect outcomes such as all-cause mortality, all-cause hospital readmission, all-cause emergency department contact, and adverse drug events.7 Given the limited evidence, the authors were unable to determine whether medication reviews were cost-effective, and given the short duration of follow-up, they could not identify any long-term effects on outcomes.7 Other strategies that have been described to help improve prescribing for older adults include a collaborative team approach, as investigated in the study by Spinewine and others.8 In that study, a clinical pharmacist conducted a medication review upon each patient’s admission to the hospital’s geriatric unit, collaborated with the multidisciplinary team to optimize pharmacotherapy, gave oral and written information on treatment changes for the patient, and provided written documentation for the patient’s general practitioner at discharge. Significant reductions in overuse, misuse, and underuse of medications were observed, which could be attributed to the structured and collaborative approach.8
At the study institution, the Acute Care for Elders (ACE) units undertook an initiative in response to the Choosing Wisely recommendation concerning medication reviews. The objective of the current study was to quantify and describe the drug therapy problems identified and interventions undertaken by pharmacists before and after implementation of collaborative medication review for high-risk older adult patients. Various quality improvement outcomes were identified and compared between the pre- and post-implementation cohorts.
METHODS
This single-centre chart review involved patients admitted to either of 2 ACE units at a large urban tertiary care hospital between July 1, 2014, and July 31, 2016. The overall study population was subdivided according to the date when a new quality improvement program—collaborative medication review—was implemented (July 1, 2015): a pre-implementation group, for patients admitted between July 1, 2014, and June 30, 2015; and a post-implementation group, for patients admitted between July 1, 2015, and July 31, 2016.
The quality improvement program was based on a comprehensive medication review for each patient, within 48 h of admission, by the clinical pharmacist assigned to that patient’s unit. The pharmacist reviewed the patient’s preadmission medications, interviewed the patient or a caregiver, and determined whether any therapeutic issues existed with the patient’s preadmission drug regimen. The pharmacist then contacted the patient’s family physician by fax to notify the physician of the comprehensive medication review and to invite participation in a collaborative care conference with the clinical pharmacist and the hospitalist to discuss the patient’s admission and drug therapy issues. If the family physician was unable to participate, documentation of the comprehensive medication review was shared with the physician by fax.
Patients eligible for collaborative medication review were older than 80 years of age and classified as being at high risk for readmission to hospital. At the study institution, high risk for readmission is assessed with the Readmission Risk Assessment Score (RRAS), a tool based on the LACE index (for length of stay, acuity of admission, Charlson comorbidity index, and number of emergency department visits in past 6 months).9 A patient with a score of 10 or higher with the RRAS tool is considered to be at high risk for readmission to hospital. The age threshold was chosen on the basis of previous work by Hohl and others,10 who showed that age over 80 years was associated with adverse drug events. In addition, before implementation of collaborative medication reviews, the previous monthly admission data for the units were reviewed to estimate the overall number of admissions that would meet the criteria for high risk of readmission. The age cutoff allowed for a reasonable number of patients to be systematically identified for collaborative medication review, without overburdening the clinical pharmacists. Patients were excluded if the medication review could not be completed within the first 48 h of admission. Possible reasons for a review not being completed included communication barrier with the patient and/or caregiver, transfer to another unit, anticipated discharge within 48 h, patient’s request for a change to palliation, or patient’s death. The same inclusion and exclusion criteria as described above were used to identify patients for the pre-implementation comparison group.
The primary outcomes were the number of drug therapy problems identified by the clinical pharmacists and the associated interventions by pharmacists, which were categorized as clinical or compliance interventions. The drug therapy problems were categorized according to the standardized definitions by Cipolle and others11 (Table 1). Compliance interventions included preparation of a medication calendar detailing the prescribed medications, administration times, and instructions for the patient; initiation of a compliance aid such as blister packs or dosettes; counselling for the patient or caregiver; and medication management, which could include liaising with a community nurse or community pharmacist. The secondary outcomes were the number of medications at discharge, the proportion of patients taking more than 5 medications, and the number of medications on the Beers list of potentially inappropriate medications for older adults that patients were taking at the time of admission and discharge. Readmission to the same hospital within 30 days of discharge and length of hospital stay were also compared before and after implementation of collaborative medication review.
Table 1.
Standardized Definitions of Drug Therapy Problems*
| Drug Therapy Problem | Definition |
|---|---|
| Drug without indication | Drug is being taken without clear indication |
| Indication without a drug | Patient has an indication for a drug but is not receiving it |
| Suboptimal dosing | Drug dose determined to be too high or too low |
| Wrong drug | Patient is receiving a drug, but it is not the most optimal drug for indication (e.g., calcium-channel blocker for hypertension instead of ACE inhibitor in a patient with diabetes mellitus) |
| Additional drug | Patient needs an additional drug for an indication |
| Adverse drug reaction | Patient is experiencing, or is at risk of experiencing, an adverse drug effect |
| Drug interaction | Medications are being taken together that have a clinically relevant interaction |
| Adherence issue | Patient is not adhering to medication (e.g., refusing, cannot swallow, forgetting to take) |
| Monitoring required | Drug therapy monitoring is required (e.g., renal function) |
| Duplicate therapy | Patient is receiving duplicate therapy for an indication |
ACE = angiotensin-converting enzyme.
Based on Cipolle and others.11
Baseline demographic characteristics collected for both groups included the following: age, sex, length of hospital admission, RRAS, living arrangements at home, medication compliance aids, number of comorbidities, and number of medications at the time of hospital admission. Comorbidity was defined as any chronic disease or condition identified by the physician in the course of obtaining the patient’s medical history. For the post-implementation group, clinical pharmacists documented their assessments and interventions using a standardized medication review form (Appendix 1, available from https://www.cjhp-online.ca/index.php/cjhp/issue/view/187/showToc). On the form, the pharmacist can include a brief summary of the patient’s reason for admission and any relevant medical history. The medication review and recommendation section of the template includes 4 columns for the following information: name of each drug, dose and frequency of the drug, indication for the drug, and the pharmacist’s recommendation for the drug. For the post-implementation group, patients’ health care records were reviewed for additional information not collected on the medication review form, such as discharge medications. For the pre-implementation group, no standardized documentation process was in place, so patients’ medical charts were reviewed for pharmacists’ entries about drug therapy problems and their recommendations. This information was then cross-referenced against the orders section of the medical chart to determine whether the recommendations had been carried out. Any verbal physician’s orders that were recorded by the pharmacist were counted as a drug therapy problem and clinical intervention even if there was no accompanying chart note, on the assumption that there would have been a discussion with the physician before the verbal order was made. Interventions involving IV medications (e.g., vancomycin dose adjustment according to trough levels) were not counted, because the focus of this project was on home medications and the potential risks of polypharmacy. Any IV medications that a patient received in hospital would have been for an acute illness and would have been stopped or interchanged to an oral equivalent before discharge home. In addition, interventions such as adjustment of the dose or formulation of a medication to match manufacturer’s availability (e.g., ciprofloxacin 400-mg tablet changed to ciprofloxacin 500-mg tablet) and therapeutic substitutions for equivalent hospital formulary medications (e.g., rabeprazole interchanged with pantoprazole) were not considered, because the pharmacist would have made such changes on the basis of product availability and hospital formulary to ensure continuity of care. Step-down from IV to oral antibiotic was considered an intervention because this change could facilitate a patient’s discharge home.
For this retrospective study, only nonidentifiable information was obtained during the chart review, and patient consent was not required. Ethics approval for the study protocol was granted by the University of British Columbia Clinical Research Ethics Board, and operational approval was granted by Vancouver Coastal Health. All data were collected from the patients’ medical charts by a single investigator (W.W.T.C.).
The data were entered into an Excel spreadsheet (Microsoft, Redmond, Washington) and analyzed using SPSS software, version 23.0 (IBM Corporation, Armonk, New York). Standard descriptive statistics were used to represent the baseline characteristics of both groups. Tests for normality were performed, and the mean or median was reported according to the distribution of the data. The independent-samples t test was used to compare the 2 groups in terms of mean numbers of drug therapy problems identified, interventions by pharmacists, medications at discharge, and medications from the Beers list at discharge. The χ2 test was used to compare the proportions of patients receiving more than 5 medications at discharge and the rate of readmission to hospital within 30 days between the 2 groups. The Mann-Whitney U test was used to compare the median length of stay between the 2 groups. Two-tailed p values less than 0.05 were considered statistically significant for all comparisons.
RESULTS
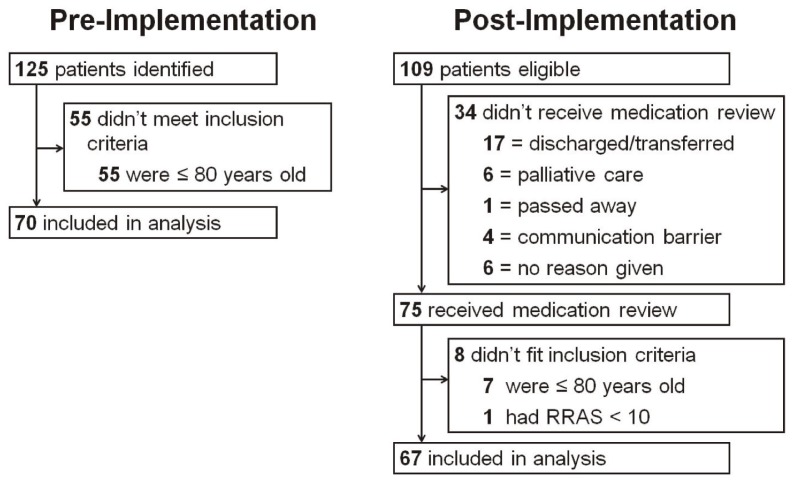
A convenience sample of 137 patients was included in the study. During the pre-implementation period, 125 patients were admitted to the ACE units and were identified as being at high risk for readmission; 55 of these were excluded because their age was 80 years or younger, leaving a total of 70 patients in the pre-implementation group. During the post-implementation period, 109 patients were eligible, of whom 75 received a medication review; after exclusions based on age and RRAS score, 67 patients were included in the post-implementation group (Figure 1).
Figure 1.
Flowchart for selection of study patients. RRAS = Readmission Risk Assessment Score.
The mean age was 88.1 years (standard deviation [SD] 4.3 years) in the pre-implementation group and 88.4 years (SD 5.1 years) in the post-implementation group. Tests for significance were done for all baseline characteristics; blister packaging was the only characteristic with a statistically significant difference. The numbers of comorbidities, medications, and Beers list medications at the time of admission were similar (Table 2). In both groups, most patients had been living at home with their families. Patients’ use of compliance aids and independence with medication administration (i.e., understands and can self-administer medications) were not well documented for the pre-implementation group and therefore could not be captured. About 49% of patients in the post-implementation group relied on blister packs, and 40% were independent with their medication administration.
Table 2.
Baseline Characteristics
| Characteristic | Study Group; No. (%) of Patients* | p Value | |
|---|---|---|---|
|
| |||
| Pre-implementation (n = 70) | Post-implementation (n = 67) | ||
| Age (years) (mean ± SD) | 88.1 ± 4.3 | 88.4 ± 5.1 | 0.75† |
|
| |||
| Sex, male | 32 (46) | 30 (45) | 0.91§ |
|
| |||
| RRAS (mean ± SD) | 11.7 ± 1.4 | 11.5 ± 1.4 | 0.52† |
|
| |||
| No. of comorbidities (mean ± SD) | 6.5 ± 2.7 | 6.5 ± 3.0 | 0.76† |
|
| |||
| Medications on admission | |||
| No. of medications on admission (mean ± SD) | 8.0 ± 3.5 | 8.3 ± 3.9 | 0.61† |
| No. of Beers list medications on admission (mean ± SD) | 0.9 ± 1.0 | 1.0 ± 1.0 | 0.56† |
| No. (%) of patients taking > 5 medications | 53 (76) | 48 (72) | 0.59§ |
|
| |||
| Living arrangements before admission | |||
| Home with family | 26 (37) | 21 (31) | 0.47§ |
| Home alone | 10 (14) | 17 (25) | 0.10§ |
| Care facility | 17 (24) | 10 (15) | 0.17§ |
| Home care | 15 (21) | 5 (7) | 0.20§ |
| Unknown | 2 (3) | 14 (21) | |
|
| |||
| Medication compliance aid | |||
| Blister pack | 13 (19) | 33 (49) | < 0.001§ |
| Vials | 7 (10) | 14 (21) | 0.08§ |
| Dosette | 4 (6) | 5 (7) | 0.68§ |
| Unknown | 46 (66) | 15 (22) | |
|
| |||
| Independence with medications | |||
| Needs assistance | 27 (39) | 30 (45) | 0.46§ |
| Independent | 19 (27) | 27 (40) | 0.10§ |
| Unknown | 24 (34) | 10 (15) | |
RRAS = Readmission Risk Assessment Score, SD = standard deviation.
Except where indicated otherwise.
By independent-samples t test.
By χ2 test.
In total, 67 drug therapy problems were identified in the pre-implementation group and 139 in the post-implementation group. There was a statistically significant increase in the mean number of drug therapy problems identified per patient after implementation of collaborative medication review (Table 3). The total number of documented pharmacist interventions increased from 58 (47 clinical, 11 compliance) for the pre-implementation group to 102 (64 clinical, 38 compliance) for the post-implementation group; however, considering the 2 categories of intervention, only the number of compliance interventions differed significantly between groups (Table 3). Among these compliance interventions, patient/caregiver counselling was the most common for the pre-implementation group and provision of a medication calendar was the most common for the post-implementation group (Table 4). The most common clinical intervention for both groups was discontinuation of a medication (Table 4).
Table 3.
Drug Therapy Problems and Pharmacist Interventions in Initial 48 h of Admission
| Outcome | Study Group; Mean ± SD | p Value* | |
|---|---|---|---|
|
| |||
| Pre-implementation (n = 70) | Post-implementation (n = 67) | ||
| No. of DTPs identified per patient | 1.0 ± 1.3 | 2.1 ± 1.4 | < 0.001 |
| No. of pharmacist interventions per patient | 0.9 ± 1.3 | 1.5 ± 1.5 | 0.004 |
| Clinical interventions | 0.7 ± 1.0 | 1.0 ± 1.1 | 0.12 |
| Compliance interventions | 0.2 ± 0.5 | 0.6 ± 0.8 | 0.001 |
Independent t test; p values less than 0.05 were deemed significant.
Table 4.
Interventions Performed by Pharmacists, in Relation to Study Group
| Intervention | Study Group; No. (%) of Interventions* | |
|---|---|---|
|
| ||
| Pre-implementation | Post-implementation | |
| Clinical | n = 47 | n = 64 |
| Discontinue a medication | 21 (45) | 24 (38) |
| Add or restart a medication | 10 (21) | 8 (12) |
| Change medication or regimen | 7 (15) | 9 (14) |
| Dose titration | 7 (15) | 17 (27) |
| Monitor therapy | 2 (4) | 6 (9) |
|
| ||
| Compliance | n = 11 | n = 38 |
| Medication calendar | 3 (27) | 18 (47) |
| Initiation of blister packing | 2 (18) | 6 (16) |
| Initiation of PharmaCare special authority | 1 (9) | 1 (3) |
| Patient/caregiver counselling | 4 (36) | 9 (24) |
| Medication management (liasing with community nursing/pharmacy) | 1 (9) | 4 (11) |
The pre-implementation group had a total of 58 interventions; the post-implementation group had a total of 102 interventions.
In the post-implementation group, 20 of the 67 patients received a positive reply from their family physicians to participate in a care conference with the clinical pharmacist and hospitalist. However, the proposed care conference was conducted for only 15 of these patients, because of a last-minute cancellation by the family physician, the patient being discharged sooner than expected, or the patient being transferred to another facility.
There were no significant differences between the 2 groups in terms of number of medications or number of Beers list medications at discharge (Table 5). There was also no significant difference in terms of readmission to hospital within 30 days. Median length of stay was significantly longer for the post-implementation group than the pre-implementation group (14 versus 8 days).
Table 5.
Secondary Outcomes, in Relation to Study Group
| Outcome | Pre-implementation Group |
Post-implementation Group |
p Value |
|---|---|---|---|
| Medications at discharge | n = 60* | n = 57† | |
| No. of medications at discharge (mean ± SD) | 8.7 ± 3.3 | 9.3 ± 3.7 | 0.30‡ |
| No. of Beers list medications at discharge (mean ± SD) | 0.8 ± 0.9 | 0.9 ± 0.9 | 0.31‡ |
| No. (%) of patients taking > 5 medications | 51 (85) | 50 (88) | 0.67§ |
|
| |||
| n = 62** | n = 58†† | ||
| No. (%) of patients with readmission within 30 days | 10 (16) | 5 (9) | 0.33§ |
| Median length of hospital stay (days) | 8 | 14 | < 0.001‡‡ |
SD = standard deviation.
Does not include 8 patients who died before discharge and 2 whose documentation for discharge medication was missing.
Does not include 8 patients who died before discharge, 1 whose documentation for discharge medication was missing, and 1 who was alive and still in hospital at the time of data analysis.
By independent-samples t test.
By χ2 test.
Does not include 8 patients who died before discharge
Does not include 8 patients who died before discharge and 1 who was alive and still in hospital at the time of data analysis.
By Mann-Whitney U test.
DISCUSSION
This study showed that implementation of collaborative medication reviews and care conferences at the study institution led to a greater number of drug therapy problems being identified by the clinical pharmacists and a greater number of resulting interventions. However, no differences were seen in the number of medications (total and Beers list) at the time of discharge or the rate of hospital readmission within 30 days of discharge.
In a previous study, Spinewine and others8 found that structured collaboration between the inpatient pharmacist and the interdisciplinary team reduced inappropriate medication use (misuse, underuse, and overuse of medications). The Choosing Wisely initiative introduced to the ACE units in this study attempted to go further by engaging each patient’s community prescriber in a care conference to both gather collateral information about the patient’s medical problems and ensure seamless care upon discharge from hospital. Kripalani and others12 performed a systematic review to characterize types of communication and the prevalence of lack of communication between hospital and community care providers; they found infrequent communication between the 2 groups of care providers. For example, patients were often seen by their primary care physician before a detailed discharge summary had been transcribed and made available.12 A care conference held during the patient’s admission would ensure that community care providers are updated with the patient’s progress. However, in the current study, it was difficult to engage family physicians to participate in the care conferences, with more than 70% opting to receive a faxed discharge summary. Unfortunately, the physicians did not provide reasons for declining to participate in care conferences, but time constraints are the most likely reason. It is the institution’s goal to achieve a higher participation rate, and as such, we have been revising our communication tools to make it easier for family physicians to respond and indicate their availability.
Similar to what other researchers have found, this study showed that a collaborative medication review may be effective in identifying drug therapy problems. Although there was no difference in clinically important outcomes such as hospital readmission, the study was not powered sufficiently to evaluate this outcome. A comprehensive medication review would detect omission of necessary medications, which might have prompted prescribers to add a medication and may explain the lack of difference in the number of medications at discharge. This supposition is supported by the finding that the most common interventions were discontinuation of a medication and adding or restarting a medication. In a study in a nursing home setting, which involved a pharmacist-led medication review, Furniss and others13 determined the number of interventions made by the pharmacist, finding that the most common pharmacist recommendation was to discontinue a medication. Their intervention resulted in a reduction in the number of medications prescribed, but there was minimal impact on morbidity and mortality.13
In the current study, the number of drug therapy problems identified was higher than the number of interventions by pharmacists in both the pre- and post-implementation groups. This result does not necessarily mean that pharmacists’ recommendations were not accepted. Hanlon and others14 noted significantly lower inappropriate prescribing scores when a clinical pharmacist was involved in seeing patients and working closely with the physician at an ambulatory care clinic. Physicians were also receptive to pharmacists’ recommendations and made more medication changes than when they were working independently. 14 These findings contrast with those of the current study, likely because of the different study setting (ambulatory care versus acute care) and consequently different patient characteristics. Some drug therapy problems are less urgent than others, and an intervention may be made by the community care provider once the patient is medically stable. Some interventions may also be more suitable for the community setting because of the need for longer follow-up. This could also explain why there was no significant reduction in the number of Beers list medications upon discharge: a patient might be reluctant to stop taking a sedative for insomnia while acutely ill with pneumonia, with tapering by the family physician required at a later date.
This study had several limitations. Given its retrospective nature, the quality of data extracted relied on the documentation available in the hospital record and the clinical pharmacists’ chart notes. The documentation was of better quality in the post-implementation phase, because standardized forms were completed during the pharmacists’ medication review of each patient (Appendix 1). For the pre-implementation group, only about half of the patients had a clinical pharmacist note documenting the assessment and pharmaceutical care plan. It was particularly difficult to identify compliance interventions, as these were not routinely documented and (according to anecdotal information) were often made in the course of verbal interactions with the patient (e.g., patient/caregiver counselling). This situation contrasts with the post-implementation group, for whom compliance interventions were documented alongside the clinical interventions. Additionally, only one investigator conducted the data collection, so the collected data could not be assessed for authenticity. The analysis was also not adjusted for confounding factors. For evaluating 30-day hospital readmission, the data were limited to one health authority site, and readmissions to other health jurisdictions might have been missed. The low frequency of care conferences might also have been a limitation, in that medication changes might not have been relayed to community care providers in a timely manner.
The increase in drug therapy problems identified by the pharmacist and in pharmacists’ compliance interventions for the post-implementation group could simply be due to introduction of a standardized medication review form, which allowed for more consistent documentation. Before implementation of this quality improvement program, pharmacists independently determined whether a patient needed a medication review. If such a medication review was conducted and recommendations were made to the physician, it was up to the pharmacist’s discretion whether any of this information was documented in the patient chart. The implementation of collaborative medication reviews did not require additional staffing on the ACE units. There were in-service sessions to inform the staff of this new program, and additional training was provided to pharmacists to assist them in using the standardized medication review form. Templates were also provided for conducting the care conference in a systematic way (including introduction of all participants, brief background on the patient, review of medications, and review of recommendations).
CONCLUSION
The implementation of collaborative medication reviews resulted in more drug therapy problems being identified and more interventions being undertaken by pharmacists. However, there is insufficient evidence to say whether the collaborative medication reviews benefited patients in terms of clinically important outcomes, such as hospital readmission and mortality. The results of this study indicate that the implementation of a structured medication review allowed for more consistent documentation by pharmacists, making it easier to identify their interventions. This documentation could be beneficial because it provides clear information for other health care professionals about the rationale for medication changes. With the eventual implementation of computerized documentation and order entry programs in institutions within our health care authority, the standardization of communication will become a forced function. In the interim, use of standardized documentation tools can help with communication between health professionals and can improve the ability to identify and solve drug-therapy problems.
Supplementary Information
Acknowledgements
The authors would like to thank Salomeh Shajari, Statistical Analyst, and Dr Tim Lau, Pharmacy Lead, of the Antimicrobial Stewardship Programme (ASPIRES) at Vancouver General Hospital, for developing the Choosing Wisely medication review form.
Footnotes
Competing interests: None declared.
Funding: None received.
References
- 1.Choosing Wisely®: promoting conversations between patients and clinicians [website] Philadelphia (PA): ABIM Foundation; 2018. [cited 2016 Jul 16]. Available from: http://www.choosingwisely.org/ [Google Scholar]
- 2.Hanlon JT, Artz MB, Pieper CF, Lindblad CI, Sloane RJ, Ruby CM, et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother. 2004;38(1):9–14. doi: 10.1345/aph.1D313. [DOI] [PubMed] [Google Scholar]
- 3.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 5.Viktil K, Blix HS, Eek AK, Davies MN, Moger TA, Reikvam A. How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461. doi: 10.1136/bmjopen-2012-001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2008;65(3):303–16. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986. doi: 10.1002/14651858.CD008986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(5):658–65. doi: 10.1111/j.1532-5415.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- 9.van Walraven C, Dhalla IA, Bell C, Etchells E, Stiell IG, Zarnke K, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–7. doi: 10.1503/cmaj.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohl CM, Yu E, Hunte GS, Brubacher JR, Hosseini F, Argent CP, et al. Clinical decision rules to improve the detection of adverse drug events in emergency department patients. Acad Emerg Med. 2012;19(6):640–9. doi: 10.1111/j.1553-2712.2012.01379.x. [DOI] [PubMed] [Google Scholar]
- 11.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice. 3rd ed. New York (NY): McGraw-Hill; 2012. [Google Scholar]
- 12.Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297(8):831–41. doi: 10.1001/jama.297.8.831. [DOI] [PubMed] [Google Scholar]
- 13.Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist’s medication review in nursing homes. Radomised controlled trial. Br J Psychiatry. 2000;176(6):563–7. doi: 10.1192/bjp.176.6.563. [DOI] [PubMed] [Google Scholar]
- 14.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.